Abstract
In the European Union, gastrointestinal disease in pigs is the main indication for the use of colistin, but large-scale epidemiologic data concerning the frequency of mobile colistin resistance (mcr) genes in pig-associated pathotypes of Escherichia coli (E. coli) are lacking. Multiplex polymerase chain reactions were used to detect virulence-associated genes (VAGs) and mcr-1–mcr-10 genes in 10,573 porcine E. coli isolates collected in Germany from July 2000 to December 2021. Whole genome sequencing was performed on 220 representative mcr-positive E. coli strains. The total frequency of mcr genes was 10.2%, the most frequent being mcr-1 (8.4%) and mcr-4 (1.6%). All other mcr genes were rarely identified (mcr-2, mcr-3, mcr-5) or absent (mcr-6 to mcr-10). The highest frequencies of mcr genes were found in enterotoxigenic and shiga toxin-encoding E. coli (ETEC/STEC hybrid) and in edema disease E. coli (EDEC) strains (21.9% and 17.7%, respectively). We report three novel mcr variants, mcr-1.36, mcr-4.8, and mcr-5.5. In 39 attaching and effacing E. coli (AEEC) isolates analyzed in our study, the eae subtype β1 was the most prevalent (71.8%). Constant surveillance for the presence of mcr genes in various sectors should consider the different frequency of mcr-positive isolates in pathogenic E. coli.
Keywords:
Escherichia coli; pathotype; mobile colistin resistance; mcr-1; mcr-4; mcr-5; plasmid; eae; swine 1. Introduction
Polymyxins are considered last-resort antibiotics in human medicine against infections caused by multidrug-resistant Gram-negative bacteria [1]. The polymyxin antibiotic colistin (polymyxin E) has been widely used for treating intestinal infections in swine caused by Escherichia coli. Enterotoxigenic E. coli (ETEC) and edema disease E. coli (EDEC) are the causative agents of enteric diseases and edema disease in piglets, resulting in significant economic losses in the swine industry worldwide [2]. ETEC are defined by the possession of at least one of the adhesive fimbriae F4 (encoded by the fae genes), F5 (fan), F6 (fas), F18 (fed), and F41 (fimF41) in combination with one of the heat-labile toxins LT-Ia or LT-Ib (eltB-Ip) or heat-stable toxins ST-Ia or ST-II (estap or estb) [3]. EDEC characteristically harbors genes for shiga toxin 2e (stx2e) and for adhesive fimbriae F18 (fed) [4]. In addition, other E. coli isolates that do not strictly apply to these pathotype definitions might be involved in porcine intestinal disorders that are commonly treated with antibiotics. According to a recent study from 2022 about the use of colistin in veterinary medicine in the European Union, it was stated that the main indication for the use of colistin was gastrointestinal disease in pigs [5].
In 2015, the plasmid-mediated mobile colistin resistance (mcr) gene mcr-1 was identified in a porcine E. coli isolate from China, followed by reports of nine additional mcr genes (mcr-2–mcr-10) and their variants [6]. A number of studies from across the globe, including studies from Germany, reported different frequencies of mcr genes in fecal E. coli isolates from healthy pigs, as recently summarized [7]. In contrast, many fewer studies were performed to determine the frequency of mcr genes among clinical isolates, i.e., obtained from pigs with post-weaning diarrhea or from enteric colibacillosis [8,9,10,11,12]. In addition, the latter studies rarely provided a detailed molecular typing of E. coli isolates regarding their affiliation to distinct intestinal pathogenic pathotypes predicted by the presence of virulence-associated genes (VAGs). This would indeed be very helpful to explore, even though it would only be based on an observational approach, if certain pathotypes are more prone to acquire mcr genes than others.
To narrow this knowledge gap, we investigated the distribution of mcr genes mcr-1 to mcr-10, which were defined at the time of writing, among a collection of more than 10,000 porcine E. coli isolates, according to their pathotype designation. The genomes of selected mcr-positive E. coli isolates were sequenced and analyzed for mcr gene variants and their location on distinct plasmids as well as for E. coli multi locus sequence types and phylogenetic groups. Finally, the presence of extended-spectrum β-lactamase (ESBL), AmpC, and carbapenemase genes among sequenced isolates was explored.
2. Materials and Methods
2.1. Sample Processing and Isolation of Putative E. coli Colonies
We investigated 9421 E. coli isolates that were obtained mainly from feces or mucosal swabs (rectum or small intestine) of piglets suffering from neonatal diarrhea, post-weaning diarrhea, or edema disease. The isolates were collected as part of routine microbiological diagnostics at the Institute for Hygiene and Infectious Diseases of Animals, Faculty of Veterinary Medicine, Justus Liebig University Giessen, Germany, from July 2000 to December 2021. Additional porcine E. coli isolates (n = 1152) were received through submissions of other veterinary diagnostic laboratories for further molecular typing in our institute. Some of these isolates have already been included in a recent study on the presence of mcr-1 and mcr-2 genes in porcine E. coli isolates [7]. Ethical review and approval were waived for this study due to the fact that the sample collection was not for research but for diagnostic purposes, and only the results obtained were used for scientific purposes. No additional pain, suffering, or harm was inflicted on the animals as a result of our study.
According to available metadata on the origin of samples and/or E. coli isolates, strains were obtained from neonatal diarrhea in piglets (i.e., isolates obtained from piglets ≤ 8 kg and/or ≤28 days with diarrhea) (n = 1473); PWD and diarrhea in elderly pigs (i.e., isolates obtained from pigs > 8 kg to ≤30 kg and/or >4 weeks to ≤12 weeks with diarrhea) (n = 3687); edema disease (i.e., isolates from pigs and/or farms, where edema disease occurred), either with (n = 702 isolates) or without diarrhea (n = 1413); diarrhea in fattening pigs (between 12 weeks and 7 months and/or >30 kg) (n = 672); diarrhea in pigs of unknown age and/or weight (n = 2605). The remaining 21 samples/isolates were provided for the typing of VAGs associated with diarrheal diseases in swine.
The maximum number of samples per farm was limited to samples from six pigs per submission. As the samples were provided for diagnostic services, they were treated immediately upon arrival at the laboratory. Fecal samples and mucosal swabs were streaked for single bacterial colonies on blood agar plates (blood agar base, Merck Chemicals, Darmstadt, Germany) containing 5% sheep blood and on Gassner agar (sifin diagnostics GmbH, Berlin, Germany). The cultures were incubated for approx. 18 h at 37 °C. Subsequently, up to six morphologically different, putative E. coli colonies were picked per sample and stored individually as pure bacterial suspensions in lysogeny broth (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) for further analysis. A single colony was regarded as putative E. coli in case the following phenotypes were observed: (i) circular, shiny, greyish diameter of 1.0–2.0 mm on blood agar or (ii) deep blue with a blue halo, diameter of 1.0–2.5 mm on Gassner agar. If hemolytic and non-hemolytic colonies of putative E. coli occurred on the same blood agar plate, representative colonies of both phenotypes were picked. Species identification was performed by matrix-assisted laser desorption time-of-flight mass spectrometry MALDI-TOF MS (Bruker Daltonics, Bremen, Germany) by applying the standard MBT Compass reference library (different versions according to the study year).
2.2. Escherichia coli Pathotyping PCR and Prediction of Pathotypes
About 3 μL of lysogeny broth cultures (about 3 × 105 CFU) were used as template DNA in a modified multiplex PCR (MP-PCR-VAGs), in total targeting 10 virulence-associated genes (VAGs), which are associated with different pathotypes of intestinal pathogenic E. coli [13,14,15]. In detail, E. coli isolates were tested for the presence of genes of adhesive fimbriae F4 (encoded by the gene faeG), F5 (fanA), F6 (fasA), F18 (fedA), and F41 (fimF41a), the afimbrial adhesin intimin (eae), heat-labile E. coli enterotoxins LT-Ia and LT-Ib (eltB-Ip), heat-stable E. coli enterotoxins ST-Ia and ST-II (estap/estb), and shiga toxin 2 (stx2). Positive controls used were E. coli strains B41 (fimF41a, fanA, estap), 987P (fasA, estap), E57 (fedA, estap, estb, stx2), G7 (faeG, eltB-Ip), and TTP-1 (eae, stx2). An E. coli K-12 laboratory strain was used as a negative control. Details regarding primers and controls used in the MP-PCR-VAGs are provided in Table S1. Each study isolate that proved positive for at least one of the tested VAGs was stored in a glycerin stock at −80 °C. If isolates from the same pig showed different VAG profiles, a representative isolate of each profile was stored. Part of the pathotyping PCRs have already been conducted as part of a recent study [7].
Pathotype prediction was conducted based on the presence of VAGs determined by PCR: Adhesive fimbriae E. coli (in the following termed AdhF-Ec), positive for at least one adhesive fimbriae gene (faeG, fanA, fasA, fedA, fimF41a); AEEC (often also referred to as atypical EPEC), positive for eae; EDEC, positive for fedA and stx2; ETEC, positive for at least one adhesive fimbriae gene (faeG, fanA, fasA, fedA, fimF41a) and at least one enterotoxin gene (eltB-Ip, estap, estb); ETEC-like, positive for at least one enterotoxin gene (eltB-Ip, estap, estb); ETEC/STEC hybrid (in the following simply termed ETEC/STEC), positive for at least one adhesive fimbriae gene (faeG, fanA, fasA, fedA, fimF41a) and at least one enterotoxin gene (eltB-Ip, estap, estb) and stx2; STEC, positive for stx2; other, positive for a combination of VAGs not covered by the previously defined pathotypes.
2.3. PCR for the Detection of Mobile Colistin Resistance Genes mcr-1 to mcr-10
Two multiplex (MP) PCRs were applied to detect mcr-1 to mcr-10 genes. The first MP-PCR enabled the detection of genes mcr-1 to mcr-5 and was mostly based on previously published primer sequences [12,16,17,18,19]. Only one primer (MCR-5-mp-fw) was newly created in this study. The second MP-PCR protocol was based on a previous protocol [19] that we modified by including two primers to amplify the novel mcr-10 gene in addition to genes mcr-6 to mcr-9. Details regarding primers and controls used in MCR MP-PCRs I and II are provided in Table S1.
2.4. Whole Genome Sequence Analysis
Genomic DNA was extracted from E. coli bacteria using the Master Pure™ DNA Purification Kit (Biozym Scientific GmbH, Hessisch Oldendorf, Germany). Bacterial genomes were sequenced using an Illumina MiSeq sequencer (MiSeq Reagent Kit V.3; Illumina Inc., San Diego, CA, USA) via multiplexing of 30 samples per flow cell using 2 × 150 bp paired-end reads to obtain an average coverage of 90-fold. Quality control, including contamination removal and adapter trimming, were performed using an in-house pipeline. De novo assemblies were generated via the SPAdes Genome Assembler (v3.15.5) with the “—isolate” flag [20]. The Bakta pipeline (v1.8.2) was employed using species-specific databases for genomic annotation of the bacterial genomes [21].
2.5. Phylogroups, Sequence Types, Clonotypes, Antimicrobial Resistance Genes, Virulence-Associated Genes
Bacterial genome sequence data were analyzed in silico to classify isolates into one of the eight E. coli phylogenetic groups (A, B1, B2, C, D, E, F, and G) or into a cryptic clade using the refined ClermonTyping method, based on the in vitro PCR assay, targeting chuA, yjaA, TspE4.C2, arpA, and trpA (http://clermontyping.iame-research.center/, accessed on 20 October 2023). MLST 2.0 (https://cge.food.dtu.dk/services/MLST/, accessed on 20 October 2023) was used to determine sequence types (STs) according to the Achtman scheme, employing seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA, and recA). The clonotyping was based on the internal 469- and 489-nucleotide sequences of the fumC and fimH genes, respectively [22]. Allele assignments for fumC and fimH and their combinations (=clonotypes) were determined using CHTyper 1.0 (https://cge.food.dtu.dk/services/CHTyper/, accessed on 28 November 2023) [23]. Sero(geno)types were determined by applying SerotypeFinder 2.0 (https://cge.food.dtu.dk/services/SerotypeFinder/, accessed on 13 November 2023). The O25b serogroup was investigated in selected isolates by comparing the papB sequence with the reference sequence of the O25b:H4-ST131 uropathogenic strain EC958 (accession number HG941718; ENA; http://www.ebi.ac.uk/ena, accessed on 13 November 2023). AMR genes and chromosomal point mutations related to antimicrobial resistance were determined using ResFinder 4.1 (https://cge.food.dtu.dk/services/ResFinder/, accessed on 12 September 2023). ST131 isolates were additionally investigated for VAGs related with extraintestinal pathogenic E. coli (ExPEC) using VirulenceFinder 2.0 (https://cge.food.dtu.dk/services/VirulenceFinder/, accessed on 28 November 2023).
2.6. Statistical Analysis
Pathotype frequencies were reported as descriptive data. Fisher’s exact tests (https://www.graphpad.com/quickcalcs/contingency1/, accessed on 19 November 2023) were used to characterize the association of specific pathotypes with the occurrence of mcr genes. In particular, we performed pairwise comparisons of the pathotype associated with the highest mcr prevalence with the mcr abundance of the other pathotypes characterized within this study. We considered p-values below 0.05 to be statistically significant. All reported p-values are two-tailed.
3. Results
3.1. E. coli Pathotypes
More than half (60.5%) of the 10,573 porcine pathogenic E. coli isolates could be clearly delineated to an intestinal E. coli pathotype following the definition provided in Material and Methods (Section 2.2) and in Table 1. They were determined as ETEC (31.9%), EDEC (12.8%), AEEC (12.4%), and STEC (3.5%). The remaining isolates were assigned to the groups of ETEC-like (25.1%), i.e., harboring at least one enterotoxin but lacking adhesive fimbriae genes, AdhF-Ec (8.1%; positive for at least one adhesive fimbriae gene), and to hybrid groups termed ETEC/STEC (5.3%) or AEEC/STEC (0.22%), fulfilling the predictive criteria for both pathotypes simultaneously. Several other VAG profiles were observed, leading to further delineation of a small proportion of the isolates (0.78%) into additional hybrid pathotypes (Table 1).

Table 1.
Pathotype distribution and occurrence of mcr genes among 10,573 porcine E. coli isolates collected from 2000 to 2021 in Germany.
E. coli isolates obtained from pigs with clinical signs or suspected of having edema disease on the farms were predominantly defined as EDEC (25.5%), ETEC (21.9%), and ETEC-like (12.0%). Isolates obtained from piglets with neonatal diarrhea were mostly assigned as ETEC (47.6%), ETEC-like (24.0%), and AEEC (17.0%), while isolates collected from cases of PWD were predominantly allocated to the pathotypes ETEC (31.6%), ETEC-like (30.2%), AEEC (11.1%), and EDEC (8.3%). Also, among the isolates obtained from diarrheic fattening pigs and from diarrheic pigs of unknown ages, pathotypes ETEC and ETEC-like E. coli were predominant (35.2%, 26.1% and 29.2%, 24.0%, respectively).
ETEC isolates revealed 25 different VAG combinations. The most frequent combination was estb, eltB-Ip, faeG (n = 1451/3369; 43.1%), and, in decreasing frequency: estb, estap, fedA (n = 513, 15.2%); estb, estap, eltB-Ip, faeG (n = 508; 15.1%), estb, estap, faeG (n = 291, 8.6%), and estb, eltB-Ip, fedA (n = 205, 6.1%). The remaining 20 VAG patterns were each present in ≤2.0% (one to 67 isolates) of the 3369 ETEC isolates (Table S2). ETEC-like isolates, which lacked all fimbrial genes investigated by PCR, predominantly harbored estb as the sole enterotoxin gene (n = 1708/2650, 64.5%). Other frequent VAG patterns among ETEC-like isolates were estb, estap (n = 766, 28.9%), and estb, eltB-Ip (n = 142, 5.4%), whereas three other patterns occurred only rarely (estap, 1.1%; eltB-Ip, 0.04%; estb, estap, eltB-Ip 0.2%). Isolates of the group of AdhF-Ec predominantly carried the F18 fimbrial gene fedA (n = 790/862, 91.6%) and less often the F4 fimbrial gene faeG (n = 44, 5.1%), fim41a (n = 23, 2.7%), or other fimbrial genes. As per definitionem, all 1348 EDEC isolates harbored fedA and stx2, all AAEC isolates carried intimin gene eae, and all STEC isolates carried shiga toxin gene stx2.
3.2. Distribution of mcr Genes, Novel mcr Gene Alleles
Out of 10,573 E. coli isolates, 10.2% (n = 1075) carried one or two mcr genes (Table 1). With regard to different pathotypes, ETEC/STEC hybrid strains and EDEC strains revealed the highest proportion of mcr-positive isolates, respectively (21.9% and 17.7%) (Figure 1). Lower percentages were identified among the groups of AdhF-Ec (10.9%) and ETEC-like (10.3%), as well as among pathotypes STEC (10.4%) and ETEC (7.6%). The prevalence of mcr-positive isolates was significantly lower in non-ETEC/STEC isolates compared to the other pathotypes (p = 0.04 compared to EDEC; p < 0.0001 for all other pathotypes, Fisher’s exact test).
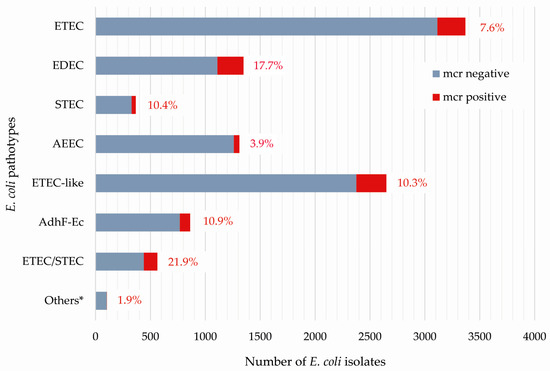
Figure 1.
Presence of mcr genes among 10,573 porcine E. coli isolates based on their association to a distinct pathotype. * Others include 104 E. coli isolates defined as ETEC-like/STEC (n = 75), AEEC/STEC (n = 23), ETEC-like/AEEC (n = 3), AdhF-Ec/EDEC (n = 2), and AdhF-Ec/AEEC (n = 1).
With respect to the different mcr genes, the group of ETEC/STEC hybrid isolates showed the highest percentages, i.e., 16.9% for mcr-1, 3.9% for mcr-4, and 1.1% for mcr-5 (Table 1), while only two ETEC-like and three EDEC isolates were positive for mcr-2 and mcr-3, respectively.
The overall frequency of mcr-1, either as a single gene or in combination with other mcr genes, was highest (8.4%), followed by mcr-4 (1.6%) and mcr-5 (0.3%) (both either as a single gene or in combination), mcr-3 (0.03%), and mcr-2 (0.02%). None of the isolates carried mcr-6, mcr-7, mcr-8, mcr-9, or mcr-10.
In terms of different time periods of sample collection and E. coli isolation, the prevalence of mcr-positive isolates differed as follows: 2000 to 2005 (0.8% among 1022 isolates obtained from this time period), 2006 to 2010 (5.9%/1266), 2011 to 2015 (15.8%/3270), and 2016 to 2021 (9.4%/5015). The earliest time points of mcr gene detection were 2001 (mcr-5, ETEC/STEC), 2005 (mcr-4, ETEC-like), 2006 (mcr-1, ETEC), 2014 (mcr-2, ETEC-like), and 2014 (mcr-3, EDEC).
Among 1075 mcr-positive porcine E. coli isolates, 220 isolates, representing different pathotypes, isolation dates, and mcr genes, were selected for whole genome sequencing. In detail, we chose (i) pathotypes (ETEC (n = 56), EDEC (n = 48), AEEC (n = 41), ETEC-like (n = 30), ETEC/STEC (n = 23), STEC (n = 14), and AdhF-Ec (n = 8)); (ii) isolation dates (2001–2005 (n = 5), 2006–2010 (n = 34), 2011–2015 (n = 113), and 2016–2021 (n = 68); and (iii) mcr genes (mcr-1 (n = 125), mcr-3 (n = 1), mcr-4 (n = 67), mcr-5 (n = 17), mcr-1 and mcr-4 (n = 6), mcr-1 and mcr-5 (n = 1), and mcr-4 and mcr-5 (n = 3) for whole genome sequencing.
Of 132 mcr-1-positive isolates, the majority (96.2%) carried the mcr-1.1 gene variant. One ST29-AEEC isolate obtained from a seven-week-old pig suffering from diarrhea in 2018 carried an mcr-1.26 allele. A novel mcr-1 variant, termed mcr-1.36 (NCBI Reference Sequence: NG_231577.1), was identified in an ST48-AEEC isolate which was provided as E. coli isolate from another laboratory in 2016. The mcr-1.36 gene variant differed from mcr-1.1 by a nucleotide substitution at position 1588 (G → T), resulting in an amino acid change at position 530 (alanine → serine) of MCR-1.36 compared to MCR-1. Another three isolates revealed mcr-1.1-like genes that were either disrupted by an IS26 element (ST29-AEEC obtained from a pig with watery diarrhea in 2015) or showed alternative start codons (ST1-EDEC, 2008; ST29-AAEC, 2016, both obtained from pigs with clinical signs of edema disease). In 53 isolates, the genomic contig carried both a plasmid replicon gene and an mcr gene, which allowed us to determine the location of mcr-1 genes on plasmids of incompatibility groups IncX4 (n = 44), IncHI2 (n = 7), and IncI2 (n = 2).
The mcr-3 gene variant shared 99.8% nucleotide sequence similarity and 100% deduced amino acid sequence identity to mcr-3.12 and MCR-3.12, respectively. The mcr-3-containing contig was 18,883 bp in length and was predicted as a plasmidial sequence (95.5%) using mlplasmids v2.1.0 (https://sarredondo.shinyapps.io/mlplasmids/, accessed on 28 November 2023). We observed co-localization of mcr-3.12 with antimicrobial resistance genes aadA5, dfrA1, sul1, tet(A), blaOXA-1, and catB3 on the same contig.
The majority of 70 sequenced mcr-4-positive isolates carried mcr-4.6 (n = 43; 61.4%) and mcr-4.2 (n = 21; 30.0%), followed by mcr-4.1 and mcr-4.3 (n = 1 each). In addition, a novel mcr-4 gene variant, termed mcr-4.8 (NCBI Reference Sequence: NG_231578.1), was identified in three ETEC and one ETEC-like isolate collected in the years 2009, 2015, 2017, and 2019. The mcr-4.8 gene differs from mcr-4.1 by a nucleotide substitution at position 706 (G → T), resulting in an amino acid change at position 331 (glutamine → arginine) (Table 2) of the gene product. In nearly all cases (97.1%), mcr-4 genes were located on ColE10 plasmids.

Table 2.
Overview of mcr-4/MCR-4 alleles and depiction of nucleotide/amino acid sequence changes compared to mcr-4.1.
Of 17 sequenced mcr-5-positive isolates, the majority revealed mcr-5.1 (94.1%). One ST29-AEEC isolate, which was obtained from an eight-week-old pig with diarrhea, carried a novel mcr-5 variant termed mcr-5.5 (NG_231579.1). As illustrated in Table 3, the mcr-5.5 gene carried one missense mutation at position 522 (T → G), in comparison to mcr-5.1, resulting in a codon change at position 498 (aspartic acid → asparagine). No plasmid-related genes were identified in the approximately 7.3 to 10.9 kb contigs containing the mcr-5 genes.

Table 3.
Overview of mcr-5/MCR-5 alleles and depiction of nucleotide/amino acid sequence changes compared with mcr-5.1.
3.3. Presence of ESBL, AmpC, Carbapenemase, and Other Antimicrobial Resistance Genes and Chromosomal Mutations among Whole Genome Sequenced mcr-Positive E. coli Isolates
Only a few (3.2%) of the 220 sequenced mcr-positive isolates co-harbored extended spectrum β-lactamase genes. ESBL gene blaCTX-M-1 was determined in AEEC (n = 2; both ST29), EDEC (n = 1; ST744), ETEC (n = 1; ST772), and ETEC/STEC (n = 1; ST10); blaCTX-M-14 in an ST29-AEEC isolate; and blaTEM-52 in an ST10-ETEC isolate. Acquired AmpC β-lactamase genes and carbapenemase genes were not identified. Two ST86-ETEC/STEC isolates revealed ampC promoter mutations (42C → T), which are known to increase ampC transcription rates and play an important role in E. coli resistance to β-lactams [24]. Broad-spectrum beta lactamase genes detected were blaTEM-1: (87.7%), blaOXA-1 (0.5%), and blaCARB-16 (1.4%).
The 220 isolates harbored several aminoglycoside resistance genes in different frequencies, such as aadA1 (63.6%), aadA2 (25.9%), aadA5 (3.6%), aadA12 (0.5%), aadA13 (2.7%), aadA24 (0.9%), aph(3′)-Ia (30.0%), aph(6)-Id (70.7%), aph(3′)-IIa (1.4%), aac(3)-IIa (3.6%), aac(3)-IV (10.9%), aph(4)-Ia (10.9%), ant(3″)-Ia (2.3%), and ant(2″)-Ia (0.5%). Moreover, the isolates carried tetracycline resistance genes tet(A) (76.8%), tet(B) (24.5%), tet(C) (4.1%), and tet(M) (3.6%), folate pathway antagonist genes sul1 (40.5%), sul2 (69.5%), sul3 (38.2%), dfrA1 (47.3%), dfrA5 (1.4%), dfrA8 (3.2%), dfrA12 (5.5%), dfrA14 (9,5%), dfrA16 (0.5%), and dfrA36 (0.5%), chloramphenicol resistance genes catA1 (19.1%), catB2 (0.9%), catB3 (3.2%), and floR (6.8%), macrolide resistance genes mph(A) (4.1%), mph(B) (7.7%), mph(E) (0.9%), mph(G) 1.4%), msr(E) (1.4%), erm(B) (1.4%), mef(B) (1.4%), and mef(C) (1.4%), as well as lincomycin resistance gene Inu(F) (2.7%). Genetic determinants associated with quinolone resistance included genes qnrB19 (0.5%) and qnrS1 (2.3%), as well as chromosomal mutations in gyrA, parC, and parE genes, which were identified in 19.1% of the isolates (gyrA S83L, 10.0%; gyrA D87N, 0.5%; gyrA D87G, 0.5%; gyrA D87Y, 1.8%; gyrA S83L and parE I355T, 2.5%; gyrA S83L and gyrA D87N and parC A56T and parC S80I, 0.5%; gyrA S83L and gyrA D87G and parC S80R, 1.4%; gyrA S83L and parE I529L, 0.5%; parE I529L, 1.4%). Eleven isolates, thereof five mcr-1.1 (ETEC and ETEC-like), four mcr-4.2 (ETEC and ETEC-like), one mcr-4.6 (ETEC), and one mcr-5.1 (ETEC/STEC) isolate, additionally revealed a mutation in the pmrB gene (V161G), which is known to confer resistance to colistin.
The majority of the isolates (55.0%) harbored between six and ten AMR genes, while almost one-third (30.0%) possessed 11–15, and 12.7% carried 1–5 AMR genes. The highest number of AMR genes, namely 16 to 19, was observed in 2.3% of the isolates, represented by mcr-1.1 positive ETEC/STEC (n = 2), EDEC (n = 1), ETEC (n = 1), and AEEC (n = 1). The number of antimicrobial resistance genes and the pathotype were not correlated.
3.4. Multi Locus Sequence Types, Phylogenetic Groups, and Clonotypes
Overall, 30 known and seven novel STs (ST15336–ST15342) were identified among the 220 whole genome sequenced E. coli isolates. Predominant STs were ST10 (n = 56), ST1 (n = 48), ST29 (n = 25), ST100 (n = 21), ST42 (n = 7), and ST86 (n = 6), as well as ST131, ST641, and ST763 (n = 4 isolates each). ETEC isolates were mainly assigned to ST100 (37.5%), ST10 (21.4%), ST42 (10.7%), and ST131 (7.1%), while EDEC isolates mostly belonged to ST1 (79.2%) and ST10 (12.5%). Predominant STs of AEEC isolates were ST29 (61.0%) and ST20 (7.3%), while most of the sequenced ETEC/STEC, STEC, and ETEC-like isolates belonged to ST10 (69.6%, 64.3%, and 33.3%, respectively).
The most frequent phylogroup was group A (n = 92), predominantly associated with ST10 (n = 56) and ST100 (n = 21), followed by D (n = 60), among others associated with ST1 (n = 48) and ST42 (n = 7), B1 (n = 51; mostly ST29 (n = 25), ST86 (n = 6), ST641 (n = 4), and ST763 (n = 4)), E (n = 7; mostly ST118 (n = 3)and ST5759 (n = 3)), B2 (n = 4; all ST131), C (n = 3; all ST23), and clade I (n = 3) (Figure 2).
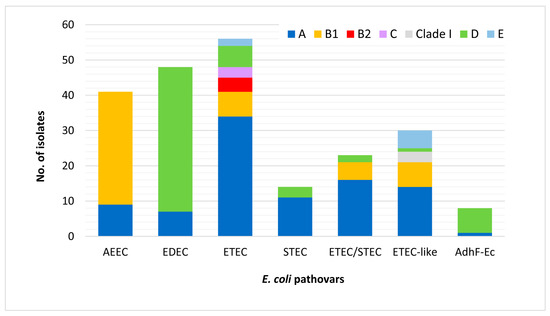
Figure 2.
Distribution of phylogenetic groups among 220 mcr-positive porcine E. coli isolates according to pathotypes.
Thirty-six different clonotypes, i.e., combinations of fumC and fimH alleles, were determined. The most frequent CH types were 2–54 (51; (ST1-ETEC), 11–24 and 11–23 (29/17) (ST10), 4–24 (23) ST29, and 27–0 (20, all ST100). ST131 ETEC isolates carrying the mcr-1.1 gene revealed CH types 40–22 (n = 1) and 40–683 (n = 3).
3.5. Sero(geno)types
Overall, 24 distinct sero(geno)types could be detected among 220 whole genome sequenced mcr-positive E. coli isolates. About half of the isolates (47.3%), almost equally distributed among the different pathotypes, were not typable (Ont). The most frequent serotype observed was O139 (16.8%; in 86.5% of the isolates associated with H-antigen H4), followed by O123:H11 (5.5%), O141:H4 (5.0%), and O149 (4.5%; 80.0% associated with H10). Genes encoding O types O8, O26, O35, O45, O50, O103, and O182 were each present in three to six of the isolates. O139 was associated with the EDEC pathotype, and O123:H11 and O149 were exclusively detected in AEEC and ETEC isolates, respectively. O141 occurred in four different pathotypes (ETEC, EDEC, ETEC/STEC, and AdhF-Ec). The four ST131 isolates belonged to the O25b:H4 serogroup.
3.6. Intimin Subtypes
The complete eae sequences were obtained from the genomes of 41 sequenced AEEC genomes; two strains which failed to yield the eae sequence were excluded from subtyping analysis. Four eae subtypes, namely β1 (n = 28), ε1 (n = 8), θ2 (n = 2), and ξ (n = 1), were assigned. Sequence polymorphisms in the eae gene, also known as genotypes (GTs), were examined to determine the diversity within each eae subtype. Subtypes that were represented by at least two isolates were explored. While ε1 and θ2 subtypes consisted only of one genotype each, the β1 subtype contained five genotypes, namely GT1 (n = 20; all ST29), GT2 (n = 4; 2 × ST29, 2 × ST20), and GT3 to GT6 (n = 1 each; 3 × ST29, 1 × ST20).
4. Discussion
E. coli neonatal and post-weaning diarrhea affecting pigs during the first weeks after birth and edema disease, an acute, often fatal enterotoxemia that affects primarily healthy, rapidly growing nursery pigs, are economically important diseases for the swine industry worldwide [25]. In the present study, we performed a comprehensive study on a collection of 10,573 fecal or intestinal E. coli isolates recovered from pigs with diarrheal disease or edema disease as well as from healthy swine sent to our laboratory to determine the presence of potentially pathogenic E. coli strains. The distribution of mcr genes among E. coli isolates linked with diarrhea or edema diseases in pigs and/or subtyped at the pathotype level has rarely been studied so far.
An epidemiological study from Spain analyzed 481 E. coli isolates obtained from 179 diarrheagenic outbreaks in pigs [26]. The most prevalent pathotypes found were ETEC (57.6%), aEPEC (32.4%, in this study referred to as AEEC), hybrid ETEC/STEC (6.9%), and STEC (3.1%). While we report similar prevalences for hybrid ETEC/STEC and STEC pathotypes in our study, the detected occurrence of ETEC and aEPEC was lower. Instead, we report the detection of additional pathotypes including EDEC, ETEC-like, and AdhF-Ec.
High prevalences of mcr-positive non-pathogenic E. coli were previously reported in the surroundings of fattening pig farms and pig slaughterhouses in Germany [27,28]. Our study is based exclusively on the investigation of mcr prevalences in pathogenic E. coli. The frequency of mcr-positive E. coli strains was 10.2%, which is lower than previous reports from other countries suggest [26,29]. Fukuda et al. (2018, 2022) found that mcr-1, mcr-3, and mcr-5 were prevalent in E. coli derived from diseased pigs in Japan [30,31]. Among 120 strains isolated from pigs with PWD on 40 farms in 2012, the mcr-1 (30.0%), mcr-3 (8.3%), mcr-5 (28.3%), and mcr-9 (0.8%) genes were detected, while mcr-2, mcr-4, mcr-6 to mcr-8 and mcr-10 were not reported. Coexistence of mcr-1 and mcr-5 (4.2%; 5/120) in the same strain was observed, but other combinations were not. Another study investigated 200 pathogenic E. coli isolated from swine enteric clinical cases between 1999 and 2018 in Spain [32]. The mcr-4 gene was the most frequently detected mobile colistin resistance gene (13%), followed by mcr-1 (7%) and mcr-5 (3%). These reports are in line with our prevalence data.
In our study, the proportion of mcr-positive E. coli was highest in ETEC/STEC hybrid isolates and in EDEC isolates. The significantly higher prevalence of mcr-positive ETEC/STEC isolates could indicate a more frequent use of colistin to treat diarrheal diseases caused by ETEC/STEC in Germany [33].
The majority of the 132 representative mcr-1-positive isolates carried the mcr-1.1 variant, which has been commonly reported worldwide. One isolated ST29-AEEC from 2018 carried the mcr-1.26 variant, which was first detected in an E. coli strain isolated from the blood culture of a 79-year-old patient with fever in Germany [34]. At present, mobile colistin resistance genes 3 (mcr-3.1–mcr-3.42) and 1 (mcr-1.1–mcr-1.36) exhibit the highest numbers of reported variants (https://www.ncbi.nlm.nih.gov/pathogens/isolates#/refgene/, accessed 3 December 2023). We here report the newest mcr-1 variant (termed mcr-1.36).
To the best of our knowledge, we report the first detection of mcr-3 in EDEC isolates which were obtained from three pigs on one farm in Germany in 2014. While the occurrence of mcr-3 has not yet been reported in pathogenic E. coli isolates from pigs in Europe, studies from Japan, Korea, and Thailand described high prevalences (Table S3) [35,36,37,38,39].
Soon after detecting and identifying the mcr-1–mcr-3 genes, the discovery of another mcr gene (mcr-4) was reported. Carattoli et al. (2017) published the finding of a novel mcr-4 gene harbored by a Salmonella enterica strain, which originated from the caecal content of a healthy pig at slaughter in Italy in 2013 [12]. This new gene was located on an 8749 bp ColE10 plasmid. Until now, seven mcr-4 variants (mcr-4.1–mcr-4.7) have been reported in several countries, including Germany, Italy, Singapore, South Korea, and Australia. While mcr-4 has been associated mainly with ColE10 or ColE10-like type plasmids, several novel or non-typeable mcr-4-harboring plasmids have been identified, e.g., in one Acinetobacter baumannii isolate obtained from frog legs in Vietnam and in a Shewanella baltica strain isolated from the gut contents of a wild Atlantic mackerel [10,40,41,42]. In this study, we report a novel mcr-4 variant (termed mcr-4.8) on a ColE10 plasmid in an ETEC-like E. coli strain isolated from a pig in April 2019. The most predominant mcr-4.6 variant found in our study was first detected in a Salmonella enterica strain originally found in a pig carcass in Spain [43]. Our study details the identification of the novel mcr-5.5 variant, which was detected in an isolate from a pig in Germany in 2006 and represents the third mcr-5 variant discovered in Germany. Our investigation revealed that an mcr-5.1-positive ETEC (ST5759) was isolated from a pig with diarrhea in 2004, making it the earliest detection of mcr genes within this study. Nevertheless, earlier observations of the mcr-5 gene were reported in diseased swine in Spain in 2000 [32].
None of the isolates tested in our study carried mcr-6 to mcr-10, which is in accordance with previous studies in commensal isolates [7]. The majority of prior studies investigating the presence of mcr genes in E. coli isolates from diseased or healthy pigs with defined E. coli pathotypes/VAG-typed E. coli did not test for mcr-6 to mcr-10 (Table S3) [44,45,46,47,48]. Only two out of seven studies investigating for mcr-6 to mcr-10 reported mcr-9-positive E. coli from pigs with diarrhea (Table S3) [49,50,51,52]. The first occurrence and distribution of mcr-1–mcr-10 were reviewed recently [6]. No specific differences of mcr-1 to mcr-5 versus mcr-6 to mcr-10 were described with regard to source or bacterial species.
The location of specific mcr genes on different plasmids was studied by García-Meniño et al. (2019). They investigated 35 E. coli isolates obtained from pigs with diarrhea from 2006 to 2016 in Spain for different mcr variants [10]. Among 18 mcr-1.1 variants located mainly on IncHI2 and IncX4-type plasmids, mcr-4.2 (n = 13) and mcr-4.5 (n = 2) were located on ColE10-like plasmids, while one mcr-4.1 variant was found on a Col8282-like plasmid. A more recent study associated plasmid ColE10 with mcr-4.6 genes isolated from eight colistin-resistant E. coli obtained from veal calves in Belgium from 2012 to 2015 [40]. These reports support our findings on the location of mcr genes in pathogenic E. coli isolates from pigs.
We observed high diversity in the phylogeny of the 220 mcr-positive isolates; however, some phylogroups and STs, including A-ST10 (25.5%), A-ST100 (9.5%), D-ST1 (21.8%), and B1-ST29 (11.4%), were predominant. Other STs detected in the present study such as ST42, ST118, and ST131 have been previously associated with intestinal pathogenic E. coli in pigs [26,53].
Four mcr-1.1-positive ST131 ETEC were isolated from three farms at four different time points between 2009 and 2014, all from pigs with post-weaning diarrhea. In addition to ETEC-typical genes fedAac, estap, and estb, they harbored genes typical of extraintestinal pathogenic E. coli (ExPEC), including chuA, fyuA, ibeA, iroN, iucD, iutA, kpsMTII, papA, papC, sitA, and yfcV (fimbrial-like gene; used by Spurbeck as UPEC-predictor, together with chuA, fyuA, vat) and belonged to clonal types (fumC-fimH allele) 40–22 (n = 1) (previously detected in mcr-1-positive ST131 from Spain, Garcia-Menino, ST131) and 40–683 (n = 3). All belonged to serogroup O25b:H4. One strain was identified as being part of subclone H22 (clade B), which is known as the predecessor to subclone H30 that is commonly linked to fluoroquinolone resistance [54,55]. This clonal type (40–22) has been previously detected in mcr-1-positive ST131 strains isolated from pigs with diarrhea in Spain [26].
Based on amino acid sequence differences at the C-terminus, at least 30 intimin subtypes have been defined, namely, α1, α2, α8, β1, β2, β3, γ1, γ2, ε1, ε2, ε3, ε4, ξ, z, z3, η, η2, θ, τ, ι1, ι2, κ, λ, μ, ν, υ, ο, π, ρ, and σ [56]. Intimin subtypes are correlated with host specificity and tissue tropism [57]. In the 39 AEEC isolates analyzed in our study, only eae genes encoding for subtypes β1, ε1, θ2, and ξ were detected. The β1 subtype was the most prevalent, consistent with previous findings of intimin subtypes in pigs, cattle, birds, and diarrheal patients [58,59,60]. The β1_GT1 genotype, which predominated in our study, was first detected in an E. coli isolate from yak feces in Qinghai, China in 2012 [60].
Our study has several limitations. Our analysis is based exclusively on the investigation of mcr prevalences in pathogenic E. coli, and therefore no statement can be made about the occurrence of colistin resistance genes in non-pathogenic isolates. We also lack information regarding pig housing conditions, hygiene standards, previous medical treatment, and animal trafficking. On the other hand, our study has several strengths. A total of 10,573 putative pathogenic porcine E. coli strains were screened for VAGs and plasmid-mediated colistin resistance genes (mcr-1 to mcr-10). Moreover, 220 representative mcr-positive isolates underwent whole genome sequencing and were examined for various aspects, including mcr-carrying plasmids, genes associated with antimicrobial resistance, and STs, which offers deep insight into the molecular characteristics of mcr-positive pig-associated E. coli pathotypes.
5. Conclusions
To the best of our knowledge, we here present for the first time prevalence data of all ten currently known mcr genes in a large set of pathogenic porcine E. coli isolates collected over a period of 20 years in Germany. Regarding the distribution of mcr genes in pig-associated pathotypes in Germany, ETEC/STEC hybrid strains revealed the highest prevalence of mcr, in particular mcr-1, -4, and -5. If this might be associated with the antimicrobial treatment strategy of diarrheal diseases in pigs or with the potential ability of these hybrid strains to acquire mobile AMR genes and/or plasmids more easily than other pathotypes remains an interesting point for future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol4010005/s1, Table S1: primers and positive control strains or DNA used for multiplex PCRs to detect virulence-associated genes and mcr-1 to mcr-10 genes [61]; Table S2: overview of all porcine E. coli isolates included in this study and their classification into pathotypes according to the VAG pattern; Table S3: occurrence of mcr genes in E. coli isolates from samples obtained from (i) diseased pigs or (ii) healthy pigs with defined E. coli pathotypes/VAG-typed E. coli. Studies involving screening of other animals/humans were included only for data as previously defined.
Author Contributions
Conceptualization, C.E.; methodology, C.E., R.B., L.G., T.S., and S.A.W.; validation, C.E. and L.G.; formal analysis, L.G. and C.E.; investigation, C.E., R.B., E.P.-B., and L.G.; resources, C.E., R.B., and T.S.; data curation, C.E., L.G., E.P.-B., T.S., and S.A.W.; writing—original draft preparation, C.E. and L.G.; writing—review and editing, C.E., L.G., and R.B.; visualization, C.E. and L.G.; supervision, C.E.; project administration, C.E. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and supplementary materials. NCBI reference sequence numbers for novel mcr variants are NG_231577.1 (mcr-1.36), NG_231578.1 (mcr-4.8), and NG_231579.1 (mcr-5.5). The sequences are available in the NCBI Reference Sequence Database (https://www.ncbi.nlm.nih.gov/refseq/, accessed on 3 December 2023). Further raw data can be made available on reasonable request.
Acknowledgments
We thank all our colleagues from the in-house microbiology diagnostic for collecting E. coli isolates. We thank Anja Schwanitz and Ursula Leidner for the excellent technical assistance. We thank the Sequencing Core Facility of the Genome Competence Centre, Robert Koch Institute, for providing excellent sequencing services. We also would like to thank Maria Borowiak from the Federal Institute for Risk Assessment, Berlin, Germany, for providing positive control DNA for the PCR amplification of genes mcr-5, mcr-6, and mcr-7.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.M.; Castro, J.; Araújo, D.; Campos, A.M.; Oliveira, R.; Silva, S.; Outor-Monteiro, D.; Almeida, C. Swine Colibacillosis: Global Epidemiologic and Antimicrobial Scenario. Antibiotics 2023, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- García-Meniño, I.; García, V.; Alonso, M.P.; Blanco, J.E.; Blanco, J.; Mora, A. Clones of enterotoxigenic and Shiga toxin-producing Escherichia coli implicated in swine enteric colibacillosis in Spain and rates of antibiotic resistance. Vet. Microbiol. 2021, 252, 108924. [Google Scholar] [CrossRef] [PubMed]
- Renzhammer, R.; Loncaric, I.; Roch, F.-F.; Pinior, B.; Käsbohrer, A.; Spergser, J.; Ladinig, A.; Unterweger, C. Prevalence of Virulence Genes and Antimicrobial Resistances in E. coli Associated with Neonatal Diarrhea, Postweaning Diarrhea, and Edema Disease in Pigs from Austria. Antibiotics 2020, 9, 208. [Google Scholar] [CrossRef]
- Jansen, W.; van Hout, J.; Wiegel, J.; Iatridou, D.; Chantziaras, I.; Briyne, N. Colistin Use in European Livestock: Veterinary Field Data on Trends and Perspectives for Further Reduction. Vet. Sci. 2022, 9, 650. [Google Scholar] [CrossRef]
- Hussein, N.H.; Al-Kadmy, I.M.S.; Taha, B.M.; Hussein, J.D. Mobilized colistin resistance (mcr) genes from 1 to 10: A comprehensive review. Mol. Biol. Rep. 2021, 48, 2897–2907. [Google Scholar] [CrossRef]
- Ewers, C.; Göpel, L.; Prenger-Berninghoff, E.; Semmler, T.; Kerner, K.; Bauerfeind, R. Occurrence of mcr-1 and mcr-2 colistin resistance genes in porcine Escherichia coli isolates (2010-2020) and genomic characterization of mcr-2-positive E. coli. Front. Microbiol. 2022, 13, 1076315. [Google Scholar] [CrossRef]
- Migura-Garcia, L.; González-López, J.J.; Martinez-Urtaza, J.; Aguirre Sánchez, J.R.; Moreno-Mingorance, A.; Perez de Rozas, A.; Höfle, U.; Ramiro, Y.; Gonzalez-Escalona, N. mcr-Colistin Resistance Genes Mobilized by IncX4, IncHI2, and IncI2 Plasmids in Escherichia coli of Pigs and White Stork in Spain. Front. Microbiol. 2019, 10, 3072. [Google Scholar] [CrossRef]
- García, V.; García-Meniño, I.; Mora, A.; Flament-Simon, S.C.; Díaz-Jiménez, D.; Blanco, J.E.; Alonso, M.P.; Blanco, J. Co-occurrence of mcr-1, mcr-4 and mcr-5 genes in multidrug-resistant ST10 Enterotoxigenic and Shiga toxin-producing Escherichia coli in Spain (2006-2017). Int. J. Antimicrob. Agents 2018, 52, 104–108. [Google Scholar] [CrossRef]
- García-Meniño, I.; Díaz-Jiménez, D.; García, V.; de Toro, M.; Flament-Simon, S.C.; Blanco, J.; Mora, A. Genomic Characterization of Prevalent mcr-1, mcr-4, and mcr-5 Escherichia coli Within Swine Enteric Colibacillosis in Spain. Front. Microbiol. 2019, 10, 2469. [Google Scholar] [CrossRef]
- Curcio, L.; Luppi, A.; Bonilauri, P.; Gherpelli, Y.; Pezzotti, G.; Pesciaroli, M.; Magistrali, C.F. Detection of the colistin resistance gene mcr-1 in pathogenic Escherichia coli from pigs affected by post-weaning diarrhoea in Italy. J. Glob. Antimicrob. Resist. 2017, 10, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Villa, L.; Feudi, C.; Curcio, L.; Orsini, S.; Luppi, A.; Pezzotti, G.; Magistrali, C.F. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017, 22, 30589. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, B.; Casey, T. Identification of toxin and pilus genes in porcine Escherichia coli using polymerase chain reaction (PCR) with multiple primer pairs. In Proceedings of the 97th General Meeting of the American Society for Microbiology, Miami Beach, CA, USA, 4–8 May 1997. [Google Scholar]
- Casey, T.A.; Bosworth, B.T. Design and evaluation of a multiplex polymerase chain reaction assay for the simultaneous identification of genes for nine different virulence factors associated with Escherichia coli that cause diarrhea and edema disease in swine. J. Vet. Diagn. Investig. 2009, 21, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Franck, S.M.; Bosworth, B.T.; Moon, H.W. Multiplex PCR for enterotoxigenic, attaching and effacing, and Shiga toxin-producing Escherichia coli strains from calves. J. Clin. Microbiol. 1998, 36, 1795–1797. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Xavier, B.B.; Lammens, C.; Ruhal, R.; Kumar-Singh, S.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill. 2016, 21, 30280. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Li, H.; Shen, Y.; Liu, Z.; Wang, S.; Shen, Z.; Zhang, R.; Walsh, T.R.; Shen, J.; Wang, Y. Novel Plasmid-Mediated Colistin Resistance Gene mcr-3 in Escherichia coli. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef]
- Borowiak, M.; Baumann, B.; Fischer, J.; Thomas, K.; Deneke, C.; Hammerl, J.A.; Szabo, I.; Malorny, B. Development of a Novel mcr-6 to mcr-9 Multiplex PCR and Assessment of mcr-1 to mcr-9 Occurrence in Colistin-Resistant Salmonella enterica Isolates From Environment, Feed, Animals and Food (2011-2018) in Germany. Front. Microbiol. 2020, 11, 80. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Schwengers, O.; Jelonek, L.; Dieckmann, M.A.; Beyvers, S.; Blom, J.; Goesmann, A. Bakta: Rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 2021, 7, 000685. [Google Scholar] [CrossRef]
- Weissman, S.J.; Johnson, J.R.; Tchesnokova, V.; Billig, M.; Dykhuizen, D.; Riddell, K.; Rogers, P.; Qin, X.; Butler-Wu, S.; Cookson, B.T.; et al. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Roer, L.; Johannesen, T.B.; Hansen, F.; Stegger, M.; Tchesnokova, V.; Sokurenko, E.; Garibay, N.; Allesøe, R.; Thomsen, M.C.F.; Lund, O.; et al. CHTyper, a Web Tool for Subtyping of Extraintestinal Pathogenic Escherichia coli Based on the fumC and fimH Alleles. J. Clin. Microbiol. 2018, 56, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Caroff, N.; Espaze, E.; Gautreau, D.; Richet, H.; Reynaud, A. Analysis of the effects of -42 and -32 ampC promoter mutations in clinical isolates of Escherichia coli hyperproducing ampC. J. Antimicrob. Chemother. 2000, 45, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, E. Colibacillosis. In Diseases of Swine, 11th ed.; Straw, B.E., Zimmerman, J.J., D’Allaire, S., Taylor, D.J., Eds.; Blackwell Publishing: Oxford, UK, 2019. [Google Scholar]
- García-Meniño, I.; García, V.; Mora, A.; Díaz-Jiménez, D.; Flament-Simon, S.C.; Alonso, M.P.; Blanco, J.E.; Blanco, M.; Blanco, J. Swine Enteric Colibacillosis in Spain: Pathogenic Potential of mcr-1 ST10 and ST131 E. coli Isolates. Front. Microbiol. 2018, 9, 2659. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Falgenhauer, L.; Semmler, T.; Imirzalioglu, C.; Chakraborty, T.; Roesler, U.; Roschanski, N. Environmental emission of multiresistant Escherichia coli carrying the colistin resistance gene mcr-1 from German swine farms. J. Antimicrob. Chemother. 2017, 72, 1289–1292. [Google Scholar] [CrossRef]
- Savin, M.; Bierbaum, G.; Blau, K.; Parcina, M.; Sib, E.; Smalla, K.; Schmithausen, R.; Heinemann, C.; Hammerl, J.A.; Kreyenschmidt, J. Colistin-Resistant Enterobacteriaceae Isolated From Process Waters and Wastewater From German Poultry and Pig Slaughterhouses. Front. Microbiol. 2020, 11, 575391. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Liao, T.-L.; Huang, W.-C.; Liu, Y.-M.; Wu, K.-M.; Lauderdale, T.-L.; Tsai, S.-F.; Kuo, S.-C.; Kuo, H.-C. Increased mcr-1 in pathogenic Escherichia coli from diseased swine, Taiwan. J. Microbiol. Immunol. Infect. 2020, 53, 751–756. [Google Scholar] [CrossRef]
- Fukuda, A.; Sato, T.; Shinagawa, M.; Takahashi, S.; Asai, T.; Yokota, S.-I.; Usui, M.; Tamura, Y. High prevalence of mcr-1, mcr-3 and mcr-5 in Escherichia coli derived from diseased pigs in Japan. Int. J. Antimicrob. Agents 2018, 51, 163–164. [Google Scholar] [CrossRef]
- Fukuda, A.; Nakano, H.; Suzuki, Y.; Nakajima, C.; Usui, M. Conjugative IncHI2/HI2A plasmids harbouring mcr-9 in colistin-susceptible Escherichia coli isolated from diseased pigs in Japan. Access Microbiol. 2022, 4, acmi000454. [Google Scholar] [CrossRef]
- Aguirre, L.; Vidal, A.; Seminati, C.; Tello, M.; Redondo, N.; Darwich, L.; Martín, M. Antimicrobial resistance profile and prevalence of extended-spectrum beta-lactamases (ESBL), AmpC beta-lactamases and colistin resistance (mcr) genes in Escherichia coli from swine between 1999 and 2018. Porc. Health Manag. 2020, 6, 8. [Google Scholar] [CrossRef]
- Khine, N.O.; Lugsomya, K.; Niyomtham, W.; Pongpan, T.; Hampson, D.J.; Prapasarakul, N. Longitudinal Monitoring Reveals Persistence of Colistin-Resistant Escherichia coli on a Pig Farm Following Cessation of Colistin Use. Front. Vet. Sci. 2022, 9, 845746. [Google Scholar] [CrossRef] [PubMed]
- Neumann, B.; Rackwitz, W.; Hunfeld, K.-P.; Fuchs, S.; Werner, G.; Pfeifer, Y. Genome sequences of two clinical Escherichia coli isolates harboring the novel colistin-resistance gene variants mcr-1.26 and mcr-1.27. Gut Pathog. 2020, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Do, K.-H.; Park, H.-E.; Byun, J.-W.; Lee, W.-K. Virulence and antimicrobial resistance profiles of Escherichia coli encoding mcr gene from diarrhoeic weaned piglets in Korea during 2007-2016. J. Glob. Antimicrob. Resist. 2020, 20, 324–327. [Google Scholar] [CrossRef]
- Mechesso, A.F.; Moon, D.C.; Kang, H.Y.; Song, H.-J.; Kim, S.-J.; Choi, J.-H.; Kim, M.H.; Na, S.H.; Kim, H.-Y.; Jung, B.Y.; et al. Emergence of mcr-3 carrying Escherichia coli in Diseased Pigs in South Korea. Microorganisms 2020, 8, 1538. [Google Scholar] [CrossRef] [PubMed]
- Trongjit, S.; Chuanchuen, R. Whole genome sequencing and characteristics of Escherichia coli with co-existence of ESBL and mcr genes from pigs. PLoS ONE 2021, 16, e0260011. [Google Scholar] [CrossRef] [PubMed]
- Trongjit, S.; Assavacheep, P.; Samngamnim, S.; My, T.H.; An, V.T.T.; Simjee, S.; Chuanchuen, R. Plasmid-mediated colistin resistance and ESBL production in Escherichia coli from clinically healthy and sick pigs. Sci. Rep. 2022, 12, 2466. [Google Scholar] [CrossRef] [PubMed]
- Nguyet, L.T.Y.; Keeratikunakorn, K.; Kaeoket, K.; Ngamwongsatit, N. Antibiotic resistant Escherichia coli from diarrheic piglets from pig farms in Thailand that harbor colistin-resistant mcr genes. Sci. Rep. 2022, 12, 9083. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, M.; Wattiau, P.; Denis, O.; Boland, C. Colistin resistance genes mcr-1 to mcr-5, including a case of triple occurrence (mcr-1, -3 and -5), in Escherichia coli isolates from faeces of healthy pigs, cattle and poultry in Belgium, 2012-2016. Int. J. Antimicrob. Agents 2021, 57, 106350. [Google Scholar] [CrossRef]
- Kalová, A.; Gelbíčová, T.; Overballe-Petersen, S.; Litrup, E.; Karpíšková, R. Characterisation of Colistin -Resistant Enterobacterales and Acinetobacter Strains Carrying mcr Genes from Asian Aquaculture Products. Antibiotics 2021, 10, 838. [Google Scholar] [CrossRef]
- Marathe, N.P.; Salvà-Serra, F.; Nimje, P.S.; Moore, E.R.B. Novel Plasmid Carrying Mobile Colistin Resistance Gene mcr-4.3 and Mercury Resistance Genes in Shewanella baltica: Insights into Mobilization of mcr-4.3 in Shewanella Species. Microbiol. Spectr. 2022, 10, e0203722. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018, 23, 17-00672. [Google Scholar] [CrossRef] [PubMed]
- Bernreiter-Hofer, T.; Schwarz, L.; Müller, E.; Cabal-Rosel, A.; Korus, M.; Misic, D.; Frankenfeld, K.; Abraham, K.; Grünzweil, O.; Weiss, A.; et al. The Pheno- and Genotypic Characterization of Porcine Escherichia coli Isolates. Microorganisms 2021, 9, 1676. [Google Scholar] [CrossRef] [PubMed]
- Malhotra-Kumar, S.; Xavier, B.B.; Das, A.J.; Lammens, C.; Butaye, P.; Goossens, H. Colistin resistance gene mcr-1 harboured on a multidrug resistant plasmid. Lancet Infect. Dis. 2016, 16, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Yang, Y.; Zhang, J.; Li, F.; Li, X.; Liu, H.; Ishfaq, M.; Xu, G.; Zhang, X. Antimicrobial Resistance and Virulence Profiles of mcr-1-Positive Escherichia coli Isolated from Swine Farms in Heilongjiang Province of China. J. Food Prot. 2020, 83, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, N.; Hikoda-Kogikuh, Y.; Tamamura-Andoh, Y.; Kusumoto, M. mcr-1 remains detectable in various Escherichia coli lineages isolated from healthy swine after withdrawal of colistin use on the farm. J. Vet. Med. Sci. 2023, 85, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Ogura, Y.; Gotoh, Y.; Iwata, T.; Hayashi, T.; Akiba, M. Colistin-Resistant mcr-1-Positive Pathogenic Escherichia coli in Swine, Japan, 2007-2014. Emerg. Infect. Dis. 2016, 22, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, J.; Wang, S.; Su, J.; Wang, X.; Zhu, Y. Genome characterization of mcr-1-Positive Escherichia coli Isolated From Pigs With Postweaning Diarrhea in China. Front. Vet. Sci. 2020, 7, 503. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Huang, X.; Xia, J.; Cui, M.; Huang, Y.; Wen, Y.; Xie, Y.; Zhao, Q.; Cao, S.; et al. Genomic traits of multidrug resistant enterotoxigenic Escherichia coli isolates from diarrheic pigs. Front. Microbiol. 2023, 14, 1244026. [Google Scholar] [CrossRef]
- Flament-Simon, S.-C.; de Toro, M.; Mora, A.; García, V.; García-Meniño, I.; Díaz-Jiménez, D.; Herrera, A.; Blanco, J. Whole Genome Sequencing and Characteristics of mcr-1-Harboring Plasmids of Porcine Escherichia coli Isolates Belonging to the High-Risk Clone O25b:H4-ST131 Clade B. Front. Microbiol. 2020, 11, 387. [Google Scholar] [CrossRef]
- Guarneri, F.; Bertasio, C.; Romeo, C.; Formenti, N.; Scali, F.; Parisio, G.; Canziani, S.; Boifava, C.; Guadagno, F.; Boniotti, M.B.; et al. First Detection of mcr-9 in a Multidrug-Resistant Escherichia coli of Animal Origin in Italy Is Not Related to Colistin Usage on a Pig Farm. Antibiotics 2023, 12, 689. [Google Scholar] [CrossRef]
- Kusumoto, M.; Hikoda, Y.; Fujii, Y.; Murata, M.; Miyoshi, H.; Ogura, Y.; Gotoh, Y.; Iwata, T.; Hayashi, T.; Akiba, M. Emergence of a Multidrug-Resistant Shiga Toxin-Producing Enterotoxigenic Escherichia coli Lineage in Diseased Swine in Japan. J. Clin. Microbiol. 2016, 54, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.-H.; Petitjean, M.; Mora, A.; Mayer, N.; Lavigne, J.-P.; Boulet, O.; Leflon-Guibout, V.; Blanco, J.; Hocquet, D. The ST131 Escherichia coli H22 subclone from human intestinal microbiota: Comparison of genomic and phenotypic traits with those of the globally successful H30 subclone. BMC Microbiol. 2017, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, N.; Sheppard, A.E.; Pankhurst, L.; de Maio, N.; Moore, C.E.; Sebra, R.; Turner, P.; Anson, L.W.; Kasarskis, A.; Batty, E.M.; et al. Evolutionary History of the Global Emergence of the Escherichia coli Epidemic Clone ST131. mBio 2016, 7, e02162. [Google Scholar] [CrossRef] [PubMed]
- Ooka, T.; Seto, K.; Kawano, K.; Kobayashi, H.; Etoh, Y.; Ichihara, S.; Kaneko, A.; Isobe, J.; Yamaguchi, K.; Horikawa, K.; et al. Clinical significance of Escherichia albertii. Emerg. Infect. Dis. 2012, 18, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Köhler, B.; Oswald, E.; Beutin, L.; Karch, H.; Morabito, S.; Caprioli, A.; Suerbaum, S.; Schmidt, H. Genetic Diversity of Intimin Genes of Attaching and Effacing Escherichia coli Strains. J. Clin. Microbiol. 2002, 40, 4486–4492. [Google Scholar] [CrossRef] [PubMed]
- Vu-Khac, H.; Holoda, E.; Pilipcinec, E.; Blanco, M.; Blanco, J.E.; Dahbi, G.; Mora, A.; López, C.; González, E.A.; Blanco, J. Serotypes, virulence genes, intimin types and PFGE profiles of Escherichia coli isolated from piglets with diarrhoea in Slovakia. Vet. J. 2007, 174, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, X.; Zhao, A.; Zhang, W.; Ba, P.; Liu, K.; Jin, Y.; Wang, H.; Guo, Q.; Sun, H.; et al. Genetic Diversity of Intimin Gene of Atypical Enteropathogenic Escherichia coli Isolated from Human, Animals and Raw Meats in China. PLoS ONE 2016, 11, e0152571. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Fan, R.; Fu, S.; Zhang, J.; Matussek, A.; Xiong, Y.; Bai, X. Genetic diversity of the intimin gene (eae) in non-O157 Shiga toxin-producing Escherichia coli strains in China. Sci. Rep. 2020, 10, 3275. [Google Scholar] [CrossRef]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017, 72, 3317–3324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).