Population Dynamics in the Biogenesis of Single-/Multi-Layered Membrane Vesicles Revealed by Encapsulated GFP-Monitoring Analysis
Abstract
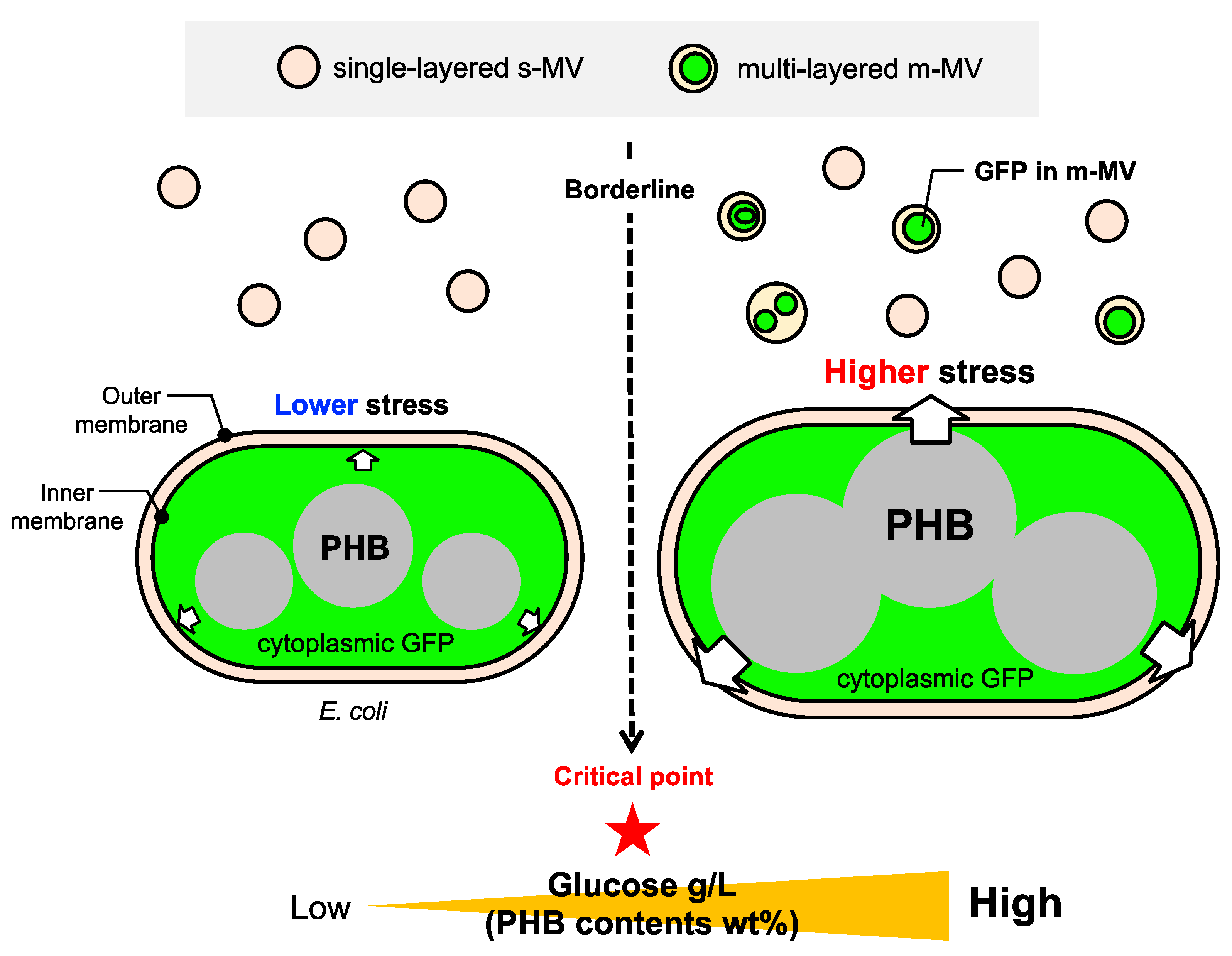
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Quantification of the Intracellular PHB Accumulation
2.3. Quantification of Extracellular MV Production and GFP Encupsulation into MVs
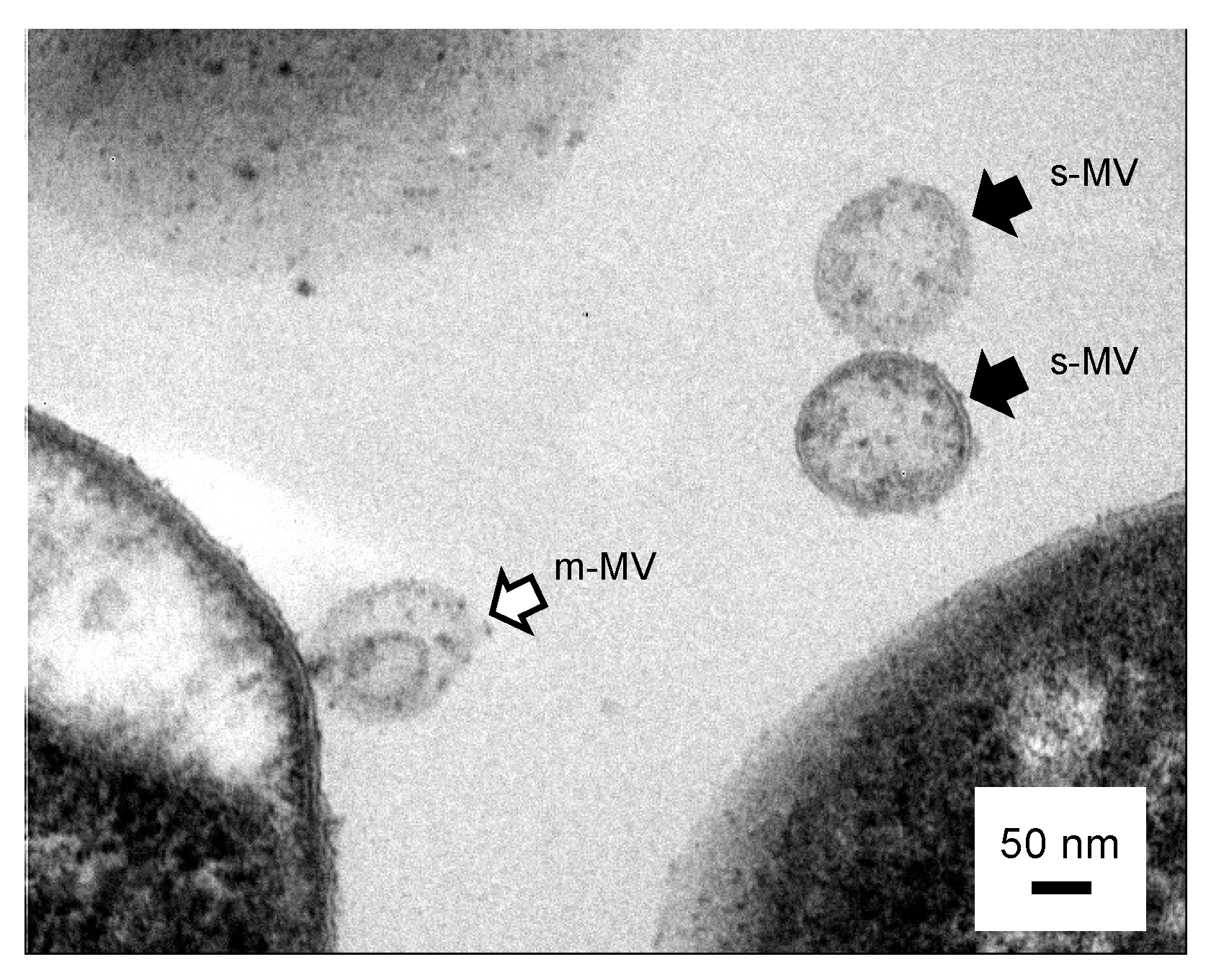
2.4. Cross-Sectional TEM Analysis
3. Results and Discussion
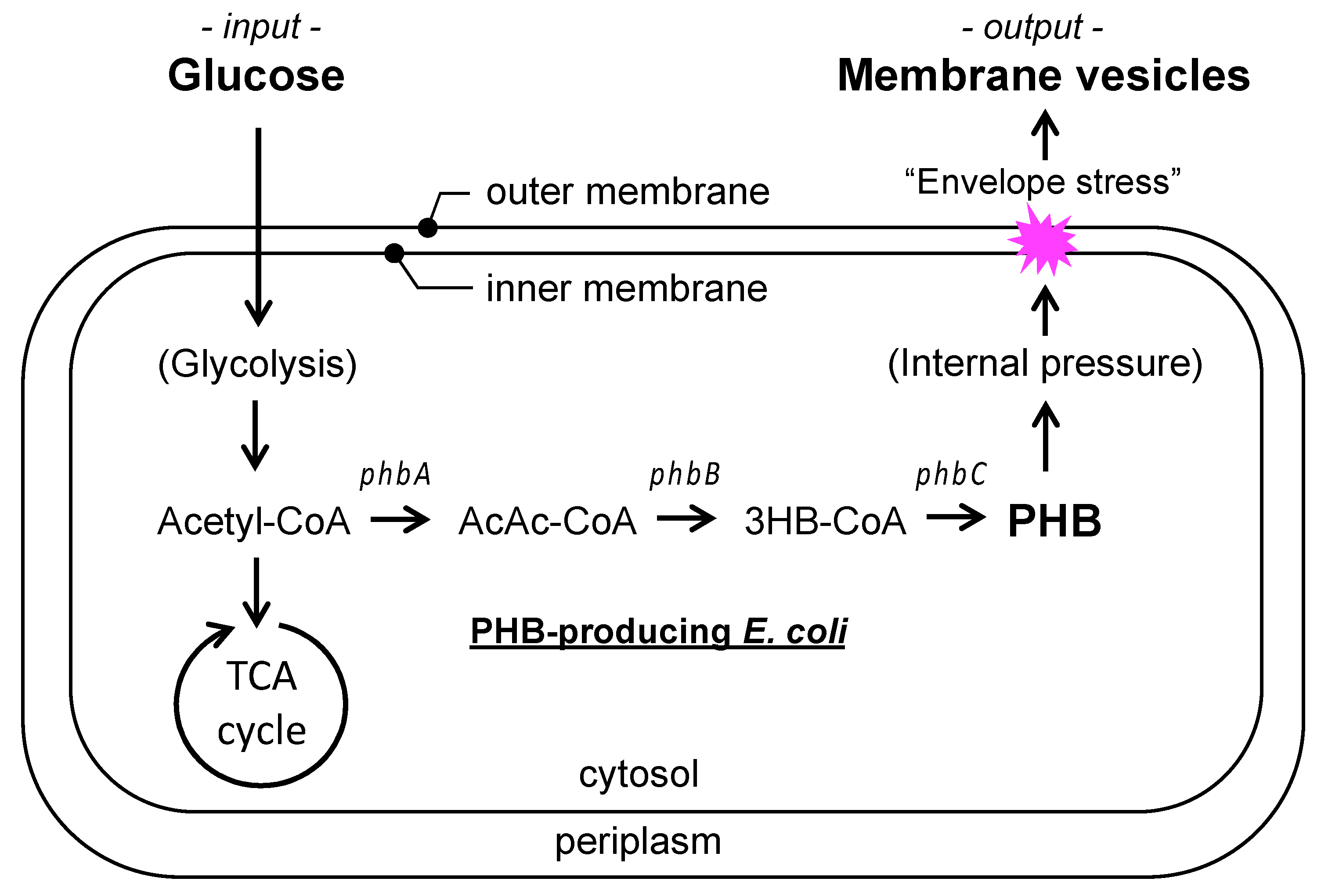
3.1. Working Hypothesis of PIA-MVP as a Regulatory Secretion Machinery of m-MVs
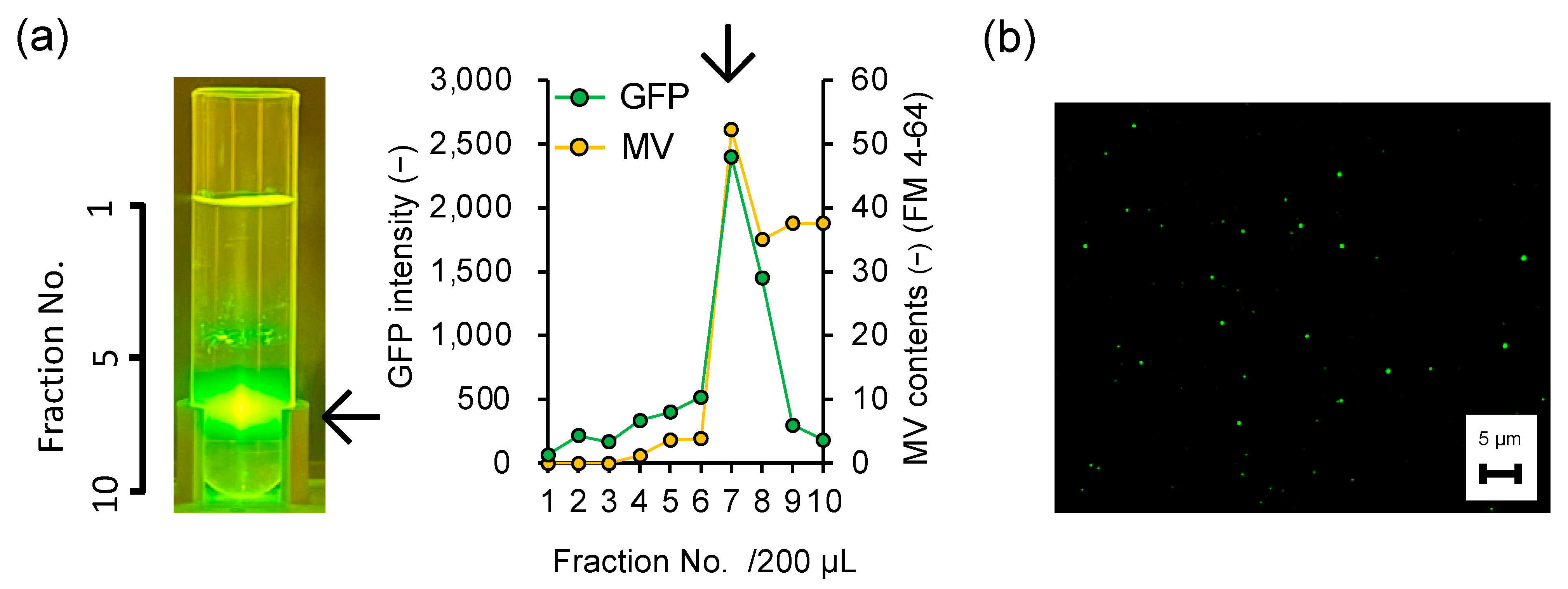
3.2. Direct Evidence of Encapsulation of GFP Reporter Protein into MVs
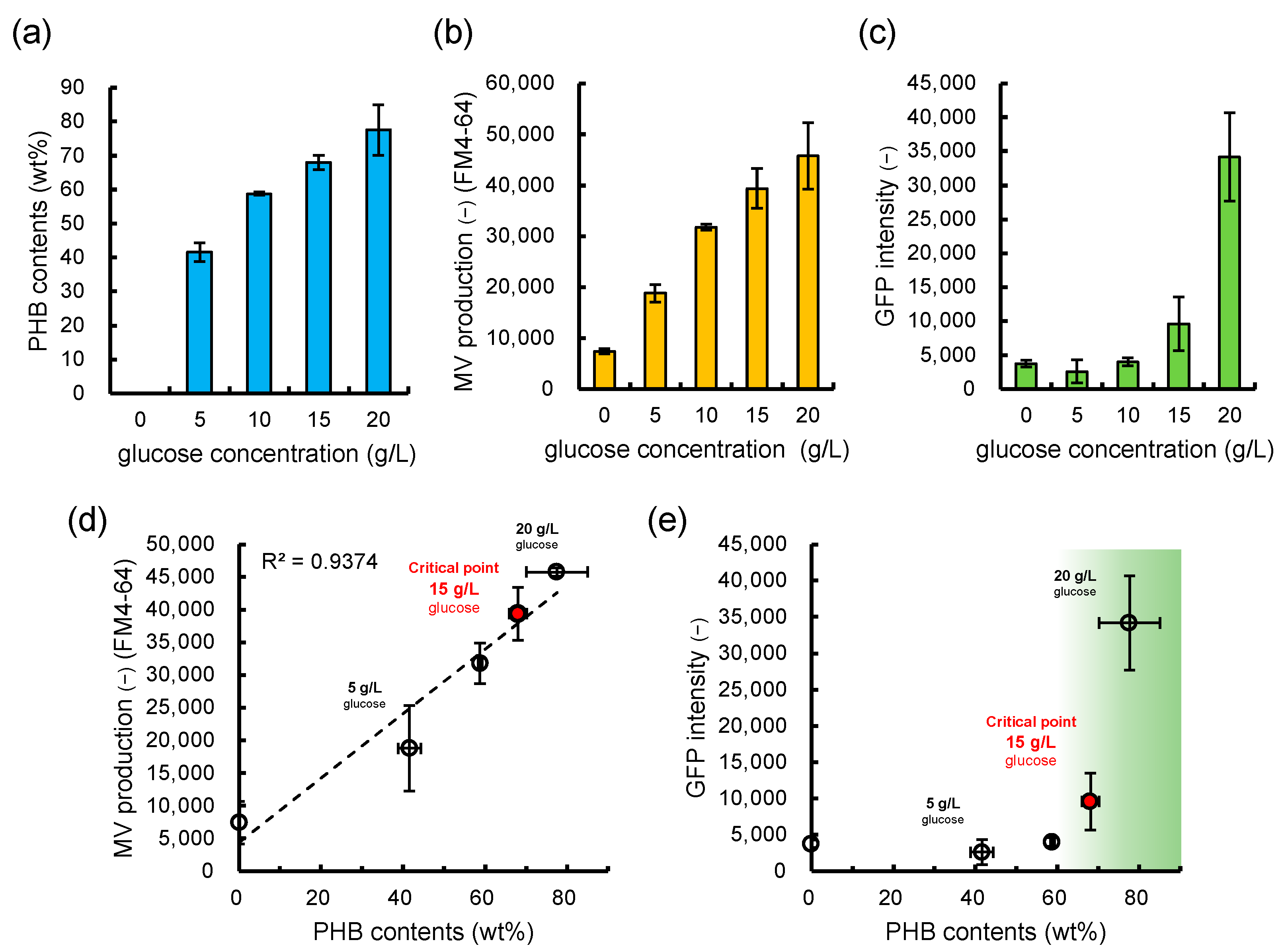
3.3. Is the Selective/regulatory Secretion of s-MV Possible?
3.4. Significance and Implication
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Schewechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, Y. Bacterial membrane vesicles with multiple lipid bilayers; vesicles harboring organelle-like structures. Biosci. Biotechnol. Biochem. 2022, 86, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence factors are released from Pseudomonus aerugiosa in association with membrane vesicles during normal growth and exposure to gentamaicin: A novel mechanism of enzyme secretion. J. Bacteriol. 1995, 177, 3998–4008. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Nakao, R. Glycine significantly enhances bacterial membrane vesicle production: A powerful approach for isolation of LPS-reduced membrane vesicles of probiotic Escherichia coli. Microb. Biotechnol. 2020, 13, 1162–1178. [Google Scholar] [CrossRef] [PubMed]
- Schewechheimer, C.; Rodriguez, D.L.; Kuehn, M.J. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli. Microbiol. Open 2015, 4, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Sawabe, T.; Nakagawa, M.; Tahara, Y.O.; Miyata, M.; Azuma, M. Aberrant membrane structures in hypervesiculating Escherichia coli strain ΔmlaEΔnlpI visualized by electron microscopy. Front. Microbiol. 2021, 12, 706525. [Google Scholar] [CrossRef] [PubMed]
- Bernadac, A.; Gavioli, M.; Lazzaroni, J.; Raina, S.; Lloubès, R. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 1998, 180, 4872–4878. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Cárcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Sato, M.; Yamashina, K.; Usukura, Y.; Toyofuku, M.; Nomura, N.; Taguchi, S. Controllable secretion of multilayer vesicles driven by microbial polymer accumulation. Sci. Rep. 2022, 12, 3393. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Slaninova, E.; Friz, I.; Daffert, C.; Meixner, K.; Sedrlova, Z.; Koller, M. Novel unexpected functions of PHA granules. Appl. Microbiol. Biotechnol. 2020, 104, 4795–4810. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A.; Hein, S. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv. Biochem. Eng. Biotechnol. 2001, 71, 81–123. [Google Scholar] [PubMed]
- Nduko, J.M.; Taguchi, S. Microbial production of biodegradable lactate-based polymers and oligomeric building blocks from renewable and waste resources. Front. Bioeng. Biotechnol. 2021, 8, 618077. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Koh, S.; Taguchi, S.; Shimada, T. Cell-growth phase-dependent promotor replacement approach for improved poly(lactate-co-3-hydroxybutyrate) production in Escherichia coli. Microb. Cell Fact. 2023, 22, 131. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 In-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef] [PubMed]
- Kesty, N.C.; Kuehn, M.J. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 2004, 279, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Ojima, Y.; Sawabe, T.; Konami, K.; Azuma, M. Construction of hypervesiculation Eshcherichia coli strains and application for secretory protein production. Biotechnol. Bioeng. 2019, 117, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A novel mechanism for the biogenesis of outer membrane vesicles in gram-negative bacteria. Nat. Commun. 2016, 7, 10515. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, D.; Slchow, O.; Hoppert, M. Poly(3-hydroxybutyrate) granules at the early stages of formation are localized close to the cytoplasmic membrane in Caryphanon latum. Appl. Environ. Microbiol. 2007, 73, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Bresan, S.; Sznajder, A.; Hauf, W.; Forchhammer, K.; Pfieffer, D.; Jendrossek, D. Polyhydroxyalkanoate (PHA) granules have no phospholipids. Sci. Rep. 2016, 6, 26612. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, S.; Noda, S.; Taguchi, S. Population Dynamics in the Biogenesis of Single-/Multi-Layered Membrane Vesicles Revealed by Encapsulated GFP-Monitoring Analysis. Appl. Microbiol. 2023, 3, 1027-1036. https://doi.org/10.3390/applmicrobiol3030070
Koh S, Noda S, Taguchi S. Population Dynamics in the Biogenesis of Single-/Multi-Layered Membrane Vesicles Revealed by Encapsulated GFP-Monitoring Analysis. Applied Microbiology. 2023; 3(3):1027-1036. https://doi.org/10.3390/applmicrobiol3030070
Chicago/Turabian StyleKoh, Sangho, Shuhei Noda, and Seiichi Taguchi. 2023. "Population Dynamics in the Biogenesis of Single-/Multi-Layered Membrane Vesicles Revealed by Encapsulated GFP-Monitoring Analysis" Applied Microbiology 3, no. 3: 1027-1036. https://doi.org/10.3390/applmicrobiol3030070
APA StyleKoh, S., Noda, S., & Taguchi, S. (2023). Population Dynamics in the Biogenesis of Single-/Multi-Layered Membrane Vesicles Revealed by Encapsulated GFP-Monitoring Analysis. Applied Microbiology, 3(3), 1027-1036. https://doi.org/10.3390/applmicrobiol3030070





