Exploring the Feasibility of Intrapartum GBS Collection to Identify Residual GBS in a Pilot Study of an Antenatal Probiotic Intervention
Abstract
:1. Introduction
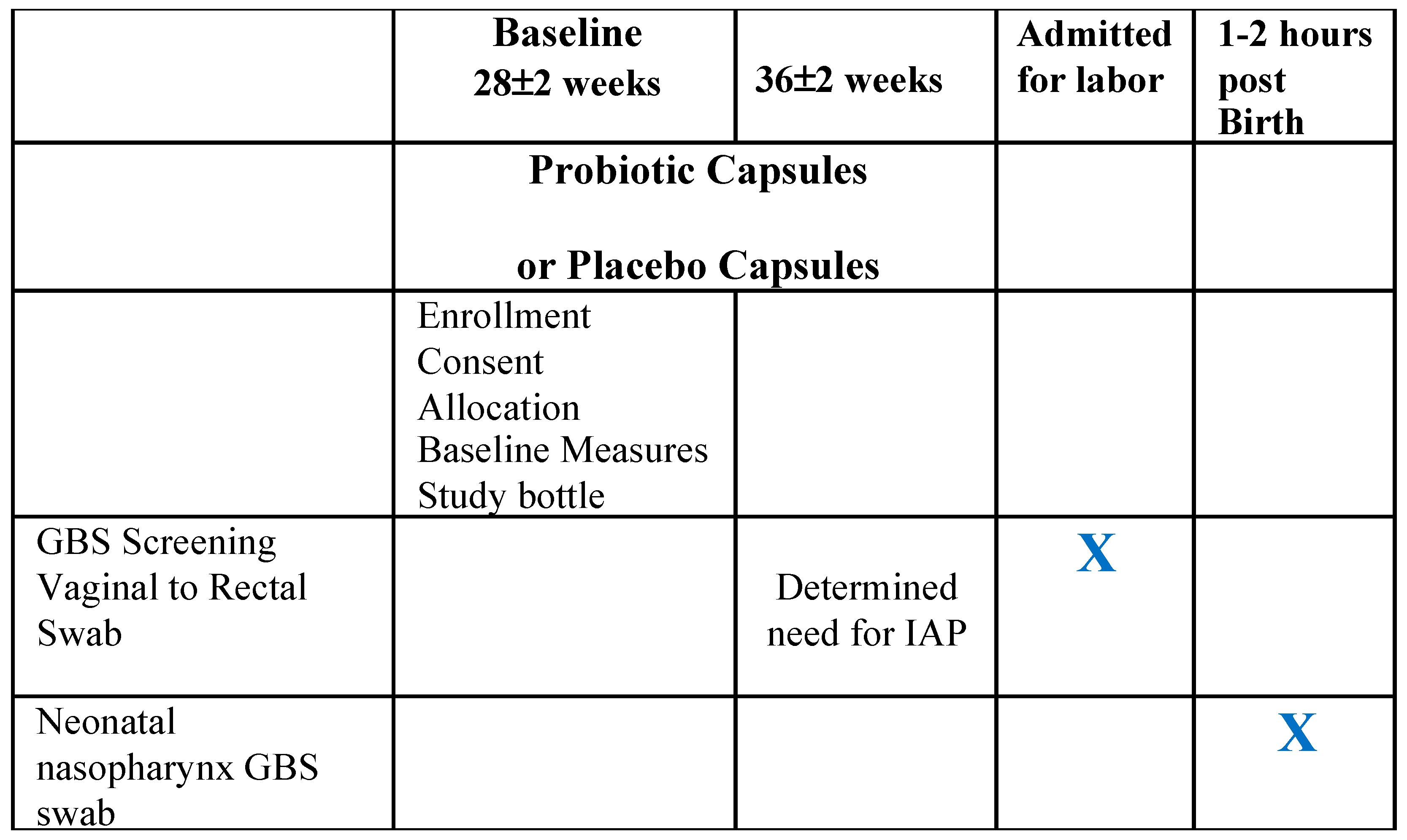
2. Materials and Methods
2.1. Relationship of the Pilot Sub-Study to Parent Double-Blind, Placebo-Controlled RCT
2.2. Data Collection
2.3. Statistical Analysis
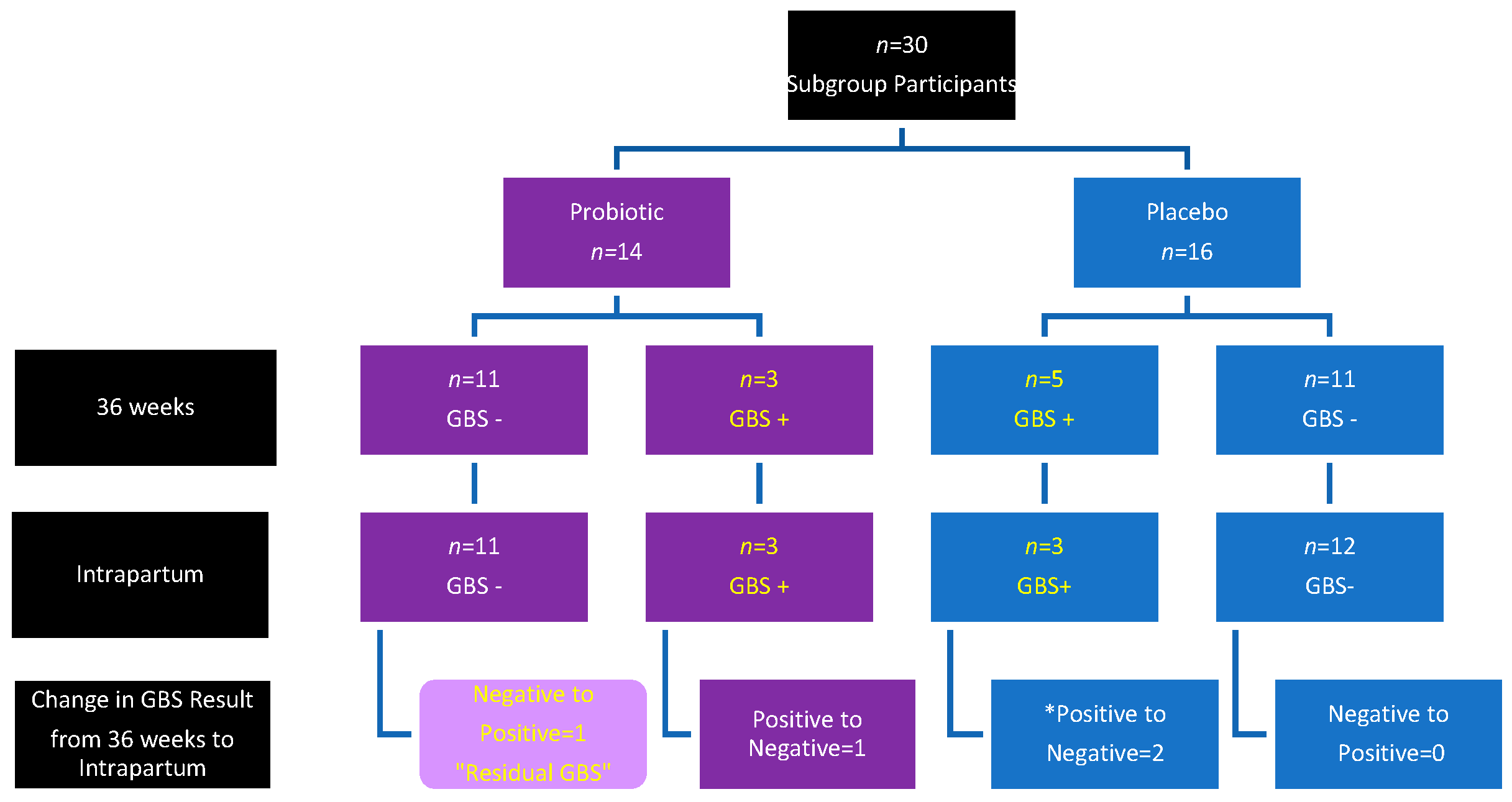
3. Results
4. Discussion
4.1. Limitations
4.2. Clinical Implications
4.3. Feasibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACOG Committee Opinion. Prevention of Group B Streptococcal Early-Onset Disease in Newborns. Obstet. Gynecol. 2019, 134, e19–e40. [Google Scholar]
- Russell, N.J.; Seale, A.C.; O’Driscoll, M.; O’Sullivan, C.; Bianchi-Jassir, F.; Gonzalez-Guarin, J. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. S2), S100–S111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virranniemi, M.; Raudaskoski, T.; Haapsamo, M.; Kauppila, J.; Renko, M.; Peltola, J.; Risteli, L.; Laatio, L. The effect of screening-to-labor interval on the sensitivity of late-pregnancy culture in the prediction of group B streptococcus colonization at labor: A prospective multicenter cohort study. Acta Obstet. Gynecol. Scand. 2019, 98, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Prevention and Control. Preventing Group B Strep Disease. 2023. Available online: https://www.cdc.gov/groupbstrep/about/prevention.html (accessed on 5 June 2023).
- Ying, Q.; Wang, S.; Lou, X.; Ding, J.; Ding, J. Burden and risk factors of invasive group B Streptococcus disease among neonates in a Chinese maternity hospital. BMC Infect. Dis. 2019, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Puopolo, K.M.; Lynfield, R.; Cummings, J.J. Management of Infants at Risk for Group B Streptococcal Disease. Pediatrics 2019, 144, e20191881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanduri, S.A.; Petit, S.; Smelser, C.; Apostol, M.; Alden, N.B.; Harrison, L.H.; Lynfield, R.; Vagnone, P.S.; Burzlaff, K.; Spina, N.L.; et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019, 173, 224–233. [Google Scholar] [CrossRef]
- Berardi, A.; Rossi, C.; Creti, R.; China, M.; Gherardi, G.; Venturelli, C.; Rumpianesi, F.; Ferrari, F. Group B streptococcal colonization in 160 mother-baby pairs: A prospective cohort study. J. Pediatr. 2013, 163, 1099–1104. [Google Scholar] [CrossRef] [Green Version]
- Furuta, A.; Brokaw, A.; Manuel, G.; Dacanay, M.; Marcell, L.; Seepersaud, R.; Rajagopal, L.; Adams Waldorf, K. Bacterial and Host Determinants of Group B Streptococcal Infection of the Neonate and Infant. Front. Microbiol. 2022, 13, 820365. [Google Scholar] [CrossRef]
- Filkins, L.; Hauser, J.R.; Robinson-Dunn, B.; Tibbetts, R.; Boyanton, B.L.; Revell, P. American Society for Microbiology Provides 2020 Guidelines for Detection and Identification of Group B Streptococcus. J. Clin. Microbiol. 2020, 59, e01230-20. [Google Scholar] [CrossRef]
- Breeding, K.M.; Ragipani, B.; Lee, K.D.; Malik, M.; Randis, T.M.; Ratner, A.J. Real-time PCR-based serotyping of Streptococcus agalactiae. Sci. Rep. 2016, 6, 38523. [Google Scholar] [CrossRef] [Green Version]
- Malloy, E.; Kates, A.; Watson, L.; VandeVusse, L.; Safdar, N.; Hanson, L. Laboratory Analysis Techniques for the Perinatal Microbiome: Implications for Studies of Probiotic Interventions. J. Perinat. Neonatal Nurs. 2020, 34, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Parente, V.; Clark, R.H.; Ku, L.; Fennell, C.; Johnson, M.; Morris, E.; Romaine, A.; Utin, U.; Benjamin, D.K.; Messina, J.A.; et al. Risk factors for group B streptococcal disease in neonates of mothers with negative antenatal testing. J. Perinatol. 2017, 37, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyke, M.K.; Phares, C.R.; Lynfield, R.; Thomas, A.R.; Arnold, K.E.; Craig, A.S.; Mohle-Boetani, J.; Gershman, K.; Schaffner, W.; Petit, S.; et al. Evaluation of Universal Antenatal Screening for Group B Streptococcus. N. Engl. J. Med. 2009, 360, 2626–2636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, M.L. Group B strep prophylaxis: What are we creating? Midwifery Today Int. Midwife 2007, 81, 18–20. [Google Scholar]
- Hanson, L.; Vandevusse, L.; Duster, M.; Warrack, S.; Safdar, N. Feasibility of oral prenatal probiotics against maternal group B Streptococcus vaginal and rectal colonization. J. Obstet. Gynecol. Neonatal Nurs. 2014, 43, 294–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO/WHO. Report on Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. Cordoba, Argentina. Available online: http://pc.ilele.hk/public/pdf/20190225/bd3689dfc2fd663bb36def1b672ce0a4.pdf (accessed on 29 November 2022).
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada. 2002. Available online: https://pdf4pro.com/amp/fullscreen/guidelines-for-the-evaluation-of-probiotics-in-food-1d59e1.html (accessed on 29 November 2022).
- Di Pierro, F.; Parolari, A.; Brundu, B.; Nigro, R. Positive clinical outcomes derived from using a proprietary mixture of selected strains during pregnancy. Acta Biomed. 2016, 87, 259–265. [Google Scholar]
- Farr, A.; Sustr, V.; Kiss, H.; Rosicky, I.; Graf, A.; Makristathis, A.; Foessleitner, P.; Petricevic, L. Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Sci. Rep. 2020, 10, 19745. [Google Scholar] [CrossRef]
- Hanson, L.; VandeVusse, L.; Forgie, M.; Malloy, E.; Singh, M.; Scherer, M.; Kleber, D.; Dixon, J.; Hryckowian, A.J.; Safdar, N. A randomized controlled trial of an oral probiotic to reduce antepartum group B Streptococcus colonization and gastrointestinal symptoms. Am. J. Obstet. Gynecol. MFM 2023, 5, 100748. [Google Scholar] [CrossRef]
- Martín, V.; Cárdenas, N.; Ocaña, S.; Marín, M.; Arroyo, R.; Beltrán, D.; Badiola, C.; Fernández, L.; Rodríguez, J.M. Rectal and Vaginal Eradication of Streptococcus agalactiae (GBS) in pregnant women by using Lactobacillus salivarius CECT 9145, a target-specific probiotic strain. Nutrients 2019, 11, 810. [Google Scholar] [CrossRef] [Green Version]
- Olsen, P.; Williamson, M.; Traynor, V.; Georgiou, C. The impact of oral probiotics on vaginal Group B Streptococcal colonisation rates in pregnant women: A pilot randomised control study. Women Birth 2018, 31, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, M.; Shah, V.; Freire-Lizama, T.; Cates, E.C.; McGrath, K.; David, I.; Cowan, S.; Letkeman, J.; Stewart-Wilson, E. Effectiveness of oral intake of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on Group B Streptococcus colonization during pregnancy: A midwifery-led double-blind randomized controlled pilot trial. J. Matern. Fetal Neonatal Med. 2019, 34, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Chang, Y.Y.; Chang, W.C.; Lin, H.C.; Wang, M.H.; Lin, W.C.; Chiu, T.H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial. Taiwan. J. Obstet. Gynecol. 2016, 5, 515–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Huang, Y.; Cai, W.; Li, D.; Zheng, W.; Xiao, Y.; Zhao, H.; Pan, S. Effect of oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on vaginal Group B Streptococcus colonization and vaginal microbiome in late pregnancy. Nan Fang Yi Ke Da Xue Xue Bao 2020, 40, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.; VandeVusse, L.; Malloy, E.; Garnier-Villarreal, M.; Watson, L.; Fial, A.; Forgie, M.; Nardini, K.; Safdar, N. Probiotic interventions to reduce antepartum Group B streptococcus colonization: A systematic review and meta-analysis. Midwifery 2022, 105, 103208. [Google Scholar] [CrossRef] [PubMed]
- Menichini, D.; Chiossi, G.; Monari, F.; De Seta, F.; Facchinetti, F. Supplementation of Probiotics in Pregnant Women Targeting Group B Streptococcus Colonization: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4520. [Google Scholar] [CrossRef]
- Sheyholislami, H.; Connor, K.L. Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2382. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Severgnini, M.; Morselli, S.; Camboni, T.; Ceccarani, C.; Laghi, L.; Zagonari, S.; Patuelli, G.; Pedna, M.F.; Sambri, V.; Foschi, C.; et al. A Deep Look at the Vaginal Environment During Pregnancy and Puerperium. Front. Cell. Infect. Microbiol. 2022, 12, 838405. [Google Scholar] [CrossRef]
- Hussain, F.N.; Al-Ibraheemi, Z.; Pan, S.; Francis, A.P.; Taylor, D.; Lam, M.C.; Lewis, D. The Accuracy of Group Beta Streptococcus Rectovaginal Cultures at 35 to 37 Weeks of Gestation in Predicting Colonization Intrapartum. AJP Rep. 2019, 09, e302–e309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foxman, B.; Gillespie, B.W.; Manning, S.D.; Marrs, C.F. Risk factors for group B streptococcal colonization: Potential for different transmission systems by capsular type. Ann. Epidemiol. 2007, 17, 854–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puopolo, K.M.; Madoff, L.C.; Eichenwald, E.C. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 2005, 115, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.L.; Cox, S.M.; Bawdon, R.E.; Gilstrap, L.C. Ampicillin for neonatal group B streptococcal prophylaxis: How rapidly can bactericidal concentrations be achieved? Am. J. Obstet. Gynecol. 1996, 175, 974–976. [Google Scholar] [CrossRef]
- Hughes, R.; Brocklehurst, P.; Steer, P.J.; Heath, P.; Stenson, B.M. on behalf of the Royal College of Obstetricians and Gynaecologists. Prevention of Early-onset Neonatal Group B Streptococcal Disease. BJOG Int. J. Obstet. Gynaecol. 2017, 124, e280–e305. [Google Scholar] [CrossRef] [Green Version]
- Center for Disease Control and Prevention. Antimicrobial Resistance:U.S. National Action Plan, 2021. Available online: https://www.cdc.gov/drugresistance/us-activities/national-action-plan.html (accessed on 5 June 2023).
- Hasperhoven, G.F.; Al-Nasiry, S.; Bekker, V.; Villamor, E.; Kramer, B. Universal screening versus risk-based protocols for antibiotic prophylaxis during childbirth to prevent early-onset group B streptococcal disease: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 680–691. [Google Scholar] [CrossRef] [Green Version]
- Vekemans, J.; Moorthy, V.; Friede, M.; Alderson, M.R.; Sobanjo-Ter Meulen, A.; Baker, C.J.; Heath, P.T.; Madhi, S.A.; Mehring-Le Doare, K.; Saha, S.K.; et al. Maternal immunization against Group B streptococcus: World Health Organization research and development technological roadmap and preferred product characteristics. Vaccine 2019, 37, 7391–7393. [Google Scholar] [CrossRef]
- Absalon, J.; Segall, N.; Block, S.L.; Center, K.J.; Scully, I.L.; Giardina, P.C.; Peterson, J.; Watson, W.J.; Gruber, W.C.; Jansen, K.U.; et al. Safety and immunogenicity of a novel hexavalent group B streptococcus conjugate vaccine in healthy, non-pregnant adults: A phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial. Lancet Infect. Dis. 2021, 21, 263–274. [Google Scholar] [CrossRef]
- Brokaw, A.; Furuta, A.; Dacanay, M.; Rajagopal, L.; Adams Waldorf, K.M. Bacterial and Host Determinants of Group B Streptococcal Vaginal Colonization and Ascending Infection in Pregnancy. Front. Cell. Infect. Microbiol. 2021, 11, 720789. [Google Scholar] [CrossRef]
| Variable | Total | Probiotics (n = 14) | Placebo (n = 16) | p Value |
|---|---|---|---|---|
| Age (Mean, SD) | 29.87 ± 5.46 | 30.0 ± 5.31 | 29.75 ± 5.77 | 0.9029 |
| Race (N, %) | 0.6237 | |||
| Black | 10 (33.33) | 4 (28.57) | 6 (37.5) | |
| White | 18 (60.0) | 10 (71.43) | 8 (50.0) | |
| Asian | 1 (3.33) | 0 (0.0) | 1 (6.25) | |
| Other | 1 (3.33) | 0 (0.0) | 1 (6.25) | |
| Parity | 0.3625 | |||
| Multiparous | 17 (56.67) | 7 (50.0) | 10 (62.5) | |
| Nulliparous | 12 (4.80) * 1 missing | 7 (50.0) | 5 (31.25) | |
| Apgar Scores, (Mean, SD) | ||||
| 1 min | 7.33 ± 1.40 | 7.43 ±1.16 | 7.25 ±1.61 | 0.7336 |
| 5 min | 8.77 ± 0.68 | 8.79 ± 0.58 | 8.75 ± 0.78 | 0.8886 |
| Mode of birth | 0.4171 | |||
| Vaginal | 22 (73.33) | 9 (64.29) | 13 (81.25) | |
| Cesarean | 8 (26.67) | 5 (35.71) | 3 (18.75) | |
| Newborn birth weight (Mean, SD), grams | 3361.10 ± 271.70 | 3386.30 ± 293.90 | 3339.10 ± 258.40 | 0.6431 |
| GBS Timing | Total (n = 30) | Probiotics (n = 14) | Placebo (n = 16) | p Value |
|---|---|---|---|---|
| 36-week SOC (n, %) | ||||
| Positive | 8 (26.67) | 3 (21.43) | 5 (31.25) | 0.6887 |
| Negative | 22 (73.33) | 11 (78.57) | 11 (68.75) | |
| Intrapartum (n, %) | ||||
| Positive | * 6 (20.0) | 3 (21.43) | * 3 (18.75) | 0.639 |
| Negative | 23 (76.67) | 11 (78.57) | 12 (75) | |
| Rate of change (n, %) | ||||
| No group change | 26 (86.67) | 12 (85.7) | 14 (87.5) | 0.8859 |
| Group change | 4 (13.33) | 2 (12.5) | 2 (14.29) | |
| • Pos to Neg | 3 | 1 | 2 | |
| • Neg to Pos | 1 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malloy, E.; Hanson, L.; VandeVusse, L.; Robinson, K.; Singh, M.; Forgie, M. Exploring the Feasibility of Intrapartum GBS Collection to Identify Residual GBS in a Pilot Study of an Antenatal Probiotic Intervention. Appl. Microbiol. 2023, 3, 752-763. https://doi.org/10.3390/applmicrobiol3030052
Malloy E, Hanson L, VandeVusse L, Robinson K, Singh M, Forgie M. Exploring the Feasibility of Intrapartum GBS Collection to Identify Residual GBS in a Pilot Study of an Antenatal Probiotic Intervention. Applied Microbiology. 2023; 3(3):752-763. https://doi.org/10.3390/applmicrobiol3030052
Chicago/Turabian StyleMalloy, Emily, Lisa Hanson, Leona VandeVusse, Karen Robinson, Maharaj Singh, and Marie Forgie. 2023. "Exploring the Feasibility of Intrapartum GBS Collection to Identify Residual GBS in a Pilot Study of an Antenatal Probiotic Intervention" Applied Microbiology 3, no. 3: 752-763. https://doi.org/10.3390/applmicrobiol3030052
APA StyleMalloy, E., Hanson, L., VandeVusse, L., Robinson, K., Singh, M., & Forgie, M. (2023). Exploring the Feasibility of Intrapartum GBS Collection to Identify Residual GBS in a Pilot Study of an Antenatal Probiotic Intervention. Applied Microbiology, 3(3), 752-763. https://doi.org/10.3390/applmicrobiol3030052






