Abstract
Livestock production in Afghanistan highly relies on grazing and clover feed, which is a key component of pastures and forage crops. This study elucidated the genetic diversity of clover-nodulating rhizobia in different ecological regions and their effects on clover growth. A total of 57 rhizobia were isolated and their genetic diversities were studied through 16S rRNA and nifD genes. The isolates were inoculated to clover (Afghan local variety), to investigate the potential of nitrogen fixation and influences of clover growth. The 16S rRNA gene analysis showed two distinct groups of Rhizobium (94.7%) and Ensifer (5.3%) species. The nifD phylogenetic relationship revealed a high similarity to Rhizobium and a novel lineage group close to Rhizobium leguminosarum species. In the plant test, different genotypes significantly (p < 0.01) exhibited an increase in plant biomass production, compared to the un-inoculated plants. Among genotypes, the highest plant biomass was recorded in PC8 (1769.0 mg/plant) and PC9 (1409.2 mg/plant) isolates as compared to un-inoculated plants (144.0 mg/plant). Moreover, these isolates showed maximum nitrogen fixation rates of 8.2 and 6.5 µM/plant, respectively. These isolates were identified as the most promising rhizobial strains for developing biofertilizers in the context of Afghanistan.
1. Introduction
Trifolium species are among the most vital and valuable forage legumes globally [1]; they belong to the world’s third-largest plant family, called Fabaceae (Leguminosae) [2]. Trifolium contains more than 250 species and plays a significant role as nitrogen fixers, enhancing pasture quality in natural and cultivated grasslands [3]. Among the species within the genus, white clover (Trifolium repens) and red clover (T. pratense) are the most extensively utilized. As legumes and valuable feed plants, they have long played a crucial role in contributing significantly to agricultural and animal production in both Europe and America [4]. Livestock production serves as a primary source of livelihood and family sustenance in Afghanistan [5]. Clover plays a significant role as one of the major feed sources, utilized both in its fresh form and as hay to nourish animals [6]. However, the cultivation area and production amount still cannot cater to the demand and soil fertility requirement. The diverse plant species within the Fabaceae family possess the capability to establish nitrogen-fixing symbiotic associations with soil bacteria, commonly known as rhizobia [7].
This process, known as biological nitrogen fixation (BNF), offers an ecological and cost-effective alternative for supplying nitrogen to legume crops. BNF reduces the reliance on synthetic nitrogen fertilizers in agriculture, thereby mitigating their negative impacts on natural ecosystems [8,9,10]. Legume crops, on average, annually fix atmospheric nitrogen from 20 to 200 kg/ha through a symbiotic relationship with rhizobia in their root nodules [11], while the symbiotic relationships are host-specific [12]. Rhizobia are distinguished by their large and complex genomes, typically ranging from 6 to 9 Mbp, comprising a chromosome alone or alongside several substantial plasmids [13,14]. These bacteria can exist as free-living organisms in the soil, plant endophytes, and endosymbionts inside legume root nodules [15].
Nitrogen (N) is a vital component of soil fertility and a primary element in chlorophyll, crucial for the photosynthesis process in plants [16]. However, the excessive use of chemical nitrogen sources has led to severe environmental contamination, adversely affecting the abundance of soil microbial communities through acidification [17] and an increased level of N in the water and air as well [17]. Also, rhizobia can promote root development, provide protection against soil-borne pathogens, increase stress tolerance, and induce systemic resistance in plants [18]. In addition to the above influences, rhizobia plays a role in promoting plant growth and productivity. This includes the synthesis of plant growth-promoting phytohormones such as indole-3-acetic acids (IAA), cytokinins, gibberellins, riboflavin, lumichrome, and Nod factors [19].
Sufficient quantities of chemical fertilizers are essential to enhance fodder production in arable field conditions and achieve a desirable biomass yield [20,21]. Given the considerable costs and environmental implications associated with nitrogenous fertilizer application, along with the aim of establishing sustainable agriculture in Afghanistan, biofertilizers, particularly promising rhizobial inoculants, are regarded as an effective alternative for optimizing fodder production [22,23]. The study of rhizobial diversity as a biological resource and identifying promising bacterial strains with exciting features is a valuable strategy to maximize agricultural productivity [24]. However, rhizobia’s genetic makeup and population composition may be linked to geographical and environmental conditions [25]. Given the significance of clover as the predominant forage crop in Afghanistan, no documentation is currently available regarding the root nodule bacteria associated with clover crops in Afghan soils and prospective biofertilizer development. Moreover, studying Afghan clover-nodulating rhizobia assists researchers and biofertilizer production companies in articulating rhizobial diversity and efficient utilization.
Hence, this is the first attempt to elucidate the physiological properties and genetic characteristics of root nodule bacteria associated with clover crops in Afghan soils. To achieve this goal, we collected six soil samples from diverse agricultural-ecological-climatic regions in Afghanistan [26], isolated their root nodulating rhizobia, and examined their genetic diversity (16S rRNA and nifD) and nitrogen fixation potential. Additionally, we assessed the isolates for abiotic tolerance, considering factors such as pH, salt, and temperature. The most promising isolates, determined by their symbiotic performances as well as tolerance to the abiotic stresses, would be selected for the development of biofertilizers tailored for clover crops in Afghanistan.
2. Materials and Methods
2.1. Soil Sampling
Various environmental factors such as climate, soil properties, and plant growth and type influence soil microorganisms’ diversity and community structure. Therefore, six soil samples were gathered at a depth of 0 to 20 cm from diverse agricultural climatic regions (hot desert climate to cold and semi-arid climate) and agricultural and forage crop fields to isolate native clover-nodulating rhizobia from Afghan soils, as reported by Habibi et al. [26]. Each soil sample was a composite sample from their sampling site, and the specifics of the soil samples can be found in Table 1. It is noteworthy that the sites where soil samples were taken have no previous history of microbial inoculation.

Table 1.
Soil sampling sites and numbers of nodules obtained from clover plants after inoculation with soil samples.
2.2. Isolation of Clover-Nodulating Rhizobia
To isolate microsymbionts associated with clover, seeds of a local Afghan clover variety (Persian clover: Trifolium resupinatum L.) underwent surface sterilization. This process involved pre-treating the seeds with 70% ethanol for 30 s, followed by immersion in a 3% sodium hypochlorite solution for 3 min. Subsequently, the seeds were thoroughly rinsed with sterilized reverse osmosis (RO) water and allowed to germinate for 2 days at 25 °C. Soil suspensions (1 g/5 mL) were prepared for each sample and utilized for the isolation of rhizobia. Each soil suspension inoculant was then applied to pots containing germinated clover seeds and vermiculite.
The pots were arranged in a growth chamber, and the plants were cultivated under controlled conditions, with a 16 h light/8 h dark photoperiod and day/night temperatures set at 25 °C/18 °C. Maintaining a soil moisture level of 60%, a sterilized nitrogen-free nutrient solution was introduced to the pots. After seven weeks, the plants were carefully uprooted from the pots, washed, and vermiculite was removed. The nodules were harvested from the plant roots and subjected to surface sterilization using 70% ethanol and 3% sodium hypochlorite. Each nodule was then macerated in a glycerol solution (15%, v/v), with 10 µL of the solution streaked onto yeast extract mannitol (YEM) agar plates. Subsequently, the plates underwent incubation for 4–7 days at 28 °C. Pure single colonies were stored for the long term in 15% glycerol at −80 °C and for the short term on slants stored at 4 °C.
2.3. Screening of Rhizobia Strains against Abiotic Stress
The growth potential of the isolate was elucidated under varied salinity, pH, and temperature conditions to assess stress tolerance, following the methodology outlined by Djedidi et al. [27]. Initially, the isolates were cultured in YEM broth medium for two days at 28 °C. Subsequently, 10 µL (106 cells mL–1) of each culture was inoculated onto YEM agar plates and incubated for three days. Temperature tolerance experiments included incubation at 25 °C, 28 °C (as a positive control), 40 °C, and 45 °C. For salinity tolerance, isolates were exposed to 0% (0.1 g L–1 NaCl) as a positive control, followed by increasing NaCl concentrations to 2%, 3%, and 4%. In terms of pH tolerance, the medium’s pH was adjusted to 4.5, 6.8, 9, and 10, with a pH of 6.8 serving as the positive control. Each of these stress-tolerance experiments was conducted in triplicate for every isolate. Based on the abiotic stress elucidation, 19 isolates were selected for further study.
2.4. Genomic DNA Extraction
Rhizobial isolates were cultured in YEM broth medium at 28 °C for a duration of 4–7 days. Following cultivation, bacterial cells were collected through centrifugation at 10,000 rpm for 10 min and subjected to two washes with TNE buffer (10 mM Tris, 0.1 M NaCl, and 1 mM EDTA, pH 8). Genomic DNA extraction was carried out using the protocol outlined by Yokoyama et al. [28]. The concentration and purity of the extracted DNA were assessed using a UV-Vis Nano Drop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Subsequently, the extracted DNA, at a specific concentration of 200–250 ng/μL, was employed for the amplification of 16S rRNA and nifD genes.
2.5. Genes Amplification and Sequencing
The PCR amplification and sequencing of 16S rRNA and nifD were performed as explained by Habibi et al. [26]. A set of 1F (5′-AGT TTG ATC CTG GCT C-3′) and 3R (5′-AAG GAG GTG ATC CAG CC-3′) was used for sequencing the 16S rRNA, and nifD 161F (TGCGRSGTRAAGTCSAAYAT) and nifD 1435R (TCCATGTCKCGSGCGAARAT) primers were used for nifD. For PCR amplification, 50 μL reaction mixtures containing a primer set of F and R (10 μM each), 0.5 μL Taq DNA polymerase (ExTaq polymerase 5 UmL-1, Takara Bio, Otsu, Japan), and 200 ng DNA/μL, 5 μL 10× reaction buffer, 4 μL dNTP mixture were used. PCR conditions were as follows: denaturation at 94 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 2 min, extension at 72 °C for 3 min, and a final extension at 72 °C for 7 min. Then, bands related to 16S rRNA and nifD were purified using a QIAEX II agarose gel extraction kit (Qiagen, Valencia, CA, USA). Eventually, according to the manufacturer’s protocols, PCR products were sequenced using an ABI PRISM 3500 genetic analyzer (Applied Biosystems, Waltham, MA, USA). The sequences of 16S rRNA and nifD have been deposited in the DNA Data Bank of Japan (DDBJ) with accession numbers LC787678–LC787696 for 16S rRNA and LC787659–LC787677 for nifD. Phylogenetic trees of sequenced genes were constructed using Molecular Evolutionary Genetics Analysis (MEGA) version 11.
2.6. Symbiotic Performance
Seed sterilization, cultivation, and growing conditions are detailed in Section 2.2. In this context, rhizobial cells were collected through centrifugation at 10,000 rpm for 10 min at 4 °C and subjected to two washes with TNE solution. Subsequently, a cell density of 108 CFU was administered to two germinated clover seeds in pots. Roots were separated from shoots, placed in jars, and assessed for nodules’ nitrogen fixation ability. Ten percent of the jar’s air was replaced with acetylene and incubated for one hour at 25 °C. The amount of ethylene in the jar was measured using a gas chromatograph (Shimadzu 2014AF, Kyoto, Japan).
2.7. Statistical Analysis
The data underwent analysis through one-way analysis of variance (ANOVA), and differences among the means of the treatments were determined using Tukey’s honestly significant difference (HSD) test at a significance level of 5%. All statistical analyses were performed using JMP Pro 16 (JMP, Cary, NC, USA).
3. Results
3.1. Isolation of Rhizobia from Clover Root Nodule
A total of fifty-seven isolates from clover nodules were obtained, as shown in Table 1. The induced root nodules by rhizobia were found in four soil samples (Nangarhar, Parwan, Baghlan, and Kunduz), while the soils of Kabul and Bamyan could not show nodule formation. High numbers of clover root nodules were found in the Baghlan and Parwan soils.
3.2. Rhizobial Tolerance for Abiotic Stresses
All clover isolates (100%) could grow at different pHs (4.5 to 10) and exhibited a high adaptation to the different soil pH (Table 2). Regarding high-temperature resistance, 40 and 45 °C influenced the growth of isolates. Only 12.5% of Baghlan isolates could survive at 45 °C, and they were the most sensitive isolates to temperature among the four soils, whereas, in Nangarhar soil, approximately 36% of isolates were able to grow (Table 2). In the salinity test, the effect of salinity was wider than the other two abiotic stresses (pH and temperature). The frequency of clover isolates obtained from three soils (Kunduz, Baghlan, and Parwan) observed the same survival ratio from 50 to 58% at 4% of NaCl condition, while the Nangarhar isolates, only 21.4% were able to survive (Table 2). At a low percent of NaCl (2%) condition, all isolates (100%) obtained from Kunduz soil could survive.

Table 2.
The growth potential of clover isolates at different pH, temperatures, and NaCl concentrations.
3.3. The 16S rRNA and nifD Genes Analysis
Based on the 16S rRNA, a total of nineteen clover isolates were divided into two groups, as shown in Figure 1. GI contained eighteen isolates (94.7% of the total), and GII consisted of only one isolate (5.3%). The GI group is divided into three subgroups [GIa (47.4%), GIb (5.3%), and GIc (42.1%)]. The GIa subgroup included nine isolates and showed a close relationship (100%) to the Rhizobium hidalgonense and Rhizobium leguminosarum species. The GIb subgroup consisted of one isolate (KC10) and was highly similar (100%) to the Rhizobium leguminosarum species. The GIc subgroup comprised eight isolates and revealed high similarity to the Rhizobium etli CFN 42. The GII group contained one isolate (KC3) and showed maximum similarity to the Ensifer meliloti 2011.
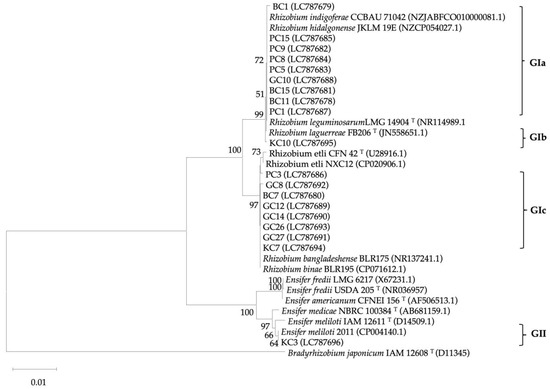
Figure 1.
Neighbor-joining phylogenetic tree based on the partial sequence of the 16S rRNA gene (1383 bp) constructed using MEGA 11.0. The clover isolates, and relevant type strains with their accession numbers exhibited in the tree represent relationships among the rhizobial strains. Bradyrhizobium japonicum IAM 12608 was used as an outgroup. Bootstrap values are displayed for each node with 1000 replicates, and values less than 50 were ignored.
All 19 clover isolates based on the nifD gene sequence, were categorized into one group (GI), as shown in Figure 2. They showed close similarity to the Rhizobium species. The GI group was divided into two subgroups: the GIa subgroup contained eleven isolates (57.9% of the total), and the GIb subgroup consisted of eight isolates (42.1%). The GIa subgroup showed close similarity to the Rhizobium leguminosarum bv. trifolii species when compared to the other references, whereas the eight isolates were separated from Rhizobium etli and Rhizobium leguminosarum bv. trifolii species. The KC3 isolate, according to the 16S rRNA gene sequence, was categorized into Ensifer meliloti 2011. In the nifD sequence, the KC3 isolate showed maximum similarity to the Rhizobium species.
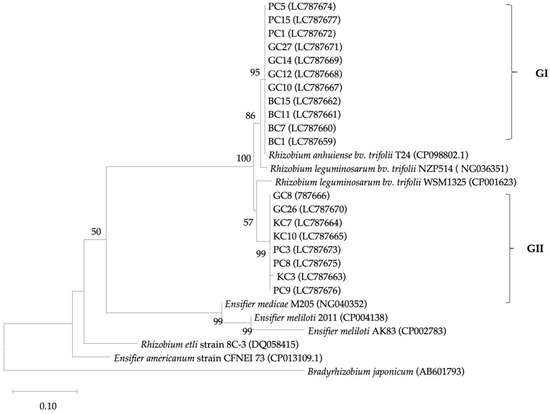
Figure 2.
The phylogenetic relationship between isolated clover strains and relevant similar species based on the partial sequence of the nifD gene (1158 bp) and the neighbor-joining method. Bootstrap values are displayed for each node with 1000 replicates. Values less than 50 were ignored.
Table 3 presents the plant growth test results for the nineteen clover isolates. All plants inoculated with rhizobia exhibited effective nodules and increased biomass. Notably, all isolates demonstrated a statistically significant increase in plant biomass compared to the control (un-inoculated plants). Specifically, among various genotypes, PC8 and PC9, associated with GIa and GIb genotypes, displayed elevated acetylene reduction activity (ARA) (8.2 µM/plant, 6.5 µM/plant) and substantial biomass production (1769.0 mg/plant and 1409.2 mg/plant) (Table 3). The most promising isolates, characterized by different genotypes and showcasing the highest biomass production, include BC15, BC7, PC8, PC9, PC15, GC8, GC14, and KC7 (Table 3). In contrast, low biomass production was observed in PC1, KC10, GC12, GC27, and KC3 isolates.

Table 3.
Growth performances of inoculated clover plants corresponding to the inoculation of 19 rhizobial isolates.
4. Discussion
Four of the six soil samples from different fields induced root nodules on clover plants. Notably, Parwan and Baghlan soils exhibited the dominance of nodules. However, the remaining two samples of Kabul and Bamyan soils failed to induce root nodulation in clover crops, possibly due to elevated soil salinity conditions (2.3–4.1 ms/cm) observed in these soils (Table 1). The challenge of nodule formation in legume crops under conditions of soil salinity has been documented in prior studies, underscoring salinity as a significant impediment to the normal growth of legume crops, especially clover [29,30,31].
The growth performance of clover isolates exhibited notable resilience across a range of pH levels (4.5–10). However, the isolates demonstrated heightened sensitivity to temperature and salinity. Elevated temperatures significantly impacted isolate growth, with approximately 50% unable to grow at 40 °C and 88% at 45 °C on the plates. Similarly, the presence of 2–4% NaCl inhibited the normal growth of colonies, with this effect being more pronounced in the Nangarhar soil sample (Mung bean field), characterized by a low electrical conductivity (EC) (Table 1). Additionally, about 95% of clover isolates displayed a close affiliation with the Rhizobium species. This suggests that Rhizobium sp. exhibits better adaptability to various pH levels, particularly at pH 4.5, compared to Ensifer or Sinorhizobium species. Despite acidic pH (4.0) tolerance, Rhizobium involves a normal nodulation process. Rhizobium strains display diverse levels of acid tolerance, with certain strains capable of thriving at pH 4.6 and below. Nevertheless, under low pH conditions, rhizobium attachment to root hairs, root colonization, and subsequent nodule formation may be adversely affected [32,33,34]. It is crucial to emphasize that distinct strains of rhizobium may demonstrate varying responses to low pH conditions, with some strains exhibiting greater tolerance than others [35]. Furthermore, elevated pH levels can influence the composition of nodulation factors produced by rhizobium, potentially impacting its capacity to form nodules and facilitate nitrogen fixation [36], while some strains can have tolerance under elevated pH conditions [33]. Moreover, in accordance with our findings, certain studies have demonstrated that high temperatures can have a detrimental impact on rhizobial growth and living conditions [37,38]. A similar effect can be observed with an increase in salt concentrations [39].
Using the 16S rRNA sequence, nineteen isolates were classified into two distinct groups, as illustrated in Figure 1. The GI group comprised eighteen isolates, constituting 94.7% of the total, while the GII group consisted of only one isolate, making up 5.3% of the total isolates. Further subdivision into three subgroups (GIa, GIb, and GIc) was observed within the GI group. Specifically, the GIa subgroup, which included nine isolates, exhibited a close relationship (100%) to the Rhizobium hidalgonense and Rhizobium leguminosarum species. The Rhizobium laguerreae FB206 seems to not only have alfalfa-nodulating rhizobia but is also able to nodulate clover (Trifolium resupinatum) effectively. GIb subgroup consisted of one isolate (KC10) and had high relatedness (100%) to the Rhizobium leguminosarum bv. viciae species. Rhizobium leguminosarum bv. viciae is a nodulating rhizobia of Vicia faba. Rhizobium leguminosarum bv. viciae showed effective nodules on clover roots. The GIc subgroup comprised eight isolates (42.1%) and revealed high similarity (100%) to different Rhizobium species (Rhizobium bangladeshense BLR175 and Rhizobium binae BLR195), including the Rhizobium etli CFN 42. Recently, Shamseldin et al. (2014) documented the capability of Rhizobium etli to form nodules on Trifolium alexandrinum L. roots in Egypt, highlighting Rhizobium etli as the predominant species responsible for nodulating Egyptian winter berseem clover (Trifolium alexandrinum L.). Furthermore, Castaingts et al. [40] identified Pvu-miR5942 as a newly discovered miRNA that plays a role in selecting Rhizobium etli strains for nodule colonization. Furthermore, Rhizobium etli CFN42 has the capacity to form a symbiotic association with the roots of Phaseolus vulgaris plants, resulting in the development of nitrogen-fixing nodules [41,42,43], in clover [44,45], and in Faba bean (Vicia faba L.), T. semipilosum, and white clover (T. repens L.). It seems that R. etli has high compatibility to nodulate various species of clover. Notably, within this investigation, the GII group included a sole isolate (KC3) demonstrating maximum similarity (100%) to Ensifer Meliloti 2011, which can nodulate some clover species [46].
The 19 clover isolates were initially grouped together (GI) based on the nifD gene sequences. Subsequently, GI was further divided into two subgroups (GIa and GIb). The GIa subgroup consisted of 11 isolates (57.9%), showing high similarity to Rhizobium leguminosarum bv. trifolii. On the other hand, the GIb subgroup comprised eight isolates (42.1%), representing a novel lineage group of Rhizobium leguminosarum bv. trifolii. Additional analysis is required to explore and understand this nifD group further. One isolate (KC3) in the GIc subgroup showed high similarity to the genus of Ensifer based on the 16S rRNA gene sequence, while in the nifD sequence, it revealed maximum similarity to the Rhizobium genus and categorized in the new lineage group of Rhizobium species. This means the nifD gene transferred from Ensifer sp. to Rhizobium sp. That horizontal gene transfer occurred in Kunduz soil in the field of Mung bean and Maize and produced effective nodules on clover roots. It could be due to the lateral or horizontal gene transfer, which is a common event among prokaryotes and can occur among the different genera and species of bacteria [27,47], including nonsymbiotic bacteria [48]. However, phylogenetic variation at nifD describes a major variation in partner quality, particularly clover [49], and may have an effective role in plant and rhizobial symbiotic and mutual relationships.
In the context of clover isolates, all plants inoculated with rhizobia exhibited the formation of effective nodules and a notable increase in biomass production. All clover isolates demonstrated statistically significant enhancements in plant biomass compared to un-inoculated plants. Increasing plant biomass varied among the different genotypes (16S rRNA and nifD). PC8 and PC9, having the same genotypes (GIa, GIb), exhibited the highest plant biomass production and ARA among the various genotypes. More stable biomass production (75%) was observed in the genotypes of GIc and GIb. However, the KC3 isolate (Ensifer meliloti) showing the transferred nifD gene from Rhizobium species, produced low plant biomass. This result is consistent with Gordon et al.’s [49] report that nifD plays an important role in partner quality issues. In our study, lateral gene transfer of nifD was not effective as compared to Rhizobium species. The inoculation effect of Rhizobium species, especially Rhizobium trifolii, was studied individually and as co-inoculation with other microbial inoculants on different clover varieties in various fields and climatic conditions [50,51,52,53,54]. Tufail et al. [50] reported that inoculation of Rhizobium trifolii seed inoculation on berseem clover (Trifolium alexandrinum) significantly increased forage biomass and quality. He found that the number of stems/m2 (348.2), plant height (24.4 cm), green forage yield (39.9 t/ha), dry matter yield (5.54 t/ha), and other parameters notably enhanced through Rhizobium seed inoculation. Also, he stated that Rhizobium inoculum increased soil fertility and gained net income compared to the non-inoculated plots. The efficacy of clover rhizobial inoculants seems to increase with the co-inoculation of other microbial inoculants. For instance, Furtak et al. [52] documented that the co-inoculation of red clover (Trifolium pratense L.) with Rhizobium leguminosarum and Azospirillum resulted in more root and shoot biomass when compared with plants inoculated with R. leguminosarum alone. Furthermore, he added that co-inoculation improved clover nodulation and growth under the condition of polycyclic aromatic hydrocarbon contamination.
Considering the environmental pollution due to excessive chemical fertilizer application and the tendency to increase organic and eco-friendly fertilizers, the application of effective microbial inoculants might be a virtuous strategy for enhancing clover green and dry biomass and feeding animals. In our study, based on the plant test results, isolates BC7, BC15, PC15, and PC8, representing various phylogenetic groups within clover, are identified as promising candidates for the formulation of biofertilizers in the Afghanistan context.
5. Conclusions
This study represents the first information on Afghanistan’s clover-nodulating rhizobial genetic diversity and its potential for increasing plant growth. Around 60% of rhizobial isolates in abiotic stress could not grow under 2–4% NaCl stress. A high-temperature effect on rhizobial isolates growth was observed at 45 °C (75.5% of total isolates), followed by 40 °C (30.1%). Genetic analysis based on 16S rRNA showed that Rhizobium species were the dominant group among the isolated rhizobia. The nifD results displayed close similarity to rhizobium species and a novel linage group to Rhizobium leguminosarum species. A lateral gene transfer of the nifD gene was observed from Rhizobium to Ensifer species in Kunduz soil. In the plant test, all isolates significantly increased plant biomass production in comparison to the negative control (un-inoculated plant). Among genotypes, PC8 and PC9 isolates displayed the height results of plant biomass and nitrogen fixation activity. All isolates, particularly PC8 and PC9, have the capacity to nodulate clover roots and enhance plant growth effectively.
Author Contributions
Conceptualization, T.Y. and S.H.; methodology, T.Y.; software, S.A.; validation, M.Y, N.O.-O. and S.A; formal analysis, S.H. and K.S.; investigation, S.H.; resources, T.Y; data curation, K.S.; writing—original draft preparation, T.Y. and N.O.-O.; writing—review and editing, N.O.-O. and M.Y.; visualization, T.Y.; supervision, T.Y. and N.O.-O.; project administration, T.Y.; funding acquisition, T.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lamont, E.-J.; Zoghlami, A.; Hamilton, R.S.; Bennett, S.J. Clovers (Trifolium L.). In Plant Genetic Resources of Legumes in the Mediterranean; Springer: Berlin/Heidelberg, Germany, 2001; pp. 79–98. [Google Scholar] [CrossRef]
- Kozieł, M.; Kalita, M.; Janczarek, M. Genetic Diversity of Microsymbionts Nodulating Trifolium pratense in Subpolar and Temperate Climate Regions. Sci. Rep. 2022, 12, 12144. [Google Scholar] [CrossRef] [PubMed]
- Egan, L.M.; Hofmann, R.W.; Ghamkhar, K.; Hoyos-Villegas, V. Prospects for Trifolium Improvement through Germplasm Characterisation and Pre-Breeding in New Zealand and Beyond. Front. Plant Sci. 2021, 12, 653191. [Google Scholar] [CrossRef] [PubMed]
- Çölgeçen, H.; Koca, U.; Büyükkartal, H.N. Use of Red Clover (Trifolium pratense L.) Seeds in Human Therapeutics. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2020; pp. 421–427. [Google Scholar] [CrossRef]
- OCHA. Rebuilding Agriculture in Afghanistan: Livestock Needs Assessment Completed—Afghanistan | ReliefWeb. Available online: https://reliefweb.int/report/afghanistan/rebuilding-agriculture-afghanistan-livestock-needs-assessment-completed (accessed on 21 November 2023).
- FAO. FAO/WFP Crop and Food Supply Assessment Mission to Afghanistan. Available online: https://www.fao.org/3/J0156e/J0156e00.htm (accessed on 21 November 2023).
- Acosta-Jurado, S.; Fuentes-Romero, F.; Ruiz-Sainz, J.E.; Janczarek, M.; Vinardell, J.M. Rhizobial Exopolysaccharides: Genetic Regulation of Their Synthesis and Relevance in Symbiosis with Legumes. Int. J. Mol. Sci. 2021, 22, 6233. [Google Scholar] [CrossRef]
- Janczarek, M.; Rachwał, K.; Marzec, A.; Grzadziel, J.; Palusińska-Szysz, M. Signal Molecules and Cell-Surface Components Involved in Early Stages of the Legume–Rhizobium Interactions. Appl. Soil Ecol. 2015, 85, 94–113. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Imran, A.; Hakim, S.; Tariq, M.; Nawaz, M.S.; Laraib, I.; Gulzar, U.; Hanif, M.K.; Siddique, M.J.; Hayat, M.; Fraz, A.; et al. Diazotrophs for Lowering Nitrogen Pollution Crises: Looking Deep Into the Roots. Front. Microbiol. 2021, 12, 637815. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Walker, L.; Lagunas, B.; Gifford, M.L. Determinants of Host Range Specificity in Legume-Rhizobia Symbiosis. Front Microbiol 2020, 11, 585749. [Google Scholar] [CrossRef]
- Young, J.P.W.; Crossman, L.C.; Johnston, A.W.B.; Thomson, N.R.; Ghazoui, Z.F.; Hull, K.H.; Wexler, M.; Curson, A.R.J.; Todd, J.D.; Poole, P.S.; et al. The Genome of Rhizobium leguminosarum Has Recognizable Core and Accessory Components. Genome Biol. 2006, 7, R34. [Google Scholar] [CrossRef]
- Kumar, N.; Lad, G.; Giuntini, E.; Kaye, M.E.; Udomwong, P.; Jannah Shamsani, N.; Peter, W.; Young, J.; Bailly, X. Bacterial Genospecies That Are Not Ecologically Coherent: Population Genomics of Rhizobium leguminosarum. Open Biol. 2015, 5, 140133. [Google Scholar] [CrossRef]
- Chi, F.; Shen, S.H.; Cheng, H.P.; Jing, Y.X.; Yanni, Y.G.; Dazzo, F.B. Ascending Migration of Endophytic Rhizobia, from Roots to Leaves, inside Rice Plants and Assessment of Benefits to Rice Growth Physiology. Appl. Environ. Microbiol. 2005, 71, 7271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Adamczyk, B.; Suseela, V.; Yin, H. The Role of Soil Organic Nitrogen in Forest Plant Nutrition. Front. For. Glob. Chang. 2023, 6, 1244102. [Google Scholar] [CrossRef]
- Lo Cascio, M.; Morillas, L.; Ochoa-Hueso, R.; Delgado-Baquerizo, M.; Munzi, S.; Roales, J.; Spano, D.; Cruz, C.; Gallardo, A.; Manrique, E.; et al. Nitrogen Deposition Effects on Soil Properties, Microbial Abundance, and Litter Decomposition across Three Shrublands Ecosystems from the Mediterranean Basin. Front. Environ. Sci. 2021, 9, 709391. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.I.; Dakora, F.D. Rhizobia as a Source of Plant Growth-Promoting Molecules: Potential Applications and Possible Operational Mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676. [Google Scholar] [CrossRef]
- Kebede, T.; Keneni, Y.G.; Senbeta, A.F.; Sime, G. Effect of Bioslurry and Chemical Fertilizer on the Agronomic Performances of Maize. Heliyon 2023, 9, e13000. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Li, Y.; Li, H. Yield and Quality of Alfalfa (Medicago sativa L.) in Response to Fertilizer Application in China: A Meta-Analysis. Front. Plant Sci. 2022, 13, 1051725. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An Ecofriendly Technology for Nutrient Recycling and Environmental Sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Ajmal, M.; Ali, H.I.; Saeed, R.; Akhtar, A.; Tahir, M.; Mehboob, M.Z.; Ayub, A. Biofertilizer as an Alternative for Chemical Fertilizers. Res. Rev. J. Agric. Allied Sci. 2018, 7, 1–7. [Google Scholar]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–Host Interactions and Symbiotic Nitrogen Fixation in Legume Crops Toward Agriculture Sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef]
- Yang, W.; Kong, Z.; Chen, W.; Wei, G. Genetic Diversity and Symbiotic Evolution of Rhizobia from Root Nodules of Coronilla varia. Syst. Appl. Microbiol. 2013, 36, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Habibi, S.; Ayubi, A.G.; Ohkama-Ohtsu, N.; Sekimoto, H.; Yokoyama, T. Genetic Characterization of Soybean Rhizobia Isolated from Different Ecological Zones in North-Eastern Afghanistan. Microbes Environ. 2017, 32, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Djedidi, S.; Yokoyama, T.; Ohkama-Ohtsu, N.; Risal, C.P.; Abdelly, C.; Sekimoto, H. Stress Tolerance and Symbiotic and Phylogenic Features of Root Nodule Bacteria Associated with Medicago Species in Different Bioclimatic Regions of Tunisia. Microbes Environ. 2011, 26, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Ando, S.; Murakami, T.; Imai, H. Genetic Variability of the Common Nod Gene in Soybean Bradyrhizobia Isolated in Thailand and Japan. Can. J. Microbiol. 1996, 42, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Al Sherif, E.A. Melilotus indicus (L.) All., a Salt-Tolerant Wild Leguminous Herb with High Potential for Use as a Forage Crop in Salt-Affected Soils. Flora—Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 737–746. [Google Scholar] [CrossRef]
- Ikeda, J. The effect of short term withdrawal of NaCl stress on nodulation of white clover. In Plant and Soil; Springer: Berlin/Heidelberg, Germany, 1994; Available online: https://www.jstor.org/stable/42939080 (accessed on 11 December 2023).
- Cekstere, G.; Karlsons, A.; Grauda, D. Salinity-Induced Responses and Resistance in Trifolium repens L. Urban For. Urban Green. 2015, 14, 225–236. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, J.-P.; Wei, S.-Q.; Zhou, Z.-Y.; Zhang, C.; Yu, Y.-X. Mechanism of Acid Tolerance in a Rhizobium Strain Isolated from Pueraria lobata (Willd.) Ohwi. Can. J. Microbiol. 2011, 57, 514–524. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Lin, M.H.; Gresshoff, P.M. Regulation of Legume Nodulation by Acidic Growth Conditions. Plant Signal. Behav. 2013, 8, e23426. [Google Scholar] [CrossRef]
- Kamran, A.; Mushtaq, M.; Arif, M.; Rashid, S. Role of Biostimulants (Ascorbic Acid and Fulvic Acid) to Synergize Rhizobium Activity in Pea (Pisum sativum L. Var. Meteor). Plant Physiol. Biochem. 2023, 196, 668–682. [Google Scholar] [CrossRef]
- Hawkins, J.P.; Oresnik, I.J. The Rhizobium-Legume Symbiosis: Co-Opting Successful Stress Management. Front. Plant Sci. 2022, 12, 796045. [Google Scholar] [CrossRef]
- Morón, B.; Soria-Díaz, M.E.; Ault, J.; Verroios, G.; Noreen, S.; Rodríguez-Navarro, D.N.; Gil-Serrano, A.; Thomas-Oates, J.; Megías, M.; Sousa, C. Low PH Changes the Profile of Nodulation Factors Produced by Rhizobium Tropici CIAT899. Chem. Biol. 2005, 12, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Vershinina, Z.R.; Chubukova, O.V.; Nikonorov, Y.M.; Khakimova, L.R.; Lavina, A.M.; Karimova, L.R.; Baymiev, A.K.; Baymiev, A.K. Effect of RosR Gene Overexpression on Biofilm Formation by Rhizobium leguminosarum. Microbiology 2021, 90, 198–209. [Google Scholar] [CrossRef]
- Irshad, A.; Rehman, R.N.U.; Dubey, S.; Khan, M.A.; Yang, P.; Hu, T. Rhizobium Inoculation and Exogenous Melatonin Synergistically Increased Thermotolerance by Improving Antioxidant Defense, Photosynthetic Efficiency, and Nitro-Oxidative Homeostasis in Medicago truncatula. Front. Ecol. Evol. 2022, 10, 945695. [Google Scholar] [CrossRef]
- Tulumello, J.; Chabert, N.; Rodriguez, J.; Long, J.; Nalin, R.; Achouak, W.; Heulin, T. Rhizobium alamii Improves Water Stress Tolerance in a Non-Legume. Sci. Total Environ. 2021, 797, 148895. [Google Scholar] [CrossRef]
- Castaingts, M.; Kirolinko, C.; Rivero, C.; Artunian, J.; Mancini Villagra, U.; Blanco, F.A.; Zanetti, M.E. Identification of Conserved and New MiRNAs That Affect Nodulation and Strain Selectivity in the Phaseolus vulgaris–Rhizobium etli Symbiosis through Differential Analysis of Host Small RNAs. New Phytol. 2022, 234, 1430–1447. [Google Scholar] [CrossRef]
- Bañuelos-Vazquez, L.A.; Cazares, D.; Rodríguez, S.; Cervantes-De la Luz, L.; Sánchez-López, R.; Castellani, L.G.; Tejerizo, G.T.; Brom, S. Transfer of the Symbiotic Plasmid of Rhizobium etli CFN42 to Endophytic Bacteria Inside Nodules. Front. Microbiol. 2020, 11, 551589. [Google Scholar] [CrossRef]
- Bañuelos-Vazquez, L.A.; Torres Tejerizo, G.; Cervantes-De La Luz, L.; Girard, L.; Romero, D.; Brom, S. Conjugative Transfer between Rhizobium etli Endosymbionts inside the Root Nodule. Environ. Microbiol. 2019, 21, 3430–3441. [Google Scholar] [CrossRef]
- Hidalgo-García, A.; Tortosa, G.; Pacheco, P.J.; Gates, A.J.; Richardson, D.J.; Bedmar, E.J.; Girard, L.; Torres, M.J.; Delgado, M.J. Rhizobium etli Is Able to Emit Nitrous Oxide by Connecting Assimilatory Nitrate Reduction with Nitrite Respiration in the Bacteroids of Common Bean Nodules. J. Plant Interact. 2023, 18. [Google Scholar] [CrossRef]
- Youseif, S.H.; Abd El-Megeed, F.H.; Mohamed, A.H.; Ageez, A.; Veliz, E.; Martínez-Romero, E. Diverse Rhizobium Strains Isolated from Root Nodules of Trifolium alexandrinum in Egypt and Symbiovars. Syst. Appl. Microbiol. 2021, 44, 126156. [Google Scholar] [CrossRef]
- Shamseldin, A.; Epstein, B.; Sadowsky, M.J.; Zhang, Q. Comparative Genomic Analysis of Diverse Rhizobia and Effective Nitrogen-Fixing Clover-Nodulating Rhizobium Strains Adapted to Egyptian Dry Ecosystems. Symbiosis 2021, 84, 39–47. [Google Scholar] [CrossRef]
- Berais-Rubio, A.; Morel-Revetria, M.; Filippi, C.V.; Reyno, R.; Monza, J. Ensifer meliloti Elite Strain U143 Used as Alfalfa Inoculant in Uruguay: Characterization and Draft Genome Sequence. Microbe 2023, 1, 100008. [Google Scholar] [CrossRef]
- Barcellos, F.G.; Menna, P.; Batista, J.S.D.S.; Hungria, M. Evidence of Horizontal Transfer of Symbiotic Genes from a Bradyrhizobium japonicum Inoculant Strain to Indigenous Diazotrophs Sinorhizobium (Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian Savannah Soil. Appl. Environ. Microbiol. 2007, 73, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Wang, E.T.; Ji, Z.J.; Zhang, J.J. Recent Development and New Insight of Diversification and Symbiosis Specificity of Legume Rhizobia: Mechanism and Application. J. Appl. Microbiol. 2021, 131, 553–563. [Google Scholar] [CrossRef]
- Gordon, B.R.; Klinger, C.R.; Weese, D.J.; Lau, J.A.; Burke, P.V.; Dentinger, B.T.M.; Heath, K.D. Decoupled Genomic Elements and the Evolution of Partner Quality in Nitrogen-Fixing Rhizobia. Ecol. Evol. 2016, 6, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.S.; Krebs, G.L.; Ahmad, J.; Southwell, A.; Piltz, J.W.; Wynn, P.C. The Effect of Rhizobium Seed Inoculation on Yields and Quality of Forage and Seed of Berseem Clover (Trifolium alexandrinum L.) and Its Impact on Soil Fertility and Smallholder Farmer’s Income. J. Anim. Plant Sci. 2018, 28, 1493–1500. [Google Scholar]
- Toukabri, W.; Ferchichi, N.; Barbouchi, M.; Hlel, D.; Jadlaoui, M.; Bahri, H.; Mhamdi, R.; Cheikh M’hamed, H.; Annabi, M.; Trabelsi, D. Enhancement of Clover (Trifolium alexandrinum L.) Shade Tolerance and Nitrogen Fixation under Dense Stands-Based Cropping Systems. Agronomy 2022, 12, 2332. [Google Scholar] [CrossRef]
- Furtak, K.; Gawryjołek, K.; Gałazka, A.; Grzadziel, J. The Response of Red Clover (Trifolium pratense L.) to Separate and Mixed Inoculations with Rhizobium leguminosarum and Azospirillum brasilense in Presence of Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2020, 17, 5751. [Google Scholar] [CrossRef]
- Seguin, P.; Sheaffer, C.C.; Ehlke, N.J.; Russelle, M.P.; Graham, P.H. Nitrogen Fertilization and Rhizobial Inoculation Effects on Kura Clover Growth. Agron. J. 2001, 93, 1262–1268. [Google Scholar] [CrossRef]
- Shi, S.; Wakelin, S.; Gerard, E.; Young, S.; Van Koten, C.; Caradus, J.; Griffiths, A.G.; Ballard, R.A.; O’Callaghan, M. Screening and Field Evaluation of White Clover Rhizobia for New Zealand Pastures. Crop. Pasture Sci. 2023, 74, 1258–1271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
