Abstract
The transformation of the food chain due to technological advances has had significant implications in regard to food safety. A noteworthy trend in this evolution relates to the emergence of new or previously unseen pathogens within products, thereby altering the landscape of foodborne illness epidemiology. The escalating frequency of these events underscores the need for a comprehensive re-evaluation of preventive strategies. The occurrence of novel species of bacteria, viruses, parasites, and unusual biotoxins from unexpected sources has challenged the previous limits that had been set to prevent foodborne illness outbreaks. The repercussions, ranging from detrimental effects on public health to economic burden, are influenced by a myriad of factors affecting the evolution of foodborne pathogens and emerging ailments. Among these factors are shifts in population demographics and behaviors, especially dietary patterns, as well as climate extremes, advances in more precise pathogen detection, microbial adaptation, evolving agricultural practices, and transformative changes within the food industry. This review critically examines the impact of technological metamorphosis along the food chain, encompassing production, processing, handling, packaging, storage, transportation, and industry demographics on the dynamics influencing the emergence of foodborne pathogens. Additionally, potential solutions to mitigate and manage this escalating issue are proposed.
1. Introduction
Foodborne illness arises primarily from the consumption of food contaminated with pathogenic microorganisms such as bacteria and their toxins, viruses, parasites, as well as chemicals and other agents. There are a wide variety of microorganisms, including viruses, bacteria, and parasites, that can contaminate human food and/or water and lead to illness when they or their toxins are ingested [1,2,3].
Every year, foodborne diseases affect millions of people worldwide. For example, in the US, the federal government estimates that there are about 48 million cases of foodborne illness yearly. According to this estimate, one in six Americans will become sick from eating contaminated food, which will cause 128,000 hospital admissions and 3000 fatalities, resulting in a significant public health commitment and economic burden despite the fact that the American food supply is among the safest in the world [4]. In Central Asia and Europe there are more than 23 million cases of foodborne disease resulting in 5000 deaths yearly [5]. On the other hand, in Australia, authorities estimate that there are 5.4 million foodborne illnesses cases annually [6,7,8].
Microbial contamination can occur in a variety of ways, such as by contact with animal feces during/post slaughtering; poor sanitation; food handlers; unclean food processing equipment as well as instruments; and through contaminated washing or irrigation water [9,10]. Among pathogenic microorganisms, viruses like norovirus and hepatitis A cause the largest number of foodborne illnesses worldwide. On the other hand, the majority of hospitalizations, as well as fatalities, are caused by bacteria, mainly strains of Campylobacter jejuni, Clostridium botulinum, Salmonella enterica, Listeria monocytogenes, Mycobacterium bovis, Shigella spp., Brucella spp., Vibrio spp. (V. cholerae, V. vulnificus, and V. parahaemolyticus), Yersinia enterocolitica and Shiga toxin-producing Escherichia coli [4,10]. Moreover, foods derived from animals (eggs, meat, milk, and shellfish), plus leafy greens, vegetables, and fruits, are examples of foods that are frequently the source of microbial hazards [10,11,12].
The intricate structure of the food supply chain is characterized by constant changes in processing, distribution, agricultural practices, and consumption patterns. These dynamic shifts, coupled with variations in food composition, have significantly contributed to the emergence or resurgence of foodborne pathogens [13,14]. In addition, the exposure of microorganisms to different environmental stresses during movement through the food chain may influence the emergence of antibiotic resistant pathogens. These can lead to failure in antibiotic therapy and increase the severity of illnesses, as well as the number and length of hospitalizations and deaths [15,16,17]. Pathogen resurgence occurs when a well-established pathogen acquires greater virulence and re-emerges as a novel threat, whereas emergence develops when previously unrecognized pathogens are identified and linked to instances of foodborne illness [13]. It is the intent of this review to explore the consequences of technological advancements on the emergence of foodborne pathogens and propose viable approaches to ensure the production of safe food products. This review was based on relevant studies that were identified through extensive literature searches using electronic databases including MEDLINE (PubMed), Web of Science, Google Scholar, and certified scientific websites with the terms and keywords of “Foodborne pathogens”, “Emerging”, “Re-emergence”, “Food industries”, “Evolving”, “Foodborne pathogens”, “Processing”, “Handling”, “Packaging”, “Production”, “Harvesting”, “Slaughtering”, and “Food chain”. The search strategy included these terms and keywords in various combinations with the Boolean phrases “AND” and “OR”. Only studies published in English were included. The titles and abstracts were screened, and then the full texts of the selected research and review articles were critically reviewed.
2. The Emergence of Foodborne Pathogens
The emergence of new or unexpected pathogens in foods is one of the most significant trends that can have an impact on food safety. There are several definitions of “emerging pathogens”. They may include a pathogen that has recently been connected to a serious public health crisis involving illness. In other instances, “emerging” has been used to define the appearance of microbial strains that have greater stress resistance and therefore have adapted to new vehicles for their transmission, or to new environments. The term “emerging foodborne pathogens” can refer to pathogens that have recently become associated with transmission through food even though they may have been previously identified as pathogens [14,18,19,20,21].
Several emerging foodborne pathogens have emerged in the past few decades. These include bacteria such as Aeromonas spp., Arcobacter butzleri, Burkholderia gladioli pathovar cocovenenans, Bacillus cereus, Campylobacter spp., Cronobacter spp., Clostridium botulinum, Clostridium difficile, Clostridium perfringens, Helicobacter pylori, Helicobacter pullorum, Helicobacter canadensis, Klebsiella pneumonia, Listeria monocytogenes, Mycobacterium paratuberculosis, Shiga toxin–producing E. coli, Salmonella spp., Shigella spp., Staphylococccus aureus, Streptococcus spp., V. cholera, Vibrio vulnificus, Vibrio parahaemolyticus, and Yersinia enterocolitica; viruses such as adenovirus, astrovirus, hepatitis A, hepatitis E, norovirus, rotavirus and sapovirus; and parasites such as Cryptosporidium, Cyclospora cayetanensis, Taenia spp., Toxoplasma gondii, Trichinella spiralis [1,14,22,23,24,25,26].
It is apparent that, even though there has been considerable effort to eliminate or control pathogenic microbes, new ones continue to emerge and appear in new vehicles. As a result, the most frequently reported foodborne illnesses have undergone significant changes with time, and this becomes evident when these illnesses cannot be explained by known pathogens. Once a new pathogen is discovered and has emerged, it becomes more prevalent or linked to novel food sources because it becomes targeted, and often this is accompanied by development of new detection methods such as restriction endonuclease analysis, pulsed-field gel electrophoresis, multilocus sequence typing and the polymerase chain reaction (PCR) technique [3,14,22,27,28]. Landmark examples include the detection of E. coli O157:H7 in Japanese radish sprouts in 1996 (with >9000 ill), a multi-state outbreak in the US caused by Salmonella Enteritidis in table eggs in 2010 (2752 illnesses), and Listeria monocytogenes in caramel-coated apples in 2014 (35 illnesses in the US and Canada) (http://www.cdc.gov/listeria/outbreaks/caramel-apples-12-14/, accessed 9 March 2024, https://www.cdc.gov/salmonella/enteritidis/archive/092010.html, accessed 8 March 2024) [29].
Additionally, humans, animals, and the environment significantly influence the emergence and spread of a variety of infections. Animals are considered to be the source of the majority of infectious diseases that affect people, with 61% of human infections being zoonotic in origin [30]. Therefore, the “zoonotic pool” is thought to be a significant and potentially rich source of emerging diseases. The frequent reports of “new” zoonosis suggest that the zoonotic pool is far from being depleted, with infection potentially spreading after introduction through other factors [31,32,33]. Even if a zoonotic agent is unable to establish itself and spread quickly from person to person, other factors, including the environment, can facilitate its spread (e.g., nosocomial infections), leading to the emergence of food as a vehicle for pathogen transmission [30,32,34,35]. Therefore, it is expected that new foodborne pathogens will emerge in the future, with it being theorized that these will be mainly zoonotic pathogens due to several factors, including the interaction between humans and animals, the consumption of raw and processed food products of animal origin, the intensive global animal production, the unsuitable disposal of waste, environmental changes, uncontrolled human population settlements, and poor sanitary conditions [22,25,36].
3. Factors Contributing to the Emergence of Foodborne Pathogens
Many factors promote the emergence of pathogens in food, and these are summarized in Figure 1. Relevant factors include technological advances during production, processing, packaging, and preparation of food; alterations in agricultural practices such as irrigation methods; microbial adaptation and the enhancement of virulence genes; modifications in human behavior, especially food consumption patterns; demographics, including migration and urbanization; the failure of public health programs; and environment-related factors, including climate change, global trade in food and travel, as well as advanced detection methods such as polymerase chain reaction (PCR) [14,27,35,37,38,39]. However, this review will be limited to the technology changes in the food industry that may have contributed to the emergence of new foodborne pathogens.
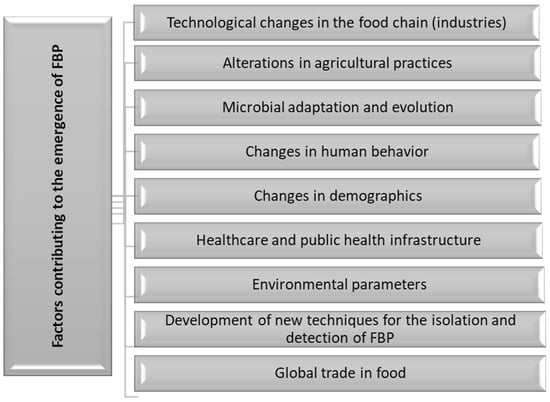
Figure 1.
Factors contributing to the emergence of foodborne pathogens.
4. Emerging Foodborne Diseases Linked to Industrial Food Technology Changes
Emerging infections include those that have recently been expressed within the population, or those that were pre-existing but are now increasing in frequency or expanding their geographic reach [32,40,41]. Furthermore, a substantial portion of newly identified infections appears to stem from pathogens that were already present in the environment but which were unmasked or gained selective advantage due to evolving circumstances that enabled the targeting of new host populations. It is important to acknowledge that the emergence of a novel variant may also occur, leading to the development of a new disease entity [42,43]. Table 1 contains examples of emerging diseases and possible factors that may have contributed to that emergence.

Table 1.
Emerging diseases and possible contributing factors.
5. Technological Changes in the Food Chain
Food processors’ response to consumer and market demand for healthful foods has created a group of minimally processed foods or foods with lower salt, sugar, and preservatives. However, new technologies, such as high pressure processing (HPP) can alter the microbial content of food in ways not typical of conventional technologies. However, the use of HPP in the food industry is limited due to the high costs of both equipment and operation [44,45]. Therefore, other emerging technologies like the use of bio-preservatives and hurdle technologies (combinations of mild processing treatments) also contribute to the transformation of the food industry [46]. Bio-preservatives, for instance, offer a natural alternative to traditional preservatives and play a crucial role in meeting the demands for clean-label products [46]. Furthermore, hurdle technology, which is an approach used in food preservation that involves combining multiple preservation methods or factors to prevent the growth of microorganisms and extend the shelf life of food, present unique challenges and opportunities, impacting the microbial content of food [47]. However, it is important to recognize that these technologies can pose serious concerns for food safety, as microorganisms may respond differently to various stressors introduced by these methods [35]. Embracing a more holistic perspective that considers a variety of processing techniques contributes to a better understanding of the complex interplay between technology, consumer demands, and food safety [48,49].
The occurrence of rapid changes in technology is an unavoidable aspect of modern life, and the positive effects seem to outweigh the negatives [50]. On the downside, technology may have been responsible for promoting the emergence and rapid spread of infectious diseases by creating environments that are more favorable to pathogen survival [50,51]. Technological development has influenced the occurrence and prevalence of infectious diseases [51,52]. The significance of these observations is dependent upon our understanding of quorum sensing, which can define virulence levels and biofilm formation, even in environments used for food manufacturing [35]. It is of interest to note that quorum sensing may influence the ability of enterohemorrhagic Escherichia coli to colonize the animal intestine, an attribute pivotal to its capability to contaminate both food products and manufacturing equipment, thus contributing to its emergence [53].
6. Impact of Contamination Steps in the Food Chain on the Emergence of New Foodborne Illnesses
Food production technology has unwittingly contributed to the emergence of new foodborne diseases, as the conversion of raw materials from agricultural production to the completed meal involves the creation of a variety of intermediate environmental niches where foci of contamination can develop. All steps of the food chain, from harvesting and slaughtering through to the consumer table, including production, processing, preparation, handling, packaging, storage, and transportation, must be continuously monitored to minimize opportunities for the development of health risks [12,54]. In order to adequately address potential problems that may emerge, steps to trace or prevent the occurrence of contaminants in finished and ready-to-eat food need to be put in place [12,54,55]. Lapses in hygienic practice at any one of these steps can have a significant effect on consumer health.
6.1. Production of Raw Materials (Harvesting and Slaughtering)
Food production involves an array of activities, from the cultivation of crops to raising animals on farms where the potential for foodborne pathogens (FBPs) to contaminate the food supply exists. It is no surprise that, during plant cultivation, fresh produce can become contaminated before harvesting, particularly if farms use contaminated water for irrigation, experience environmental extremes, or if fertilizers and pesticides are improperly applied [12]. Consequences resulting from failures at one or more steps in the food chain can be catastrophic, providing opportunistic pathogens the chance to adapt, grow and cause problems in foods not previously implicated in causing illness. An example of this would be a series of outbreaks that emerged at multiple North American locations in 1996 encompassing the District of Columbia and 20 states within the US, plus two provinces in Canada. This outbreak involved 1465 illnesses, characterized by symptoms such as diarrhea and pronounced fatigue. The root cause was fresh raspberries harboring Cyclospora cayetanensis, a pathogenic parasite not previously associated with food. Regrettably, investigators were unable to pinpoint the precise source of berry contamination with this pathogen [3]. It is notable that Cyclospora outbreaks have been reported recently in the US and linked to fresh produce, mainly involving basil, cilantro, lettuce, raspberries, and snow peas [56].
It Is widely understood that animals and animal farms are environments normally colonized by pathogenic microorganisms [57]. For example, a new serotype of Vibrio parahaemolyticus O3:K6, a marine organism that can be found in raw seafood, such as raw oysters and shellfish, was somewhat recently spread from Southeast Asia to Japan and the United States. Early in the 1990s, this new V. parahaemolyticus serotype emerged in Southeast Asia; later in the decade it caused numerous outbreaks in Japan linked to seafood, while significant outbreaks were linked to oysters in the US in 1997 and 1998. It seems oysters were harvested from the sea near shipping lanes where oil tankers from the east discharged contaminated ballast water before being filled with oil and gasoline for Japan [3,58]. Another potential pathogen, Arcobacter, formerly classified as Campylobacter and now more frequently isolated from water, has also been found in a variety of livestock carcasses. It is associated with gastrointestinal illness in humans and has been isolated from food [59,60]. Although regarded as a nosocomial pathogen, Clostridium difficile is found in 6–8% of food samples, with as many as 10% of seafood samples being positive for spores of the organism. It is also regarded as an emerging foodborne pathogen, and spores in hog or beef cattle feces may contaminate meat products during slaughter [61,62,63]. In addition, during slaughter, infectious agents can spread quickly in poultry farms with large bird populations. Hot water dips used to slaughter birds aid in feather removal but may spread intestinal contents to subsequently processed carcasses [64,65]. It is notable that at least 90% of chicken sold at retail stores in different regions is contaminated with Campylobacter spp. This group is thought to be responsible for causing the largest proportion of bacterial foodborne illness in countries monitoring the causative agents responsible, although the illness they cause is generally mild, resolves spontaneously, and is substantially underreported [66]. It is evident that the major source of Campylobacter infections in humans is poultry [64]. The control measures in the United States for Campylobacter have focused on the chlorination of water baths and chiller tanks in addition to slaughter sanitation [34].
6.2. Processing
Conventional processing of foods involves a wide array of techniques including, but not limited to, the pasteurization of milk, roasting and grinding of nuts, smoking, drying, and salting [12]. The contamination of food products may occur during processing due to mistreatment or is most commonly a result of post-process contamination, which occurs in the factory environment, potentially originating with workers, the floors, walls, equipment, or even the ingredients used in processing or other sources [67].
As mentioned earlier, Arcobacter is an example of an emerging pathogen. It is represented by a genus containing 33 species, including Arcobacter nitrofigilis, which is isolated from plant roots, while Arcobacter skirrowii, Arcobacter butzleri, and Arcobacter cryaerophilus have been isolated from animals. These pathogens belong to the Epsilobacteria group that also includes Helicobacter spp. and Campylobacter spp. [59,68]. Arcobacter is usually associated with poultry carcasses, although controlling Arcobacter on the farm should reduce contamination during processing. However, there are also studies that report Arcobacter may be found in processing environments, and it can survive outside or within carcasses [69]. Indeed, it was pointed out that Arcobacter butzleri survived in pen litter for long periods [68].
Bovine spongiform encephalopathy (BSE), which first appeared in Britain, is a further example that has emerged in the recent past. BSE most likely developed following the interspecies transfer of scrapie from sheep to cattle, which happened when modifications to the rendering processes enabled the scrapie agent in sheep byproducts to survive, with contaminated ingredients then being fed to cattle [70]. Another example occurred in 1985 following a failure in the pasteurization of milk at a dairy plant that led to over 150,000 infections with Salmonella enetrica [71]. In addition, significant cholera outbreaks have been associated with municipal water systems in developing countries, often stemming from inadequate sewage treatment, the mixing of sewage and drinking water because of inadvertent plumbing cross-connections, or improper equipment operation [72]. Similarly, contaminated water was responsible for an incident involving canned mangoes, where product contamination with Salmonella enetrica resulted from the infiltration of tainted cooling and cleaning water through minuscule leaks in the can walls [35].
6.3. Preparation and Handling
Food preparation commonly occurs in restaurants, homes, or processing establishment kitchens, and it might simply involve cooking, heating, and serving food on a plate. Contamination here could originate with food handlers if they are sick, do not follow accepted hygienic rules associated with good hand washing, or result from cross-contamination [12]. For instance, E. coli O157:H7 infection was first identified in 1982 after two outbreaks in the US were linked to eating undercooked hamburgers from a fast-food restaurant chain. Subsequently, this pathogen has emerged to become a major cause of outbreaks of bloody diarrhea, with cases of the infection being reported in Japan, Canada, the UK, and the US [40,73]. In addition, due to the lack of adequate hygiene and sanitation, Aeromonas spp. among other pathogens, may be found in food-processing equipment, which may then become a source for food cross-contamination [14].
Of late, a variety of plant-derived and dried food ingredients have been found to contain Cronobacter spp., particularly C. sakazakii, which can be present intrinsically within the manufacturing process following failures to follow suitable hygiene standards. Contamination may also occur extrinsically during the preparation of powdered infant formula at hospital neonatal units or in the home. This can occur after the hydration of infant formula prior to use, if kept under improper or prolonged storage that allows for multiplication and the growth of bacteria [35,74,75]. Another notable finding, especially in Southeast Asia, was that Streptococcus suis became linked to handling or consuming uncooked or undercooked pork and the slaughter of swine [76,77,78,79]. Additionally, an outbreak of gastroenteritis in North America was linked to St. suis contamination of cantaloupe on the farm because of failure to thoroughly clean or wash melons before they were cut [51].
6.4. Storage
Maintenance of suitable storage conditions is a major factor influencing the success of efforts to protect the safety of food and maximize its shelf life. In most jurisdictions, use of proper storage conditions is mandatory at the industrial level and widely promoted for use at home. Guidelines prescribe acceptable ranges of times and temperatures for refrigerated and frozen storage, environmental conditions like humidity, gaseous atmosphere, and flow rates [54]. Additionally, food storage practices have led to the emergence of some foodborne pathogens, particularly in undercooked meats, cheeses, and vegetables, that have been processed or held at unsuitable temperatures for extended periods, even when held in a refrigerator. For instance, meningitis outbreaks caused by Listeria monocytogenes have been linked to contaminated food because this psychrotroph can grow at 0 °C [35,80,81]. Another emerging human pathogen, the motile mesophile Aeromonas, particularly A. hydrophila, has recently been receiving growing public health recognition as a potential cause of both gastrointestinal and extra-intestinal infections, particularly in immunocompromised people, and multiple factors can result in serious health complications [14,82]. One of the most significant factors influencing the public health impact of Aeromonas in foods is their ability to grow at temperatures from 4 °C to 51 °C. Drinking contaminated water or eating food that has been processed with contaminated water poses greater risks for Aeromonas [14,83].
6.5. Packaging
Food packaging provides benefits, including advertising and branding, protection from physical damage or atmospheric protection, facilitating the preservation food and promoting shelf life [54]. Additives, including antioxidants, stabilizers, and plasticizers, are frequently added to monomer ingredients during the production of packaging material to enhance film properties. Under some conditions, the migration of substances from the packaging to wrapped food has been reported [55]. It has been considered that packaging could contribute to the emergence of pathogenic organisms, although there have been no reported outbreaks related directly to packaging materials [54,84].
6.6. Transportation/Distribution
Transportation and distribution of food from its origin at farms, large and small suppliers or industrial complexes to wholesalers, retailers, food service operations at restaurants, schools and cafeterias, or the consumer at home represent vulnerable intervals for perishable products. The importance of this issue rises from the number of unfavorable events that can occur during this step, and these may involve physical damage, temperature abuse, and exposure to poor sanitation such as use of unclean vehicles and careless handling, which can increase the risk of biological, chemical, or physical hazards causing contamination of the food being moved [1,12,85,86]. For instance, in 1994, an outbreak of foodborne illness due to S. Enteritidis was confirmed in 80 persons who ate ice cream distributed nationally after a pre-mix was transported in an unclean tanker truck [51].
7. Food Industry Demographics (Globalization)
In many food industries, there is a trend toward increasing market size and the wider geographic distribution of food products [35,39,87]. Moreover, modern production techniques enable increased efficiency and deliver lower costs for food production. However, for processes that use products of a biological nature, they can also increase the risk of accidental contamination and amplify its effects [32,55,88]. Globalization has made the issue worse by making it possible to introduce agents from distant locations, which occurred when large batches of hamburger meat contaminated by Shiga toxigenic E. coli strains (E. coli O157) were widely distributed [32,89,90].
8. Outbreaks Associated with Emerging Foodborne Pathogens Related to Technology and Industry
The epidemiology of foodborne illnesses has changed in recent years since new pathogens have emerged. Table 2 includes selected outbreaks in the United States, China, France, and South Korea that are associated with emerging foodborne pathogens related to technology and industry.

Table 2.
Outbreaks associated with emerging foodborne pathogens related to technology and industry.
9. Future of Work and Recommendations
It is crucial to emphasize the significance of careful tracking and managing of outbreaks caused by new pathogens or new food vehicles. It is essential to adapt or adopt measures to address emerging pathogens through an in-depth understanding of the organisms’ biology. This should include development of suitable analytical and typing techniques to characterize novel pathogen incidence in raw components and ingredients, the niches they occupy, their behavior during processing and handling of foods, as well as their ecology in food processing settings. These are in addition to the planning and implementation of improvements and modifications needed to achieve their control. This missing information will facilitate the evaluation and improvement of preventive controls currently employed by food producers. It should be kept in mind that manufacturers face many challenges in regard to conveying safe food products to the consumer and that they have a major role in preventing contamination and reducing the presence of known pathogenic microbes, as well as in reducing the emergence of new organisms by applying the safety and hygienic practices at the manufacturer level, or even in the home or restaurant, right up to the dining table, which can be achieved by the implementation the phenomenon of food safety culture. Further studies are required to identify factors that may trigger the emergence or re-emergence of foodborne pathogens. International cooperation on research studies should be endorsed and coordinated to resolve the problem of emerging foodborne pathogens. In addition, further studies of the factors affecting the resistance of foodborne pathogens to antibiotic and natural antimicrobials are required.
10. Conclusions
Microbial hazards have the potential to affect the food chain in a variety of places, including production, processing, preparation, handling, storage, and transportation. It is of interest that alterations in food production and handling practices anywhere from farm to plate can significantly contribute to the emergence of foodborne pathogens. In response, emerging pathogens can become associated with specific segments of the food chain. Simultaneously, global transformation and shifts in how food is processed, packaged, stored, and distributed throughout the 20th century have given rise to new foodborne pathogens and alterations in the landscape of foodborne illness epidemiology. Climate change and weather extremes may play a more important role in influencing the pattern of new pathogen evolution. These factors underscore the importance of conscientious, consistent surveillance and effective management of outbreaks originating from novel pathogens or unconventional food conduits. It is essential to understand the principle and mechanisms of action of modern technologies that are currently used in the food industry to control foodborne pathogens and their possible roles in the emergence and re-emergence of foodborne pathogens. Further, it is crucial to implement good agricultural, manufacturing, and hygienic practices, as is applying the Food Safety Management System (FSMS) based on the principles of Hazard Analysis and Critical Control Point (HACCP) and quality control (QC) as a preventive approach for recognizing, preventing, and decreasing foodborne pathogens to ensure the microbial safety of food products.
Author Contributions
S.H.: writing—original draft, Data curation. A.N.O.: Formal analysis; Conceptualization; supervision, writing—original draft; writing—review and editing. M.A.A.-H.: writing—review and editing. A.A.: Data curation. writing—original draft. H.M.S.: Writing—original draft. M.A. (Mahmoud Abughoush): Writing—review and editing. A.A.-N.: Writing—review and editing. T.O.: Writing—review and editing. M.A. (Mutamed Ayyash): Writing—review and editing. R.A.H.: Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef]
- Tauxe, R.V. Emerging Foodborne Pathogens. Int. J. Food Microbiol. 2002, 78, 31–41. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne Illness Acquired in the United States—Major Pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, Y. Etiological Agents Implicated in Foodborne Illness World Wide. Food Sci. Anim. Resour. 2021, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kirk, M.; Ford, L.; Glass, K.; Hall, G. Foodborne Illness, Australia, circa 2000 and circa 2010. Emerg. Infect. Dis. 2014, 20, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). FAO, WHO Set an Example of Collaborative Action for Safe Food with a Systems Approach. 2021. Available online: https://www.who.int/europe/news/item/08-06-2021-fao-who-set-an-example-of-collaborative-action-for-safe-food-with-a-systems-approach (accessed on 14 December 2023).
- De Sousa, C.P. The Impact of Food Manufacturing Practices on Food Borne Diseases. Braz. Arch. Biol. Technol. 2008, 51, 615–623. [Google Scholar] [CrossRef]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Editorial: Foodborne Pathogens: Hygiene and Safety. Front. Microbiol. 2019, 10, 1974. [Google Scholar] [CrossRef]
- Beeton-Kempen, N. Technology Networks Emerging Technologies in Combating Foodborne Illness. Technology Networks, Applied Sciences. 2019. Available online: https://www.technologynetworks.com/applied-sciences/articles/emerging-technologies-in-combating-foodborne-illness-315787 (accessed on 10 December 2023).
- Belina, D.; Hailu, Y.; Gobena, T.; Hald, T.; Njage, P.M.K. Prevalence and Epidemiological Distribution of Selected Foodborne Pathogens in Human and Different Environmental Samples in Ethiopia: A Systematic Review and Meta-Analysis. One Health Outlook 2021, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- CDC. How Food Gets Contaminated—The Food Production Chain. Available online: https://www.cdc.gov/foodsafety/production-chain.html (accessed on 10 December 2023).
- Smith, J.L.; Fratamico, P.M. Emerging and Re-Emerging Foodborne Pathogens. Foodborne Pathog. Dis. 2018, 15, 737–757. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Lianou, A.; Sofos, J.N. Food Safety: Emerging Pathogens. Encycl. Agric. Food Syst. 2014, 2014, 250–272. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of Antibiotic Resistance InListeria MonocytogenesIsolated from Food Products: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, J.F.; Grace, D. The Consequences of Human Actions on Risks for Infectious Diseases: A Review. Infect. Ecol. Epidemiol. 2015, 5, 30048. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging Human Infectious Diseases and the Links to Global Food Production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Saber, T.; Samir, M.; El-Mekkawy, R.M.; Ariny, E.; El-Sayed, S.R.; Enan, G.; Abdelatif, S.H.; Askora, A.; Merwad, A.M.A.; Tartor, Y.H. Methicillin- and Vancomycin-Resistant Staphylococcus Aureus from Humans and Ready-To-Eat Meat: Characterization of Antimicrobial Resistance and Biofilm Formation Ability. Front. Microbiol. 2022, 12, 735494. [Google Scholar] [CrossRef] [PubMed]
- Tartor, Y.H.; Gharieb, R.M.A.; Abd El-Aziz, N.K.; El Damaty, H.M.; Enany, S.; Khalifa, E.; Attia, A.S.A.; Abdellatif, S.S.; Ramadan, H. Virulence Determinants and Plasmid-Mediated Colistin Resistance Mcr Genes in Gram-Negative Bacteria Isolated from Bovine Milk. Front. Cell. Infect. Microbiol. 2021, 11, 761417. [Google Scholar] [CrossRef] [PubMed]
- Behravesh, C.B.; Williams, I.T.; Tauxe, R.V. Emerging Foodborne Pathogens and Problems: Expanding Prevention Efforts before Slaughter or Harvest. In Improving Food Safety through a One Health Approach: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK114501/ (accessed on 30 November 2023).
- Gabriël, S.; Dorny, P.; Saelens, G.; Dermauw, V. Foodborne Parasites and Their Complex Life Cycles Challenging Food Safety in Different Food Chains. Foods 2022, 12, 142. [Google Scholar] [CrossRef]
- Han, D.; Chen, J.; Chen, W.; Wang, Y. Bongkrekic Acid and Burkholderia Gladioli Pathovar Cocovenenans: Formidable Foe and Ascending Threat to Food Safety. Foods 2023, 12, 3926. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Taybeh, A.O.; Al-Nabulsi, A.; Al-Holy, M.; Hatmal, M.M.; Alzyoud, J.; Aolymat, I.; Abughoush, M.H.; Shahbaz, H.; Alzyoud, A.; et al. Common and Potential Emerging Foodborne Viruses: A Comprehensive Review. Life 2024, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-Borne Diseases—The Challenges of 20 years Ago Still Persist While New Ones Continue to Emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Swerdlow, D.L.; Wells, S.J. Factors in the Emergence of Food Borne Diseases. Vet. Clin. N. Am. Food Anim. Pract. 1998, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tack, D.M.; Marder, E.P.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Hurd, S.; Scallan, E.; Lathrop, S.; Muse, A.; Ryan, P.; et al. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2015–2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Michino, H.; Araki, K.; Minami, S.; Takaya, S.; Sakai, N.; Miyazaki, M.; Ono, A.; Yanagawa, H. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 1999, 150, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.A. Emerging Zoonoses: The Challenge for Public Health and Biodefense. Prev. Vet. Med. 2008, 86, 216–223. [Google Scholar] [CrossRef]
- Morse, S.S. Factors in the Emergence of Infectious Diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef]
- Richard, L.; Aenishaenslin, C.; Zinszer, K. Zoonoses and Social Determinants of Health: A Consultation of Canadian Experts. One Health 2020, 12, 100199. [Google Scholar] [CrossRef]
- Institute of Medicine (US). Improving Food Safety through a One Health Approach: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2012. [Google Scholar]
- Smoot, L.; Cordier, J.-L. Emerging Foodborne Pathogens and the Food Industry; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Seimenis, A.; Battelli, G. Main Challenges in the Control of Zoonoses and Related Foodborne Diseases in the South Mediterranean and Middle East Region. Vet. Ital. 2018, 54, 97–106. [Google Scholar] [CrossRef]
- Badiane, O.; Hendriks, S.L.; Glatzel, K.; Abdelradi, F.; Admassie, A.; Adjaye, J.A.; Ayieko, M.; Bekele, E.; Chaibi, T.; Hag, M.; et al. Policy Options for Food System Transformation in Africa and the Role of Science, Technology and Innovation; Springer Ebooks: Berlin/Heidelberg, Germany, 2023; pp. 713–735. [Google Scholar] [CrossRef]
- Sahoo, M.; Panigrahi, C.; Aradwad, P. Management Strategies Emphasizing Advanced Food Processing Approaches to Mitigate Food Borne Zoonotic Pathogens in Food System. Food Front. 2022, 3, 641–665. [Google Scholar] [CrossRef]
- Tauxe, R.V.; Doyle, M.P.; Kuchenmüller, T.; Schlundt, J.; Stein, C.E. Evolving Public Health Approaches to the Global Challenge of Foodborne Infections. Int. J. Food Microbiol. 2010, 139, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Swerdlow, D.L. The Changing Epidemiology of Foodborne Diseases. Am. J. Med. Sci. 1996, 311, 23–29. [Google Scholar] [CrossRef] [PubMed]
- McArthur, D.B. Emerging Infectious Diseases. Nurs. Clin. N. Am. 2019, 54, 297–311. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Seal, S.; Dharmarajan, G.; Khan, I. Evolution of Pathogen Tolerance and Emerging Infections: A Missing Experimental Paradigm. eLife 2021, 10, e68874. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D.J. Cost Analysis and Environmental Impact of Pulsed Electric Fields and High Pressure Processing in Comparison with Thermal Pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- Shahbaz, H.M.; Javed, F.; Park, J. Current Challenges and Future Applications of HPP; Springer: Cham, Switzerland, 2023; pp. 71–72. [Google Scholar] [CrossRef]
- Muthuvelu, K.S.; Ethiraj, B.; Pramnik, S.; Raj, N.; Venkataraman, S.; Rajendran, D.S.; Bharathi, P.; Palanisamy, E.; Narayanan, A.; Vaidyanathan, V.K.; et al. Biopreservative Technologies of Food: An Alternative to Chemical Preservation and Recent Developments. Food Sci. Biotechnol. 2023, 32, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P. Recent Approaches in Food Bio-Preservation—A Review. Open Vet. J. 2018, 8, 104. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A.; Simal-Gandara, J. Technological Strategies Ensuring the Safe Arrival of Beneficial Microorganisms to the Gut: From Food Processing and Storage to Their Passage through the Gastrointestinal Tract. Food Res. Int. 2020, 129, 108852. [Google Scholar] [CrossRef]
- Schneider, S.A. Examining Food Safety from a Food Systems Perspective: The Need for a Holistic Approach. Wis. Law Rev. 2014, 2014, 397–419. Available online: https://ssrn.com/abstract=3493366 (accessed on 10 January 2024).
- Palmer, J. The Pros and Cons of Emerging Technology in Our Food System. Available online: https://ccafs.cgiar.org/news/pros-and-cons-emerging-technology-our-food-system (accessed on 10 November 2023).
- Breiman, R.F. Impact of Technology on the Emergence of Infectious Diseases. Epidemiol. Rev. 1996, 18, 4–9. [Google Scholar] [CrossRef]
- Foxman, B.; Mehta, S. Impact of Technological Developments on Infectious Disease Epidemiology: Lessons from the First 100 Years of AJE. Am. J. Epidemiol. 2023, 192, 1820–1826. [Google Scholar] [CrossRef]
- Skovgaard, N. New Trends in Emerging Pathogens. Int. J. Food Microbiol. 2007, 120, 217–224. [Google Scholar] [CrossRef]
- Nerín, C.; Aznar, M.; Carrizo, D. Food Contamination during Food Process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- Lebelo, K.; Malebo, N.; Mochane, M.J.; Masinde, M. Chemical Contamination Pathways and the Food Safety Implications along the Various Stages of Food Production: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5795. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Cyclosporiasis Illnesses in the United States. 2023. Available online: https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/2023/index.html (accessed on 5 December 2023).
- Doyle, M.P.; Erickson, M.C. Opportunities for Mitigating Pathogen Contamination during On-Farm Food Production. Int. J. Food Microbiol. 2012, 152, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. Infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ghaju Shrestha, R.; Tanaka, Y.; Haramoto, E. A Review on the Prevalence of Arcobacter in Aquatic Environments. Water 2022, 14, 1266. [Google Scholar] [CrossRef]
- Houf, K.; Stephan, R. Isolation and characterization of the emerging food-borne pathogen Arcobacter from human stool. J. Microbiol. Methods 2007, 68, 408–413. [Google Scholar] [CrossRef]
- Borji, S.; Kadivarian, S.; Dashtbin, S.; Kooti, S.; Abiri, R.; Motamedi, H.; Moradi, J.; Rostamian, M.; Alvandi, A. Global Prevalence of Clostridioides difficile in 17,148 Food Samples from 2009 to 2019: A Systematic Review and Meta-Analysis. J. Health Popul. Nutr. 2023, 42, 36. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.E. Raw Animal Meats as Potential Sources of Clostridium Difficile in Al-Jouf, Saudi Arabia. Food Sci. Anim. Resour. 2021, 41, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Thitaram, S.N.; Frank, J.F.; Lyon, S.A.; SIiragusa, G.R.; Bailey, J.S.; Lombard, J.E.; Haley, C.A.; Wagner, B.A.; Dargatz, D.A.; Fedorka-Cray, P.J. Clostridium Difficile from Healthy Food Animals: Optimized Isolation and Prevalence. J. Food Prot. 2011, 74, 130–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- US Food and Drug Administration. Foodborne Pathogens. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/foodborne-pathogens (accessed on 17 December 2023).
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, M.J.; Lu, X. Survival and Control of Campylobacter in Poultry Production Environment. Front. Cell. Infect. Microbiol. 2021, 10, 615049. [Google Scholar] [CrossRef]
- Kornacki, J.L. Principles of Microbiological Troubleshooting in the Industrial Food Processing Environment; Springer: New York, NY, USA, 2010; ISBN 9781441955173. [Google Scholar]
- Behling, R.G.; Eifert, J.; Erickson, M.C.; Gurtler, J.B.; Kornacki, J.L.; Line, E.; Radcliff, R.; Ryser, E.T.; Stawick, B.; Yan, Z. Selected Pathogens of Concern to Industrial Food Processors: Infectious, Toxigenic, Toxico-Infectious, Selected Emerging Pathogenic Bacteria. In Principles of Microbiological Troubleshooting in the Industrial Food Processing Environment; Springer: New York, NY, USA, 2010; pp. 5–61. [Google Scholar] [CrossRef]
- Giacometti, F.; Lucchi, A.; Di Francesco, A.; Delogu, M.; Grilli, E.; Guarniero, I.; Stancampiano, L.; Manfreda, G.; Merialdi, G.; Serraino, A. Arcobacter Butzleri, Arcobacter Cryaerophilus, and Arcobacter Skirrowii Circulation in a Dairy Farm and Sources of Milk Contamination. Appl. Environ. Microbiol. 2015, 81, 5055–5063. [Google Scholar] [CrossRef] [PubMed]
- Swire, E.; Colchester, A. Out of Sight, out of Mind? BSE 30 Years On: Continuing Environmental Risks to Human Health. Land Use Policy 2023, 126, 106521. [Google Scholar] [CrossRef]
- Olsen, S.J.; Ying, M.; Davis, M.F.; Deasy, M.; Holland, B.; Iampietro, L.; Baysinger, C.M.; Sassano, F.; Polk, L.D.; Gormley, B.; et al. Multidrug-Resistant Salmonella Typhimurium Infection from Milk Contaminated after Pasteurization. Emerg. Infect. Dis. 2004, 10, 932–935. [Google Scholar] [CrossRef]
- Fredrick, T.; Ponnaiah, M.; Murhekar, M.V.; Jayaraman, Y.; David, J.K.; Vadivoo, S.; Joshua, V. Cholera Outbreak Linked with Lack of Safe Water Supply Following a Tropical Cyclone in Pondicherry, India, 2012. J. Health Popul. Nutr. 2015, 33, 31–38. [Google Scholar]
- Travert, B.; Rafat, C.; Mariani, P.; Cointe, A.; Dossier, A.; Coppo, P.; Joseph, A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: Specificities of Adult Patients and Implications for Critical Care Management. Toxins 2021, 13, 306. [Google Scholar] [CrossRef]
- Drudy, D.; Mullane, N.R.; Quinn, T.; Wall, P.G.; Fanning, S. Enterobacter Sakazakii: An Emerging Pathogen in Powdered Infant Formula. Clin. Infect. Dis. 2006, 42, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, Z.E.; Hunt, K.; Koolman, L.; Butler, F.; Fanning, S. Cronobacter Species in the Built Food Production Environment: A Review on Persistence, Pathogenicity, Regulation and Detection Methods. Microorganisms 2023, 11, 1379. [Google Scholar] [CrossRef] [PubMed]
- Boonyong, N.; Kaewmongkol, S.; Khunbutsri, D.; Satchasataporn, K.; Meekhanon, N. Contamination of Streptococcus Suis in Pork and Edible Pig Organs in Central Thailand. Vet. World 2019, 12, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kerdsin, A.; Segura, M.; Fittipaldi, N.; Gottschalk, M. Sociocultural Factors Influencing Human Streptococcus Suis Disease in Southeast Asia. Foods 2022, 11, 1190. [Google Scholar] [CrossRef] [PubMed]
- Segura, M. Streptococcus Suis: An Emerging Human Threat. J. Infect. Dis. 2009, 199, 4–6. [Google Scholar] [CrossRef]
- Wangsomboonsiri, W.; Luksananun, T.; Saksornchai, S.; Ketwong, K.; Sungkanuparph, S. Streptococcus Suis Infection and Risk Factors for Mortality. J. Infect. 2008, 57, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria Monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Schlech, W.F.; Acheson, D. Foodborne Listeriosis. Clin. Infect. Dis. 2000, 31, 770–775. [Google Scholar] [CrossRef]
- Hoel, S.; Vadstein, O.; Jakobsen, A. The Significance of Mesophilic aeromonas spp. In Minimally Processed Ready-To-Eat Seafood. Microorganisms 2019, 7, 91. [Google Scholar] [CrossRef]
- Praveen, P.K.; Debnath, C.; Shekhar, S.; Dalai, N.; Ganguly, S. Incidence of Aeromonas spp. Infection in Fish and Chicken Meat and Its Related Public Health Hazards: A Review. Vet. World 2016, 9, 6–11. [Google Scholar] [CrossRef]
- Collins, J. Impact of Changing Consumer Lifestyles on the Emergence/Reemergence of Foodborne Pathogens. Emerg. Infect. Dis. 1997, 3, 471–479. [Google Scholar] [CrossRef]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Gapud, V. Driving Best Practices in Distribution. Food Safety Magazine. 2006. Available online: https://www.food-safety.com/articles/4621-driving-best-practices-in-distribution (accessed on 15 November 2023).
- Wood, B.; Williams, O.; Nagarajan, V.; Sacks, G. Market Strategies Used by Processed Food Manufacturers to Increase and Consolidate Their Power: A Systematic Review and Document Analysis. Glob. Health 2021, 17, 17. [Google Scholar] [CrossRef]
- Hodson, E.; Niggli, U.; Kitajima, K.; Lal, R.; Sadoff, C. Boost Nature-Positive Production; Springer Ebooks: Cham, Switzerland, 2023; pp. 319–340. [Google Scholar] [CrossRef]
- Fukuda, K. Food Safety in a Globalized World. Bull. World Health Organ. 2015, 93, 212. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Waterson, P. Global Food Safety as a Complex Adaptive System: Key Concepts and Future Prospects. Trends Food Sci. Technol. 2019, 91, 409–425. [Google Scholar] [CrossRef]
- Besser, R.E.; Lett, S.M.; Weber, J.T.; Doyle, M.P.; Barrett, T.J.; Wells, J.G.; Griffin, P.M. An Outbreak of Diarrhea and Hemolytic Uremic Syndrome from Escherichia coli O157:H7 in Fresh-Pressed Apple Cider. JAMA 1993, 269, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Berkelman, R.L.; Bryan, R.T.; Osterholm, M.T.; LeDuc, J.W.; Hughes, J.M. Infectious Disease Surveillance: A Crumbling Foundation. Science 1994, 264, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, T.W.; Hedberg, C.W.; Slutsker, L.; White, K.E.; Besser-Wiek, J.M.; Moen, M.E.; Feldman, J.; Coleman, W.W.; Edmonson, L.M.; MacDonald, K.L.; et al. A National Outbreak of Salmonella Enteritidis Infections from Ice Cream. The Investigation Team. N. Engl. J. Med. 1996, 334, 1281–1286. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Hepatitis A Associated with Consumption of Frozen Strawberries—Michigan, March 1997. MMWR Morb. Mortal. Wkly. Rep. 1997, 46, 288–295. [Google Scholar]
- Burr, R.; Effler, P.; Kanenaka, R.; Nakata, M.; Holland, B.; Angulo, F.J. Emergence of Salmonella Serotype Enteritidis Phage Type 4 in Hawaii Traced to Locally-Produced Eggs. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2005, 9, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Daniels, N.A.; Bergmire-Sweat, D.A.; Schwab, K.J.; Hendricks, K.A.; Reddy, S.; Rowe, S.M.; Fankhauser, R.L.; Monroe, S.S.; Atmar, R.L.; Glass, R.I.; et al. A Foodborne Outbreak of Gastroenteritis Associated with Norwalk-like Viruses: First Molecular Traceback to Deli Sandwiches Contaminated during Preparation. J. Infect. Dis. 2000, 181, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Haeghebaert, S.; Sulem, P.; Deroudille, L.; Vanneroy-Adenot, E.; Bagnis, O.; Bouvet, P.; Grimont, F.; Brisabois, A.; Hervy, C.; Espié, E.; et al. Two Outbreaks of Salmonella Enteritidis Phage Type 8 Linked to the Consumption of Cantal Cheese Made with Raw Milk, France, 2001. Eurosurveillance 2003, 8, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, G.-Q.; Tang, G.-P.; Zou, Z.-T.; Yao, G.-H.; Zeng, G. A Foodborne Outbreak of Aeromonas Hydrophila in a College, Xingyi City, Guizhou, China, 2012. West. Pac. Surveill. Response J. WPSAR 2012, 3, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Oh, S.-S.; Oh, K.-H.; Park, J.-H.; Jang, E.J.; Chung, G.T.; Yoo, C.-K.; Bae, G.-R.; Cho, S.-H. An Outbreak of Foodborne Illness Caused by Enteroaggregative Escherichia coli in a High School in South Korea. Jpn. J. Infect. Dis. 2015, 68, 514–519. [Google Scholar] [CrossRef]
- Park, J.-H.; Jung, S.; Shin, J.; Lee, J.S.; Joo, I.S.; Lee, D.-Y. Three Gastroenteritis Outbreaks in South Korea Caused by the Consumption of Kimchi Tainted by Norovirus GI.4. Foodborne Pathog. Dis. 2015, 12, 221–227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).