Sugar-Induced Cell Death in the Yeast S. cerevisiae Is Accompanied by the Release of Octanoic Acid, Which Does Not Originate from the Fatty Acid Synthesis Type II Mitochondrial System
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Growth
2.2. 1,2,3,-Dihydrorhodamine (DHR) Staining
2.3. SICD Assay and Flow Cytometry
2.4. Preparation of FAME for Gas Chromatography
2.5. Mass Spectrometry and Gas Chromatography
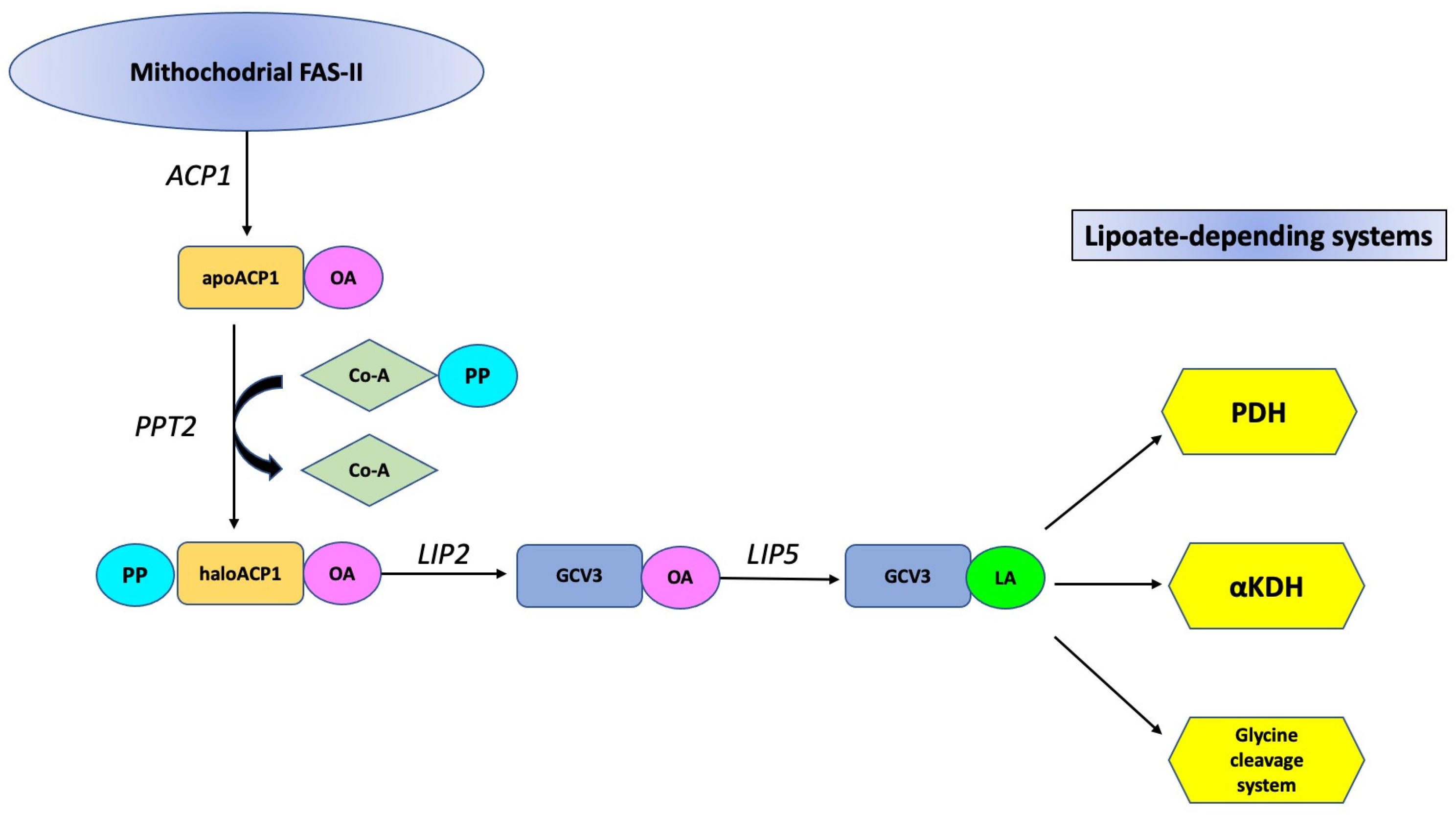
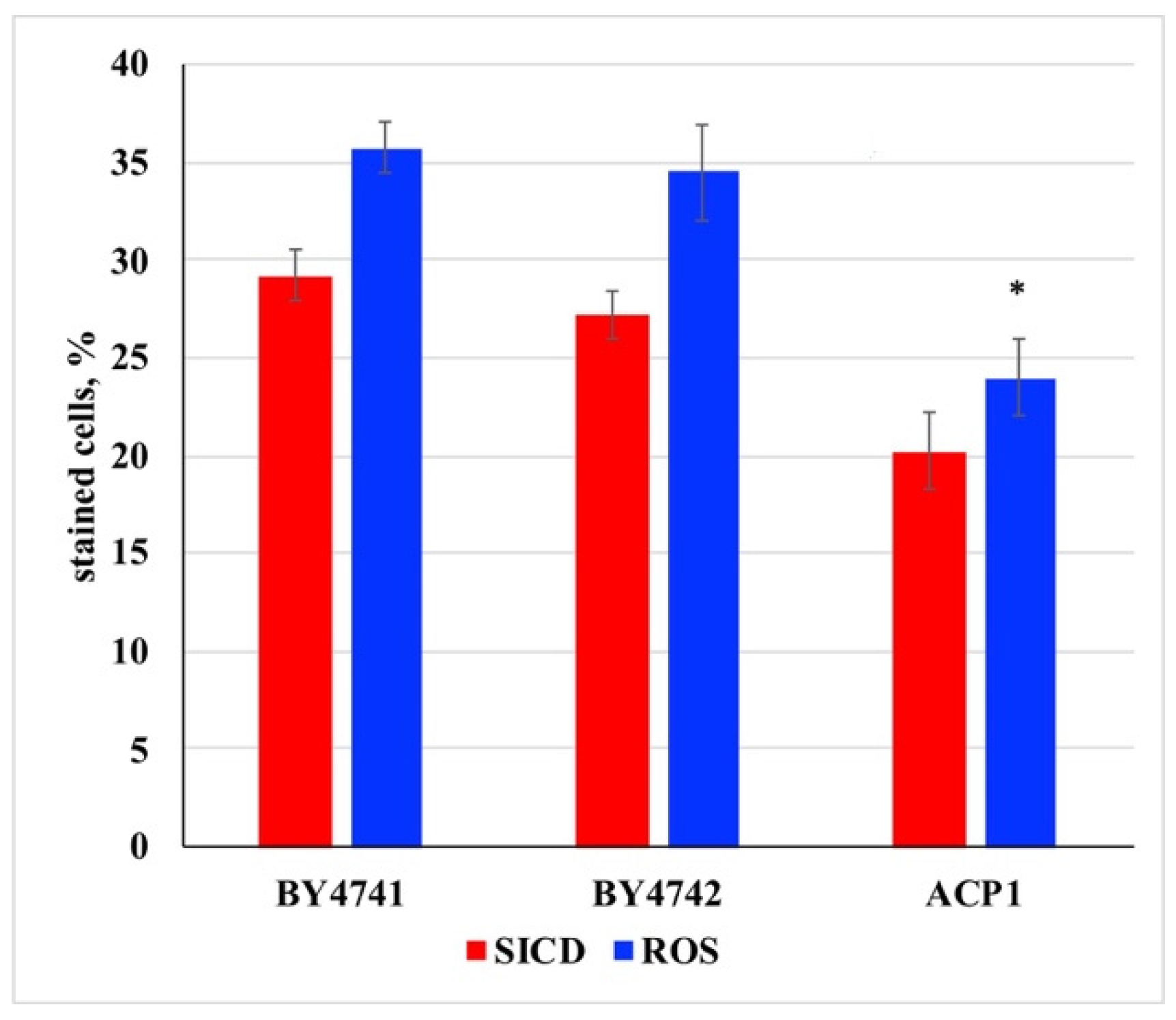
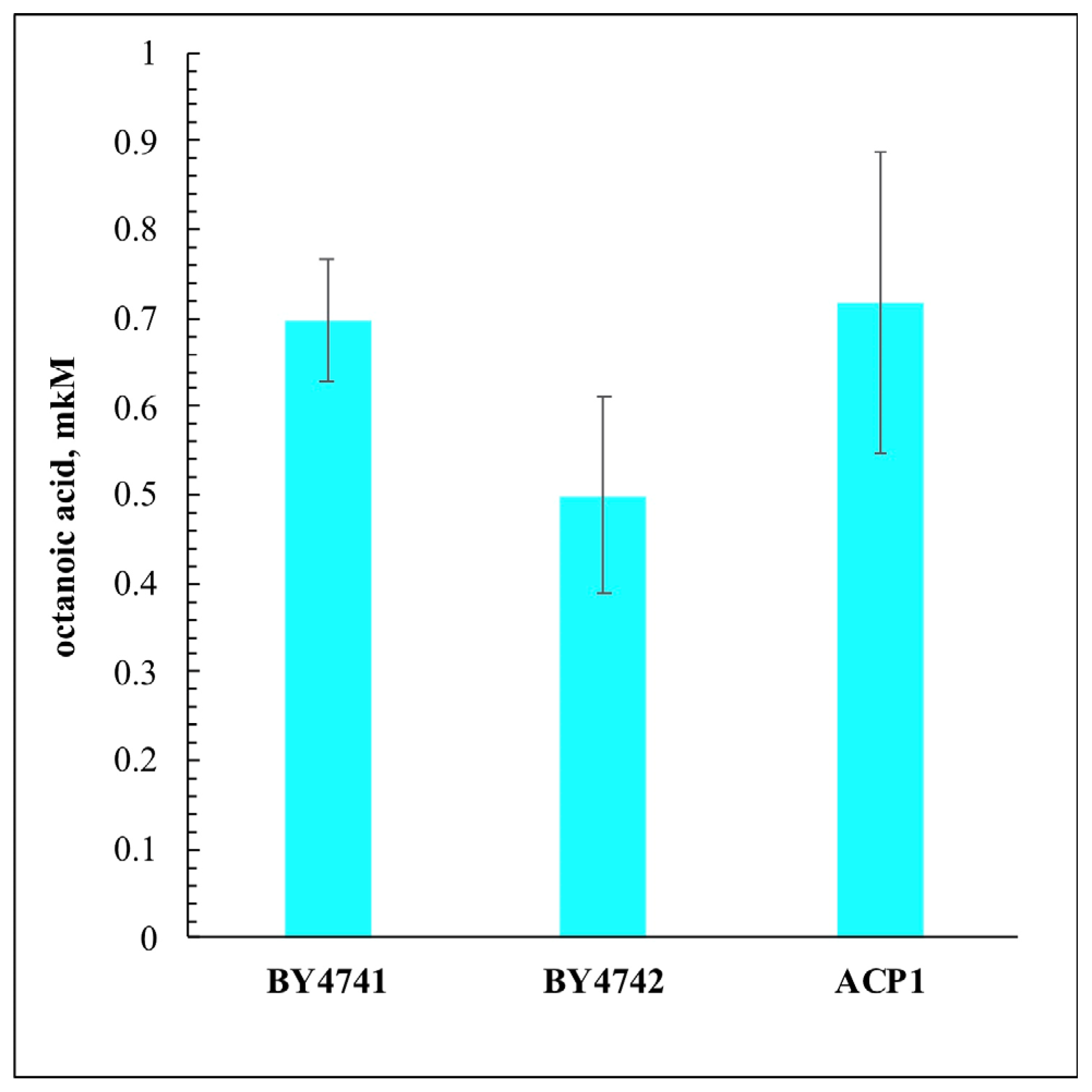
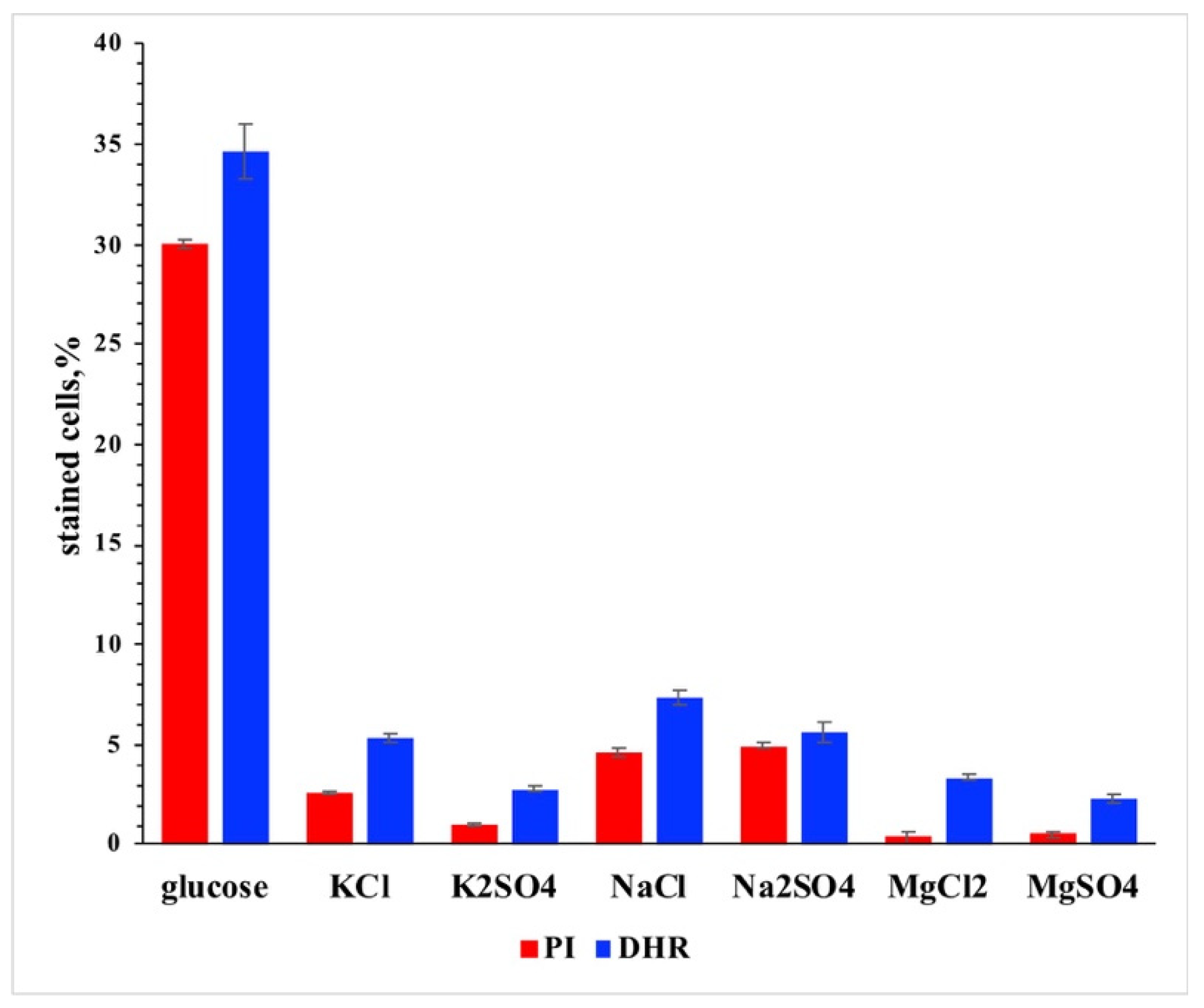
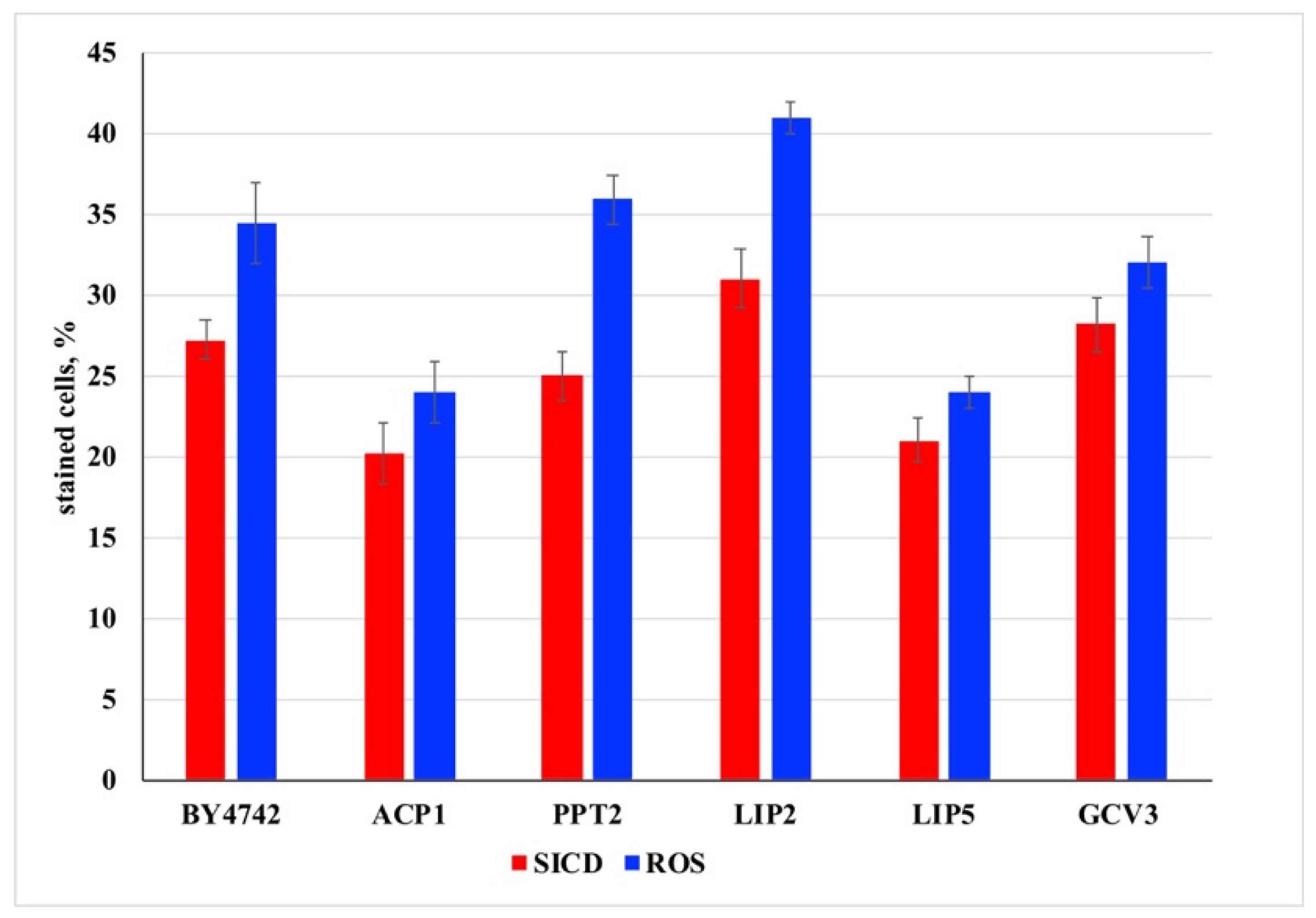
3. Results and Discussion
4. Conclusions
- (1)
- OA originated from mitochondrial FAS-II systems is not the cause of SICD;
- (2)
- mitochondria are not the site of initiation of primary necrosis in the form of SICD.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madeo, F.; Frohlich, E.; Frohlich, K.U. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 1997, 139, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Snyder, M. Glucose induces cAMP-independent growth-related changes in stationary-phase cells of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1991, 88, 5724–5728. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Levine, A.; Dor-Hefetz, E. Sugar-induced apoptosis in yeast cells. FEMS Yeast Res. 2003, 4, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Dai, N. Sugar induced cell death in yeast is dependent on the rate of sugar phosphorylation as determined by Arabidopsis thaliana hexokinase. Cell Death Differ. 1997, 4, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Snyder, M. Carbon source induces growth of stationary phase yeast cells, independent of carbon source metabolism. Yeast 1993, 9, 465–479. [Google Scholar] [CrossRef]
- Lee, Y.J.; Burlet, E.; Galiano, F.; Circu, M.L.; Aw, T.Y.; Williams, B.J.; Witt, S.N. Phosphate and succinate use different mechanisms to inhibit sugar-induced cell death in yeast: Insight into the Crabtree effect. J. Biol. Chem. 2011, 286, 20267–20274. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Ohuchi, R.; Ikado, K.; Yoshida, S.; Minato, T.; Ishiguro, T.; Mizutani, S.; Kobayashi, O. Sugar induces death of the bottom fermenting yeast Saccharomyces pastorianus. J. Biosci. Bioeng. 2009, 108, 60–62. [Google Scholar] [CrossRef]
- Valiakhmetov, A.Y.; Kuchin, A.V.; Suzina, N.E.; Zvonarev, A.N.; Shepelyakovskaya, A.O. Glucose causes primary necrosis in exponentially grown yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2019, 19, foz019. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Bauer, M.A.; Zimmermann, A.; Aguilera, A.; Austriaco, N.; Ayscough, K.; Balzan, R.; Bar-Nun, S.; Barrientos, A.; Belenky, P.; et al. Guidelines and recommendations on yeast cell death nomenclature. Microb. Cell 2018, 5, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Bidiuk, V.A.; Alexandrov, A.I.; Valiakhmetov, A.Y. Extracellular pH and high concentration of potassium regulate the primary necrosis in the yeast Saccharomyces cerevisiae. Arch. Microbiol. 2021, 204, 35. [Google Scholar] [CrossRef]
- Lapathitis, G.; Kotyk, A. Different sources of acidity in glucose-elicited extracellular acidification in the yeast Saccharomyces cerevisiae. Biochem. Mol. Biol. Int. 1998, 46, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Kotyk, A.; Lapathitis, G.; Krenkova, S. Glucose- and K(+)-induced acidification in different yeast species. Folia Microbiol. 1999, 44, 295–298. [Google Scholar] [CrossRef]
- Martins, V.M.; Fernandes, T.R.; Lopes, D.; Afonso, C.B.; Domingues, M.R.M.; Corte-Real, M.; Sousa, M.J. Contacts in Death: The Role of the ER-Mitochondria Axis in Acetic Acid-Induced Apoptosis in Yeast. J. Mol. Biol. 2019, 431, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, P.; Rodrigues, F.; Almeida, A.; Silva, M.T.; Barrientos, A.; Corte-Real, M. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell 2002, 13, 2598–2606. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, P.; Sousa, M.J.; Silva, M.T.; Leao, C.; Corte-Real, M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 2001, 147, 2409–2415. [Google Scholar] [CrossRef]
- Viegas, C.A.; Rosa, M.F.; Sa-Correia, I.; Novais, J.M. Inhibition of Yeast Growth by Octanoic and Decanoic Acids Produced during Ethanolic Fermentation. Appl. Environ. Microbiol. 1989, 55, 21–28. [Google Scholar] [CrossRef]
- Taylor, G.; Kirsop, B. The origin of the medium chain length fatty acids present in beer. J. Inst. Brew. 1977, 83, 241–243. [Google Scholar] [CrossRef]
- Lafon-Lafourcade, S.; Geneix, C.; Ribéreau-Gayon, P. Inhibition of Alcoholic Fermentation of Grape Must by Fatty Acids Produced by Yeasts and Their Elimination by Yeast Ghosts. Appl. Environ. Microbiol. 1984, 47, 1246–1249. [Google Scholar] [CrossRef]
- Liu, P.; Chernyshov, A.; Najdi, T.; Fu, Y.; Dickerson, J.; Sandmeyer, S.; Jarboe, L. Membrane stress caused by octanoic acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2013, 97, 3239–3251. [Google Scholar] [CrossRef]
- Borrull, A.; Lopez-Martinez, G.; Poblet, M.; Cordero-Otero, R.; Rozes, N. New insights into the toxicity mechanism of octanoic and decanoic acids on Saccharomyces cerevisiae. Yeast 2015, 32, 451–460. [Google Scholar] [CrossRef]
- Legras, J.L.; Erny, C.; Le Jeune, C.; Lollier, M.; Adolphe, Y.; Demuyter, C.; Delobel, P.; Blondin, B.; Karst, F. Activation of two different resistance mechanisms in Saccharomyces cerevisiae upon exposure to octanoic and decanoic acids. Appl. Environ. Microbiol. 2010, 76, 7526–7535. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.A.; Sa-Correia, I. Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J. Gen. Microbiol. 1991, 137, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.A.; Almeida, P.F.; Cavaco, M.; Sa-Correia, I. The H(+)-ATPase in the plasma membrane of Saccharomyces cerevisiae is activated during growth latency in octanoic acid-supplemented medium accompanying the decrease in intracellular pH and cell viability. Appl. Environ. Microbiol. 1998, 64, 779–783. [Google Scholar] [CrossRef]
- Stevens, S.; Hofmeyr, J.-H.S. Effects of ethanol, octanoic and decanoic acids on fermentation and the passive influx of protons through the plasma membrane of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993, 38, 656–663. [Google Scholar] [CrossRef]
- Alexandre, H.; Mathieu, B.; Charpentier, C. Alteration in membrane fluidity and lipid composition, and modulation of H(+)-ATPase activity in Saccharomyces cerevisiae caused by decanoic acid. Microbiology 1996, 142 Pt 3, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Besada-Lombana, P.B.; Fernandez-Moya, R.; Fenster, J.; Da Silva, N.A. Engineering Saccharomyces cerevisiae fatty acid composition for increased tolerance to octanoic acid. Biotechnol. Bioeng. 2017, 114, 1531–1538. [Google Scholar] [CrossRef]
- Cabral, M.G.; Viegas, C.A.; Sa-Correia, I. Mechanisms underlying the acquisition of resistance to octanoic-acid-induced-death following exposure of Saccharomyces cerevisiae to mild stress imposed by octanoic acid or ethanol. Arch. Microbiol. 2001, 175, 301–307. [Google Scholar] [CrossRef]
- Wernig, F.; Baumann, L.; Boles, E.; Oreb, M. Production of octanoic acid in Saccharomyces cerevisiae: Investigation of new precursor supply engineering strategies and intrinsic limitations. Biotechnol. Bioeng. 2021, 118, 3046–3057. [Google Scholar] [CrossRef]
- Baumann, L.; Wernig, F.; Born, S.; Oreb, M. Engineering Saccharomyces cerevisiae for Production of Fatty Acids and Their Derivatives. In Genetics and Biotechnology; Benz, J.P., Schipper, K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 339–368. [Google Scholar] [CrossRef]
- Henritzi, S.; Fischer, M.; Grininger, M.; Oreb, M.; Boles, E. An engineered fatty acid synthase combined with a carboxylic acid reductase enables de novo production of 1-octanol in Saccharomyces cerevisiae. Biotechnol. Biofuels 2018, 11, 150. [Google Scholar] [CrossRef]
- Schneider, R.; Massow, M.; Lisowsky, T.; Weiss, H. Different respiratory-defective phenotypes of Neurospora crassa and Saccharomyces cerevisiae after inactivation of the gene encoding the mitochondrial acyl carrier protein. Curr. Genet. 1995, 29, 10–17. [Google Scholar] [CrossRef]
- Miinalainen, I.J.; Chen, Z.J.; Torkko, J.M.; Pirilä, P.L.; Sormunen, R.T.; Bergmann, U.; Qin, Y.M.; Hiltunen, J.K. Characterization of 2-enoyl thioester reductase from mammals. An ortholog of YBR026p/MRF1’p of the yeast mitochondrial fatty acid synthesis type II. J. Biol. Chem. 2003, 278, 20154–20161. [Google Scholar] [CrossRef]
- Zhang, L.; Joshi, A.K.; Smith, S. Cloning, expression, characterization, and interaction of two components of a human mitochondrial fatty acid synthase. Malonyltransferase and acyl carrier protein. J. Biol. Chem. 2003, 278, 40067–40074. [Google Scholar] [CrossRef]
- Zhang, L.; Joshi, A.K.; Hofmann, J.; Schweizer, E.; Smith, S. Cloning, expression, and characterization of the human mitochondrial beta-ketoacyl synthase. Complementation of the yeast CEM1 knock-out strain. J. Biol. Chem. 2005, 280, 12422–12429. [Google Scholar] [CrossRef]
- Brody, S.; Oh, C.; Hoja, U.; Schweizer, E. Mitochondrial acyl carrier protein is involved in lipoic acid synthesis in Saccharomyces cerevisiae. FEBS Lett. 1997, 408, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Schonauer, M.S.; Kastaniotis, A.J.; Kursu, V.A.S.; Hiltunen, J.K.; Dieckmann, C.L. Lipoic Acid Synthesis and Attachment in Yeast Mitochondria *. J. Biol. Chem. 2009, 284, 23234–23242. [Google Scholar] [CrossRef] [PubMed]
- Marvin, M.E.; Williams, P.H.; Cashmore, A.M. The isolation and characterisation of a Saccharomyces cerevisiae gene (LIP2) involved in the attachment of lipoic acid groups to mitochondrial enzymes. FEMS Microbiol. Lett. 2001, 199, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Pietikäinen, L.P.; Rahman, M.T.; Hiltunen, J.K.; Dieckmann, C.L.; Kastaniotis, A.J. Genetic dissection of the mitochondrial lipoylation pathway in yeast. BMC Biol. 2021, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Schonauer, M.S.; Kastaniotis, A.J.; Hiltunen, J.K.; Dieckmann, C.L. Intersection of RNA processing and the type II fatty acid synthesis pathway in yeast mitochondria. Mol. Cell. Biol. 2008, 28, 6646–6657. [Google Scholar] [CrossRef]
- Della Croce, C.; Bronzetti, G.; Cini, M.; Caltavuturo, L.; Poi, G. Protective effect of lipoic acid against hydrogen peroxide in yeast cells. Toxicol. Vitr. 2003, 17, 753–759. [Google Scholar] [CrossRef]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid. Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Almeida, B.; Ohlmeier, S.; Almeida, A.J.; Madeo, F.; Leao, C.; Rodrigues, F.; Ludovico, P. Yeast protein expression profile during acetic acid-induced apoptosis indicates causal involvement of the TOR pathway. Proteomics 2009, 9, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Volkov, V. Quantitative description of ion transport via plasma membrane of yeast and small cells. Front. Plant Sci. 2015, 6, 425. [Google Scholar] [CrossRef]
- Mikolajczyk, S.; Brody, S. De novo fatty acid synthesis mediated by acyl-carrier protein in Neurospora crassa mitochondria. Eur. J. Biochem. 1990, 187, 431–437. [Google Scholar] [CrossRef]
- Chen, B.; Foo, J.L.; Ling, H.; Chang, M.W. Mechanism-Driven Metabolic Engineering for Bio-Based Production of Free R-Lipoic Acid in Saccharomyces cerevisiae Mitochondria. Front. Bioeng. Biotechnol. 2020, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; Binbin, C.; FOO, J.L.; Hua, L. Metabolic engineering for production of lipoic acid. Google Patents: WO2021177896A1, 10 September 2021. [Google Scholar]
- White, R.H. Stable isotope studies on the biosynthesis of lipoic acid in Escherichia coli. Biochemistry 1980, 19, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Booker, S.J.; Cicchillo, R.M.; Grove, T.L. Self-sacrifice in radical S-adenosylmethionine proteins. Curr. Opin. Chem. Biol. 2007, 11, 543–552. [Google Scholar] [CrossRef]
- Stuible, H.-P.; Meier, S.; Wagner, C.; Hannappel, E.; Schweizer, E. A Novel Phosphopantetheine:Protein Transferase Activating Yeast Mitochondrial Acyl Carrier Protein *. J. Biol. Chem. 1998, 273, 22334–22339. [Google Scholar] [CrossRef]
- Sulo, P.; Martin, N.C. Isolation and characterization of LIP5. A lipoate biosynthetic locus of Saccharomyces cerevisiae. J. Biol. Chem. 1993, 268, 17634–17639. [Google Scholar]
- Nagarajan, L.; Storms, R.K. Molecular characterization of GCV3, the Saccharomyces cerevisiae gene coding for the glycine cleavage system hydrogen carrier protein. J. Biol. Chem. 1997, 272, 4444–4450. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avtukh, A.; Baskunov, B.; Keshelava, V.; Valiakhmetov, A. Sugar-Induced Cell Death in the Yeast S. cerevisiae Is Accompanied by the Release of Octanoic Acid, Which Does Not Originate from the Fatty Acid Synthesis Type II Mitochondrial System. Appl. Microbiol. 2023, 3, 722-734. https://doi.org/10.3390/applmicrobiol3030050
Avtukh A, Baskunov B, Keshelava V, Valiakhmetov A. Sugar-Induced Cell Death in the Yeast S. cerevisiae Is Accompanied by the Release of Octanoic Acid, Which Does Not Originate from the Fatty Acid Synthesis Type II Mitochondrial System. Applied Microbiology. 2023; 3(3):722-734. https://doi.org/10.3390/applmicrobiol3030050
Chicago/Turabian StyleAvtukh, Alexander, Boris Baskunov, Varlam Keshelava, and Airat Valiakhmetov. 2023. "Sugar-Induced Cell Death in the Yeast S. cerevisiae Is Accompanied by the Release of Octanoic Acid, Which Does Not Originate from the Fatty Acid Synthesis Type II Mitochondrial System" Applied Microbiology 3, no. 3: 722-734. https://doi.org/10.3390/applmicrobiol3030050
APA StyleAvtukh, A., Baskunov, B., Keshelava, V., & Valiakhmetov, A. (2023). Sugar-Induced Cell Death in the Yeast S. cerevisiae Is Accompanied by the Release of Octanoic Acid, Which Does Not Originate from the Fatty Acid Synthesis Type II Mitochondrial System. Applied Microbiology, 3(3), 722-734. https://doi.org/10.3390/applmicrobiol3030050




