Exopolysaccharides Synthesized by Rhizospheric Bacteria: A Review Focused on Their Roles in Protecting Plants against Stress
Abstract
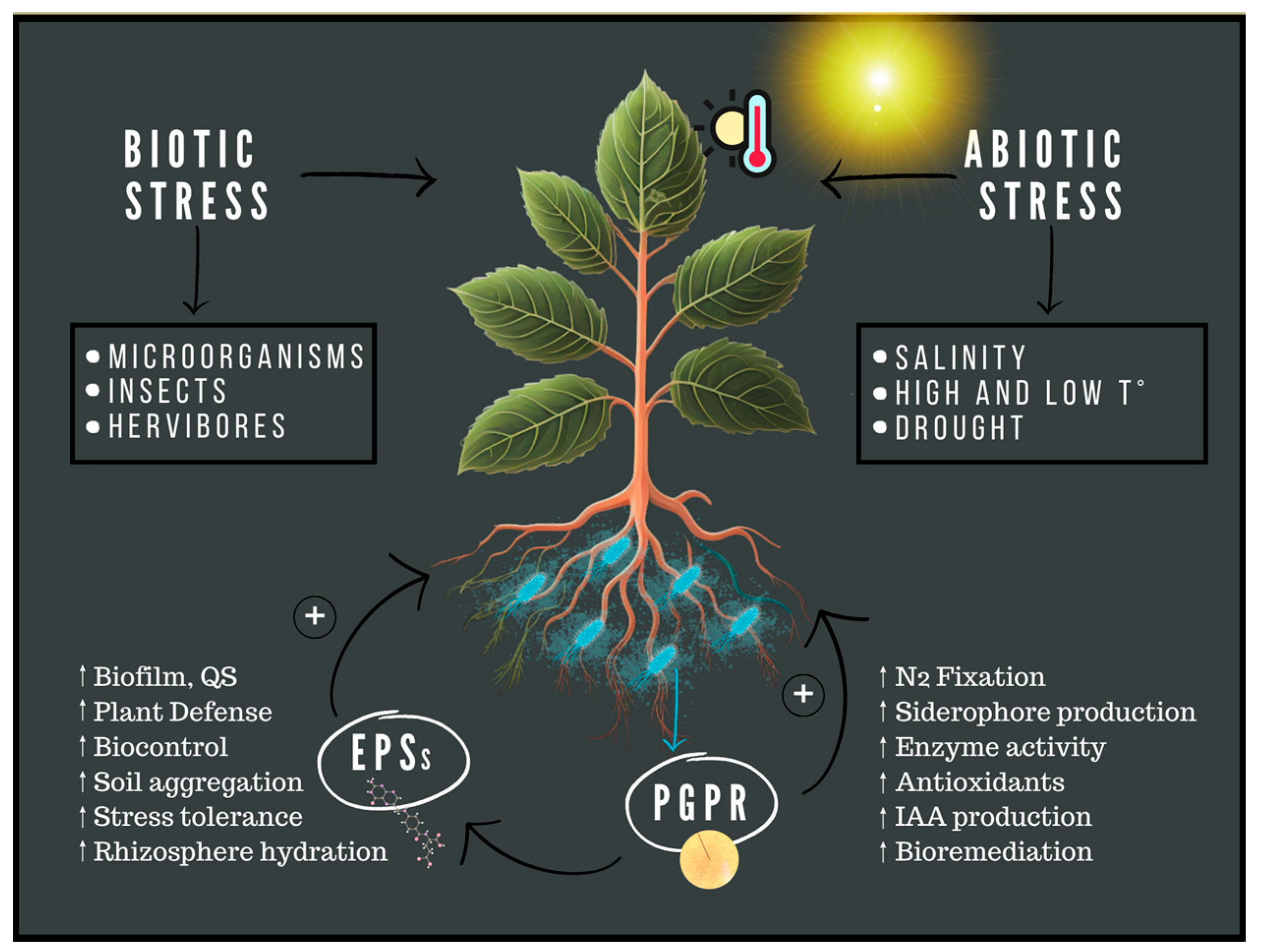
:1. Introduction
2. EPSs in Bacterial Biofilms
3. EPSs Synthesized by Plant Growth-Promoting Rhizobacteria
4. PGPR Activity and the Role of EPSs during Abiotic Stress
4.1. Salt Stress
4.2. Drought Stress
4.3. Temperature Stress
4.4. Stress by Metal(loid)s
5. PGPR Activity and the Role of EPSs during Biotic Stress
6. Beneficial Rhizobacteria and Sustainable Agriculture
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, R.; Shah, M.D.; Shah, L.; Lee, P.; Khan, I. Bacterial polysaccharides-A big source for prebiotics and therapeutics. Front. Nutr. 2022, 9, 1031935. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, L.; Li, Z.; Cheng, Y. The role of cyanobacterial external layers in mass transfer: Evidence from temperature shock experiments by noninvasive microtest technology. Microorganisms 2020, 8, 861. [Google Scholar] [CrossRef] [PubMed]
- Moradali, M.F.; Rehm, B.H. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Jurado, S.; Fuentes-Romero, F.; Ruiz-Sainz, E.; Janczarek, M.; Vinardell, M. Rhizobial exopolysaccharides: Genetic regulation of their synthesis and relevance in symbiosis with legumes. Int. J. Mol. Sci. 2021, 22, 6233. [Google Scholar] [CrossRef] [PubMed]
- Carezzano, M.E.; Paletti Rovey, M.F.; Sotelo, J.P.; Giordano, M.; Bogino, P.; Oliva, M.d.l.M.; Giordano, W. Inhibitory potential of Thymus vulgaris essential oil against growth, biofilm formation, swarming, and swimming in Pseudomonas syringae isolates. Process. 2023, 11, 933. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.; Ivanova, E. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Nadell, C.D.; Xavier, J.B.; Foster, K.R. The sociobiology of biofilms. FEMS Microbiol. 2009, 33, 206–224. [Google Scholar] [CrossRef]
- Heindl, J.E.; Wang, Y.; Heckel, B.C.; Mohari, B.; Feirer, N.; Fuqua, C. Mechanisms and regulation of surface interactions and biofilm formation in Agrobacterium. Front. Plant Sci. 2014, 6, 176. [Google Scholar] [CrossRef]
- Jamal, M.; Tasneem, U.; Hussain, T.; Andleeb, S. Bacterial biofilm: Its composition, formation and role in human infections. J. Microbiol. Biotechnol. 2015, 4, 1–14. [Google Scholar]
- Bogino, P.C.; Oliva, M.D.L.M.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Carezzano, M.E.; Paletti Rovey, M.F.; Cappellari, L.D.R.; Gallarato, L.A.; Bogino, P.; Oliva, M.D.L.M.; Giordano, W. Biofilmforming ability of phytopathogenic bacteria: A review of its involvement in plant stress. Plants 2023, 12, 2207. [Google Scholar] [CrossRef]
- Mangwani, N.; Dash, H.R.; Chauhan, A.; Das, S. Bacterial quorum sensing: Functional features and potential applications in biotechnology. J. Mol. Microbiol. 2012, 22, 215–227. [Google Scholar] [CrossRef]
- McNear, D.H. The Rhizosphere-Roots, Soil and Everything In Between. Nat. Educ. Knowl. 2013, 4, 1. Available online: https://www.nature.com/scitable/knowledge/library/the-rhizosphereroots-soil-and-67500617/ (accessed on 3 October 2023).
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 422679. [Google Scholar] [CrossRef]
- Kumar, U.; Sheleke, R.M.; Singh, R. Editorial: Soil-plant-microbe interactions: An innovative approach towards improving soil health and plant growth. Front. Agron. 2023, 5, 1165328. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: A review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Morcillo, R.J.; Manzanera, M. The Effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Pandey, S. Plant growth promoting rhizobacteria to mitigate biotic and abiotic stress in plants. In Sustainable Agriculture Reviews 60; Singh, N., Chattopadhyay, A., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2023; Volume 60, pp. 47–68. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A promising source of plant growth-promoting molecules and their non-legume interactions: Examining applications and mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Nevo, E. Plant Root Associated Biofilms: Perspectives for Natural Product Mining. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 3, pp. 285–300. [Google Scholar] [CrossRef]
- Granados, C.B. Rhizobacteria and their contribution to plant tolerance to drought and salinity. Cuba. J. Agric. Sci. 2022, 56, 69–87. Available online: https://www.cjascience.com/index.php/CJAS/article/view/1051 (accessed on 16 August 2023).
- Dar, A.; Zahir, Z.A.; Iqbal, M.; Mehmood, A.; Javed, A.; Hussain, A.; Bushra; Ahmad, M. Efficacy of rhizobacterial exopolysaccharides in improving plant growth, physiology, and soil properties. Environ. Monit. Assess 2021, 193, 515. [Google Scholar] [CrossRef]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045–1059. [Google Scholar] [CrossRef]
- Velasco-Jiménez, A.; Castellanos-Hernández, O.; Acevedo-Hernández, G.; Aarland, R.C.; Rodríguez-Sahagún, A. Bacterias rizosféricas con beneficios potenciales en la agricultura. Terra Latinoam. 2020, 38, 333–345. [Google Scholar] [CrossRef]
- Omae, N.; Tsuda, K. Plant-microbiota interactions in abiotic stress environments. Mol. Plant-Microbe Interact. 2022, 35, 511–526. [Google Scholar] [CrossRef]
- Bello, S.K.; Alayafi, A.H.; AL-Solaimani, S.G.; Abo-Elyousr, K.A.M. Mitigating soil salinity stress with gypsum and bio-organic amendments: A review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, T.; Zhang, B.; Zhang, T.; Cheng, Y.; Wang, C.; Luo, M.; Feng, H.; Siddique, K.H.M. The higher relative concentration of K+ to Na+ in saline water improves soil hydraulic conductivity, salt-leaching efficiency and structural stability. Soil 2023, 9, 339–349. [Google Scholar] [CrossRef]
- Osman, K.T. Soils on steep slopes. In Management of Soil Problems; Osman, K.T., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 185–217. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Gavelienė, V.; Jurkonienė, S.; Švegždienė, D. Effects of elevated temperature on root system development of two lupine species. Plants 2022, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in french bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 442191. [Google Scholar] [CrossRef]
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. PGPR-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J. Basic Microbiol. 2016, 56, 1274–1288. [Google Scholar] [CrossRef]
- Arora, N.K.; Khare, E.; Maheshwari, D.K. Plant growth promoting rhizobacteria: Constraints in bioformulation, commercialization, and future strategies. In Plant Growth and Health Promoting Bacteria. Microbiology Monographs; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 18, pp. 97–116. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Y.; Wang, R.; Li, S.; Qiu, Y.; Lei, P.; Gao, J.; Xu, H.; Zhang, F.; Lv, Y. Effects of exopolysaccharide derived from Pantoea alhagi NX-11 on drought resistance of rice and its efficient fermentation preparation. Int. J. Biol. Macromol. 2020, 162, 946–955. [Google Scholar] [CrossRef]
- Valverde-Otárola, J.C.; Arias, D. Effects of water stress on growing and physiological development of Gliricidia sepium (Jacq.) Kunth ex Walp. Colomb. For. 2020, 23, 20–34. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Fetsiukh, A.; Conrad, J.; Bergquist, J.; Timmusk, S. Silica particles trigger the exopolysaccharide production of harsh environment isolates of growth-promoting rhizobacteria and increase their ability to enhance wheat biomass in drought-stressed soils. Int. J. Mol. Sci. 2021, 22, 6201. [Google Scholar] [CrossRef]
- Zheng, W.; Zeng, S.; Bais, H.; LaManna, J.M.; Hussey, D.S.; Jacobson, D.L.; Jin, Y. Plant growth-promoting rhizobacteria (PGPR) reduce evaporation and increase soil water retention. Water Resour. Res. 2018, 54, 3673–3687. [Google Scholar] [CrossRef]
- Naseem, H.; Bano, A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014, 9, 689–701. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Atouei, M.T.; Pourbabaee, A.A.; Shorafa, M. Alleviation of salinity stress on some growth parameters of wheat by exopolysaccharide-producing bacteria. Iran J. Sci. Technol. Trans. Sci. 2019, 43, 2725–2733. [Google Scholar] [CrossRef]
- Primo, E.; Bogino, P.; Cossovich, S.; Foresto, E.; Nievas, F.; Giordano, W. Exopolysaccharide II is relevant for the survival of Sinorhizobium meliloti under water deficiency and salinity stress. Molecules 2020, 25, 4876. [Google Scholar] [CrossRef]
- Lavee, H.; Sarah, P.; Imeson, A.C. Aggregate stability dynamics as affected by soil temperature and moisture regimes. Geogr. Ann. A Phys. Geogr. 1996, 78, 73–82. [Google Scholar] [CrossRef]
- Kelishadi, H.; Mosaddeghi, M.; Ayoubi, S.; Mamedov, A. Effect of temperature on soil structural stability as characterized by high energy moisture characteristic method. Catena 2018, 170, 290–304. [Google Scholar] [CrossRef]
- Li, H.; Chang, L.; Wei, Y.; Li, Y. Interacting effects of land use type, soil attributes, and environmental factors on aggregate stability. Land 2023, 12, 1286. [Google Scholar] [CrossRef]
- Nievola, C.C.; Carvalho, C.P.; Carvalho, V.; Rodrigues, E. Rapid responses of plants to temperature changes. Temp. Multidiscip. Biomed. J. 2017, 4, 371–405. [Google Scholar] [CrossRef]
- Kai, H.; Iba, K. Temperature Stress in Plants. In eLS; John Wiley & Sons Ltd.: Chichester, UK, 2014; pp. 1–7. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2022, 41, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Khan, M.A.; Wani, S.H.; Pande, A.; Imran, M.; Kang, S.; Rahim, W.; Khan, S.A.; Bhatta, D.; Kwon, E.; et al. A Review on the role of endophytes and plant growth promoting rhizobacteria in mitigating heat stress in plants. Microorganisms 2022, 10, 1286. [Google Scholar] [CrossRef]
- Mukhtar, T.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Ali, S.; Chaudhary, H.J.; Solieman, T.H.; Ibrahim, A.A.; Saad, M.A. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Zainab, N.; Din, B.U.; Javed, M.T.; Afridi, M.S.; Mukhtar, T.; Kamran, M.A.; Khan, A.A.; Ali, J.; Jatoi, W.N.; Hussain Munis, M.F.; et al. Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol. Biochem. 2020, 152, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I. Heavy metal toxicity effects on plants. Toxics 2022, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef]
- Berger, S. Plant physiology meets phytopathology: Plant primary metabolism and plant-pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Motta Escobar, S.; Salazar Cabezas, L.D.; Sánchez Leal, L.C. Perspectiva del uso de Pseudomonas spp. como biocontrol de fitopatógenos en cultivos de hortalizas en Colombia: Una revisión sistemática. Mutis 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; You, M.P.; Barbetti, M.J.; Chen, Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPR): Role of bacterial diversity. Microorganisms 2021, 9, 1988. [Google Scholar] [CrossRef]
- Huang, R.; Feng, Z.; Chi, X.; Sun, X.; Lu, Y.; Zhang, B.; Lu, R.; Luo, W.; Wang, Y.; Miao, J.; et al. Pyrrolnitrin is more essential than phenazines for Pseudomonas chlororaphis G05 in its suppression of Fusarium graminearum. Microbiol. Res. 2018, 215, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Kasotia, A.; Jain, S.; Vaishnav, A.; Kumari, S.; Sharma, K.P.; Varma, A. Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J. Plant Growth Regul. 2016, 35, 276–300. [Google Scholar] [CrossRef]
- Tewari, S.; Arora, N.K. Multifunctional exopolysaccharides from Pseudomonas aeruginosa PF23 Involved in plant growth stimulation, biocontrol and stress amelioration in sunflower under saline conditions. Curr. Microbiol. 2014, 69, 484–494. [Google Scholar] [CrossRef]
- Foley, J.A.; De Fries, R.; Asner, G.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Firbank, L.G.; Petit, S.; Smart, S.; Blain, A.; Fuller, R.J. Assessing the impacts of agricultural intensification on biodiversity: A British perspective. Philos. Trans. R Soc. Lond. B Biol. Sci. 2008, 363, 777–787. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C. Biodiversidad y Manejo de Plagas en Agroecosistemas; Icaria Editorial: Catalonia, España, 2007. [Google Scholar]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutiérrez, C.; López, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef]
- Nastis, S.A.; Michailidis, A.; Mattas, K. Crop biodiversity repercussions of subsidized organic farming. Land Use Policy 2013, 32, 23–26. [Google Scholar] [CrossRef]
- Relyea, R.A. The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol. Appl. 2005, 15, 618–627. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Phys. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Villaamil Lepori, E.; Bovi Mitre, G.; Nassetta, M. Situación actual de la contaminación por plaguicidasen Argentina. Rev. Int. Contam. Ambient. 2013, 29, 25–43. [Google Scholar]
- Kumar, S.; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Vara-Sánchez, I.; Cuéllar-Padilla, M. Biodiversidad cultivada: Una cuestión de coevolución y transdisciplinariedad. Ecosistemas 2013, 22, 5–9. [Google Scholar] [CrossRef]
- Beltrán-Pineda, M.E.; Bernal-Figueroa, A.A. Biofertilizantes: Alternativa biotecnológica para los agroecosistemas. Mutis 2022, 12, 1–18. [Google Scholar] [CrossRef]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef]
- Duke, S. The history and current status of glyfhosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Bacteria and fungi controlling plant growth by manipulating auxin: Balance between development and defense. J. Plant Physiol. 2015, 172, 4–12. [Google Scholar] [CrossRef]
- Christeson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carezzano, M.E.; Alvarez Strazzi, F.B.; Pérez, V.; Bogino, P.; Giordano, W. Exopolysaccharides Synthesized by Rhizospheric Bacteria: A Review Focused on Their Roles in Protecting Plants against Stress. Appl. Microbiol. 2023, 3, 1249-1261. https://doi.org/10.3390/applmicrobiol3040086
Carezzano ME, Alvarez Strazzi FB, Pérez V, Bogino P, Giordano W. Exopolysaccharides Synthesized by Rhizospheric Bacteria: A Review Focused on Their Roles in Protecting Plants against Stress. Applied Microbiology. 2023; 3(4):1249-1261. https://doi.org/10.3390/applmicrobiol3040086
Chicago/Turabian StyleCarezzano, María Evangelina, Florencia Belén Alvarez Strazzi, Verónica Pérez, Pablo Bogino, and Walter Giordano. 2023. "Exopolysaccharides Synthesized by Rhizospheric Bacteria: A Review Focused on Their Roles in Protecting Plants against Stress" Applied Microbiology 3, no. 4: 1249-1261. https://doi.org/10.3390/applmicrobiol3040086
APA StyleCarezzano, M. E., Alvarez Strazzi, F. B., Pérez, V., Bogino, P., & Giordano, W. (2023). Exopolysaccharides Synthesized by Rhizospheric Bacteria: A Review Focused on Their Roles in Protecting Plants against Stress. Applied Microbiology, 3(4), 1249-1261. https://doi.org/10.3390/applmicrobiol3040086





