Probiotic Lactic Acid Bacteria for Vaginal Application. Optimization of Biomass Production and Freeze-Drying Conditions
Abstract
1. Introduction
2. Material and Methods
2.1. Micro-Organisms and Culture Conditions
2.2. Optimization of Growth Conditions for VLB Biomass Production
2.3. Optimization of Freeze Drying Conditions
2.4. Stability of Freeze-Dried VLB during Storage
2.4.1. Encapsulation and Storage at Different Temperatures
2.4.2. Maintenance of VLB Probiotic Properties
2.5. Statistics
3. Results
3.1. Optimization of Growth Conditions of VLB Probiotics
3.2. Growth Parameters
3.3. Bacterial Biomass Yield
3.4. Optimization of Freeze-Drying Conditions and Effect of Cryoprotectants on VLB
3.5. Stability of Encapsulated VLB during Storage at Different Temperatures
3.5.1. Stability of VLB Stored at 25 °C
3.5.2. Stability of Lyophilized VLB Stored at 4 °C
3.6. Maintenance of Surface Properties after VLB Freeze Drying
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, X.C.; Segata, N.; Huttenhower, C. Biodiversity and functional genomics in the human microbiome. Trends Genet. 2013, 29, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Food and Agricultural Organization of the United Nation and the WHO. Curr. Pharm. Des. 2005, 11, 11–16. [Google Scholar] [CrossRef]
- FAO/WHO. Evaluation of Health and Nutritional Properties of Powder Milk and Live Lactic Acid Bacteria, Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation Report. 2001. Available online: https://scirp.org/reference/referencespapers.aspx?referenceid=1456307 (accessed on 3 March 2021).
- ISAPP; Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics [ISAPP] consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.K.; Shah, N.P. Effect of various encapsulating materials on the stability of probiotic bacteria. J. Food Sci. 2009, 74, 100–107. [Google Scholar] [CrossRef]
- Mastromarino, P.; Vitali, B.; Mosca, L. Bacterial vaginosis: A review on clinical trials with probiotics. New Microbiol. 2013, 36, 229–238. [Google Scholar] [PubMed]
- Maresca, D.; De Filippis, F.; Robertiello, A.; Mauriello, G. Metabolic Profiling and Cold-Starvation Stress Response of Oxygen-Tolerant Lactobacillus gasseri Strains Cultured in Batch Bioreactor. Microorganisms 2019, 7, 200. [Google Scholar] [CrossRef]
- Ren, H.; Zentek, J.; Vahjen, W. Optimization of Production Parameters for Probiotic Lactobacillus Strains as Feed Additive. Molecules 2019, 24, 3286. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn–Hägerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Qureshi, N.; Annous, B.A.; Ezeji, T.C.; Karcher, P.; Maddox, I.S. Biofilm reactors for industrial bioconversion processes: Employing potential of enhanced reaction rates. Microb. Cell Fact. 2005, 4, 24. [Google Scholar] [CrossRef]
- Vinderola, G.; Champagne, C.P.; Desfossès-Foucailt, E. The production of lactic acid bacteria starters and probiotics. An industrial perspective. In Lactic Acid Bacteria. Microbiological and Functional Aspects; Vinderola, G., Ouwehand, A.C., Salminen, S., von Wright, A., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Chapter 20; pp. 317–336. [Google Scholar]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004, 14, 835–847. [Google Scholar] [CrossRef]
- Fonseca, F.; Cenard, S.; Passot, S. Freeze-drying of lactic acid bacteria. In Cryopreservation abd Freeze-Drying Protocols. Methods in Molecular Biology (Methods and protocols); Wolkers, W., Oldemhof, H., Eds.; Springer: New York, NY, USA, 2015; Volume 1257. [Google Scholar]
- Gautier, J.; Passot, S.; Pénicaud, C.; Guillemin, H.; Cenard, S.; Lieben, P.; Fonseca, F. A low membrane lipid phase transition temperature is associated with a high cryotolerance of Lactobacillus delbrueckii subspecies bulgaricus CFL1. J. Dairy Sci. 2013, 96, 5591–5602. [Google Scholar] [CrossRef] [PubMed]
- Montel Mendoza, G.; Pasteris, S.E.; Otero, M.C.; Nader-Macías, M.E.F. Survival and beneficial properties of lactic acid bacteria from raniculture subjected to freeze-drying and storage. J. Appl. Microbiol. 2013, 116, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.P.; Teixeira, P.M.; Kirby, R. Storage of lyophilized cultures of Lactobacillus bulgaricus under different relative humidities and atmospheres. Appl. Microbiol. Biotechnol. 1995, 44, 172–176. [Google Scholar] [CrossRef]
- Martos, G.I.; Minahk, C.J.; Font de Valdez, G.; Morero, R. Effects of protective agents on membrane fluidity of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Lett. Appl. Microbiol. 2007, 45, 282–288. [Google Scholar] [CrossRef]
- Pyar, H.; Peh, K.K. Cost effectiveness of cryoprotective agents and modified De Man Rogosa Sharpe medium on growth of Lactobacillus acidophilus. Pak. J. Biol. Sci. 2014, 17, 462–471. [Google Scholar] [CrossRef]
- Bagad, M.; Pande, R.; Dubey, V.; Ghosh, A.S. Survivability of freeze-dried probiotic Pediococcus pentosaceus strains GS4, GS17 and Lactobacillus gasseri (ATCC 19992) during storage with commonly used pharmaceutical excipients within a period of 120 days. Asian Pac. J. Trop. Biomed. 2017, 7, 921–929. [Google Scholar] [CrossRef]
- Ocaña, V.S.; Bru, E.; De Ruiz Holgado, A.A.; Nader-Macias, M.E. Surface characteristics of lactobacilli isolated from human vagina. J. Gen. Appl. Microbiol. 1999, 45, 203–212. [Google Scholar] [CrossRef]
- Juárez Tomás, M.S.; Zonenschain, D.; Morelli, L.; Nader-Macías, M.E. Characterization of potentially probiotic vaginal lactobacilli isolated from Argentinean women. Br. J. Biomed. Sci. 2005, 62, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Juárez Tomás, M.S.; Saralegui Duhart, C.I.; De Gregorio, P.R.; Vera Pingitore, E.; Nader-Macías, M.E. Urogenital pathogen inhibition and compatibility between vaginal Lactobacillus strains to be considered as probiotic candidates. Eur. J. Obstet. Gynecol. Reprod Biol. 2011, 159, 399–406. [Google Scholar] [CrossRef]
- De Gregorio, P.R.; Juárez Tomás, M.S.; Leccese Terraf, M.C.; Nader-Macías ME, F. In vitro and in vivo effects of beneficial vaginal lactobacilli on pathogens responsible for urogenital tract infections. J. Med. Microbiol. 2014, 63, 685–696. [Google Scholar] [CrossRef]
- Leccese Terraf, M.C.; Juarez Tomás, M.S.; Rault, L.; Le Loir, Y.; Even, S.; Nader-Macías ME, F. Biofilms of vaginal Lactobacillus reuteri CRL 1324 and Lactobacillus rhamnosus CRL 1332: Kinetics of formation and matrix characterization. Arch. Microbiol. 2016, 198, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Leccesse Terraf, M.L.; Mendoza, L.M.; Tomás, M.J.; Silva, C.; Nader-Macías ME, F. Phenotypic surface properties (aggregation, adhesion and biofilm formation) and presence of related genes in beneficial vaginal lactobacilli. J. Appl. Microbiol. 2014, 117, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, V.S.; Nader-Macías, M.E. Vaginal lactobacilli: Self-and co-aggregating ability. Br. J. Biomed. Sci. 2002, 59, 183–190. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, P.R.; Tomás MS, J.; Santos, V.; Nader-Macías ME, F. Beneficial lactobacilli: Effects on the vaginal tract in a murine experimental model. Antonie Van Leeuwenhoek 2012, 102, 569–580. [Google Scholar] [CrossRef]
- Silva, J.A.; Marchesi, A.; Wiese, B.; Nader-Macias, M.E.F. Screening of autochthonous vaginal beneficial lactobacilli strains by their growth at high temperatures for technological applications. Antonie Van Leeuwenhoek 2020, 113, 1393–1409. [Google Scholar] [CrossRef]
- Marchesi, A.; Silva, J.; Wiese, B.; Nader-Macías, M.E.F. Survival of Beneficial Vaginal Lactobacilli (VLB) to Different Gastrointestinal Tract Conditions. Curr. Pharm. Des. 2020, 26, 3608–3618. [Google Scholar] [CrossRef]
- Marchesi, A.; Salva, J.; Ficoseco, C.A.; Wiese, B.; Nader-Macias ME, F. Effect of phytoderivatives on the growth of homologous beneficial vaginal lactobacilli (VLB) strains and their compatibility for the design of phytobiotics for the vaginal tract health. World J. Pharm. Pharm. Sci. 2020, 9, 2278–4357. [Google Scholar]
- De Gregorio, P.R.; Maldonado, N.C.; Pingitore, E.V.; Terraf, M.C.; Tomás, M.S.; de Ruiz, C.S.; Nader, M.F. Intravaginal administration of gelatine capsules containing freeze-dried autochthonous lactobacilli: A double-blind, randomised clinical trial of safety. Benef. Microbes. 2020, 11, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, V.S.; de Ruiz Holgado, A.A.P.; Nader-Macías, M.E. Characterization of a bacteriocin-like substance produced by a vaginal Lactobacillus salivarius strain. Appl. Environ. Microbiol. 1999, 65, 5631–5635. [Google Scholar] [CrossRef]
- Juárez Tomás, M.S.; Wiese, B.; Nader-Macías, M.E. Effects of culture conditions on the growth and auto-aggregation ability of vaginal Lactobacillus johnsonii CRL1294. J. Appl. Microbiol. 2005, 99, 1383–1391. [Google Scholar] [CrossRef]
- Nader-Macías ME, F.; Juárez Tomás, M.S. Profiles and technological requirements of urogenital probiotics. Adv. Drug Deliv. Rev. 2015, 92, 84–104. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, P.R.; Juárez Tomás, M.S.; Nader-Macías ME, F. Immunomodulation of Lactobacillus reuteri CRL1324 on Group B Streptococcus Vaginal Colonization in a Murine Experimental Model. Am. J. Reprod. Immunol. 2016, 75, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Geshnizgani, A.M.; Onderdonk, A.B. Defined medium simulating genital tract secretions for growth of vaginal microflora. J. Clin. Microbiol. 1992, 30, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Aristimuño Ficoseco, C.; Mansilla, F.I.; Vignolo, G.M.; Nader-Macías, M.E.F. Optimization of Probiotic Lactobacilli Production for In-Feed Supplementation to Feedlot Cattle. Appl. Microbiol. 2023, 3, 339–357. [Google Scholar] [CrossRef]
- Śliżewska, K.; Chlebicz-Wójcik, A. Growth kinetics of probiotic Lactobacillus strains in the alternative, cost-efficient semi-solid fermentation medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef]
- Juarez Tomás, M.S.; Bru, E.; Wiese, B.; de Ruiz Holgado, A.A.P.; Nader-Macías, M.E.F. Influence of pH, temperature and culture media on the growth and bacteriocin production by vaginal Lactobacillus salivarius CRL 1328. J. Appl. Microbiol. 2002, 93, 714–724. [Google Scholar] [CrossRef]
- Pancheniak, E.F.R.; Maziero, M.T.; Rodriguez-León, J.A.; Parada, J.L.; Spier, M.R.; Soccol, C.R. Molecular characterisation and biomass and metabolite production of Lactobacillus reuteri LPB P01–001: A potential probiotic. Braz. J. Microbiol. 2012, 43, 135–147. [Google Scholar] [CrossRef]
- Lim, C.M.; Rahim, R.A.; Ho, Y.W.; Arbakariya, B.A. Optimization of Growth Medium for Efficient Cultivation of Lactobacillus salivarius i 24 using Response Surface Method. Malays. J. Microbiol. 2007, 3, 41–47. [Google Scholar] [CrossRef]
- Salih, N.; Hutari, A.; Gaseem, W.; Yusoff, W.M.W. Maximization of growth and storage of locally isolated Lactobacillus salivarius subsp salivarius with high stability and functionality. In Proceedings of Southern Biomedical Engineering 2009, Miami, FL, USA, 15–17 May 2009; Springer: Berlin/Heidelberg, Germany, 2009; pp. 175–178. [Google Scholar]
- Lavari, L.; Ianniello, R.; Pàez, R.; Zotta, T.; Cuatrin, A.; Reihemer, J.; Parente, E.; Vinderola, G. Growth of Lactobacillus rhamnosus and the study of the effect of mildstresses on survival to spray drying. LWT Food Sci. Technol. 2015, 63, 322–330. [Google Scholar] [CrossRef]
- Maresca, D.; Zotta, T.; Mauriello, G. Adaptation to Aerobic Environment of Lactobacillus johnsonii/gasseri Strains. Front. Microbiol. 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, S.; Abd Malek, R.; Ramli, S.; Dailin, D.J.; Gupta, V.J.; Sukmawati, D.; El-Adawi, H.I.; Leng, O.M.; El Enshasy, H. Improvement of biomass production by Lactobacillus reuteri using double-carbon source cultivation strategy. AIP Conf. Proc. 2021, 2331, 050023. [Google Scholar] [CrossRef]
- Zotta, T.; Ricciardi, A.; Ianniello, R.G.; Parente, E.; Reale, A.; Rossi, F.; Iacumin, L.; Comi, G.; Coppola, R. Assessment of Aerobic and Respiratory Growth in the Lactobacillus casei Group. PLoS ONE 2014, 9, e99189. [Google Scholar] [CrossRef]
- Li, B.; Tian, F.; Liu, X.; Zhao, J.; Zhangm, H.; Chen, W. Effects of cryoprotectants on viability of Lactobacillus reuteri CICC6226. Appl. Microbiol. Biotechnol. 2011, 92, 609–616. [Google Scholar] [CrossRef]
- Miao, S.; Mills, S.; Stanton, C.; Fitzgerald, G.F.; Roos, Y.; Ross, R.P. Effect of disaccharides on survival during storage of freeze dried probiotics. Dairy Sci. Technol. 2008, 88, 19–30. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Oliver, A.E.; Tsvetkova, N.; Wolkers, W.; Tablin, F. The Trehalose Myth Revisited: Introduction to a Symposium on Stabilization of Cells in the Dry State. Cryobiology 2001, 43, 89–105. [Google Scholar] [CrossRef]
- Jalali, M.; Abedi, D.; Varshosaz, J.; Najjarzadeh, M.; Mirlohi, M.; Tavakoli, N. Stability evaluation of freeze-dried Lactobacillus paracasei subsp. tolerance and Lactobacillus delbrueckii subsp. bulgaricus in oral capsules. Res. Pharm. Sci. 2012, 7, 31–36. [Google Scholar]
- Jofré, A.; Aymerich, T.; Garriga, M. Impact of different cryoprotectants on the survival of freeze-dried Lactobacillus rhamnosus and Lactobacillus casei/paracasei during long-term storage. Benef. Microbes 2015, 6, 381–386. [Google Scholar] [CrossRef]
- Savedboworn, W.; Teawsomboonkit, K.; Surichay, S.; Riansa-ngawong, W.; Rittisak, S.; Charoen, R.; Phattayakorn, K. Impact of protectants on the storage stability of freeze-dried probiotic Lactobacillus plantarum. Food Sci. Biotechnol. 2019, 28, 795–805. [Google Scholar] [CrossRef]
- Juárez Tomás, M.S.; Bru, E.; Martos, G.; Nader-Macías, M.E.F. Stability of freeze-dried vaginal Lactobacillus strains in the presence of different lyoprotectors. Can. J. Microbiol. 2009, 55, 544–552. [Google Scholar] [CrossRef]


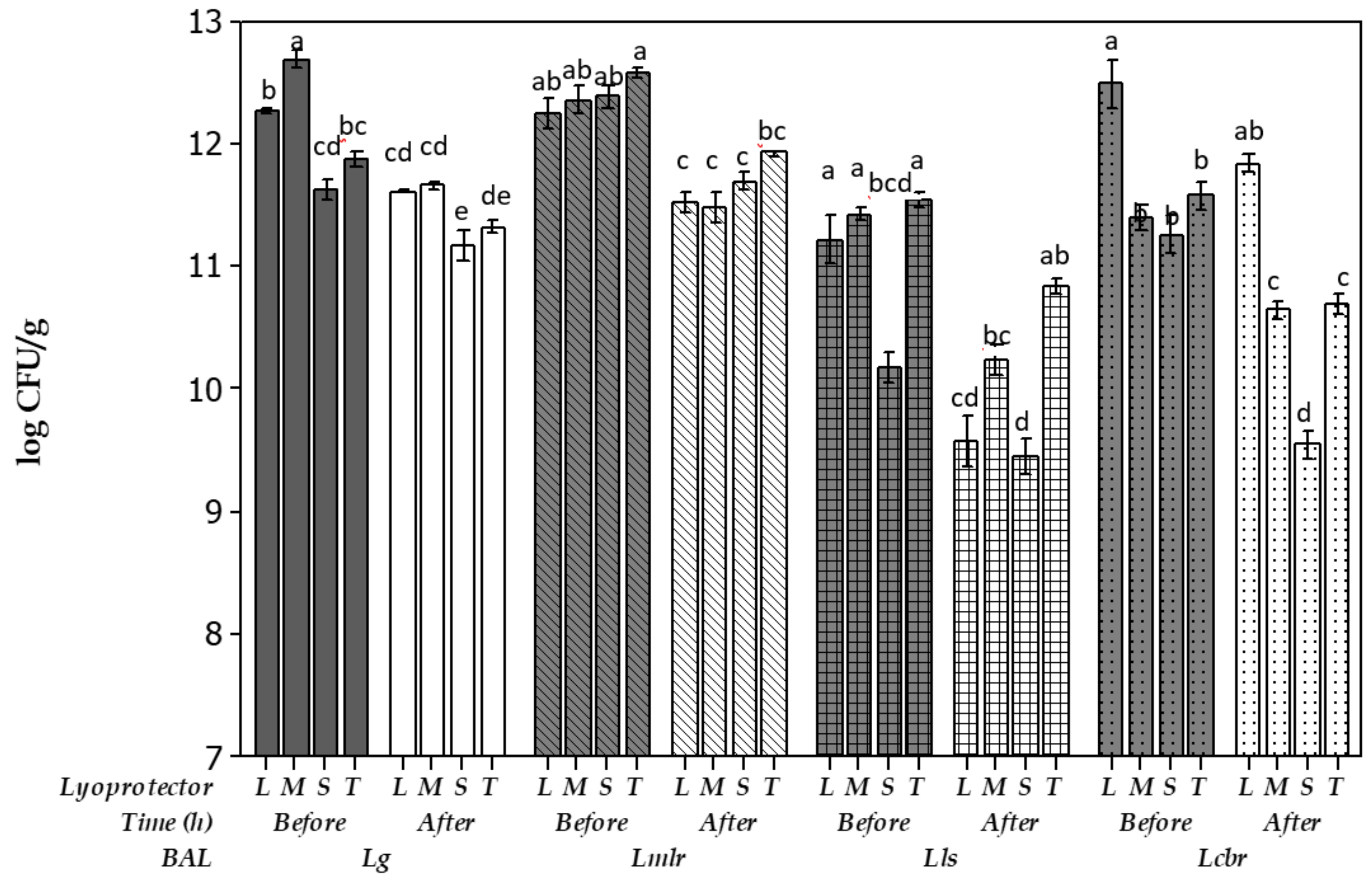
 ), Limosilactobacillus reuteri CRL1324 (oblique line bars
), Limosilactobacillus reuteri CRL1324 (oblique line bars  ), Ligilactobacillus salivarius CRL1328 (squared bars
), Ligilactobacillus salivarius CRL1328 (squared bars  ) and Lacticaseibacillus rhamnosus CRL1332 (dotted bars
) and Lacticaseibacillus rhamnosus CRL1332 (dotted bars  ). Different letters indicate statistical differences between strains at different fermentation condicions (p < 0.05).
). Different letters indicate statistical differences between strains at different fermentation condicions (p < 0.05).
 ), Limosilactobacillus reuteri CRL1324 (oblique line bars
), Limosilactobacillus reuteri CRL1324 (oblique line bars  ), Ligilactobacillus salivarius CRL1328 (squared bars
), Ligilactobacillus salivarius CRL1328 (squared bars  ) and Lacticaseibacillus rhamnosus CRL1332 (dotted bars
) and Lacticaseibacillus rhamnosus CRL1332 (dotted bars  ). Different letters indicate statistical differences between strains at different fermentation condicions (p < 0.05).
). Different letters indicate statistical differences between strains at different fermentation condicions (p < 0.05).
 ), L. reueri CRL1324 (oblique lines bars
), L. reueri CRL1324 (oblique lines bars  ), L. salivarius CRL1328 (squeared bars
), L. salivarius CRL1328 (squeared bars  ) and L. rhamnosus CRL1332 (dotted bars
) and L. rhamnosus CRL1332 (dotted bars  ).
).
 ), L. reueri CRL1324 (oblique lines bars
), L. reueri CRL1324 (oblique lines bars  ), L. salivarius CRL1328 (squeared bars
), L. salivarius CRL1328 (squeared bars  ) and L. rhamnosus CRL1332 (dotted bars
) and L. rhamnosus CRL1332 (dotted bars  ).
).
 ), Limosilactobacillus reuteri CRL1324 (oblique lines bars
), Limosilactobacillus reuteri CRL1324 (oblique lines bars  ), Ligilactobacillus alivarius CRL1328 (squeared bars
), Ligilactobacillus alivarius CRL1328 (squeared bars  ) and Lacticaseibacillus rhamnosus CRL 1332 (dotted bars
) and Lacticaseibacillus rhamnosus CRL 1332 (dotted bars  ).
).
 ), Limosilactobacillus reuteri CRL1324 (oblique lines bars
), Limosilactobacillus reuteri CRL1324 (oblique lines bars  ), Ligilactobacillus alivarius CRL1328 (squeared bars
), Ligilactobacillus alivarius CRL1328 (squeared bars  ) and Lacticaseibacillus rhamnosus CRL 1332 (dotted bars
) and Lacticaseibacillus rhamnosus CRL 1332 (dotted bars  ).
).
 );, Limosilactobacillus reuteri CRL1324 (oblique lines bars
);, Limosilactobacillus reuteri CRL1324 (oblique lines bars  ), Ligilactobacillus salivarius CRL1328 (squeared bars
), Ligilactobacillus salivarius CRL1328 (squeared bars  ) and Lacticaseibacillus rhamnosus CRL1332 (doted bars
) and Lacticaseibacillus rhamnosus CRL1332 (doted bars  ).
).
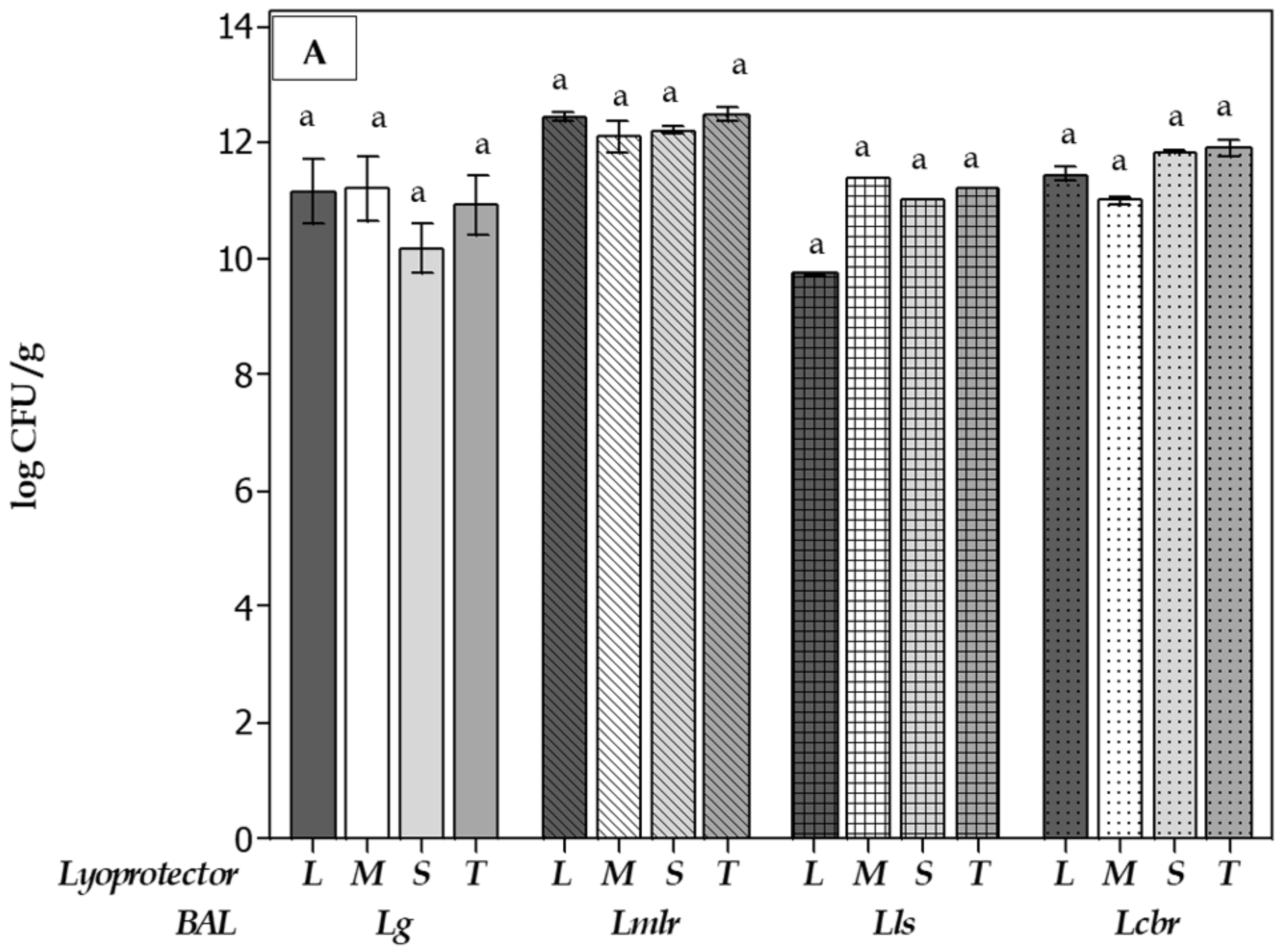
 );, Limosilactobacillus reuteri CRL1324 (oblique lines bars
);, Limosilactobacillus reuteri CRL1324 (oblique lines bars  ), Ligilactobacillus salivarius CRL1328 (squeared bars
), Ligilactobacillus salivarius CRL1328 (squeared bars  ) and Lacticaseibacillus rhamnosus CRL1332 (doted bars
) and Lacticaseibacillus rhamnosus CRL1332 (doted bars  ).
).

| VLB Strain * | Incubation (pH/rpm) | µ (h−1) | Lag Phase (h) | Amax (log UFC) |
|---|---|---|---|---|
| Lactobacillus gasseri (L. gasseri CRL 1320) | 5.5/150 | 0.87 ± 0.07 | 0 | 11.09 ± 0.02 |
| 5.5/75 | 1.00± 0.01 | 1 | 10.21 ± 0.01 | |
| 6.5/150 | 1.62 ± 0.05 | 6 | 10.64 ± 0.09 | |
| MRS | 0.40 ± 0.10 | 5 | 9.96 ± 0.03 | |
| Lacticaseibacillus rhamnosus (L. rhamnosus CRL 1332) | 5.5–150 | 0.64 ± 0.01 | 0 | 10.74 ± 0.04 |
| 5.5–75 | 1.44 ± 0.01 | 2 | 10.74 ± 0.06 | |
| 6.5–150 | 0.86 ± 0.01 | 2 | 10.75 ± 0.08 | |
| MRS | 0.44 ± 0.04 | 0 | 9.78 ± 0.04 | |
| Limosilactobacillus reuteri (L. reuteri CRL 1324) | 5.5–150 | 0.92 ± 0.02 | 0 | 9.92 ± 0.55 |
| 6.5–150 | 0.92 ± 0.03 | 2 | 10.83 ± 0.03 | |
| MRS | 0.48 ± 0.09 | 1 | 10.61 ± 0.01 | |
| Ligilactobacillus salivarius (L. salivarius CRL 1328) | 5.5–150 | 0.82 ± 0.04 | 0 | 10.47 ± 0.07 |
| 6.5–150 | 0.93 ± 0.01 | 8 | 8.24 ± 0.01 | |
| MRS | 0.75 ± 0.08 | 1 | 10.5 ± 0.08 |
| BVL | T °C | Lyoprotectant | % Hydrophobicity | % Autoagreggation | ||
|---|---|---|---|---|---|---|
| Pre-Lyofilization | Post-Lyofilization | Pre-Lyofilization | Post-Lyofilization | |||
| Lactobacillus gasseri (L. gasseri CRL 1320) | 4 °C | Lactose | 97.00 | 71.40 | 10.00 | 50.00 |
| Sucrose | 97.00 | 10.00 | 10.00 | 54.16 | ||
| Trehalose | 97.00 | 62.50 | 10.00 | 55.83 | ||
| Mixture | 97.00 | 83.30 | 10.00 | 62.50 | ||
| 25 °C | Lactose | 97.00 | 10.00 | 10.00 | 68.69 | |
| Sucrose | 97.00 | 10.00 | 10.00 | 72.30 | ||
| Trehalose | 97.00 | 10.00 | 10.00 | 82.75 | ||
| Mixture | 97.00 | 61.00 | 10.00 | 74.13 | ||
| Limosilactobacillus reuteri (L. reuteri CRL 1324) | 4 °C | Lactose | 96.00 | 41.60 | 23.00 | 62.50 |
| Sucrose | 96.00 | 10.00 | 23.00 | 54.16 | ||
| Trehalose | 96.00 | 78.90 | 23.00 | 35.83 | ||
| Mixture | 96.00 | 10.00 | 23.00 | 39.16 | ||
| 25 °C | Lactose | 96.00 | 10.00 | 23.00 | 2.00 | |
| Sucrose | 96.00 | 10.00 | 23.00 | 2.00 | ||
| Trehalose | 96.00 | 10.00 | 23.00 | 2.00 | ||
| Mixture | 96.00 | 10.00 | 23.00 | 2.00 | ||
| Ligilactobacillus salivarius (L. salivarius CRL 1328) | 4 °C | Lactose | 10.00 | 23.07 | 80.00 | 6.00 |
| Sucrose | 10.00 | 25.50 | 80.00 | 69.16 | ||
| Trehalose | 10.00 | 10.00 | 80.00 | 39.19 | ||
| Mixture | 10.00 | 2.00- | 80.00 | 40.00 | ||
| 25 °C | Lactose | 10.00 | 2.00 | 80.00 | 2.00 | |
| Sucrose | 10.00 | 2.00 | 80.00 | 2.00 | ||
| Trehalose | 10.00 | 2.00 | 80.00 | 2.00 | ||
| Mixture | 10.00 | 2.00 | 80.00 | 2.00 | ||
| Lacticaseibacillus rhamnosus (L. rhamnosus CRL 1332) | 4 °C | Lactose | 42.00 | 5.00 | 18.00 | 2.00 |
| Sucrose | 42.00 | 5.00 | 18.00 | 10.83 | ||
| Trehalose | 42.00 | 5.00 | 18.00 | 2.00 | ||
| Mixture | 42.00 | 5.00 | 18.00 | 2.00 | ||
| 25 °C | Lactose | 42.00 | 2.00 | 18.00 | 2.00 | |
| Sucrose | 42.00 | 5.00 | 18.00 | 2.00 | ||
| Trehalose | 42.00 | 5.00 | 18.00 | 2.00 | ||
| Mixture | 42.00 | 5.00 | 18.00 | 2.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchesi, A.; Nader-Macías, M.E.F. Probiotic Lactic Acid Bacteria for Vaginal Application. Optimization of Biomass Production and Freeze-Drying Conditions. Appl. Microbiol. 2023, 3, 519-535. https://doi.org/10.3390/applmicrobiol3020037
Marchesi A, Nader-Macías MEF. Probiotic Lactic Acid Bacteria for Vaginal Application. Optimization of Biomass Production and Freeze-Drying Conditions. Applied Microbiology. 2023; 3(2):519-535. https://doi.org/10.3390/applmicrobiol3020037
Chicago/Turabian StyleMarchesi, Antonella, and María Elena Fátima Nader-Macías. 2023. "Probiotic Lactic Acid Bacteria for Vaginal Application. Optimization of Biomass Production and Freeze-Drying Conditions" Applied Microbiology 3, no. 2: 519-535. https://doi.org/10.3390/applmicrobiol3020037
APA StyleMarchesi, A., & Nader-Macías, M. E. F. (2023). Probiotic Lactic Acid Bacteria for Vaginal Application. Optimization of Biomass Production and Freeze-Drying Conditions. Applied Microbiology, 3(2), 519-535. https://doi.org/10.3390/applmicrobiol3020037





