Abstract
Macrofungi have been previously studied for their nutritional value and medicinal properties. However, despite wild mushrooms being a great source of beneficial bioactive compounds, the literature exploring their biotechnological application as nutraceuticals, cosmeceuticals and nutricosmetics is scarce. In this work, the species Butyriboletus regius, Ganoderma lucidum, Inonotus hispidus, Lanmaoa fragrans, Pisolithus tinctorius, Suillellus luridus, Suillellus mendax and Xerocomus subtomentosus were characterized according to their phenolic composition, antioxidant capacity, antimicrobial activity and cosmeceutical potential. For that purpose, dried and ground mushrooms were extracted with ethanol (40% v/v) using ultrasound-assisted extraction. Of the eight mushrooms analyzed, I. hispidus and P. tinctorius stood out for their high content of phenolic compounds, high antioxidant capacity and anti-hyaluronidase activity. Regarding antimicrobial activity, both mushrooms showed good inhibition of bacterial growth and bactericidal activity, especially on Gram-positive bacteria; however, L. fragans obtained the best results. Cream formulations with I. hispidus and P. tinctorius extracts in their composition improved their antioxidant activity. These results indicate that I. hispidus and P. tinctorius can be proposed as a new potential source of natural compounds with application in the cosmetic industry.
1. Introduction
Mushrooms have been used as both food and medicine for centuries. Regarding wild mushrooms, they can offer a wide variety of beneficial compounds, since environmental stress benefits the production of secondary metabolites often associated with medicinal properties [1]. Phenolic compounds are secondary metabolites that can be found in plants and mushrooms, and possess biological activities, usually attributed to their antioxidant capacity [2,3]. For instance, the edible wild mushroom Butyriboletus regius (=Boletus regius) is rich in phenolic compounds, tocopherols and citric acid, and also presents a high antioxidant activity [4]. Ganoderma lucidum is one of the most studied wild mushrooms. G. lucidum is an edible mushroom and has been shown to have some medicinal benefits such as antibiotic, anti-HIV, anti-metastatic, anti-tumor and anti-viral properties, immunomodulatory effect, hepatic protection, and cholesterol synthesis inhibition [5,6,7]. Inonotus hispidus is a fungal plant pathogen of deciduous trees of the genera Fraxinus, Malus, Quercus and Sorbus [8]. I. hispidus has been used as a medicinal mushroom with health benefits such as immunomodulatory and anticancer activities [9,10,11]. This mushroom possesses a high phenolic content and a great antioxidant capacity [12]. Lanmaoa fragrans (=Boletus fragrans), another edible mushroom, has a high antioxidant potential, mostly due to the presence of polar antioxidants such as phenolics and sugars [13]. Pisolithus tinctorius is a mushroom poorly studied regarding its health benefits but, recently, it was shown to have a great antioxidative and NO scavenging potential [14]. Suillellus luridus (=Boletus luridus), an edible mushroom, has been shown to have high antioxidant and antihyperglycemic activities [15]. The polysaccharide extract of this mushroom showed the highest antioxidant capacity compared to other boletus mushrooms [16]. Regarding the wild mushrooms Suillellus mendax (=Boletus mendax) and Xerocomus subtomentosus (=Boletus subtomentosus), no studies regarding their potential health effects have been published.
The literature focus on the characterization and evaluation of the beneficial effects of wild mushrooms is scarce, especially the ones that are not traditionally used in gastronomy. The seasonality, the difficult cultivation of some wild species due to their symbiotic associations, the difficult access to these mushrooms and the correct identification of the species are some of the reasons that could contribute to the scarcity of studies on this matter. Thus, the aim of the present study was to evaluate the phenolic profile as well as the antioxidant, antimicrobial and cosmeceutical potential of the wild mushrooms B. regius, G. lucidum, I. hispidus, L. fragrans, P. tinctorius, S. luridus, S. mendax and X. subtomentosus collected from Portugal.
2. Materials and Methods
2.1. Mushroom Material
The mushrooms B. regius, G. lucidum, L. fragrans, S. luridus, S. mendax and X. subtomentosus were collected in July 2021, at Sabugal, Guarda (coordinates: 40.347500; −7.058333), located at the Center of Portugal. I. hispidus was collected in July 2022, at Lamego (coordinates: 41.044998; −7.760772), north of Portugal, and the mushroom P. tinctorius was collected in June 2022, at Vila Real (coordinates: 41.289444; −7.740750), north of Portugal. After taxonomic identification, the mushrooms were cut, dried at 40 °C in a drying oven (Termaks, Nordic Labtech AB, Germany) and then ground to a fine powder. The samples were kept in the dark in hermetically sealed plastic bags up to analysis.
2.2. Mushroom Extracts
Ultrasound-assisted extraction was carried out as previously described [12]. The solid–liquid extractions were performed by mixing 1 g of dried powder with 75 mL of 40% (v/v) ethanol in a pulsed mode (5 s on/5 s off cycles) for 20 min, using an ultrasonic processor device (Hielscher UP400St Berlin, Germany), with a sonotrode of 14 mm diameter, 400 Watts, 24 kHz, and adjustable amplitude (1:2.55). Upon completion of the extractions, the samples were centrifuged, filtered, collected and stored at −20 °C until analysis. All the experiments were performed in triplicate. The total extracted volume was concentrated in a vacuum rotary evaporator (IKA-RV 10, IKA, Staufen im Breisgau, Germany) at 38 °C to remove ethanol and then stored at −20 °C before lyophilization.
2.3. Phenolic Composition
The phenolic composition of the extracts was determined by colorimetric and spectrophotometric approaches according to the literature [17]. All the assays were adapted to microscale and read on a microplate reader (Multiskan GO Microplate Photometer, TermoFisher Scientific, Vantaa, Finland).
2.3.1. Total Phenol Content (TPC)
To quantify the total phenols in mushroom extracts, Folin–Ciocalteu reagent (Sigma Aldrich, St. Louise, MO, USA) was used with gallic acid (Sigma Aldrich, St. Louise, MO, USA) as standard. Briefly, 20 μL of each sample and 100 μL of 10% (v/v) Folin–Ciocalteu reagent were mixed. After that, 80 μL of 7.5% (w/v) aqueous sodium carbonate (Sigma Aldrich, St. Louise, MO, USA) was added and the reaction was incubated in an oven at 40–45 °C for 30 min protected from the light. The absorbance was read at 750 nm and the results were expressed as milligrams of gallic acid per gram of dry weight (mg GA/g dw).
2.3.2. Ortho-Diphenol Content
The ortho-diphenol content in mushroom extracts was evaluated by mixing 40 μL of 5% (w/v) sodium molybdate solution (Sigma Aldrich, St. Louise, MO, USA) with 160 μL of the diluted extracts. The plates were kept protected from the light and incubated at room temperature for 15 min. Caffeic acid (Sigma Aldrich, St. Louise, MO, USA) was used as standard. The absorbance was read at 375 nm and the results were expressed as milligrams of caffeic acid per gram of dry weight (mg CA/g dw).
2.3.3. Flavonoid Content
The flavonoid content in mushroom extracts was measured by mixing 24 μL of the diluted extracts with 28 μL of 5% (w/v) sodium nitrite (Merck, Darmstadt, Germany). After incubating for 5 min at room temperature, 28 μL of a 10% (w/v) aluminum chloride (Merck, Darmstadt, Germany) solution was added and the mixture was left to react for another 6 min. Finally, 120 μL of 1 M sodium hydroxide (Merck, Darmstadt, Germany) were added and the mixture was shaken for 30 s before reading the absorbance at 520 nm. Catechin was used as standard and the results were expressed as milligrams of catechin per gram of dry weight (mg Catechin/g dw).
2.4. In Vitro Antioxidant Capacity
The radical scavenging activity of sample extracts was assessed using the methodologies of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH) according to Mena et al. [18], with minor modifications. Additionally, the ferric reducing antioxidant power (FRAP) was assessed according to methodologies previously used [19,20], with some modifications. All the assays were adapted to microscale and read on a microplate reader (Multiskan GO Microplate Photometer, TermoFisher Scientific, Vantaa, Finland). For the three assays, 6-hydroxy-2,5,7,8-tetramethlychroman-2-carboxylic acid (Trolox, Sigma Aldrich) was used as standard and the results were expressed as micromoles of Trolox per gram of dry weight (µmol Trolox/g dw).
2.4.1. DPPH Radical Scavenging Activity
The assessment of the DPPH antioxidant activity was performed by diluting DPPH (Sigma Aldrich, St. Louise, MO, USA) in methanol (99.9%, v/v) to obtain a DPPH solution (8.87 mM). Then, 190 μL of the DPPH solution and 10 μL of sample dilutions or 70% methanol (v/v, blank) were added to each well of the microplate. The plate was incubated in the dark for 15 min at room temperature and the absorbance was measured at 520 nm. The inhibition of DPPH● was determined as follow:
2.4.2. ABTS Radical Scavenging Activity
To evaluate ABTS radical scavenging activity, an ABTS stock solution (7.0 mM) was prepared using ABTS salt (Sigma Aldrich, St. Louise, MO, USA). The ABTS stock solution was mixed with a 148 mM solution of potassium persulfate (Sigma Aldrich, St. Louise, MO, USA) and then diluted with sodium acetate buffer (20 mM, pH 4.5) to obtain the final working solution. Finally, 12 μL of sample dilutions or distilled water (blank) and 188 μL of the ABTS working solution were mixed, and the plate was left to react, protected from the light, for 30 min at room temperature. The absorbance was measured at 734 nm and the inhibition of ABTS●+ radicals was determined using Formula (1) above.
2.4.3. FRAP
To assess FRAP activity, a fresh working solution was prepared by mixing sodium acetate buffer (300 mM, pH 3.6) with 10 mM 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ, Sigma Aldrich) solution (40 mM hydrochloric acid as solvent) and 20 mM iron (III) chloride solution in a ratio of 10:1:1, respectively. After incubating the mixture for 10 min at 37 °C, 20 μL of sample dilutions were added to each well of the microplate, followed by the addition of 280 μL of FRAP working solution. The reaction was incubated for 30 min at 37 °C in the dark and the absorbance was recorded at 593 nm.
2.5. Polyphenolic Analysis by High-Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD)
The profile and content of phenolic compounds of the mushroom extracts were analyzed by HPLC-DAD, as previously reported [21] with minor modifications. Briefly, 10 μL of each extract was injected into a C18 column (250 × 4.6 mm, 5 μm particle size; ACE HPLC Columns, Advanced Chromatography Technologies Ltd., Aberdeen, Scotland, UK) with an eluent composed of water with 0.1% of trifluoroacetic acid (TFA) (solvent A) and acetonitrile with 0.1% TFA (solvent B). The elution was performed at a flow rate of solvent of 1 mL/min, with a gradient starting from 0% solvent B at 0 min, 0% solvent B at 5 min, 20% solvent B at 15 min, 50% solvent B at 30 min, 100% solvent B at 45 min, 100% solvent B at 50 min, 0% solvent B at 55 min and 0% solvent B at 60 min. Chromatograms were recorded in the 200–600 nm range and analyzed at 254, 280, 320 and 370 nm. All samples were injected in triplicate and the individual polyphenols were identified using peak retention time, UV spectra and UV maximum absorbance bands, external standards, and through comparison with the literature. Naringin (internal standard; Extrasynthese) was run simultaneously with the samples. All standards were freshly prepared in 70% (v/v) methanol at a concentration of 1 mg/mL and injected in HPLC before the samples. The chromatograms were analyzed with Xcalibur (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The amount of each compound was calculated using the internal standard method and the results were expressed as µg/100 g dw.
2.6. Antimicrobial Activity
The multidrug-resistant Gram-positive and Gram-negative bacteria used in this study were collected from human patients hospitalized in the Hospital Center of Trás-os-Montes and Alto Douro (CHTMAD), north of Portugal, according to a research collaboration protocol with the University of Trás-os-Montes and Alto Douro (UTAD) signed in 2004. These strains belong to MJH and MJMC collections stored at the Medical Microbiology Laboratory at the Department of Veterinary Sciences—Antimicrobials, Biocides and Biofilms Unit at UTAD. The antimicrobial activity of the mushroom extracts was evaluated against several Gram-positive and Gram-negative bacteria isolated from wound exudates, namely Enterococcus faecium (MJMC 531-B), methicillin-sensitive (MS) Staphylococcus aureus (MJMC 109), methicillin-resistant (MR) Staphylococcus aureus (MJMC 534-B and MJMC 565-A), Acinetobacter baumannii (MJMC 525), Enterobacter aerogenes (MJMC 534-A), Klebsiella pneumoniae (MJH 513) and Pseudomonas aeruginosa (MJH 540). The identification of the strains was performed by morphological and biochemical assays (morphological identification of colonies, Gram staining, standard biochemical identification methodologies, and MicroScan WalkAway identification panels), followed by Kirby–Bauer antibiotic sensitivity assays using different antibiotics. Once identified, the strains were stored at −70 °C in aliquots of brain heart infusion medium with 15% (v/v) glycerol. The reference strains Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were obtained from the American Type Culture Collection.
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MICs of the mushroom extracts were determined by the microtiter broth dilution method as previously reported by Taofiq et al. [22], with slight modifications (Figure 1). Stock solutions in 50% dimethyl sulfoxide (DMSO) of each mushroom extract were prepared to a final concentration of 220 mg/mL. The serial dilutions from the stock solution ranged from 20 mg/mL to 0.156 mg/mL using Mueller–Hinton broth (MHB; Oxoid, Basingstoke, UK) medium in sterile 96-well microplates. Afterwards, the bacterial suspensions (approximately 1.5 × 108 CFU/mL) were prepared from a 24 h culture plate. Then, 100 μL of the previous inoculum were diluted in 9.9 mL of MHB medium and 10 μL of the suspension were inoculated into each well. A negative (prepared with MHB 5% DMSO) and a positive (gentamicin) control were included in the assay. The microtiter plates were incubated at 38 °C for 24 h. After incubation, 40 μL of 0.2 mg/mL 2,3,5-triphenyltetrazolium chloride (TTC) solution was added to each well as an indicator of microbial growth. The plates were incubated at 38 °C for 2 h and the MIC values were visually determined. In the presence of viable microorganisms, the colorless TTC turn to red (formation of formazan). Therefore, the MIC was defined as the lowest concentration of the extract that prevented the color change (colorless to red), showing complete bacterial growth inhibition [23]. The determination of MBC was assessed as previously described by Garcia et al. [24] (Figure 1). Briefly, the content of the wells with no color change determined from the previous assay was plated on Mueller–Hinton agar (MHA; Oxoid, Basingstoke, UK) medium and the plates were incubated at 38 °C for 24 h. The MBC values were visually determined and corresponded to the lowest concentration that yielded no growth after this subculturing.
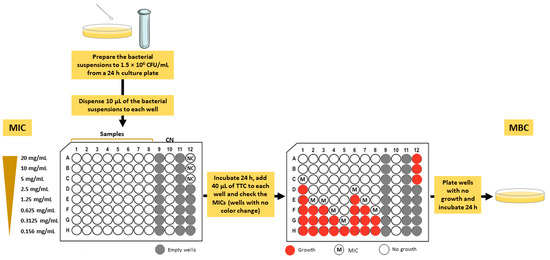
Figure 1.
Experimental design for the determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). CN, gentamicin; NC, negative control (MHB 5% DMSO); TTC, 2,3,5-triphenyltetrazolium chloride.
2.7. Determination of Anti-Hyaluronidase Activity
The hyaluronidase inhibitory activity was evaluated by a turbidimetric method adapted to the microscale as previously described [25], with some modifications. First, 20 µL of the samples (0.1, 1 or 10 mg/mL) was mixed with 20 µL of enzyme diluent (20 mM sodium phosphate with 77 mM NaCl and 0.1 mg/mL of albumin (Sigma Aldrich; pH 7; 37 °C)) and 20 µL of hyaluronidase (Sigma Aldrich, Type I-S from bovine testes). The reaction mixture was left for 10 min at 37 °C. Then, 20 µL of 0.5 mg/mL hyaluronic acid (Sigma Aldrich) was added. After incubating for 45 min at 37 °C, undigested hyaluronic acid was precipitated with 100 µL of 2 mg/mL acid albumin solution. After 10 min at room temperature, the turbidance of the resulting mixture was read at 600 nm using a microplate reader (Multiskan GO 1510, Thermo Fisher Scientific, Vantaa, Finland). For the assay, 4 blanks (B1 to B4) were prepared according to Paczkowska-Walendowska et al. [26]. An additional control was performed (20 µL of distilled water + 20 µL of enzyme diluent + 20 µL of enzyme diluent + 20 µL of hyaluronic acid solution + 100 µL of acid albumin solution). The percentage of hyaluronidase inhibition was determined using Equation (2):
where
AS: absorbance of the sample;
AB1: absorbance of blank 1 (20 µL distilled water + 20 µL enzyme diluent + 20 µL enzyme diluent + 20 µL 300 mM sodium phosphate buffer + 100 µL acid albumin solution);
AB2: absorbance of blank 2 (20 µL distilled water + 20 µL enzyme diluent + 20 µL hyaluronidase enzyme solution + 20 µL hyaluronic acid + 100 µL acid albumin solution);
AB3: absorbance of blank 3 (20 µL sample + 20 µL enzyme diluent + 20 µL hyaluronidase enzyme solution + 20 µL 300 mM sodium phosphate buffer + 100 µL acid albumin solution);
AB4: absorbance of blank 4 (20 µL sample + 20 µL enzyme diluent + 20 µL enzyme diluent + 20 µL hyaluronic acid solution + 100 µL acid albumin solution).
Three independent experiments repeated in triplicate were performed.
2.8. Determination of Anti-Tyrosinase Activity
The inhibitory activity of the mushroom extracts against tyrosinase was performed using a spectrophotometric assay according to No et al. [27], with slight modifications. L-tyrosine (Sigma Aldrich) was used as substrate for the tyrosinase enzyme. Each sample or control was accompanied by a blank containing all the reaction mixture components except the tyrosinase enzyme, which was replaced by phosphate buffer (50 mM, pH 6.5). For the assay, 50% DMSO was used as a negative control and 1 mg/mL kojic acid (Sigma Aldrich) (dissolved in distilled water) was used as a positive control. Briefly, three concentrations of the mushroom extracts (0.1, 1 and 10 mg/mL) were prepared by dissolution in 50% DMSO. Then, 40 µL of mushroom tyrosinase enzyme (Sigma Aldrich, 1000 U/mL) or phosphate buffer, 80 µL of phosphate buffer, and 40 µL of the extracts or controls were added to the microplate. After incubating at 30 ± 1 °C for 10 min, 40 µL of 0.2 mg/mL L-tyrosine solution was added. The reaction was incubated in the oven at 30 ± 1 °C for 60 min and the absorbance of the resulting mixture was read at 475 nm in a microplate reader (Multiskan GO 1510, Thermo Fisher Scientific, Vantaa, Finland). The percentage of tyrosinase inhibition was calculated as follows:
where
C1: absorbance of control with enzyme;
C2: absorbance of control without enzyme;
S1: absorbance of sample with enzyme;
S2: absorbance of sample without enzyme.
Three independent experiments repeated in triplicate were performed.
2.9. Preparation of Cosmetic Creams
The hydroethanolic extracts that showed better cosmeceutical properties were incorporated in a commercial cream made with natural ingredients (Kit Crema Facial Antiedad, purchased from Gran Velada, Zaragoza, Spain). The cream without extract (0%) was used as control (C). Accordingly, the selected hydroethanolic extracts were added to the cream individually or in combination (two different extracts) at a percentage of 0.2% and 0.1%, respectively.
2.10. Stability Studies
To evaluate the stability of the prepared creams, different batches were prepared for the tests described below.
2.10.1. Centrifuging Test
The physical stability of the creams was assessed as previously described [28]. Briefly, the creams were subjected to centrifugation with a Hettich Benchtop centrifuge UNIVERSAL 320 (Tuttlingen, Germany), at 3000 rpm for 30 min. The stability was visually evaluated before and after the thermal tests described below, and the occurrence of phase separation and color changes was recorded.
2.10.2. Thermal Test
An accelerated thermal stability test was performed using heating and cooling cycles according to a method described by Salem and co-workers [29], with slight modifications. The creams were stored at 4 °C for 24 h and then placed at 40 °C for another 24 h (1 cycle) for 4 cycles (8 consecutive days). For the storage test, the creams were stored at 4 °C, 25 °C and 40 °C for 30 days. Prior to evaluation, all the creams were allowed to reach room temperature.
2.10.3. pH Test
The pH values of the samples previously diluted in distilled water were recorded at room temperature using a pH meter (VWR pHenomenal™ MU 6100 L, Darmstadt, Germany). The measurements were taken before and after the thermal tests.
2.11. Phenolic Contents and Antioxidant Activity of the Cosmetic Creams
The cream samples were prepared as described elsewhere [30], with some modifications. Briefly, 1 g of cream was diluted in 10 mL of ethanol 40% (v/v). The samples were mixed, centrifuged at 4500 rpm for 30 min and then filtered. The measurements of TPC and DPPH free radical scavenging activity of the creams were determined as described, respectively, in Section 2.3.1 and Section 2.4.1. above. The TPC was shown as milligrams of GA per 1 g of cream and the antioxidant activity was shown as µM of Trolox per 1 g of cream. The measurements were taken in triplicate, 24 h after the preparation of the cream.
2.12. Statistical Analysis
All the measurements were conducted in triplicate. Statistical analyses were performed using GraphPad Prism software (San Diego, CA, USA) for Windows (version 7). The results of the samples are presented as mean ± standard deviation (SD) (n = 3). Differences between samples were evaluated with the non-parametric Mann–Whitney U test considering a significance level of p < 0.05.
3. Results and Discussion
3.1. Phenolic Composition and Antioxidant Activity
The phenolic composition and the antioxidant activity of the hydroethanolic extracts from the studied wild mushrooms are shown in Table 1 and Table 2, respectively. The total phenols of the mushroom extracts ranged from 7.87 ± 0.51 to 84.30 ± 5.27 mg GA/g dw, the ortho-diphenols ranged from 4.97 ± 0.53 to 190.60 ± 2.08 mg CA/g dw and the flavonoids ranged from 2.37 ± 0.15 to 96.03 ± 4.69 mg Catechin/g dw. In the ABTS, DPPH and FRAP assays, the values ranged from 73.22 ± 47.49 to 929.70 ± 88.54 µM Trolox/g dw, from 40.22 ± 3.27 to 1291.00 ± 240.00 µM Trolox/g dw and from 55.00 ± 2.87 to 1292.00 ± 84.03 µM Trolox/g dw, respectively.

Table 1.
Phenolic composition of hydroethanolic extracts from wild mushrooms.

Table 2.
Antioxidant activity of hydroethanolic extracts from wild mushrooms.
Both in phenolic composition and in antioxidant activity, for all evaluated parameters, the I. hispidus extract always showed the highest values, being significantly different (p < 0.05) from the other mushroom species under analysis, except in the DPPH assay. In the latter, the values are similar to those obtained for the extract of P. tinctorius and significantly different from all the other studied species. In a recent study from our group [12], we demonstrated that the ethanolic extract of I. hispidus presents high contents of hispidin, a yellow polyphenol pigment with the capacity to neutralize free radicals [31,32]. Thus, the high levels of phenolic compounds present in the hydroethanolic extract of I. hispidus and its strong antioxidant and reducing power probably result mainly from the presence of hispidin. Shomali and co-workers [33] investigated the polyphenolic contents and biological activities of four wild mushrooms and found that the ethanolic extracts of I. hispidus demonstrated the highest total phenolic contents and total flavonoid contents, as well as the highest DPPH radical scavenging activity compared to the other mushroom species. In general, the P. tinctorius extract was second on the list with the highest values in phenolic composition and antioxidant activity. This mushroom has been mainly used on forestry inoculation programs and in commercial ectomycorrhizas inoculum production [34], since it can establish associations with several hosts, including important genera of the temperate forests [35]. In a recent study, Pringle and co-workers [14] reported a great antioxidant potential of ethanolic extracts from P. tinctorius compared with other mushroom species. Accordingly, our results also suggest that this mushroom could be a source of natural antioxidants and used in other functional areas with purposes different from the current ones. Regarding the other mushroom species, these present values are considerably lower than those observed for the extracts of I. hispidus and P. tinctorius. In general, the values obtained for phenolic composition and antioxidant activity were higher in mushrooms belonging to the Suillellus genus, followed by the mushrooms B. regius and L. fragrans, and finally by the mushrooms G. lucidum and X. subtomentosus, which globally presented the lowest values.
Our results are in accordance with other studies [36,37,38] that also observed the presence of phenolic compounds with antioxidant activity in alcoholic extracts from wild mushrooms. These bioactive compounds are beneficial to human health and could have a great potential in medical, food and cosmetic applications.
3.2. Phenolic Profile
The phenolic compounds identified by HPLC-DAD in the eight mushroom species of the study and their respective quantification are depicted in Table 3. In general, among the phenolic compounds identified, gallic acid and p-hydroxybenzoic acid were present in all mushroom species in the ranges of 2.49 ± 0.47 to 34.96 ± 7.38 µg/100 g dw and 0.91 ± 0.06 to 41.03 ± 8.49 µg/100 g dw, respectively, except in P. tinctorius wherein the latter was not detected. Moreover, the presence of a gallic acid isomer was also verified in six out of eight mushroom species under analysis. As reported in a recent review [2], and in accordance with our results, p-hydroxybenzoic and gallic acids are two of the most common phenolic acids found in mushroom extracts. Palacios et al. [39] identified and quantified phenolic compounds in eight edible mushrooms and reported gallic acid as the second main component of the phenolic acids in the studied mushrooms. Contrary to our findings, the same authors showed the presence of high levels of homogentisic acid in all samples. This phenolic compound was only identified in L. fragrans (57.58 ± 14.96 µg/100 g dw) and some of its derivatives were found in S. luridus and S. mendax. Caffeic acid and ellagic acid, more often the former, were previously found in mushrooms [40]. We identified low quantities of caffeic acid in I. hispidus (3.06 ± 0.26 µg/100 g dw) and ellagic acid in P. tinctorius (8.64 ± 1.65 µg/100 g dw). Among the catechin group, we described the presence of epigallocatechin gallate in P. tinctorius (89.93 ± 16.26 µg/100 g dw) and X. subtomentosus (13.61 ± 1.34 µg/100 g dw), and reduced levels of catechin gallate in L. fragrans (6.03 ± 3.91 µg/100 g dw). In another study [36], other members belonging to this group, such as catechin and epicatechin, were described in different wild mushroom species. In our analysis we detected the presence of flavonols, such as isorhamnetin (I. hispidus and P. tinctorius), kaempferol-7-O-glucoside (P. tinctorius), myricetin (I. hispidus and S. luridus), morin (B. regius, L. fragrans, S. luridus and S. mendax) and rutin (B. regius and S. mendax), and these compounds were also previously described in mushrooms [12,36,40,41]. Still in the flavonoid category, we identified the presence of small amounts of diosmetin (Suillellus spp.), glycitin (I. hispidus), luteolin-7-O-glucoside (I. hispidus and L. fragrans) and luteolin-4′-O-glucoside (I. hispidus), and naringenin (G. lucidum and X. subtomentosus).

Table 3.
Phenolic compounds identified and quantified (µg/100 g dw) from wild mushrooms.
From our analysis two phenolic compounds stand out, namely hispidin (along with hispidin-like compounds) and norbadione A, identified in I. hispidus at a concentration of 482.10 ± 26.67 µg/100 g dw and in P. tinctorius at 351.30 ± 24.60 µg/100 g dw, respectively. Hispidin is a yellow pigment that, as the name implies, was identified and isolated for the first time in I. hispidus [42]. On the other hand, norbadione A is a dark brown dye and it was previously reported as the major pulvinic acid derivative produced by P. tinctorius [43]. The biological effects of both pigments have been demonstrated in previous studies [31,32,42,44,45,46]. For instance, hispidin displays anti-cancer, anti-platelet, antioxidant, anti-diabetic, anti-inflammatory and antiviral activities, while norbadione A shows anti-radiation and antioxidant effects. Accordingly, it is possible that the high values obtained for both total phenols and antioxidant activity in the mushrooms I. hispidus and P. tinctorius result from the presence of these two compounds.
3.3. Antibacterial Activity
The ESKAPE pathogens, most of them multidrug-resistant, contribute to a high number of nosocomial infections and comprise a list of six bacteria species, namely Acinetobacter baumannii, Enterobacter spp., Enterococcus faecium, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa [47].
In this study, we determined the effect of hydroethanolic extracts obtained from wild mushrooms against ESKAPE isolates from clinical wound infections. The MIC and MBC of each mushroom extract against Gram-positive and Gram-negative bacterial isolates are shown in Table 4 and Table 5, respectively. It can be observed that all the hydroethanolic extracts under analysis had an antibacterial effect against the isolates tested, except X. subtomentosus which failed to inhibit one of the MR S. aureus (MJMC 565-A) tested and E. aerogenes and I. hispidus that had no effect on P. aeruginosa. The MIC values ranged from 20 mg/mL to <0.156 mg/mL for Gram-positive isolates and from 20 mg/mL to 0.625 mg/mL for Gram-negative strains. The first global observation is that Gram-positive bacteria were more susceptible to the extracts than Gram-negative and this is in accordance with data available from the literature [48]. In general, for both Gram-positive and Gram-negative isolates, the extracts of I. hispidus, L. fragrans and P. tinctorius stood out from the others, with lower MIC and MBC values, which indicate, respectively, a greater antibacterial and bactericidal effect. Among these species, I. hispidus is the most studied in terms of antibacterial activity. Angelini et al. [49] assessed the antimicrobial activity of methanolic extracts from I. hispidus against different bacterial strains and found that I. hispidus was effective against S. aureus, E. coli and P. aeruginosa. In our study, I. hispidus had no effect against P. aeruginosa, which could be explained by the fact that clinical isolates are more resistant than ATCC strains, as described in other studies [50,51,52]. In another investigation, Pala and co-workers [53] also demonstrated the antibacterial activity of I. hispidus extracts against laboratory strains of S. aureus, E. coli, K. pneumoniae and P. aeruginosa, with MIC values ranging from 3.2 mg/mL to 6.4 mg/mL. To our knowledge, there are no studies in the literature addressing the antibacterial effect of the wild mushrooms L. fragrans and P. tinctorius, which, according to our results, proved to be quite effective in inhibiting and killing the multidrug-resistant bacterial strains in this study.

Table 4.
Minimum inhibitory concentration (MIC; mg/mL) and minimum bactericidal concentration (MBC; mg/mL) of hydroethanolic extracts from wild mushrooms against Gram-positive bacterial isolates.

Table 5.
Minimum inhibitory concentration (MIC; mg/mL) and minimum bactericidal concentration (MBC; mg/mL) of hydroethanolic extracts from wild mushrooms against Gram-negative bacterial isolates.
The role of phenolic compounds as natural antibacterial agents is well described in the literature [54,55,56]. Moreover, plants rich in these compounds could be of interest against antibiotic resistance since their mechanism of action is different from that of conventional antibiotics [57]. In our study, the extracts showing the best antibacterial properties also present increased levels of phenolic compounds. Therefore, the bioactive compounds derived from these extracts could be considered potential candidates in novel therapeutic approaches against antibiotic resistance.
3.4. Cosmeceutical Properties
The cosmetic industry is in a constant search for bioactive ingredients to design novel cosmeceuticals with lower toxicity, providing an additional health-related function or benefit [58,59]. Cosmeceuticals contain bioingredients that confer different effects, such as antiaging, anti-inflammatory, antioxidant and photoprotective [58]. Wild mushrooms are resources of bioactive molecules, some of those acting as enzyme-inhibitory compounds [60,61]. In this study, we assessed the effect of the hydroethanolic extracts from wild mushrooms on two skin-related enzymes, hyaluronidase and tyrosinase. During aging, as hyaluronidase activity increases, hyaluronic acid levels decrease, and the moisture and tension of the skin are reduced. Therefore, natural inhibitors of this enzyme could be useful as anti-wrinkle and anti-aging agents. On the other hand, tyrosinase is responsible for the melanin synthesis process and, even though melanin has an important role in protecting the skin from UV rays, its overproduction is associated with hyperpigmentation-related disorders. During aging, an increase in tyrosinase activity and melanin production is responsible for typical age spots on the skin. Thus, natural tyrosinase inhibitors could be used as skin-whitening agents in skin-aging disorders [62,63].
The anti-hyaluronidase and anti-tyrosinase activities of different concentrations of the extracts are shown as percentage of inhibition in Table 6 and Table 7, respectively. As shown in Table 6, none of the mushroom extracts inhibit the activity of hyaluronidase at the minimum concentration tested (0.1 mg de/mL). Of the eight extracts under study, only P. tinctorius was able to inhibit hyaluronidase activity by 95.2 ± 3.8% at the concentration of 1.0 mg de/mL, being significantly different from the other extracts. At 10 mg de/mL, the levels were above the limit of detection, which did not allow a value to be determined. At the maximum concentration (10 mg de/mL), the extracts of G. lucidum, I. hispidus, S. luridus, S. mendax and X. subtomentosus had an inhibitory effect on hyaluronidase with a percentage of inhibition of 71.3 ± 7.5%, 91.1 ± 11.2%, 76.8 ± 8.2%, 77.9 ± 8.6% and 54.6 ± 6.5%, respectively. Therefore, for the first time, it was demonstrated that hydroethanolic extracts of wild mushrooms could be attractive natural anti-aging agents, since they prevent hyaluronic acid degradation by inhibiting the hyaluronidase enzyme, thus retarding the skin aging process.

Table 6.
Anti-hyaluronidase activity of hydroethanolic extracts from wild mushrooms (0.1–10.0 mg/mL) expressed in percentage (%) of inhibition.

Table 7.
Anti-tyrosinase activity of hydroethanolic extracts from wild mushrooms (0.1–10.0 mg/mL) expressed in percentage (%) of inhibition.
Regarding inhibition of the tyrosinase enzyme (Table 7), it was observed that none of the extracts was able to inhibit its activity at all the concentrations tested. However, for the mushrooms I. hispidus and P. tinctorius, at 10 mg de/mL, no value was measured, as the levels were above the limit of detection and, therefore, no conclusion can be made regarding the effect of these extracts on the tyrosinase enzyme. According to some studies [64,65,66], bioactive compounds from G. lucidum exert an inhibitory effect on tyrosinase and, for that reason, this mushroom has been used in skin-whitening products. In our study, the G. lucidum extract only inhibited tyrosinase by 40.4 ± 2.2% at the maximum concentration tested and, in this condition, its activity was similar to that exerted by S. luridus, which inhibited tyrosinase enzyme by 42.0 ± 4.4%. The differences between the literature and the obtained results may be due to several factors, which include different growing conditions and locations, ripening stages, storage and processing conditions, and extraction methodologies, as well as UV radiation exposure and extreme temperatures, among others [67].
3.5. Incorporation of Extracts from I. hispidus and P. tinctorius in a Cosmetic Cream
Several species of macrofungi are currently used in different formulations of cosmeceuticals as a source of natural bioactive compounds to confer on the products antioxidant, antiaging, anti-wrinkle, skin whitening and moisturizing effects [58,59]. Considering the previous described results, in general, the extracts of I. hispidus and P. tinctorius stood out from the others in all the evaluated parameters, being good cosmeceutical candidates. Accordingly, cosmetic creams were made by incorporating extracts from I. hispidus (IH) and P. tinctorius (PT), and the combination of both extracts (IH + PT) was also evaluated. An extract-free cream (C) was also prepared and serves as a control.
3.5.1. Cream Stability Studies
The quality and safety of the creams were determined by stability studies and the results are shown in Table 8. The physicochemical properties of the creams were visually monitored after subjecting them to thermal stress and centrifugation. Compared to the initial formulations, no changes were observed after the thermal stability tests regarding the color, homogeneity, feel on skin, phase separation or pH. After the heating and cooling cycles, and 30 days at 40 °C, the pH of the creams slightly increased but remained in the range considered safe for the skin [68]. Srisuksomwong et al. [69] also reported a similar increase in pH after subjecting the formulations to higher temperatures. Accordingly, our results confirmed that the incorporation of the extracts from I. hispidus and P. tinctorius did not altered the stability and properties of the final formulation.

Table 8.
Results of the stability tests of the creams without (C) and with 0.2% I. hispidus (IH), 0.2% P. tinctorius (PT), and the combination of 0.1% I. hispidus and 0.1% P. tinctorius (IH + PT) extracts under different conditions.
3.5.2. Phenolic Contents and In Vitro Antioxidant Capacity
The search by consumers of natural antioxidants in skincare products is increasing and that may restrict the use of synthetic substances in the cosmetic industry [70,71]. Accordingly, the effect of the incorporation in the cream of I. hispidus and P. tinctorius extracts on the phenolic content and antioxidant activity was assessed and compared with the cream used as control, in which no extract was added. The results are given in Table 9 and show that IH and IH + PT present total phenol values significantly higher than C and PT, with PT displaying higher values than C. Regarding the antioxidant activity measured through the DPPH assay, IH stood out from the other formulations, followed by IH + PT and, finally, no differences were observed between C and PT. Therefore, the incorporation of I. hispidus extract (individually or combined with P. tinctorius extract) significantly increased the antioxidant power of the cream, improving the final formulation.

Table 9.
Total phenols and antioxidant activity (measured by DPPH) of the studied creams without (C) and with 0.2% I. hispidus (IH), 0.2% P. tinctorius (PT), and the combination of 0.1% I. hispidus and 0.1% P. tinctorius (IH + PT) extracts.
4. Conclusions
Wild mushrooms are good candidates as new sources of natural compounds for application in the cosmetic industry. In the present study, I. hispidus and P. tinctorius were demonstrated to be a good example of this potential. Their beneficial properties are probably due to their phenolic composition, especially their specific pigments. The mushroom L. fragrans also showed to be suitable for microbiology applications since its antibiotic activity obtained the best results among the species studied. Some of the mushrooms evaluated in this work are poorly investigated. Therefore, the results presented in this investigation are important findings for the applicability of wild mushrooms in several functional areas and enrich the current knowledge in the field of macrofungi.
Author Contributions
Conceptualization, G.M., L.M.-C., M.J.S. and T.M.; methodology, A.A., G.M., L.M.-C., M.J.S. and T.M.; formal analysis, A.A., L.M.-C. and T.M.; investigation, G.M., L.M.-C. and T.M.; resources, A.A., G.M. and M.J.S.; data curation, L.M.-C. and T.M.; writing—original draft preparation, L.M.-C. and T.M.; writing—review and editing, A.A., G.M., L.M.-C., M.J.S. and T.M.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Fungi4Health project, no. NORTE-01-0247-FEDER-070171, financed by the European Regional Development Fund (ERDF) through NORTE 2020 (North Regional Operational Program 2014/2020).
Data Availability Statement
Dataset will be provided upon reasonable request.
Acknowledgments
This work was supported by National Funds by FCT—Portuguese Foundation for Science and Technology, under the project UIDB/04033/2020.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 253–271. [Google Scholar]
- Leal, A.R.; Barros, L.; Barreira, J.C.M.; Sousa, M.J.; Martins, A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Portuguese wild mushrooms at the “pharma–nutrition” interface: Nutritional characterization and antioxidant properties. Food Res. Int. 2013, 50, 1–9. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Walton, E.L. Buried treasure: Unlocking the secrets of medicinal mushrooms. Biomed. J. 2014, 37, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Zhao, W.; Zhong, J.J. Biotechnological production and application of ganoderic acids. Appl. Microbiol. Biotechnol. 2010, 87, 457–466. [Google Scholar] [CrossRef]
- Ryvarden, L.; Gilbertson, R.L. European Polypores, Part 1; Fungiflora: Oslo, Norway, 1993; Volume 6. [Google Scholar]
- Wang, Z.-X.; Feng, X.-l.; Liu, C.; Gao, J.-m.; Qi, J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes. Inonotus Hispidus. Antibiot. 2022, 11, 1097. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Qu, Y.; Li, L.; Li, Y.; Wang, D. Anti-Colorectal Cancer Effects of Inonotus hispidus (Bull.: Fr.) P. Karst. Spore Powder through Regulation of Gut Microbiota-Mediated JAK/STAT Signaling. Nutrients 2022, 14, 3299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, J.; Liu, Z.; Li, Z.; Teng, L.; Wang, D. Inonotus hispidus Protects against Hyperlipidemia by Inhibiting Oxidative Stress and Inflammation through Nrf2/NF-κB Signaling in High Fat Diet Fed Mice. Nutrients 2022, 14, 3477. [Google Scholar] [CrossRef] [PubMed]
- Machado-Carvalho, L.; Martins, T.; Aires, A.; Marques, G. Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites 2023, 13, 524. [Google Scholar] [CrossRef]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Pringle, N.A.; van de Venter, M.; Boukes, G.J.; Koekemoer, T.C. Therapeutic potential of selected South African macrofungi in diabetic wound healing: An in vitro evaluation. S. Afr. J. Bot. 2021, 138, 337–347. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, D.; You, Y.; Zeng, S.; Li, Y.; Tang, Q.; Han, G.; Liu, A.; Feng, C.; Li, C.; et al. Nutritional composition of boletus mushrooms from Southwest China and their antihyperglycemic and antioxidant activities. Food Chem. 2016, 211, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Dominguez-Perles, R.; Rodrigues, M.; Barros, A.I.R.N.A. Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crop. Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.A.S.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Teixeira-Guedes, C.I.; Oppolzer, D.; Barros, A.I.; Pereira-Wilson, C. Impact of cooking method on phenolic composition and antioxidant potential of four varieties of Phaseolus vulgaris L. and Glycine max L. LWT 2019, 103, 238–246. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R. Kiwi fruit residues from industry processing: Study for a maximum phenolic recovery yield. J. Food Sci. Technol. 2020, 57, 4265–4276. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Barreiro, M.F.; González-Paramás, A.M.; Ferreira, I.C. Development of Mushroom-Based Cosmeceutical Formulations with Anti-Inflammatory, Anti-Tyrosinase, Antioxidant, and Antibacterial Properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef]
- Veiga, A.; Toledo, M.; Rossa, L.S.; Mengarda, M.; Stofella, N.C.F.; Oliveira, L.J.; Gonçalves, A.G.; Murakami, F.S. Colorimetric microdilution assay: Validation of a standard method for determination of MIC, IC(50%), and IC(90%) of antimicrobial compounds. J. Microbiol. Methods 2019, 162, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Rodrigues, F.; Castro, F.; Aires, A.; Marques, G.; Saavedra, M.J. Antimicrobial, Antibiofilm, and Antioxidant Properties of Boletus edulis and Neoboletus luridiformis Against Multidrug-Resistant ESKAPE Pathogens. Front. Nutr. 2022, 8, 773346. [Google Scholar] [CrossRef] [PubMed]
- Łyko, L.; Olech, M.; Nowak, R. LC-ESI-MS/MS Characterization of Concentrated Polyphenolic Fractions from Rhododendron luteum and Their Anti-Inflammatory and Antioxidant Activities. Molecules 2022, 27, 827. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Cielecka-Piontek, J. Chitosan as a Functional Carrier for the Local Delivery Anti-Inflammatory Systems Containing Scutellariae baicalensis radix Extract. Pharmaceutics 2022, 14, 2148. [Google Scholar] [CrossRef] [PubMed]
- No, J.K.; Soung, D.Y.; Kim, Y.J.; Shim, K.H.; Jun, Y.S.; Rhee, S.H.; Yokozawa, T.; Chung, H.Y. Inhibition of tyrosinase by green tea components. Life Sci. 1999, 65, PL241–PL246. [Google Scholar] [CrossRef]
- Rodrigues Ueoka, A.; Pedriali Moraes, C.A. Development and Stability Evaluation of Liquid Crystal-Based Formulations Containing Glycolic Plant Extracts and Nano-Actives. Cosmetics 2018, 5, 25. [Google Scholar] [CrossRef]
- Salem, Y.; Rajha, H.N.; Franjieh, D.; Hoss, I.; Manca, M.L.; Manconi, M.; Castangia, I.; Perra, M.; Maroun, R.G.; Louka, N. Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams. Antioxidants 2022, 11, 1348. [Google Scholar] [CrossRef]
- Adeel, S.; Habiba, M.; Kiran, S.; Iqbal, S.; Abrar, S.; Hassan, C.M. Utilization of Colored Extracts for the Formulation of Ecological Friendly Plant-Based Green Products. Sustainability 2022, 14, 11758. [Google Scholar] [CrossRef]
- Zan, L.F.; Qin, J.C.; Zhang, Y.M.; Yao, Y.H.; Bao, H.Y.; Li, X. Antioxidant hispidin derivatives from medicinal mushroom Inonotus hispidus. Chem. Pharm. Bull. 2011, 59, 770–772. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, H.; Lu, T.; Zhao, Y.; Zheng, W. Ultraviolet radiation promotes the production of hispidin polyphenols by medicinal mushroom Inonotus obliquus. Fungal Biol. 2022, 126, 775–785. [Google Scholar] [CrossRef]
- Shomali, N.; Onar, O.; Alkan, T.; Demirtaş, N.; Akata, I.; Yildirim, Ö. Investigation of the Polyphenol Composition, Biological Activities, and Detoxification Properties of Some Medicinal Mushrooms from Turkey. Turk. J. Pharm. Sci. 2019, 16, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Sebastiana, M.; Pereira, V.T.; Alcântara, A.; Pais, M.S.; Silva, A.B. Ectomycorrhizal inoculation with Pisolithus tinctorius increases the performance of Quercus suber L. (cork oak) nursery and field seedlings. New For. 2013, 44, 937–949. [Google Scholar] [CrossRef]
- Cairney, J.W.G.; Chambers, S.M. Interactions between Pisolithus tinctorius and its hosts: A review of current knowledge. Mycorrhiza 1997, 7, 117–131. [Google Scholar] [CrossRef]
- Butkhup, L.; Samappito, W.; Jorjong, S. Evaluation of bioactivities and phenolic contents of wild edible mushrooms from northeastern Thailand. Food Sci. Biotechnol. 2018, 27, 193–202. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT 2014, 59, 689–694. [Google Scholar] [CrossRef]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Şahin, H.; Malkoç, M. Wild Edible Mushrooms as a Natural Source of Phenolics and Antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Barros, L.; Abreu, R.M. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef]
- Yaltirak, T.; Aslim, B.; Ozturk, S.; Alli, H. Antimicrobial and antioxidant activities of Russula delica Fr. Food Chem. Toxicol. 2009, 47, 2052–2056. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Gill, M.; Lally, D.A. A naphthalenoid pulvinic acid derivative from the fungus Pisolithus tinctorius. Phytochemistry 1985, 24, 1351–1354. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, I.K.; Seok, S.J.; Lee, H.J.; Kim, Y.H.; Yun, B.S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Martinčič, R.; Mravljak, J.; Švajger, U.; Perdih, A.; Anderluh, M.; Novič, M. In Silico Discovery of Novel Potent Antioxidants on the Basis of Pulvinic Acid and Coumarine Derivatives and Their Experimental Evaluation. PLoS ONE 2015, 10, e0140602. [Google Scholar] [CrossRef] [PubMed]
- Meunier, S.; Desage-El Murr, M.; Nowaczyk, S.; Le Gall, T.; Pin, S.; Renault, J.P.; Boquet, D.; Créminon, C.; Saint-Aman, E.; Valleix, A.; et al. A powerful antiradiation compound revealed by a new high-throughput screening method. Chembiochem 2004, 5, 832–840. [Google Scholar] [CrossRef]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic resistance in hospital-acquired ESKAPE-E infections in low- and lower-middle-income countries: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Angelini, P.; Girometta, C.; Tirillini, B.; Moretti, S.; Covino, S.; Cipriani, M.; D’Ellena, E.; Angeles, G.; Federici, E.; Savino, E.; et al. A comparative study of the antimicrobial and antioxidant activities of Inonotus hispidus fruit and their mycelia extracts. Int. J. Food Prop. 2019, 22, 768–783. [Google Scholar] [CrossRef]
- Fux, C.A.; Shirtliff, M.; Stoodley, P.; Costerton, J.W. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 2005, 13, 58–63. [Google Scholar] [CrossRef]
- Hume, E.B.; Flanagan, J.; Masoudi, S.; Zhu, H.; Cole, N.; Willcox, M.D. Soft contact lens disinfection solution efficacy: Clinical Fusarium isolates vs. ATCC 36031. Optom. Vis. Sci. 2009, 86, 415–419. [Google Scholar] [CrossRef]
- Mohammadinia, M.; Rahmani, S.; Eslami, G.; Ghassemi-Broumand, M.; Aghazadh Amiri, M.; Aghaie, G.; Tabatabaee, S.M.; Taheri, S.; Behgozin, A. Contact lens disinfecting solutions antibacterial efficacy: Comparison between clinical isolates and the standard ISO ATCC strains of Pseudomonas aeruginosa and Staphylococcus aureus. Eye 2012, 26, 327–330. [Google Scholar] [CrossRef]
- Pala, S.A.; Wani, A.H.; Ganai, B.A. Antimicrobial potential of some wild Macromycetes collected from Kashmir Himalayas. Plant Sci. Today 2019, 6, 137–146. [Google Scholar] [CrossRef]
- Ecevit, K.; Barros, A.A.; Silva, J.M.; Reis, R.L. Preventing Microbial Infections with Natural Phenolic Compounds. Future Pharmacol. 2022, 2, 460–498. [Google Scholar] [CrossRef]
- Pancu, D.F.; Scurtu, A.; Macasoi, I.G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics: Conventional Therapy and Natural Compounds with Antibacterial Activity-A Pharmaco-Toxicological Screening. Antibiotics 2021, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Barkhudaryan, A.; Rapior, S. Medicinal Macrofungi as Cosmeceuticals: A Review. Int. J. Med. Mushrooms 2022, 24, 1–13. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom Cosmetics: The Present and Future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Hyde, K.D.; Bahkali, A.H.; Moslem, M.A. Fungi—An unusual source for cosmetics. Fungal Divers. 2010, 43, 1–9. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, elastase, hyaluronidase, inhibitory and antioxidant activity of Sri Lankan medicinal plants for novel cosmeceuticals. Ind. Crop. Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- Pintus, F.; Floris, S.; Fais, A.; Era, B.; Porcedda, C.; Tuberoso, C.I.G.; Caddeo, C. Euphorbia characias Extract: Inhibition of Skin Aging-Related Enzymes and Nanoformulation. Plants 2022, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.C.; Tsai, M.L.; Chen, C.C.; Chang, S.J.; Tseng, C.H. Effects on tyrosinase activity by the extracts of Ganoderma lucidum and related mushrooms. Mycopathologia 2008, 166, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, H.I.; Kim, J.H.; Kwon, O.C.; Son, E.S.; Lee, C.S.; Park, Y.J. Effects of Ganodermanondiol, a New Melanogenesis Inhibitor from the Medicinal Mushroom Ganoderma lucidum. Int. J. Mol. Sci. 2016, 17, 1798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, Z.; Xu, P.; Wang, Y.; Gu, Z.; Qian, Z.; Shi, G.; Zhang, K. Methyl lucidenate F isolated from the ethanol-soluble-acidic components of Ganoderma lucidum is a novel tyrosinase inhibitor. Biotechnol. Bioprocess. Eng. 2011, 16, 457–461. [Google Scholar] [CrossRef]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. The Relation of pH and Skin Cleansing. Curr. Probl. Dermatol. 2018, 54, 132–142. [Google Scholar] [CrossRef]
- Srisuksomwong, P.; Kaenhin, L.; Mungmai, L. Collagenase and Tyrosinase Inhibitory Activities and Stability of Facial Cream Formulation Containing Cashew Leaf Extract. Cosmetics 2023, 10, 17. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).