Abstract
Partially hydrolyzed guar gum (PHGG) is a water-soluble, prebiotic fiber that is used in foods and supplements. The effects of PHGG and its role in gut health are still being studied. The purpose of this study was to evaluate changes in the gut microbiome composition of healthy individuals in response to low-dose PHGG supplementation compared with a low fiber diet. A randomized, double-blind, placebo-controlled crossover study was performed on 33 healthy subjects (17 males, 16 females). Each subject completed three 14-day treatment periods with a 2-week washout between each period. Treatments included supplementation with 3 g PHGG, 6 g PHGG, or a placebo. During all periods, the participants followed a low fiber diet (≤14 g/day). Stools were collected on days 0 and 14 of each period. Gut microbiome profiling was performed using 16S rRNA sequencing. Stools were assessed by investigators with the Bristol Stool Form Scale as a secondary outcome. Saliva cortisol was also measured as a secondary outcome. Supplementation of 3 g and 6 g PHGG significantly increased Verrucomicrobia on day 14 when compared to the placebo (p = 0.0066 and p = 0.0068, respectively). On the genus level, Akkermansia was significantly increased on day 14 with both the 3 g and 6 g PHGG doses (p = 0.0081 and p = 0.0083). Faecalibacterium was significantly decreased on day 14 with 3 g PHGG (p = 0.0054). Supplementing with low doses of PHGG has the potential to cause shifts in the gut microbiome composition. By increasing beneficial microbes, PHGG can improve the microbiome composition of healthy individuals and may play a role in the treatment of inflammatory gastrointestinal diseases.
1. Introduction
As changes in the gut microbiome and their relationship to human health are continuously being studied, many different types of pre- and probiotics have emerged as potential modulating agents [1]. It is well-known that soluble fiber has the ability to fuel changes to the composition of the gut microbiome, and a variety of such fibers have been explored in research to determine the extent of change they may be able to produce and the dose that may be required to effect that change. Partially hydrolyzed guar gum (PHGG) is a water-soluble, prebiotic fiber that has been of interest over the years due to its potential to cause shifts in bacterial taxa that may be beneficial to human health [2].
Guar gum is derived from the seeds of the guar plant (Cyamopsis tetragonolobus), and for many years, it has been safely used as a food additive for thickening and stabilization [1]. Beyond its use in food products for these purposes, guar gum has also been found to carry properties beneficial to human health such as blood lipid-lowering effects and potential glycemic control for individuals with type 2 diabetes mellitus [3,4]. However, because guar gum itself is highly viscous and gel-forming, it tends to be less appealing than other fibers when added to certain foods or when taken as a supplement.
The partial hydrolysis of guar gum creates a much less viscous fiber, PHGG, which is preferable to traditional guar gum for use as a dietary supplement for a number of reasons. Because PHGG is less viscous and non-gelling, it is more palatable than guar gum when added to foods [2,5]. PHGG also ferments to a lesser degree than other types of soluble fiber, making it less likely to cause the undesirable GI symptoms traditionally associated with the use of fiber supplements such as gas and bloating [5].
Several studies have analyzed the effects of PHGG on individuals with GI disorders including irritable bowel syndrome (IBS) [6,7,8]. Because PHGG stimulates the production of Bifidobacteria and short chain fatty acids (SCFAs) such as butyrate, it may be used to improve the gut bacterial environment of those with dysbiosis [9,10,11,12,13].
PHGG has also been studied for its ability to relieve constipation [6,14,15,16,17]. Constipation remains a prevalent issue in both children and adults, and a key factor that many attribute to this high prevalence of constipation is the historically low fiber consumption in the majority of the U.S. population [18]. These studies found that PHGG alleviated constipation in the subjects and conferred additional benefits such as increasing the frequency of bowel movements [16], improving stool consistency [16,17], and relieving abdominal pain [17].
While existing studies largely support the hypothesis that PHGG can positively influence the gut microbiome composition of individuals with GI disorders and help to alleviate the symptoms associated with those disorders, questions remain about the impact that PHGG may have on the gut microbiome of healthy individuals. Some studies have sought to answer this question, however, the current research is limited [9,11,12,19]. This lack of research opens the door to further explore microbial changes in the gut as a result of PHGG intake and any possible associated physiological benefits. Therefore, this study aimed to explore the effects of a 2-week supplementation of low-dose PHGG on the gut microbiome of healthy individuals in comparison with a low-fiber diet.
2. Materials and Methods
2.1. Participants
This study was a randomized, placebo-controlled, double-blind dietary intervention study conducted at the University of Minnesota, registered at clinicaltrials.gov as NCT03722862. The trial was conducted between April and September 2019. Forty-seven subjects were initially screened with 33 healthy adult volunteers (17 males, 16 females) between the ages of 20 and 49 meeting the eligibility requirements and choosing to participate in the study. Subjects were recruited by phone to determine their eligibility. Those who met the eligibility requirements were invited to participate in the study. Inclusion criteria included a BMI of ≥18.5 and ≤30 kg/m2, weight stable for the last 6 months, no pre-existing health conditions that would prevent the subject from fulfilling the study, and a low fiber consumer (≤14 g/day). In addition, the subject had to be willing to stick to their normal habitual diet excluding the consumption of any energy-rich or fat-rich meals, or prolonged fasting throughout the duration of the study. They were expected to maintain habitual physical activity patterns during the study period and be willing to follow the study procedures and dietary restrictions. Participants completed a food frequency questionnaire of common high-fiber foods, administered by research staff, to determine the subject’s baseline fiber intake. Subjects were excluded from the study if they met any of the following exclusion criteria:
- History of a gastrointestinal disorder;
- Lactose intolerant;
- High fiber consumer (≥15 g per day);
- Use of pre-and probiotics in the past 90 days;
- High protein consumer (i.e., vegetarians or those who follow diets high in protein such as paleo);
- History of psychological illness or conditions that may interfere with the subject’s ability to understand the study directions;
- Use of antibiotics or signs of active systemic infection in the last 6 months. Subjects who are on hypo/hypercaloric diet aiming for weight loss or weight gain;
- History or presence of cancer in the prior 2 years (except for non-melanoma skin cancer);
- Currently pregnant, lactating, or planning to be pregnant during the study period;
- Regular use of dietary supplements (ex: fish oil, riboflavin, etc.), 90 days prior to study inclusion;
- Exposure to any non-registered drug product within the last 30 days prior to screening visit;
- History of or strong potential for alcohol or substance abuse (within 12 months of screening visit). Alcohol abuse is defined as >60 g (men)/40 g (women) pure alcohol per day (1.5 L/1 L beer, resp. 0.75 L/0.5 L wine);
- Current smoker or use of tobacco products in the past 90 days;
- Concurrent or recent participation (30 days) in a dietary intervention trial.
All research was conducted following the ethical principles for medical research involving human subjects set forth in the Helsinki Declaration. Written consent was obtained from all participating study subjects, and all study protocols were submitted to and approved by the University of Minnesota Institutional Review Board (Study CON0000000742323).
2.2. Material and Characteristics
A commercial formulation of PHGG (Sunfiber, Taiyo International, Minneapolis, MN, USA) was used as the treatment in this study. Previous studies have used doses between 5 and 10 g PHGG and found positive effects on the gut microbiome and on IBS symptoms [8,11,13,18,19,20]. Dosages of 3 g PHGG and 6 g PHGG were chosen for this study with the intention of discovering whether low doses of PHGG are capable of producing similar effects in healthy individuals. The placebo was maltodextrin. Both PHGG treatments and the placebo were in powdered form to be mixed with water or any non-alcoholic beverage for consumption. Subjects were not instructed on any specific liquid to mix the treatment, but were advised that carbonated beverages were more difficult to mix with.
2.3. Supplementation and Dosages
Subjects completed all three treatments throughout the duration of the study including a daily dietary supplementation of 3 g PHGG, 6 g PHGG, and a placebo. All treatments were provided in plain packaging, labeled only with “A”, “B”, or “C” to indicate which study arm the treatment belonged to. The 3 g PHGG treatment was combined with 3 g maltodextrin, and the placebo contained 6 g maltodextrin, so the weights of the packages were equivalent for all treatments. Subjects were instructed to consume one package daily, mixed with water or another beverage. Subjects were instructed to consume their daily treatment at a consistent time throughout each treatment period. During the study period, subjects were asked to continue with their usual diet and to avoid prolonged fasting or the unusual consumption of any high-energy, high-fat, or high-fiber meals. To ensure that the participants did not change their regular food intake pattern, food diaries were collected with 24-h food records from the subjects on days 1, 7, and 14 of each treatment.
2.4. Study Design and Protocol
The study performed was a randomized, double-blind, placebo-controlled, crossover study (Figure 1). Three treatment periods of 14 days each were separated by two 14-day washout periods. Participants were randomly allocated to study arms where each arm consisted of a sequence of three treatments given consecutively. Randomization was stratified by gender. For each of the three treatment arms, subjects collected stool samples at the baseline (Day 0) and on the final two days of treatment (Day 13, Day 14), with the subjects being asked to submit the final stool sample they were able to collect from either day for analysis.
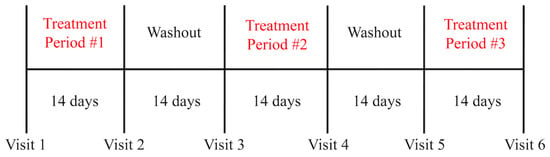
Figure 1.
Timeline of the PHGG treatment delivery and washout periods.
2.5. 16S rRNA Sequencing
16S ribosomal RNA sequencing was performed for the analysis of the gut microbiome composition. This method serves as an efficient and cost-effective means to visualize changes in gut bacteria [21]. Current technologies for 16S rRNA sequencing constitute the most widely accepted and functional method for evaluating gut microbial diversity [22]. 16S analysis was completed using the following steps:
Sample processing: Samples were boiled to lyze bacteria, and ethanol was added to the preservative binding buffer to a final concentration of 30%. The total volume was placed on a silica column to bind DNA, and washed 2×. Final elutions of DNA were carried out using 40 μL of water.
16S sequencing: The libraries were prepared using an Illumina 16S Metagenomic Sequencing Kit (Illumina, Inc., San Diego, CA, USA) according to the manufacturer’s protocol. The V3–V4 region of the bacterial 16S rRNA gene sequences were amplified using the primer pair containing the gene-specific sequences and Illumina adapter overhang nucleotide sequences. Amplicon PCR was performed to amplify the template out of input DNA samples. Briefly, each 25 μL of polymerase chain reaction (PCR) reaction contained 12.5 ng of sample DNA as the input as well as 12.5 μL 2× KAPA HiFi HotStart ReadyMix (Kapa Biosystems, Wilmington, MA, USA) and 5 μL of 1 μM of each primer. PCR reactions were carried out using the following protocol: an initial denaturation step performed at 95 °C for 3 min followed by 25 cycles of denaturation (95 °C, 30 s), annealing (55 °C, 30 s), and extension (72 °C, 30 s), and a final elongation of 5 min at 72 °C. The PCR product was then cleaned up from the reaction mix with Mag-Bind RxnPure Plus magnetic beads (Omega Bio-tek, Norcross, GA, USA). A second index PCR amplification, used to incorporate barcodes and sequencing adapters into the final PCR product, was performed in 25 μL reactions, using the same master mix conditions as described above. Cycling conditions were as follows: 95 °C for 3 min, followed by 8 cycles of 95 °C for 30″, 55 °C for 30”, and 72 °C for 30″. A final 5 minute elongation step was performed at 72 °C. The libraries were normalized with the Mag-Bind® EquiPure Library Normalization Kit (Omega Bio-tek, Norcross, GA, USA) and then pooled. The pooled library, ~600 bases in size, was checked using an Agilent 2200 TapeStation and sequenced (2 × 300 bp paired-end read setting) on the MiSeq (Illumina, San Diego, CA, USA).
Metagenomics analysis: The Metagenomics workflow on BaseSpace (Illumina) was used for the analysis of 16S ribosomal RNA. In MiSeq Reporter, a naïve Bayesian classifier has been implemented that has been optimized for Illumina paired-end reads [23]. The analysis used the reference database from the May 2011 release of the Greengenes 16S rRNA database. The main output of the workflow is a classification of reads at several taxonomic levels (kingdom, phylum, class, order, family).
2.6. Salivary Cortisol
Salivary cortisol was measured as a secondary objective of this study to evaluate potential connections between PHGG and stress. Previous studies have evaluated the effect of prebiotics on depression, anxiety, stress, and sleep quality [24,25,26,27]. While cortisol may be measured in the blood, urine, or saliva, multiple studies have found salivary cortisol to be a noninvasive and effective means of quantifying the stress response [25,26]. Subjects were provided with simple collection kits that included a sterile phial and funnel to simplify the collection of saliva. Upon entry to the study, subjects were given instructions on best practices for saliva collection including a recommended saliva amount.
2.7. Bristol Stool Form Scale
The Bristol Stool Form Scale (BSFS) visually depicts and describes seven different levels, or types, of stool ranging from the hardest (type 1) to softest (type 7). Images with accompanying definitions were used by research staff to match the subject’s stool consistency to one of the descriptions listed [28,29]. Types 1–2 are more indicative of constipation, types 3–4 indicate normal stool consistency, and levels 5–7 indicate loose stools or diarrhea [28]. BSFS ratings were assigned to each stool by research staff immediately upon receipt of each stool sample.
2.8. Statistical Analysis
Descriptive statistics were calculated and presented by treatment. Treatments were compared using mixed-effects models (SAS Proc Mixed). Models included the fixed effects of sequence, period, and treatment, and a random effect of subject (nested within sequence) to account for the within-subject correlation among repeated measurements. The interaction between treatment and period was tested and dropped from the model as it was not significant. If the overall F test for the effect of treatment was significant, pairwise comparisons were conducted to examine which treatment was different from which. No adjustment was made for multiple testing. Analyses were performed in SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). p values of less than 0.05 were considered statistically significant.
3. Results
3.1. Microbiome Composition
For the microbiome analysis, both the differences between Day 0 and Day 14 for each treatment were measured as well as the differences across treatments on Day 14 (Table 1). The concentration of Verrucomicrobia was significantly greater with both 3 g and 6 g of PHGG supplementation on Day 14 (p = 0.0066 and p = 0.0068, respectively) when compared with the placebo. Verrucomicrobia was also significantly increased from Day 0 to Day 14 with 6 g PHGG compared to the placebo (p = 0.0102). Notable changes on the genus level were those of Akkermansia, Dorea, and Sutterella. Akkermansia was significantly increased from Day 0 to Day 14 with 6 g PHGG (p = 0.0116), and the concentration of Akkermansia was greater on Day 14 with both 3 g (p = 0.0081) and 6 g (p = 0.0083) PHGG supplementation compared to the placebo. Concentrations of Sutterella and Dorea were significantly less with 3 g and 6 g PHGG than with the placebo on Day 14. Counts of Erysipelotrichi were significantly decreased over the course of 14-day supplementation with 6 g PHGG compared with the placebo (p = 0.0092) (Table 2). Finally, the concentration of Faecalibacterium was decreased with 3 g PHGG, 6 g PHGG, and the placebo, but the results were only significant with 3 g PHGG (p = 0.0054).

Table 1.
Significant differences in relative change between Day 0 and Day 14.

Table 2.
Significant differences in count change between Day 0 and Day 14.
3.2. Salivary Cortisol
No significant differences were observed between the mean change of salivary cortisol from Day 0 to Day 14 across treatments. However, the salivary cortisol did decrease with 6 g PHGG (−85.68 ± 681.4), while it increased with both 3 g PHGG and the placebo. Salivary cortisol was increased to a lesser degree with 3 g PHGG (21.52 ± 345.32) than with the placebo (68.88 ± 407.41), although was not statistically significant.
3.3. Stool Consistency
Participants rated their stool consistency at the end of each 14-day treatment period using the Bristol Stool Form Scale. Results are displayed in Table 3 With the 3 g PHGG treatment, the percentage of stools rated as “normal” on the BSFS were similar to subjects that were receiving the placebo. However, when subjects were consuming 6 g PHGG, a greater percentage stools were rated “normal” (50%) compared to both 3 g PHGG (36.4%) and the placebo (39.4%).

Table 3.
Rating of stool consistency for each treatment using the Bristol Stool Form Scale.
3.4. Safety Aspects
No serious adverse events were reported during this study, suggesting that PHGG is safe to consume. This indicates that PHGG taken as a supplement in doses of 3 to 6 g will likely not cause any adverse effects.
4. Discussion
Dietary fiber is beneficial to gut microbiome stability and richness, and dietary fiber intake is linked to improved health status and disease prevention. The fermentation of fiber in the gut does more than change the gut microbiota including regulating gut transit and producing metabolites including short chain fatty acids that not only provide energy, but also have effects in the gut systemically. Human microbiome research has expanded in the past few years, but Bifidobacteria were described in 1899 and beneficial gut microorganisms were discussed in the early 1900s [30]. Methods to measure changes in the gut microbiota have also evolved over time, making it difficult to compare studies from classical microbiological methods to more advanced techniques [30].
Additionally, results on changes in the microbiota do not show that the functionality of the microbiota has changed. It has been suggested that the gut microbiome is functionally redundant, supported by the finding that the taxonomic composition of human metagenomes vary hugely, but functional gene prediction profiles remain consistent [22]. It is also suggested that the metabolites in the gut such as the short chain fatty acids are more relevant biomarkers than the gut microbiota. As the short chain fatty acids are quickly absorbed and metabolized from the gut, they are difficult to study in humans, and few published human feeding studies have provided information on both changes in the gut microbiota in short chain fatty acid production with fiber interventions.
Our study was designed to determine whether small doses of PHGG would alter the gut microbiota, with a particular interest in the prebiotic properties of PHGG. Fibers and oligosaccharides are known to alter the gut microbiota. While fructooligosaccharides (FOS) and galactooligosaccharides (GOS) are generally the most well-studied, the search for which fermentable fibers are most successful in changing the gut microbiota continues, as does determining which microbial shifts are most linked to health outcomes [22]. Prebiotics are defined by the fact that they are non-digestible, fermented by intestinal microbes, and that they stimulate changes in the composition of gut bacteria to provide benefits to the host. While all prebiotics are fiber, not all types of fiber fall under the prebiotic category [31]. As PHGG has been shown to fuel positive changes to the human gut microbiome, it functions under the umbrella of a prebiotic fiber, along with FOS and GOS [32]. Furthermore, it may be better tolerated [8].
In the present study, both Verrucomicrobia and Akkermansia increased with PHGG supplementation. The mucin-degrading microbe, Akkermansia muciniphila, is a bacteria of the phylum Verrucomicrobia, and it is found in higher concentrations in healthy individuals [33,34]. It has been shown that the concentration of Verrucomicrobia present in individuals with obesity is lower [35], and decreased concentrations of Verrucomicrobia may even serve as an indicator of insulin resistance or type 2 diabetes (T2DM) [36]. In individuals with GI diseases such as IBD, the abundance of A. muciniphila was significantly lower, suggesting that the decreased concentration of this bacteria may function as an indicator of dysbiosis [37,38].
Conversely, Dorea and Sutterella were both decreased in this study with PHGG consumption. Dorea belongs to the Lachnospiraceae family, which has been shown to produce some beneficial metabolites [39]. However, increased Lachnospiraceae is also associated with certain diseases, systemic inflammation, and IBS [19]. Some studies have demonstrated how Dorea may support the production of IFNγ, leading to increased inflammation [40,41]. Therefore, it is thought that lower concentrations of Dorea may be important for better health outcomes [19]. Sutterella is another bacteria whose abundance is correlated with IBD and inflammation [42]. Increases in Erysipelotrichi were also positively correlated with inflammatory GI disorders and colorectal cancer, though more research needs to be undertaken to establish differences between human and animal models [43,44]. In this case, the decreased presence of these microbes with PHGG supplementation in our study was likely a beneficial shift.
Faecalibacterium was decreased in our study with 3 g PHGG supplementation. Because of its anti-inflammatory properties and ability to produce butyrate, increased Faecalibacterium is largely accepted as an indicator of a healthy gut. It also tends to be found in decreased concentrations in individuals with inflammatory GI diseases [45]. The results of a previous study, wherein subjects consumed 5 g PHGG up to three times daily, showed a subsequent increase in the level of Faecalibacterium [32]. This may suggest that higher doses of PHGG are needed to increase the concentration of Faecalibacterium. Further research needs to be conducted to explore the impact of low dose PHGG on Faecalibacterium.
Secondary measures of this study included stool consistency and salivary cortisol. Neither the placebo nor the 3 g treatment of PHGG showed an efficacy in improving stool consistency, but 6 g PHGG supplementation did appear to result in an improvement in stool firmness. Salivary cortisol was decreased with 6 g PHGG supplementation, suggesting that PHGG may have a positive effect on the body’s stress response. However, this poses an area of research that requires much further exploration. Future studies should focus on specific measures of the gut–brain connection with PHGG as a microbiome modulator. This includes determining the best methods for evaluating the effects of PHGG and other probiotics on stress and mental health.
One major limitation of this study is the variability in subject diets and lifestyle factors beyond the control of the research team. While participants were asked to complete a baseline dietary fiber intake questionnaire and keep a food diary to ensure their diet did not change dramatically throughout the course of the study, it is impossible to control for diet. Beyond differences in fiber consumption, other components of the diet may also influence changes in the gut microbial composition, which may not be fully accounted for [46]. Salivary cortisol was used in this study as a measure of stress response. However, it is possible that urinary cortisol may be a better measure, as it more accurately reflects the cortisol levels in the blood. Values for salivary cortisol may also be affected by the time of day the sample is taken, introducing the potential for skewed results [26].
Because PHGG increased the concentration of microbes in this study such as Verrucomicrobia and Akkermansia, it is possible that low doses of PHGG may be a potential treatment option in the future for improving the gut microbiome composition of individuals with inflammatory GI diseases. Other types of soluble fiber have been studied with respect to their use as a method for managing GI disorders such as IBS [47,48,49]. Similar to other fibers, PHGG may work to improve dysbiosis, while triggering less undesirable symptoms given its non-viscous, lower-fermenting properties. The dose–response effects of PHGG should be further explored with respect to GI disorders to determine the most effective dose for both beneficial microbiome shifts and the management of symptoms.
We also measured changes in stool consistency and stool weight in our study. We did not expect to see changes as the doses we fed in the study were small and not likely to alter the fecal weight or consistency. No changes were found in the Bristol Stool Form Scale (BSFS) with the small doses of PHGG fed.
Additionally, we measured the saliva cortisol across the three treatments based on other work showing differences in saliva cortisol with fiber treatment [26,49,50]. It is well-accepted that the brain and gut communicate, and that changes in gut physiology will alter the mental state. Past work has found that prebiotic consumption reduced stress response as measured by saliva cortisol levels [49]. Other recent papers have found that prebiotics can alter stress-induced mood states [26], while another paper reported that FOS + GOS did not affect the biological markers of stress and inflammation or mental health symptoms in healthy adults, despite an increase in Bifidobacterium [50]. Agreement on the best practices to measure the gut microbiota and mental health markers in clinical studies will be necessary to design studies that determine the links between gut health and mental health measures [27].
The present study demonstrated that even small doses of PHGG, as little as 3 g, can influence the composition of the human gut microbiome in healthy subjects including positive shifts in beneficial microbes. Individual variability in the human gut microbiome poses challenges in determining how selective changes to bacterial taxa may result in health outcomes. Additional research should be focused on how changes in the gut microbiome composition are associated with resulting GI symptoms and other physiological benefits.
In conclusion, our results on changes in microbiota with fiber intervention are limited by methodology challenges for gut microbiota. We do not have information on changes in diversity with PHGG fiber addition. Thus, the changes in the microbiota do not imply that the functionality of the microbiota has changed. Variation in microbiota composition must be accompanied by assessments of the relationships between microbes with the host as well as assessments of the metabolites. Future studies are needed on dietary fiber interventions and changes in gut metabolites that have links to health and disease. Many host factors beyond diet contribute to disease and gut microbiota composition such as age, medications, host metabolites, and immune responses. Working across disciplines including nutrition, microbiology, gastroenterology, and immunity will be required to keep this field moving forward.
Author Contributions
Conceptualization, Q.W., R.A. and J.S.; Software, Q.W.; Validation, M.E., Q.W. and R.A.; Formal analysis, M.E. and Q.W.; Investigation, R.A. and J.S.; Resources, J.S.; Data curation, M.E., Q.W., R.A. and J.S.; Writing—original draft, M.E.; Writing—review & editing, Q.W. and J.S.; Visualization, R.A.; Supervision, J.S.; Project administration, J.S.; Funding acquisition, J.S.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Taiyo International.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Slavin, J.L.; Greenberg, N.A. Partially hydrolyzed guar gum: Clinical nutrition uses. Nutrition 2003, 19, 549–552. [Google Scholar] [CrossRef]
- Setayesh, L.; Pourreza, S.; Khosroshahi, M.Z.; Asbaghi, O.; Bagheri, R.; Kelishadi, M.R.; Wong, A.; Clark, C.C.; Larky, D.A.; Suzuki, K.; et al. The effects of guar gum supplementation on lipid profile in adults: A GRADE-assessed systematic review, meta-regression and dose–response meta-analysis of randomised placebo-controlled trials. Br. J. Nutr. 2022, 129, 1703–1713. [Google Scholar] [CrossRef]
- Alaeian, M.J.; Pourreza, S.; Yousefi, M.; Golalipour, E.; Setayesh, L.; Khosroshahi, M.Z.; Bagheri, R.; Ashtary-Larky, D.; Wong, A.; Zamani, M.; et al. The effects of guar gum supplementation on glycemic control, body mass and blood pressure in adults: A GRADE-assessed systematic review and meta-analysis of randomized clinical trials. Diabetes Res. Clin. Pract. 2023, 199, 110604. [Google Scholar] [CrossRef]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef]
- Cuomo, R.; Russo, L.; Sarnelli, G.; Savino, I.; Vozzella, L.; Zito, F.; Andreozzi, P. Partially hydrolyzed guar gum in the treatment of irritable bowel syndrome with constipation: Effects of gender, age, and body mass index. Saudi J. Gastroenterol. 2015, 21, 104. [Google Scholar] [CrossRef]
- Niv, E.; Halak, A.; Tiommny, E.; Yanai, H.; Strul, H.; Naftali, T.; Vaisman, N. Randomized clinical study: Partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr. Metab. 2016, 13, 10. [Google Scholar] [CrossRef]
- Parisi, G.C.; Zilli, M.; Miani, M.P.; Carrara, M.; Bottona, E.; Verdianelli, G.; Battaglia, G.; Desideri, S.; Faedo, A.; Marzolino, C.; et al. High-Fiber Diet Supplementation in Patients with Irritable Bowel Syndrome (IBS): A Multicenter, Randomized, Open Trial Comparison Between Wheat Bran Diet and Partially Hydrolyzed Guar Gum (PHGG). Dig. Dis. Sci. 2002, 47, 1697–1704. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Koido, M.; Kawaguchi, M.; Timm, D.; Ozeki, M.; Yamada, M.; Mitsuya, T.; Okubo, T. Lifestyle related changes with partially hydrolyzed guar gum dietary fiber in healthy athlete individuals—A randomized, double-blind, crossover, placebo-controlled gut microbiome clinical study. J. Funct. Foods 2020, 72, 104067. [Google Scholar] [CrossRef]
- Ohashi, Y.; Sumitani, K.; Tokunaga, M.; Ishihara, N.; Okubo, T.; Fujisawa, T. Consumption of partially hydrolysed guar gum stimulates Bifidobacteria and butyrate-producing bacteria in the human large intestine. Benef. Microbes 2015, 6, 451–455. [Google Scholar] [CrossRef]
- Okubo, T.; Ishihara, N.; Takahashi, H.; Fujisawa, T.; Kim, M.; Yamamoto, T.; Mitsuoka, T. Effects of Partially Hydrolyzed Guar Gum Intake on Human Intestinal Microflora and Its Metabolism. Biosci. Biotechnol. Biochem. 1994, 58, 1364–1369. [Google Scholar] [CrossRef]
- Yasukawa, Z.; Inoue, R.; Ozeki, M.; Okubo, T.; Takagi, T.; Honda, A.; Naito, Y. Effect of Repeated Consumption of Partially Hydrolyzed Guar Gum on Fecal Characteristics and Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Clinical Trial. Nutrients 2019, 11, 2170. [Google Scholar] [CrossRef]
- Pylkas, A.M.; Juneja, L.R.; Slavin, J.L. Comparison of Different Fibers for In Vitro Production of Short Chain Fatty Acids by Intestinal Microflora. J. Med. Food 2005, 8, 113–116. [Google Scholar] [CrossRef]
- Inoue, R.; Sakaue, Y.; Kawada, Y.; Tamaki, R.; Yasukawa, Z.; Ozeki, M.; Ueba, S.; Sawai, C.; Nonomura, K.; Tsukahara, T.; et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J. Clin. Biochem. Nutr. 2019, 64, 217–223. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Sugita, M.; Fukuzawa, Y.; Okubo, T. Impact of partially hydrolyzed guar gum (PHGG) on constipation prevention: A systematic review and meta-analysis. J. Funct. Foods 2017, 33, 52–66. [Google Scholar] [CrossRef]
- Polymeros, D.; Beintaris, I.; Gaglia, A.; Karamanolis, G.; Papanikolaou, I.S.; Dimitriadis, G.; Triantafyllou, K. Partially Hydrolyzed Guar Gum Accelerates Colonic Transit Time and Improves Symptoms in Adults with Chronic Constipation. Dig. Dis. Sci. 2014, 59, 2207–2214. [Google Scholar] [CrossRef]
- Ustundag, G.; Kuloglu, Z.; Kirbas, N.; Kansu, A. Can partially hydrolyzed guar gum be an alternative to lactulose in treatment of childhood constipation? Turk. J. Gastroenterol. 2010, 21, 360–364. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis RHJr Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Morishima, S.; Kapoor, M.P.; Inoue, R.; Tsukahara, T.; Naito, Y.; Ozeki, M. Partially hydrolyzed guar gum is associated with improvement in gut health, sleep, and motivation among healthy subjects. J. Clin. Biochem. Nutr. 2023, 72, 189–197. [Google Scholar] [CrossRef]
- Williams, L.; Slavin, J.L. Dietary Fiber and Other Alternative Therapies and Irritable Bowel Syndrome. Top. Clin. Nutr. 2009, 24, 262. [Google Scholar] [CrossRef]
- Kwa, W.T.; Sundarajoo, S.; Toh, K.Y.; Lee, J. Application of emerging technologies for gut microbiome research. Singap. Med. J. 2023, 64, 45–52. [Google Scholar]
- Swanson, K.S.; De Vos, W.M.; Martens, E.C.; Gilbert, J.A.; Menon, R.S.; Soto-Vaca, A.; Hautvast, J.; Meyer, P.D.; Borewicz, K.; Vaughan, E.E.; et al. Effect of fructans, prebiotics and fibres on the human gut microbiome assessed by 16S rRNA-based approaches: A review. Benef. Microbes 2020, 11, 101–129. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Taylor, A.M.; Holscher, H.D. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr. Neurosci. 2020, 23, 237–250. [Google Scholar] [CrossRef]
- Haarhuis, J.E.; Kardinaal, A.; Kortman, G.M. Probiotics, prebiotics and postbiotics for better sleep quality: A narrative review. Benef. Microbes 2022, 13, 169–182. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.J.; Harmer, C.J.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2015, 232, 1793–1801. [Google Scholar] [CrossRef]
- Mysonhimer, A.R.; Cannavale, C.N.; Bailey, M.A.; Khan, N.A.; Holscher, H.D. Prebiotic Consumption Alters Microbiota but Not Biological Markers of Stress and Inflammation or Mental Health Symptoms in Healthy Adults: A Randomized, Controlled, Crossover Trial. J. Nutr. 2023, 153, 1283–1296. [Google Scholar] [CrossRef]
- Shokouhi, N.; Mohammadi, S.; Ghanbari, Z.; Montazeri, A. Development of a new version of the Bristol Stool Form Scale: Translation, content validity, face validity, and reliability of the Persian version. BMJ Open Gastroenterol. 2022, 9, e001017. [Google Scholar] [CrossRef]
- Peng, Z.; Yi, J.; Liu, X. A Low-FODMAP Diet Provides Benefits for Functional Gastrointestinal Symptoms but Not for Improving Stool Consistency and Mucosal Inflammation in IBD: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2072. [Google Scholar] [CrossRef]
- Walker, A.W.; Hoyles, L. Human Microbiome Myths and Misconceptions. Nat. Microbiol. 2023, 8, 1392–1396. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef] [PubMed]
- Reider, S.J.; Moosmang, S.; Tragust, J.; Trgovec-Greif, L.; Tragust, S.; Perschy, L.; Przysiecki, N.; Sturm, S.; Tilg, H.; Stuppner, H.; et al. Prebiotic Effects of Partially Hydrolyzed Guar Gum on the Composition and Function of the Human Microbiota—Results from the PAGODA Trial. Nutrients 2020, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.Y.; Kostopoulos, I.; de Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Belzer, C.; de Vos, W.M. Microbes inside—From diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Shanahan, F.; Guarner, F.; de Vos, W.M. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm. Bowel. Dis. 2013, 19, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic Bacteria With Increased Prevalence in IBD Mucosa AugmentIn VitroUtilization of Mucin by Other Bacteria. Off. J. Am. Coll. Gastroenterol. ACG 2010, 105, 2420. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Crost, E.H.; Tailford, L.E.; Le Gall, G.; Fons, M.; Henrissat, B.; Juge, N. Utilisation of Mucin Glycans by the Human Gut Symbiont Ruminococcus gnavus Is Strain-Dependent. PLoS ONE 2013, 8, e76341. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1897. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Sutterella Species, IgA-degrading Bacteria in Ulcerative Colitis. Trends Microbiol. 2020, 28, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Laursen, R.P.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Frøkiær, H.; Bahl, M.I.; Licht, T.R. Faecalibacterium Gut Colonization Is Accelerated by Presence of Older Siblings. mSphere 2017, 2, e00448-17. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.I.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.; Spiegel, B.M.R.; Ford, A.C. The Effect of Fiber Supplementation on Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Off. J. Am. Coll. Gastroenterol. ACG 2014, 109, 1367. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.; Morden, A.; Bischof, D.; King, E.A.; Kosztowski, M.; Wick, E.C.; Stein, E.M. The Role of Fiber Supplementation in the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1002. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Gibson, P.R.; Muir, J.G.; Yao, C.K. Dietary fibres and IBS: Translating Functional Characteristics to cCinical Value in the Era of Personalised Medicine. Gut 2021, 70, 2383–2394. [Google Scholar] [CrossRef]
- Jackson, P.P.J.; Wifeyesekera, A.; Williams, C.M.; Theis, S.; van Harsselaar, J.; Rastall, R.A. Inulin-type Fructans and 2-Fucosyllactose Alter Both Microbial Composition and Appear To Alleviate Stress-induced Mood State in a Working Population Compared to Placebo (maltodextrin): The EFFICAD Trial, A Randomized, Controlled Trial. Am. J. Clin. Nutr. 2023, 118, 938–955. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).