Fungal Pigments: Their Diversity, Chemistry, Food and Non-Food Applications
Abstract
1. Introduction
2. Fungi as a Source of Pigments
3. Chemistry of Fungal Pigments
| Compound | Color | Chemical Structure |
|---|---|---|
| Carotenoids | Yellow Yellowish orange | 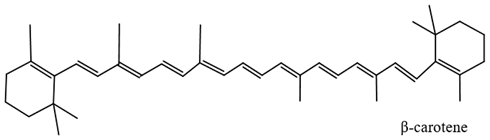 |
| Anthraquinones and hydroxyanthraquinones | Orange Bronze Maroon | 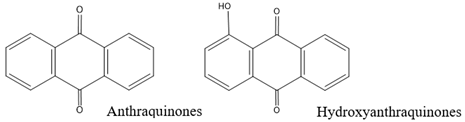 |
| Oxopolyene and azaphilones | Yellow Red Purple red |  |
| Naphthoquinone | Red Purple | 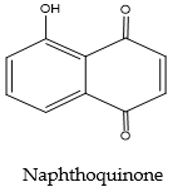 |
| Melanin | Black Greyish black | 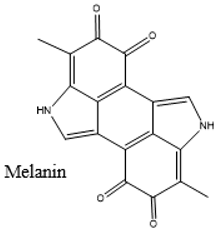 |
| Fungal Species | Pigment Name | Color | Reference |
|---|---|---|---|
| Monascus purpureus | Monascin | Yellow | Hsu et al. [28]; Mukherjee et al. [46]; Srianta et al. [47] |
| Monapurone A–C | Yellow | ||
| Ankaflavin | Yellow | ||
| Monasphilone A and B | Yellow | ||
| Monopilol A–D | Yellow | ||
| Citrinin | Yellow | ||
| Monascorubrin | Orange | ||
| Rubropunctatin | Orange | ||
| Monascorubramine | Red | ||
| Rubropunctamine | Magenta | ||
| Monascus ruber | Monascin | Yellow | Mapari et al. [29]; Loret and Morel [48] |
| Citrinin | Yellow | ||
| Ankaflavin | Yellow | ||
| Monarubrin | Yellow | ||
| Rubropunctin | Yellow | ||
| Rubropunctatin | Orange | ||
| Monascorubrin | Orange | ||
| Monascorubramine | Red | ||
| N–glucosylrubropunctamine | Red | ||
| N–glucosylmonascorubramine | Red | ||
| Rubropunctamine | Purple-red | ||
| Trichoderma harzianum | Pachybasin | Yellow | Caro et al. [30] |
| Emodin | Yellow | ||
| Chrysophanol | Orange-red | ||
| Aspergillus ruber | Asperflavin | Yellow | Caro et al. [30] |
| Guestin | Yellowish orange | ||
| Emodin | Orange | ||
| 3–O–(α–D–ribofuranosyl)–questin | Orange | ||
| Catenarin | Red | ||
| Rubrocristin | Red | ||
| Eurorubrin | Brown | ||
| Talaromyces purpureogenus | Mitorubrin | Yellow | Mapari et al. [29]; Ogbonna et al. [36] |
| Purpurogenone | Yellowish orange | ||
| Mitorubrinol | Orange-red | ||
| Rubropunctatin | Red | ||
| Azaphilones | Red | ||
| Penicillium viridicatum | Viomellein | reddish-brown | Mapari et al. [29]; Ogbonna et al. [36] |
| Xanthomegnin | Orange | ||
| Penicillium oxalicum | Secalonic acid D | Yellow | Mapari et al. [29]; Caro et al. [30] |
| Arpink red™ | Red | ||
| Anthraquinone derivative | Red | ||
| Anthraquinones | Red | ||
| Ophiocordyceps unilateralis | Erythrostominone | Red | Caro et al. [30] |
| Deoxyerythrostominone | Red | ||
| deoxyerythrostominol | Red | ||
| 4–O–methyl erythrostominone | Red | ||
| Epierythrostominol | Red | ||
| Naphthoquinones | Bloody red | ||
| Cerioporus squamosus | Melanin | Black | Tudor [49] |
| Fomes fomentarius | Melanin | Black | Tudor [49]; Tudor et al. [50] |
| Chaetomium globosum | Chaetoviridins A–D | Yellow | Caro et al. [30] |
| Chaetoglobin A–B | Purple | ||
| Chaetomugilins A–F | Purple | ||
| Cochliodinol | Purple | ||
| Epicoccum nigrum | Carotenoids | Yellow | Mapari et al. [29]; da Costa Souza et al. [44] |
| Chromanone | Yellow | ||
| Orevactaene | Yellow | ||
| Epicoccarines A–B | Fluorescent yellow | ||
| Epicocconone | Fluorescent yellow | ||
| Epipyridone | Red | ||
| Flavipin | Brown | ||
| Isobenzofuran | Brownish yellow | ||
| Fusarium fujikuroi | Bikaverin | Red | Mapari et al. [29]; Frandsen et al. [51]; Avalos et al. [52] |
| Norbikaverin | Red | ||
| O–demethylanhydrofusarubin | Red | ||
| 8–O–methybostrycoidin, 2–(4–((3E,5E)–14–aminotetradeca–3,5–dienyloxy) butyl)–1,2,3,4–tetrahydroisoquinolin–4–ol (ATDBTHIQN) | Pink | ||
| Neurosporaxanthin | Orange | ||
| β–carotene | Orange-red | ||
| Fusarubin | Red | ||
| O–methylsolaniol | Orange-red | ||
| Fusarium oxysporum | 2,7–dimethoxy–6–(acetoxyethyl)juglone | Yellow | Medentsev et al. [53]; Avalos et al. [52]; Lebeau et al. [54] |
| Nectriafurone | Yellow | ||
| O–methyl–6– hydroxynorjavanicin | Yellow | ||
| Bikaverin | Red | ||
| Bostrycoidin | Red | ||
| Norjavanicin | Red | ||
| O–methylfusarubin | Red | ||
| O–methylanhydrofusarubin | Orange-red | ||
| Neurosporaxanthin | Orange | ||
| β–carotene | Orange-red | ||
| Naphthaquinones | Purple | ||
| Beauveria basiana | Tenellin | Yellow | Wat et al. [55]; Caro et al. [30] |
| Bassianin | Yellow | ||
| Pyridovericin | Yellow | ||
| Pyridomacrolidin | Yellow | ||
| Oosporein | Red | ||
| Curvularia lunata | Chrysophanol | Red | Mapari et al. [29]; Caro et al. [30] |
| Erythroglaucin | Red | ||
| Catenarin | Red | ||
| Cynodontin | Bronze | ||
| Helminthosporin | Maroon | ||
| Pyrenophora species | Catenarin | Red | Mapari et al. [29]; Caro et al. [30] |
| Erythroglaucin | Red | ||
| Cynodontin | Bronze | ||
| Helminthosporin | Maroon | ||
| Tritisporin | Reddish brown | ||
| Alternaria alternate | Alternariol | Red | Devi et al. [56] |
| Alternarienoic acid | Red | ||
| Alterperylenol | Red | ||
| Altenuene | Violet-red | ||
| Alternariol-5-methyl ether | Brownish red | ||
| Tenuazoic acid | Orange-red | ||
| Stemphyperylenol | Yellow–orange-red | ||
| Neurospora crassa | Neurosporaxanthin | Yellow-orange | Avalos et al. [57]; Caro et al. [30] |
| Phytoene | Yellow-orange | ||
| Neurosporen | Yellow-orange | ||
| β–carotene | Red-orange-yellow | ||
| Lycopene | Red | ||
| Spirilloxanthin | Violet | ||
| γ–carotene | Yellow-orange |
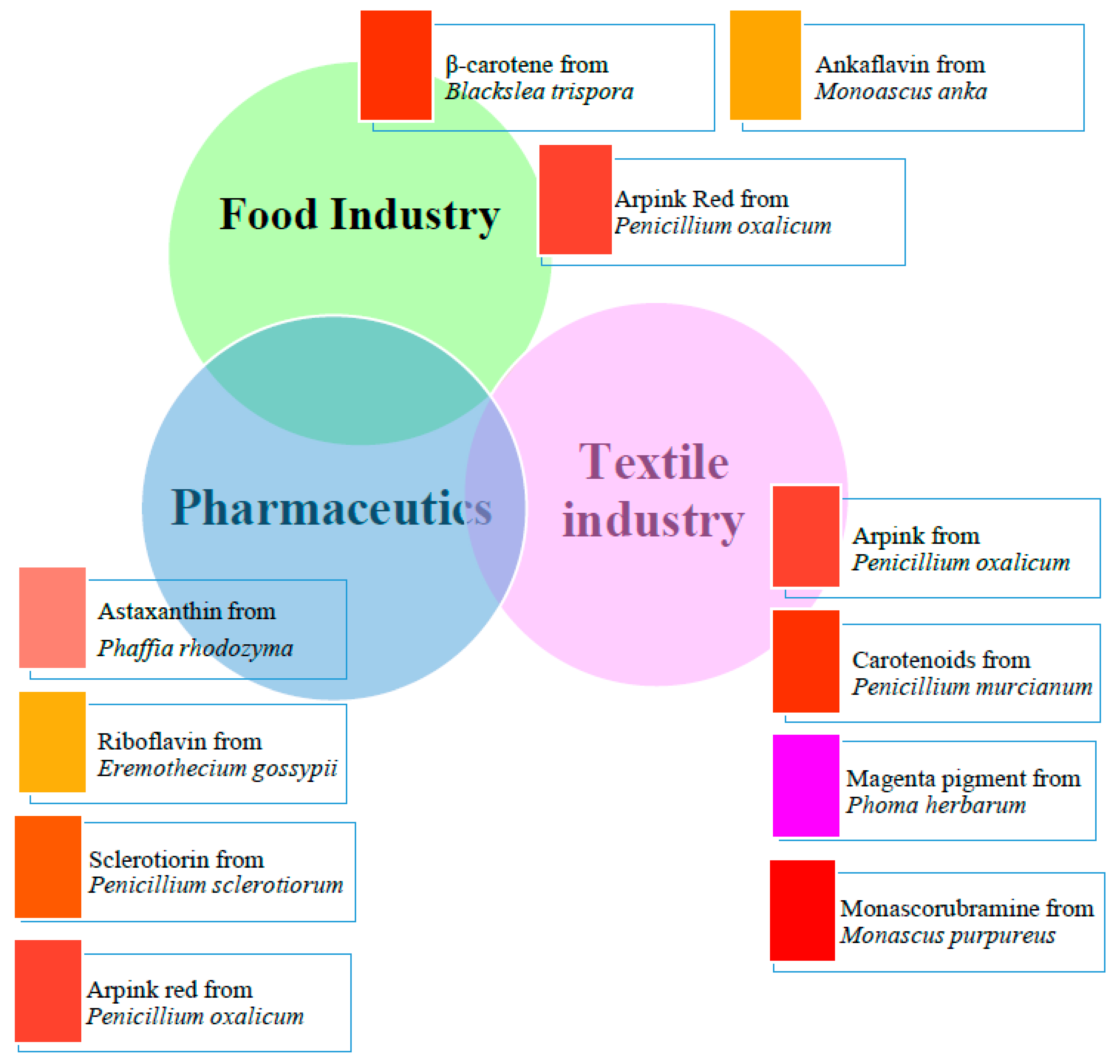
4. Application of Fungal Pigments in Different Industries
4.1. Fungal Pigment Applications in Food Industry
4.2. Non-Food Applications of Fungal Pigments
5. Epicoccum as an Example of Pigment-Producing Fungi
6. Extraction and Optimization of Pigment Production
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ho, S. Low-Cost Adsorbents for the Removal of Phenol/Phenolics, Pesticides, and Dyes from Wastewater Systems: A Review. Water 2022, 14, 3203. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S.; Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and pigments: Their structure and properties. In Dyes and Pigments; Springer: Cham, Switzerland, 2016; pp. 13–29. [Google Scholar]
- International Agency for Research on Cancer. General introduction to the chemistry of dyes. In Some Aromatic Amines, Organic Dyes, and Related Exposures; IARC Press: Lyon, France, 2010; Volume 99. [Google Scholar]
- Garfield, S. Mauve: How One Man Invented a Colour That Changed the World; Canongate Books: Edinburgh, UK, 2018; Volume 81. [Google Scholar]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Galaffu, N.; Bortlik, K.; Michel, M. An industry perspective on natural food colour stability. In Colour Additives for Foods and Beverages; Woodhead Publishing: Sawston, UK, 2015; pp. 91–130. [Google Scholar]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.W.F.; de Menezes, G.C.A.; e Silva, T.R.; Bicas, J.L.; Oliveira, V.M.; Rosa, L.H. Antarctic fungi as producers of pigments. In Fungi of Antarctica: Diversity, Ecology and Biotechnological Applications; Springer: Cham, Switzerland, 2019; pp. 305–318. [Google Scholar]
- Prabowo, C.P.S.; Eun, H.; Yang, D.; Huccetogullari, D.; Jegadeesh, R.; Kim, S.J.; Lee, S.Y. Production of natural colorants by metabolically engineered microorganisms. Trends Chem. 2022, 4, 608–626. [Google Scholar] [CrossRef]
- Mohammad Azmin, S.N.H.; Sulaiman, N.S.; Mat Nor, M.S.; Abdullah, P.S.; Abdul Kari, Z.; Pati, S. A Review on Recent Advances on Natural Plant Pigments in Foods: Functions, Extraction, Importance and Challenges. Appl. Biochem. Biotechnol. 2022, 194, 4655–4672. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Kolkar, K.P.; Chalannavar, R.K. Plant Natural Pigment Colorants-Health Benefits: Toxicity of Synthetic or Artificial Food Colorants. Int. J. Innov. Sci. Res. Rev. 2022, 4, 3418–3429. [Google Scholar]
- Echegaray, N.; Guzel, N.; Kumar, M.; Guzel, M.; Hassoun, A.; Lorenzo, J.M. Recent advancements in natural colorants and their application as coloring in food and in intelligent food packaging. Food Chem. 2023, 404, 134453. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Garcia, J.A.A.; Correa, V.G.; Vieira, T.F.; Bracht, A.; Peralta, R.M. Pigments and vitamins from plants as functional ingredients: Current trends and perspectives. Adv. Food Nutr. Res. 2019, 90, 259–303. [Google Scholar]
- Heer, K.; Sharma, S. Microbial pigments as a natural color: A review. Int. J. Pharm. Sci. Res. 2017, 8, 1913–1922. [Google Scholar]
- Hejazi, M.A.; Wijffels, R.H. Milking of microalgae. Trends Biotechnol. 2004, 22, 189–194. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Deshmukh, S.K.; Johri, B.N. (Eds.) Developments in Fungal Biology and Applied Mycology; Springer: Singapore, 2017. [Google Scholar]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [PubMed]
- Manikprabhu, D.; Lingappa, K. γ Actinorhodin a natural and attorney source for synthetic dye to detect acid production of fungi. Saudi J. Biol. Sci. 2013, 20, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ashok, G.; Mohan, U.; Boominathan, M.; Ravichandiran, V.; Viswanathan, C.; Senthilkumar, V. Natural Pigments from Filamentous Fungi: Production and Applications. In Industrially Important Fungi for Sustainable Development: Volume 2: Bioprospecting for Biomolecules; Springer: Cham, Switzerland, 2021; pp. 651–678. [Google Scholar]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Renuka Devi, P.; Veera Ravi, A. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef]
- Gmoser, R.; Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol. Biotechnol. 2017, 4, 4. [Google Scholar] [CrossRef]
- Dufosse, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef]
- Shcherba, V.V.; Babitskaya, V.G.; Kurchenko, V.P.; Ikonnikova, N.V.; Kukulyanskaya, T.A. Antioxidant properties of fungal melanin pigments. Appl. Biochem. Microbiol. 2000, 36, 491–495. [Google Scholar] [CrossRef]
- Jia, L.; Tu, X.; He, K.; Wang, C.; Yin, S.; Zhou, Y.; Chen, W. Monascorubrin and rubropunctatin: Preparation and reaction characteristics with amines. Dye. Pigment. 2019, 170, 107629. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Molnár, I.; Chen, F. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572. [Google Scholar] [CrossRef]
- De Santis, D.; Moresi, M.; Gallo, A.M.; Petruccioli, M. Assessment of the dyeing properties of pigments from Monascus purpureus. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 1072–1079. [Google Scholar]
- Hsu, Y.W.; Hsu, L.C.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. Monaphilones A−C, three new antiproliferative azaphilone derivatives from Monascus purpureus NTU 568. J. Agric. Food Chem. 2010, 58, 8211–8216. [Google Scholar] [CrossRef]
- Mapari, S.A.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Factories 2009, 8, 24. [Google Scholar] [CrossRef]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and colorants from filamentous fungi. In Fungal Metabolites; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 499–568. [Google Scholar]
- Pandey, N.; Jain, R.; Pandey, A.; Tamta, S. Optimisation and characterisation of the orange pigment produced by a cold adapted strain of Penicillium sp.(GBPI_P155) isolated from mountain ecosystem. Mycology 2018, 9, 81–92. [Google Scholar] [CrossRef]
- Sardaryan, E. Strain of the Microorganism Penicillium Oxalicum var. Armeniaca and Its Application. U.S. Patent 6,340,586, 22 January 2002. [Google Scholar]
- Teixeira, M.F.; Martins, M.S.; Da Silva, J.C.; Kirsch, L.S.; Fernandes, O.C.; Carneiro, A.L.; Da Conti, R.; Durán, N. Amazonian biodiversity: Pigments from Aspergillus and Penicillium-characterizations, antibacterial activities and their toxicities. Curr. Trends Biotechnol. Pharm. 2012, 6, 300–311. [Google Scholar]
- Tam, E.W.; Tsang, C.C.; Lau, S.K.; Woo, P.C. Polyketides, toxins and pigments in Penicillium marneffei. Toxins 2015, 7, 4421–4436. [Google Scholar] [CrossRef]
- Sethi, B.K.; Parida, P.; Sahoo, S.L.; Behera, B.C. Extracellular production and characterization of red pigment from Penicillium purpurogenum BKS9. Alger. J. Nat. Prod. 2016, 4, 379–392. [Google Scholar]
- Ogbonna, C.N.; Aoyagi, H.; Ogbonna, J.C. Isolation and identification of Talaromyces purpurogenus and preliminary studies on its pigment production potentials in solid state cultures. Afr. J. Biotechnol. 2017, 16, 672–682. [Google Scholar]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef]
- Arai, T.; Koganei, K.; Umemura, S.; Kojima, R.; Kato, J.; Kasumi, T.; Ogihara, J. Importance of the ammonia assimilation by Penicillium purpurogenum in amino derivative Monascus pigment, PP-V, production. AMB Express 2013, 3, 19. [Google Scholar] [CrossRef]
- Keekan, K.K.; Hallur, S.; Modi, P.K.; Shastry, R.P. Antioxidant activity and role of culture condition in the optimization of red pigment production by Talaromyces purpureogenus KKP through response surface methodology. Curr. Microbiol. 2020, 77, 1780–1789. [Google Scholar] [CrossRef]
- Bell, P.J.; Karuso, P. Epicocconone, A Novel Fluorescent Compound from the Fungus Epicoccum nigrum. J. Am. Chem. Soc. 2003, 125, 9304–9305. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Veal, D.A.; Karuso, P. Epicocconone, a new cell-permeable long Stokes’ shift fluorescent stain for live cell imaging and multiplexing. J. Fluoresc. 2006, 16, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.; Duplais, C. Fluorescent natural products as probes and tracers in biology. Nat. Prod. Rep. 2017, 34, 161–193. [Google Scholar] [CrossRef]
- Velmurugan, P.; Lee, Y.H.; Nanthakumar, K.; Kamala-Kannan, S.; Dufossé, L.; Mapari, S.A.; Oh, B.T. Water-soluble red pigments from Isaria farinosa and structural characterization of the main colored component. J. Basic Microbiol. 2010, 50, 581–590. [Google Scholar] [CrossRef] [PubMed]
- da Costa Souza, P.N.; Grigoletto, T.L.B.; de Moraes, L.A.B.; Abreu, L.M.; Guimarães, L.H.S.; Santos, C.; Galvão, L.R.; Cardoso, P.G. Production and chemical characterization of pigments in filamentous fungi. Microbiology 2016, 162, 12–22. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Díaz-Godínez, G. Mushroom Pigments and Their Applications. In Biomolecules from Natural Sources: Advances and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 82–100. [Google Scholar]
- Mukherjee, G.; Singh, S.K. Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process Biochem. 2011, 46, 188–192. [Google Scholar] [CrossRef]
- Srianta, I.; Zubaidah, E.; Estiasih, T.; Yamada, M. Comparison of Monascus purpureus growth, pigment production and composition on different cereal substrates with solid state fermentation. Biocatal. Agric. Biotechnol. 2016, 7, 181–186. [Google Scholar] [CrossRef]
- Loret, M.O.; Morel, S. Isolation and structural characterization of two new metabolites from Monascus. J. Agric. Food Chem. 2010, 58, 1800–1803. [Google Scholar] [CrossRef]
- Tudor, D. Fungal Pigment Formation in Wood Substrate. Doctoral Dissertation, University of Toronto, Toronto, ON, Canada, 2013. [Google Scholar]
- Tudor, D.; Robinson, S.C.; Cooper, P.A. The influence of pH on pigment formation by lignicolous fungi. Int. Biodeterior. Biodegrad. 2013, 80, 22–28. [Google Scholar] [CrossRef]
- Frandsen, R.J.; Rasmussen, S.A.; Knudsen, P.B.; Uhlig, S.; Petersen, D.; Lysøe, E.; Gotfredsen, C.H.; Giese, H.; Larsen, T.O. Black perithecial pigmentation in Fusarium species is due to the accumulation of 5-deoxybostrycoidin-based melanin. Sci. Rep. 2016, 6, 26206. [Google Scholar] [CrossRef]
- Avalos, J.; Pardo-Medina, J.; Parra-Rivero, O.; Ruger-Herreros, M.; Rodríguez-Ortiz, R.; Hornero-Méndez, D.; Limón, M.C. Carotenoid biosynthesis in Fusarium. J. Fungi 2017, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Medentsev, A.G.; Arinbasarova, A.Y.; Akimenko, V.K. Biosynthesis of naphthoquinone pigments by fungi of the genus Fusarium. Appl. Biochem. Microbiol. 2005, 41, 503–507. [Google Scholar] [CrossRef]
- Lebeau, J.; Petit, T.; Clerc, P.; Dufossé, L.; Caro, Y. Isolation of two novel purple naphthoquinone pigments concomitant with the bioactive red bikaverin and derivates thereof produced by Fusarium oxysporum. Biotechnol. Prog. 2019, 35, e2738. [Google Scholar] [CrossRef] [PubMed]
- Wat, C.K.; Mcinnes, A.G.; Smith, D.G.; Wright, J.L.; Vining, L.C. The yellow pigments of Beauveria species. Structures of tenellin and bassianin. Can. J. Chem. 1977, 55, 4090–4098. [Google Scholar] [CrossRef]
- Devi, S.; AK Kumar, H.; Ramachandran, G.; Subramanian, C.; Karuppan, P. Growth and mass spectrometry profile of Alternaria alternata pigment grown in maize grain extract. J. Microbiol. Biotechnol. Food Sci. 2014, 4, 179–184. [Google Scholar] [CrossRef]
- Avalos, J.; Prado-Cabrero, A.; Estrada, A.F. Neurosporaxanthin production by Neurospora and Fusarium. Microb. Carotenoids Fungi: Methods Protoc. 2012, 898, 263–274. [Google Scholar]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food colors: Existing and emerging food safety concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef]
- Chung, K.T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Dufossé, L.; Kannan, J. Safety evaluation of fungal pigments for food applications. J. Fungi 2021, 7, 692. [Google Scholar] [CrossRef]
- Jonnalagadda, P.R.; Rao, P.; Bhat, R.V.; Nadamuni Naidu, A. Type, extent and use of colours in ready-to-eat (RTE) foods prepared in the non-industrial sector–a case study from Hyderabad, India. Int. J. Food Sci. Technol. 2004, 39, 125–131. [Google Scholar] [CrossRef]
- Narendrababu, B.N.; Shishupala, S. Spectrophotometric detection of Pigments from Aspergillus and Penicillium isolatesbn. J. Appl. Biol. Biotechnol. 2017, 5, 53–58. [Google Scholar] [CrossRef]
- Sanjay, K.R.; Kumaresan, N.; Naidu, K.A.; Viswanatha, S.; Narasimhamurthy, K.; Kumar, S.U.; Vijayalakshmi, G. Safety evaluation of pigment containing Aspergillus carbonarius biomass in albino rats. Food Chem. Toxicol. 2007, 45, 431–439. [Google Scholar] [CrossRef]
- Somasundaram, T.; Rao, S.S.; Maheshwari, R. Pigments in Thermophilic fungi. Curr. Sci. 1986, 55, 957–960. [Google Scholar]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]
- Cambaza, E. Comprehensive description of Fusarium graminearum pigments and related compounds. Foods 2018, 7, 165. [Google Scholar] [CrossRef]
- Menezes, B.S.; Solidade, L.S.; Conceição, A.A.; Santos Junior, M.N.; Leal, P.L.; de Brito, E.S.; Canuto, K.M.; Mendonça, S.; de Siqueira, F.G.; Marques, L.M. Pigment production by Fusarium solani BRM054066 and determination of antioxidant and anti-inflammatory properties. AMB Express 2020, 10, 117. [Google Scholar] [CrossRef]
- Santos, M.C.D.; Mendonça, M.D.L.; Bicas, J.L. Modeling bikaverin production by Fusarium oxysporum CCT7620 in shake flask cultures. Bioresour. Bioprocess. 2020, 7, 13. [Google Scholar] [CrossRef]
- Shi, K.; Tang, R.; Huang, T.; Wang, L.; Wu, Z. Pigment fingerprint profile during extractive fermentation with Monascus anka GIM 3.592. BMC Biotechnol. 2017, 17, 46. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Almoallim, H.S.; Alharbi, S.A.; Oh, Y.K. Isolation and optimization of Monascus ruber OMNRC45 for red pigment production and evaluation of the pigment as a food colorant. Appl. Sci. 2020, 10, 8867. [Google Scholar] [CrossRef]
- Mohankumari, H.P.; Naidu, K.A.; Narasimhamurthy, K.; Vijayalakshmi, G. Bioactive pigments of Monascus purpureus attributed to antioxidant, HMG-CoA reductase inhibition and anti-atherogenic functions. Front. Sustain. Food Syst. 2021, 5, 590427. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Poorniammal, R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr. J. Biotechnol. 2008, 7, 1894–1898. [Google Scholar] [CrossRef]
- Kojima, R.; Arai, T.; Matsufuji, H.; Kasumi, T.; Watanabe, T.; Ogihara, J. The relationship between the violet pigment PP-V production and intracellular ammonium level in Penicillium purpurogenum. AMB Express 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.C.; Takahashi, J.A. Sequential fungal fermentation-biotransformation process to produce a red pigment from sclerotiorin. Food Chem. 2016, 210, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.G.; Puttananjaih, M.H.; Harohally, N.V.; Dhale, M.A. Functional attributes of a new molecule-2-hydroxymethyl-benzoic acid 2′-hydroxy-tetradecyl ester isolated from Talaromyces purpureogenus CFRM02. Food Chem. 2018, 255, 89–96. [Google Scholar] [CrossRef]
- Patil, S.; Sivanandhan, G.; Thakare, D. Effect of physical and chemical parameters on the production of red exopigment from Penicillium purpurogenum isolated from spoilt onion and study of its antimicrobial activity. Int. J. Curr. Microbiol. Appl. Sci 2015, 4, 599–609. [Google Scholar]
- Sibero, M.T.; Triningsih, D.W.; Radjasa, O.K.; Sabdono, A.; Trianto, A. Evaluation of antimicrobial activity and identification of yellow pigmented marine sponge-associated fungi from Teluk Awur, Jepara, Central Java. J. Biotechnol. 2016, 21, 1–11. [Google Scholar] [CrossRef]
- Saravanan, D.; Radhakrishnan, M. Antimicrobial activity of pigments produced by fungi from Western Ghats. J. Chem. Pharm. Res. 2016, 8, 634–638. [Google Scholar]
- Wang, W.; Liao, Y.; Chen, R.; Hou, Y.; Ke, W.; Zhang, B.; Gao, M.; Shao, Z.; Chen, J.; Li, F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar. Drugs 2018, 16, 61. [Google Scholar] [CrossRef]
- Parthiban, M.; Thilagavathi, G.; Viju, S. Development of antibacterial silk sutures using natural fungal extract for healthcare applications. J. Text. Sci. Eng. 2016, 6, 249. [Google Scholar]
- Elkhateeb, W.; Elnahas, M.O.; Daba, G. Wide Range Applications of Fungal Pigments in Textile Dyeing. In Fungi and Fungal Products in Human Welfare and Biotechnology; Springer Nature: Singapore, 2023; pp. 289–304. [Google Scholar]
- Poorniammal, R.; Balachandar, D.; Gunasekaran, S. Evaluation of antioxidant property of some fungal pigments by DNA protection assay. Ann. Phytomedicine 2018, 7, 106–111. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Sakthi, A.R. Evaluation of in vitro antioxidant activity of fungal pigments. Pharma Innov. J. 2019, 8, 326–330. [Google Scholar]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Kiyota, A.; Yasukawa, K.; Sakamoto, N.; Kimura, Y.; Suzuki, T.; Takayasu, J.; Nishino, H. Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers. 2005, 2, 1305–1309. [Google Scholar] [CrossRef]
- Afroz Toma, M.; Rahman, M.H.; Rahman, M.S.; Arif, M.; Nazir, K.N.H.; Dufossé, L. Fungal Pigments: Carotenoids, Riboflavin, and Polyketides with Diverse Applications. J. Fungi 2023, 9, 454. [Google Scholar] [CrossRef]
- Meruvu, H.; Dos Santos, J.C. Colors of life: A review on fungal pigments. Crit. Rev. Biotechnol. 2021, 41, 1153–1177. [Google Scholar] [CrossRef]
- Hernández, V.A.; Galleguillos, F.; Thibaut, R.; Müller, A. Fungal dyes for textile applications: Testing of industrial conditions for wool fabrics dyeing. J. Text. Inst. 2019, 110, 61–66. [Google Scholar] [CrossRef]
- Pieckenstain, F.L.; Bazzalo, M.E.; Roberts, A.M.; Ugalde, R.A. Epicoccum purpurascens for biocontrol of Sclerotinia head rot of sunflower. Mycol. Res. 2001, 105, 77–84. [Google Scholar] [CrossRef]
- Brown, A.E.; Finlay, R.; Ward, J.S. Antifungal compounds produced by Epicoccum purpurascens against soil-borne plant pathogenic fungi. Soil Biol. Biochem. 1987, 19, 657–664. [Google Scholar] [CrossRef]
- Madrigal, C.; Tadeo, J.L.; Melgarejo, P. Relationship between flavipin production by Epicoccum nigrum and antagonism against Monilinia laxa. Mycol. Res. 1991, 95, 1375–1381. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Fisch, K.M.; Wright, A.D.; Konig, G.M. A new antioxidant isobenzofuranone derivative from the algicolous marine fungus Epicoccum sp. Planta Med. 2003, 69, 831–834. [Google Scholar]
- Kemami Wangun, H.V.; Hertweck, C. Epicoccarines A, B and epipyridone: Tetramic acids and pyridone alkaloids from an Epicoccum sp. associated with the tree fungus Pholiota squarrosa. Org. Biomol. Chem. 2007, 5, 1702–1705. [Google Scholar] [CrossRef] [PubMed]
- El Amrani, M.; Lai, D.; Debbab, A.; Aly, A.H.; Siems, K.; Seidel, C.; Schnekenburger, M.; Gaigneaux, A.; Diederich, M.; Feger, D.; et al. Protein kinase and HDAC inhibitors from the endophytic fungus Epicoccum nigrum. J. Nat. Prod. 2014, 77, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Gloer, J.B.; Wicklow, D.T. Isolation of chromanone and isobenzofuran derivatives from a fungicolous isolate of Epicoccum purpurascens. Bull. Korean Chem. Soc. 2007, 28, 877–879. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Dittrich, B.; Schuffler, A.; Sun, H.; Laatsch, H. Epicoccolides: Antimicrobial and antifungal polyketides from an endophytic fungus Epicoccum sp. associated with Theobroma cacao. Eur. J. Org. Chem. 2013, 2013, 3174–3180. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Melong, R.; Tsamo, A.T.; Maffo, T.; Kapche, D.G.W.F.; Ngadjui, B.T.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, antioxidant and antibacterial activity of four compounds produced by an endophytic fungus Epicoccum nigrum associated with Entada abyssinica. Braz. J. Pharmacogn. 2017, 27, 251–253. [Google Scholar] [CrossRef]
- Shu, Y.Z.; Ye, Q.; Li, H.; Kadow, K.F.; Hussain, R.A.; Huang, S.; Gustavson, D.R.; Lowe, S.E.; Chang, L.P.; Pirnik, D.M.; et al. Orevactaene, 1 a novel binding inhibitor of HIV-1 rev protein to rev response element (RRE) from Epicoccum nigrum WC47880. Bioorganic Med. Chem. Lett. 1997, 7, 2295–2298. [Google Scholar] [CrossRef]
- Kemami Wangun, H.V.; Ishida, K.; Hertweck, C. Epicoccalone, a coumarin-type chymotrypsin inhibitor, and isobenzofuran congeners from an Epicoccum sp. associated with a tree fungus. Eur. J. Org. Chem. 2008, 22, 3781–3784. [Google Scholar] [CrossRef]
- Foppen, F.H.; Gribanovski-Sassu, O. Lipids produced by Epicoccum nigrum in submerged culture. Biochem. J. 1968, 106, 97–100. [Google Scholar] [CrossRef]
- Stricker, R.; Romailler, G.; Turian, G.; Tzanos, D. Production and food applications of a mold hydrosoluble yellow pigment (Epicoccum nigrum Link). Leb. Wiss Technol. 1981, 14, 18–20. [Google Scholar]
- Mapari, S.A.; Meyer, A.S.; Thrane, U. Evaluation of Epicoccum nigrum for growth, morphology and production of natural colorants in liquid media and on a solid rice medium. Biotechnol. Lett. 2008, 30, 2183–2190. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Urreta, I.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E.; Suárez-Alvarez, S. Optimization of clean extraction methods to isolate carotenoids from the microalga Neochloris oleoabundans and subsequent chemical characterization using liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4607–4616. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Sin, H.N.; Yusof, S.; Hamid, N.S.; Rahman, R.A. Optimization of hot water extraction for sapodilla juice using response surface methodology. J. Food Eng. 2006, 74, 352–358. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S. Response surface modeling and optimization of process parameters for aqueous extraction of pigments from prickly pear (Opuntia ficus-indica) fruit. Dye. Pigment. 2012, 95, 465–472. [Google Scholar] [CrossRef]
- Wickerham, L.J.; Flickinger, M.H.; Johnsten, R.M. The production of riboflavin by Ashbya gossypii. Arch. Biochem. 1946, 9, 95–98. [Google Scholar]
- Mantzouridou, F.; Tsimidou, M.Z. Lycopene formation in Blakeslea trispora. Chemical aspects of a bioprocess. Trends Food Sci. Technol. 2008, 19, 363–371. [Google Scholar] [CrossRef]
- Commission regulation (EC) No 721/2006 of 23 October 2006. Authorising the Placing on the Market of Lycopene from Blakeslea trispora as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (Notified under Document Number C (2006) 4973) Official Journal of the European Union, L 296. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006D0721&from=DA (accessed on 15 June 2023).
- Mukherjee, G.; Mishra, T.; Deshmukh, S.K. Fungal pigments: An overview. In Developments in Fungal Biology and Applied Mycology; Springer: Cham, Switzerland, 2017; pp. 525–541. [Google Scholar]
- Wang, T.H.; Lin, T.F. Monascus rice products. Adv. Food Nutr. Res. 2007, 53, 123–159. [Google Scholar]
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–323. [Google Scholar]
- Perveen, I.; Raza, M.A.; Iqbal, T.; Naz, I.; Sehar, S.; Ahmed, S. Isolation of anticancer and antimicrobial metabolites from Epicoccum nigrum; endophyte of Ferula sumbul. Microb. Pathog. 2017, 110, 214–224. [Google Scholar] [CrossRef]
- Aguilar, D.; Morales-Oyervides, L.; Contreras-Esquivel, J.C.; Méndez-Zavala, A.; Raso, J.; Montañez, J. Effect of ozone processing conditions on stability of fungal pigments. Innov. Food Sci. Emerg. Technol. 2018, 45, 255–263. [Google Scholar] [CrossRef]
| Compound | Type | Pigment Color | References |
|---|---|---|---|
| Flavipin | Polyketide | Yellow pigment | Brown et al. [90]; Madrigal et al. [91] |
| Epicoccones A and B | Polyketide | Brown pigment | Abdel-lateff et al. [92]; Kemami et al. [93]; El Amrani et al. [94] |
| 3-methoxy epicoccone | Polyketide | Yellow pigment | El Amrani et al. [94] |
| 3-methoxy epicoccone B | Polyketide | Yellow pigment | El Amrani et al. [94] |
| 2,3,4-trihydroxy-6-(methoxymethyl)-5-methylbenzaldehyde | Polyketide | Brown pigment | El Amrani et al. [94] |
| 7-methoxy-4-oxo-chroman-5-carboxy acid methyl ester | Polyketide | Pale yellow pigment | Lee et al. [95] |
| 1,3-dihydro-5-methoxy-7-methyl isobenzofuran | Polyketide | Light brown pigment | Lee et al. [95] |
| Epicoccalone | Polyketide | Yellow pigment | Kemami Wangun et al. [93] |
| Epicocconone | Polyketide | Pigment of high orange-red fluorescent in the presence of proteins | Bell and Karuso [40] |
| Acetosellin | Polyketide | Yellow pigment | Talontsi et al. [96] |
| Quinizarin | Polyketide | Red pigment | Dzoyem et al. [97] |
| Orevactaene | Polyketide | Orange pigment | Shu et al. [98] |
| Epipyridone | Polyketide–nonribosomal peptide hybrid | Red pigment | Kemami Wangun and Hertweck [99] |
| Epicoccarines A and B | Polyketide–nonribosomal peptide hybrid | Antibacterial and red pigment | Kemami Wangun and Hertweck [99] |
| β-Carotene | Carotenoid | Antioxidant and yellow pigment | Foppen and Gribanovski-Sassu [100] |
| γ-Carotene | Carotenoid | Orange pigment | Foppen and Gribanovski-Sassu [100] |
| Rhodoxanthin | Carotenoid | Red pigment | Foppen and Gribanovski-Sassu [100] |
| Torularhodin | Carotenoid | Violet pigment | Foppen and Gribanovski-Sassu [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkhateeb, W.; Daba, G. Fungal Pigments: Their Diversity, Chemistry, Food and Non-Food Applications. Appl. Microbiol. 2023, 3, 735-751. https://doi.org/10.3390/applmicrobiol3030051
Elkhateeb W, Daba G. Fungal Pigments: Their Diversity, Chemistry, Food and Non-Food Applications. Applied Microbiology. 2023; 3(3):735-751. https://doi.org/10.3390/applmicrobiol3030051
Chicago/Turabian StyleElkhateeb, Waill, and Ghoson Daba. 2023. "Fungal Pigments: Their Diversity, Chemistry, Food and Non-Food Applications" Applied Microbiology 3, no. 3: 735-751. https://doi.org/10.3390/applmicrobiol3030051
APA StyleElkhateeb, W., & Daba, G. (2023). Fungal Pigments: Their Diversity, Chemistry, Food and Non-Food Applications. Applied Microbiology, 3(3), 735-751. https://doi.org/10.3390/applmicrobiol3030051







