Meaning of the Decreased HPV Normalized Viral Load Marker in Clinical Evolution of Women with HPV Infection
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Genotype
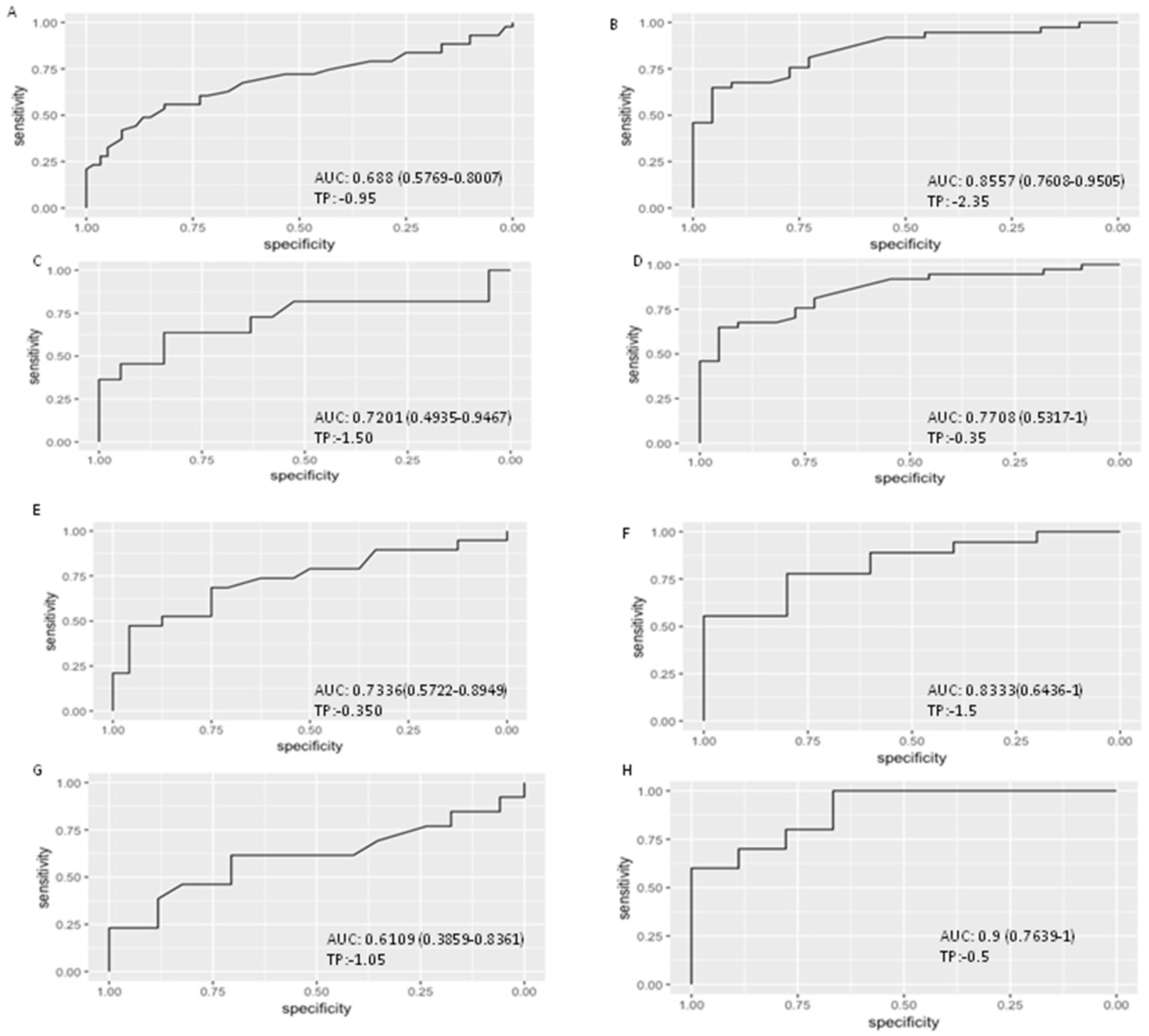
3.2. Viral Load
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rosa, M.; Medeiros, L.; Dormelles, D.; Bozzeti, M.; Rosa, F.; Rosa, B. Human papillomavirus and cervical naoplasia. Cad. Saúde Pública 2009, 25, 953–964. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Brusselaers, N.; Shrestha, S.; van de Wijgert, J.; Verstraelen, H. Vaginal dysbiosis, and the risk of human papillomavirus and cervical cancer: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2019, 221, 9–18. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H.; Gissmann, L.; Steiner, W.; Dreger, I. Human papilloma viruses and cancer. Bibl. Haematol. 1975, 43, 569–571. [Google Scholar]
- zur Hausen, H. Human papillomavirusesand their possible role in squamous cell carcinomas. Cur. Top. Microbiol. Immunol. 1977, 78, 1–30. [Google Scholar]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, F12–F23. [Google Scholar] [CrossRef] [PubMed]
- Wolrd Health Organization. Comprehensive Cervical Cancer Control: A guide to Essential Practice; Wolrd Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Moscicki, A.B.; Ma, Y.; Wibbelsman, C.; Darragh, T.M.; Powers, A.; Farhat, S.; Shiboski, S. Rate of and risks for regression of CIN-2 in adolescents and young women. Obstet. Gynecol. 2010, 116, 1373. [Google Scholar] [CrossRef]
- Verhelst, S.; Poppe, W.A.; Bogers, J.J.; Depuydt, C.E. Serial measurement of type-specific human papillomavirus load enables classification of cervical intraepithelial neoplasia lesions according to occurring human papillomavirus-induced pathway. Eur. J. Cancer Prev. 2017, 26, 156–164. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Criel, A.M.; Benoy, I.H.; Arbyn, M.; Vereecken, A.J.; Bogers, J.J. Changes in type-specific human papillomavirus load predict progression to cervical cancer. J. Cell. Mol. Med. 2012, 16, 3096–3104. [Google Scholar] [CrossRef]
- Álvarez-Argüelles, M.E.; de Oña-Navarro, M.; Rojo-Alba, S.; Torrens-Muns, M.; Junquera-Llaneza, M.L.; Antonio-Boga, J.; Melón-García, S. Quantification of human papilloma virus (HPV) ADN using the Cobas 4800 system in women with and without pathological alterations attributable to the virus. J. Virol. Methods 2015, 222, 95–102. [Google Scholar] [CrossRef]
- Perez, S.; Cid, A.; Araujo, A.; Lamas, M.J.; Saran, M.T.; Alvarez, M.J.; Melon, S. A novel real-time genotyping assay for detection of the E6-350G HPV16 variant. J. Virol. Methods 2011, 173, 357–363. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 1 March 2018).
- Woodman, C.B.; Collins, S.; Winter, H.; Bailey, A.; Ellis, J.; Prior, P.; Young, L.S. Natural history of cervical human papillomavirus infection in young women: A longitudinal cohort study. Lancet 2001, 357, 1831–1836. [Google Scholar] [CrossRef]
- Gravitt, P.E. The known unknowns of HPV natural history. J. Clin. Investig. 2011, 121, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.M.D.; Silva, N.N.T.; Pitol, B.C.V.; Silva, I.D.C.G.D.; Lima-Filho, J.L.D.; Carvalho, R.F.D.; Lima, A.A. Persistence or clearance of human papillomavirus infections in women in Ouro Preto, Brazil. BioMed Res. Int. 2013, 2013, 578276. [Google Scholar] [CrossRef] [PubMed]
- Banura, C.; Sandin, S.; van Doorn, L.J.; Quint, W.; Kleter, B.; Wabwire-Mangen, F.; Weiderpass, E. Type-specific incidence, clearance and predictors of cervical human papillomavirus infections (HPV) among young women: A prospective study in Uganda. Infect. Agents Cancer 2010, 5, 7. [Google Scholar] [CrossRef]
- Woo, Y.L.; Sterling, J.; Damay, I.; Coleman, N.; Crawford, R.; van der Burg, S.H.; Stanley, M. Characterising the local immune responses in cervical intraepithelial neoplasia: A cross-sectional and longitudinal analysis. BJOG 2008, 115, 1616–1622. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Gravitt, P.E.; Winer, R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef]
- Adebamowo, S.N.; Befano, B.; Cheung, L.C.; Rodriguez, A.C.; Demarco, M.; Rydzak, G.; Chen, X.; Porras, C.; Herrero, R.; Kim, J.J.; et al. Different human papillomavirus types share early natural history transitions in immunocompetent women. Int. J. Cancer 2022, 151, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.T.; Shvetsov, Y.B.; McDuffie, K.; Wilkens, L.R.; Zhu, X.; Thompson, P.J.; Ning, L.; Killeen, J.; Kamemoto, L.; Hernandez, B.Y. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii Human Papillomavirus Cohort Study. Cancer Res. 2008, 68, 8813–8824. [Google Scholar] [CrossRef] [PubMed]
- Kjær, S.K.; Munk, C.; Junge, J.; Iftner, T. Carcinogenic VPH prevalence and age-specific type distribution in 40,382 women with normal cervical cytology, ASCUS/LSIL, HSIL, or cervical cancer: What is the potential for prevention? Cancer Causes Control 2014, 25, 179–189. [Google Scholar] [CrossRef]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.-R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Joura, E.A.; Ault, K.A.; Bosch, F.X.; Brown, D.; Cuzick, J.; Ferris, D.; Garland, S.M.; Giuliano, A.R.; Hernandez-Avila, M.; Huh, W.; et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Howell-Jones, R.; Li, N.; Bruni, L.; de Sanjose, S.; Franceschi, S.; Clifford, G.M. Human papillomavirus types in 115,789 VPH positive women: A meta-analysis from cervical infection to cancer. Int. J. Cancer 2012, 131, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Mejlhede, N.; Bonde, J.; Fomsgaard, A. High frequency of multiple HPV types in cervical specimens from Danish women. APMIS 2009, 117, 108–114. [Google Scholar] [CrossRef]
- Vaccarella, S.; Franceschi, S.; Herrero, R.; Schiffman, M.; Rodriguez, A.C.; Hildesheim, A.; Burk, R.D.; Plummer, M. Clustering of multiple human papillomavirus infections in women from a population-based study in Guanacaste, Costa Rica. J. Infect. Dis. 2011, 204, 385–390. [Google Scholar] [CrossRef]
- Wentzensen, N.; Nason, M.; Schiffman, M.; Dodd, L.; Hunt, W.C.; Wheeler, C.M. No evidence for synergy between human papillomavirus genotypes for the risk of high-grade squamous intraepithelial lesions in a large population-based study. J. Infect. Dis. 2014, 209, 855–864. [Google Scholar] [CrossRef]
- Salazar, K.L.; Zhou, H.S.; Xu, J.; Peterson, L.E.; Schwartz, M.R.; Mody, D.R.; Ge, Y. Multiple Human Papilloma Virus Infections and Their Impact on the Development of High-Risk Cervical Lesions. Acta Cytol. 2015, 59, 391–398. [Google Scholar] [CrossRef]
- Del Río-Ospina, L.; Soto-De León, S.C.; Camargo, M.; Moreno-Pérez, D.A.; Sánchez, R.; Pérez-Prados, A.; Patarroyo, M.A. The ADN load of six high-risk human papillomavirus types and its association with cervical lesions. BMC Cancer 2015, 15, 1. [Google Scholar] [CrossRef]
- Quint, W.; Jenkins, D.; Molijn, A.; Struijk, L.; van de Sandt, M.; Doorbar, J.; Mols, J.; van Hoof, C.; Hardt, K.; Struyf, F.; et al. One virus, one lesion--individual components of CIN lesions contain a specific HPV type. J Pathol. 2012, 227, 62–71. [Google Scholar] [CrossRef]
- Kovacic, M.B.; Castle, P.E.; Herrero, R.; Schiffman, M.; Sherman, M.E.; Wacholder, S.; Rodriguez, A.C.; Hutchinson, M.L.; Bratti, M.C.; Hildesheim, A.; et al. Relationships of human papillomavirus type, qualitative viral load, and age with cytologic abnormality. Cancer Res. 2006, 66, 10112–10119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, B.; Sun, P.; Ruan, G.; Huang, W.; Mao, X.; Kang, Y.; Pan, D.; Lin, F. Type-specific high-risk human papillomavirus viral load as a viable triage indicator for high-grade squamous intraepithelial lesion: A nested case–control study. Cancer Manag. Res. 2018, 10, 4839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, H.; Xiao, A.; Zhang, W.; Wang, C.; Huang, X.; Qu, X.; Wang, J.; Wu, R. Verification of the association of the cycle threshold (Ct) values from HPV testing on Cobas4800 with the histologic grades of cervical lesions using data from two population-based cervical cancer screening trials. Infect. Agents Cancer 2022, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Oton-Gonzalez, L.; Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Tognon, M.; Martini, F. Simultaneous Detection and Viral DNA Load Quantification of Different Human Papillomavirus Types in Clinical Specimens by the High Analytical Droplet Digital PCR Method. Front. Microbiol. 2020, 11, 591452. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Jonckheere, J.; Berth, M.; Salembier, G.M.; Vereecken, A.J.; Bogers, J.J. Serial type-specific human papillomavirus (HPV) load measurement allows differentiation between regressing cervical lesions and serial virion productive transient infections. Cancer Med. 2015, 4, 1294–1302. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Thys, S.; Beert, J.; Jonckheere, J.; Salembier, G.; Bogers, J.J. Linear viral load increase of a single HPV-type in women with multiple HPV infections predicts progression to cervical cancer. Int. J. Cancer 2016, 139, 2021–2032. [Google Scholar] [CrossRef]

| Patient | Age | Lesion | Genotype(s) | Genotype Group | Variant T350G | VL0 | VL1 | VL2 | VL3 | VL4 | GROUP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | NILM | 53 | Nonα9 | 4.4 | 3.5 | CI | ||||
| 2 | 65 | NILM | 18 | Nonα9 | 3.7 | 4.3 | 6.4 | 2.7 | NCI | ||
| 3 | 45 | HSIL | 31/33 | α9 | 4.8 | 4.9 | 3.9 | CI | |||
| 4 | 61 | LSIL | 44/66 | Nonα9 | 5.1 | 4.9 | 4.2 | CI | |||
| 5 | 39 | HSIL | 16 | HPV16 | YES | 5.1 | 2 | CI | |||
| 6 | 30 | LSIL | 16/51 | HPV16/α9 | NO | 4.8 | 8 | 8.7 | 3.8 | 0 | NCI |
| 7 | 37 | LSIL | 52 | α9 | 4 | 4.1 | 6.4 | CI | |||
| 8 | 30 | HSIL | 33/31 | α9 | 5.3 | 6.1 | CI | ||||
| 9 | 23 | LSIL | 53/66 | Nonα9 | 2.4 | 3.1 | 0 | NCI | |||
| 10 | 63 | NILM | 16 | HPV16 | YES | 4 | 3.8 | 5.9 | 0 | NCI | |
| 11 | 30 | HSIL | 16 | HPV16 | YES | 4.2 | 4 | CI | |||
| 12 | 57 | HSIL | 66 | Nonα9 | 6 | 4.5 | 4.5 | CI | |||
| 13 | 44 | LSIL | 31 | α9 | 5.4 | 4.9 | 0 | 0 | NCI | ||
| 14 | 49 | HSIL | 16 | HPV16 | NO | 3.7 | 3.4 | 3.1 | 4.4 | 4.1 | CI |
| 15 | 38 | HSIL | 33 | α9 | 5.3 | 5.3 | CI | ||||
| 16 | 33 | NILM | 31 | α9 | 3.1 | 4.3 | 0 | NCI | |||
| 17 | 44 | LSIL | 16/66 | HPV16 | YES | 9 | 8.6 | 8.5 | 7.8 | CI | |
| 18 | 29 | NILM | 31 | α9 | 4.9 | 6.1 | 3.8 | 0 | NCI | ||
| 19 | 38 | NILM | 16/45 | HPV16/nonα9 | NO | 5.2 | 7.3 | 5.9 | CI | ||
| 20 | 64 | NILM | 52 | α9 | 3.4 | 3.5 | 4.2 | CI | |||
| 21 | 60 | NILM | 16 | HPV16 | NO | 4.2 | 3.6 | 3.9 | 3.4 | NCI | |
| 22 | 39 | NILM | 18 | Nonα9 | 4.2 | 4 | 4.6 | CI | |||
| 23 | 68 | HSIL | 16/52 | HPV16/α9 | YES | 6.5 | 10.8 | 6.4 | CI | ||
| 24 | 56 | LSIL | 53/56 | Nonα9 | 5.4 | 5.8 | 5.6 | 4.8 | CI | ||
| 25 | 57 | NILM | 31 | α9 | 3.8 | 2.4 | 3.3 | 0 | NCI | ||
| 26 | 30 | NILM | 52/56 | α9 | 5.1 | 5.2 | 3.6 | 0 | 0 | NCI | |
| 27 | 43 | NILM | 31 | α9 | 5.6 | 5.7 | 5.4 | 0 | 0 | NCI | |
| 28 | 26 | NILM | 52 | α9 | 3 | 3.8 | 4.6 | 4.3 | CI | ||
| 29 | 35 | NILM | 52 | α9 | 4.7 | 4.8 | 5.2 | 0 | 0 | NCI | |
| 30 | 27 | NILM | 56 | Nonα9 | 4.9 | 3.9 | 4.1 | 4.6 | CI | ||
| 31 | 35 | HSIL | 16 | HPV16 | NO | 5 | 4 | CI | |||
| 32 | 24 | LSIL | 16 | HPV16 | NO | 4.9 | 5.8 | 0 | 0 | NCI | |
| 33 | 67 | NILM | 31 | α9 | 3.6 | 3.2 | 3.4 | CI | |||
| 34 | 46 | HSIL | 16 | HPV16 | YES | 3.8 | 3.9 | 4.3 | CI | ||
| 35 | 37 | NILM | 16 | HPV16 | YES | 4 | 2.7 | CI | |||
| 36 | 52 | NILM | 16 | HPV16 | YES | 3.9 | 2.5 | 2.5 | 2.7 | 0 | NCI |
| 37 | 34 | LSIL | 31 | α9 | 4.2 | 2.9 | 4.7 | 4.1 | 0 | NCI | |
| 38 | 32 | NILM | 16/59 | HPV16/nonα9 | YES | 10.9 | 9.2 | 11.5 | 9.6 | CI | |
| 39 | 27 | LSIL | 31 | α9 | 6.7 | 3.4 | 3.2 | 0 | NCI | ||
| 40 | 58 | HSIL | 16 | HPV16 | YES | 3.5 | 3.5 | CI | |||
| 41 | 43 | LSIL | 56 | Nonα9 | 4.9 | 5.2 | CI | ||||
| 42 | 62 | NILM | 52 | α9 | 4.6 | 3.6 | CI | ||||
| 43 | 37 | NILM | 33 | α9 | 4.9 | 4.6 | 3.6 | CI | |||
| 44 | 39 | NILM | 31 | α9 | 3.1 | 5.3 | 6 | 5.3 | 3.6 | NCI | |
| 45 | 26 | LSIL | 16 | HPV16 | NO | 4.4 | 4.5 | 0 | 0 | NCI | |
| 46 | 36 | NILM | 58/66 | α9 | 5.6 | 5.3 | 5.2 | NCI | |||
| 47 | 30 | ASCUS | 31/66 | α9 | 6.9 | 6.1 | CI | ||||
| 48 | 37 | NILM | 45/52 | α9 | 3.2 | 3.7 | 5.5 | CI | |||
| 49 | 23 | ASCUS | 58 | α9 | 5.5 | 6.3 | 3.9 | 0 | 0 | NCI | |
| 50 | 31 | HSIL | 16 | HPV16 | NO | 5 | 3.9 | 3.1 | 3.1 | CI | |
| 51 | 28 | NILM | 35 | α9 | 5.7 | 4.8 | CI | ||||
| 52 | 30 | HSIL | 18 | Nonα9 | 5.1 | 6.2 | 5.4 | 5.2 | CI | ||
| 53 | 49 | LSIL | 52 | α9 | 5.4 | 3.6 | 5.1 | CI | |||
| 54 | 35 | NILM | 59 | Nonα9 | 4.2 | 5.7 | 4.5 | 2.7 | CI | ||
| 55 | 56 | LSIL | 16 | HPV16 | NO | 4.6 | 4.8 | CI | |||
| 56 | 53 | NILM | 51 | Nonα9 | 5.3 | 2 | CI | ||||
| 57 | 59 | HSIL | 31 | α9 | 4.1 | 2.8 | CI | ||||
| 58 | 49 | HSIL | 16/66 | HPV16/nonα9 | NO | 8.4 | 10.4 | CI | |||
| 59 | 38 | NILM | 39 | Nonα9 | 6.5 | 6 | 0 | NCI | |||
| 60 | 36 | LSIL | 16 | HPV16 | YES | 2.9 | 5.4 | 5.8 | 3.3 | CI | |
| 61 | 40 | NILM | 51 | Nonα9 | 4.8 | 4.7 | 3.4 | 0 | NCI | ||
| 62 | 26 | LSIL | 16 | HPV16 | YES | 5.4 | 5.4 | 5.3 | 0 | 0 | NCI |
| 63 | 28 | ASCUS | 56 | Nonα9 | 4.2 | 5.4 | 0 | NCI | |||
| 64 | 26 | NILM | 31/33 | α9 | 5.6 | 6 | 3.7 | 0 | 0 | NCI | |
| 65 | 47 | HSIL | 16 | HPV16 | YES | 2.8 | 4 | CI | |||
| 66 | 53 | NILM | 31 | α9 | 7.1 | 5.8 | 5.7 | 3 | CI | ||
| 67 | 37 | LSIL | 35 | α9 | 5.7 | 4.4 | CI | ||||
| 68 | 22 | ASCUS | 16 | HPV16 | NO | 4.9 | 4.7 | 4.4 | CI | ||
| 69 | 30 | HSIL | 33 | α9 | 5 | 4.2 | CI | ||||
| 70 | 39 | ASCUS | 18 | Nonα9 | 3.3 | 5.9 | 0 | NCI | |||
| 71 | 30 | LSIL | 52 | α9 | 3.8 | 4.5 | 4 | 3.2 | 0 | NCI | |
| 72 | 47 | NILM | 39 | Nonα9 | 6.1 | 6.1 | 3.8 | 0 | NCI | ||
| 73 | 40 | NILM | 16 | HPV16 | YES | 2.1 | 3 | 2.1 | CI | ||
| 74 | 33 | NILM | 18 | Nonα9 | 4.9 | 4.2 | 3.2 | 3.7 | NCI | ||
| 75 | 28 | NILM | 16 | HPV16 | NO | 4.7 | 3.5 | 3.5 | 4.3 | CI | |
| 76 | 37 | HSIL | 35 | α9 | 4.2 | 3 | 3.3 | CI | |||
| 77 | 29 | NILM | 35 | α9 | 5.6 | 5.7 | 5.8 | CI | |||
| 78 | 45 | NILM | 35 | α9 | 3 | 4.6 | 4.6 | CI | |||
| 79 | 40 | NILM | 31 | α9 | 6.3 | 3.1 | 2.7 | 0 | 0 | NCI | |
| 80 | 48 | NILM | 16 | HPV16 | NO | 4.6 | 5.2 | 4.1 | CI | ||
| 81 | 42 | NILM | 31/70 | α9 | 3.4 | 3.3 | NCI | ||||
| 82 | 33 | LSIL | 51 | Nonα9 | 2.1 | 2.2 | 5 | 0 | NCI | ||
| 83 | 34 | LSIL | 66 | Nonα9 | 5.7 | 5.9 | 5.6 | CI | |||
| 84 | 51 | NILM | 16 | HPV16 | NO | 4.2 | 4.2 | 4.5 | 6.7 | CI | |
| 85 | 33 | NILM | 51 | Nonα9 | 2.8 | 6.8 | 5.2 | 3.2 | CI | ||
| 86 | 24 | NILM | 52 | α9 | 2.5 | 2.7 | CI | ||||
| 87 | 59 | HSIL | 58 | α9 | 2.7 | 2.6 | CI | ||||
| 88 | 39 | NILM | 16 | HPV16 | NO | 3.7 | 4 | 3.5 | CI | ||
| 89 | 53 | NILM | 16 | HPV16 | NO | 5.1 | 5.4 | 4.4 | 4.7 | 4.5 | NCI |
| 90 | 42 | HSIL | 39/58 | α9 | 4.1 | 4.7 | 4.7 | 3.8 | 4.3 | CI | |
| 91 | 27 | LSIL | 16/52 | HPV16/α9 | YES | 11.2 | 10.5 | 8.6 | 8.1 | 4.6 | NCI |
| 92 | 50 | NILM | 31 | α9 | 2.9 | 4.5 | 3 | CI | |||
| 93 | 42 | LSIL | 51 | Nonα9 | 5.6 | 5.4 | 5.6 | 6.6 | 4.6 | CI | |
| 94 | 55 | NILM | 51 | Nonα9 | 4.6 | 7.1 | 7.6 | CI | |||
| 95 | 60 | NILM | 35 | α9 | 4 | 2.9 | 3.3 | 3.6 | NCI | ||
| 96 | 34 | NILM | 31 | α9 | 5.4 | 3 | 2.7 | CI | |||
| 97 | 54 | NILM | 16 | HPV16 | YES | 4.4 | 4.5 | CI | |||
| 98 | 33 | LSIL | 18 | Nonα9 | 6.9 | 6 | 6 | 6.9 | 6.9 | CI | |
| 99 | 31 | NILM | 16 | HPV16 | YES | 2.9 | 3.3 | 3.8 | CI | ||
| 100 | 30 | ASCUS | 33/61 | α9 | 3.9 | 4.3 | 6 | 0 | NCI | ||
| 101 | 27 | HSIL | 16 | HPV16 | NO | 5.8 | 6.2 | 5.8 | 4 | CI | |
| 102 | 33 | NILM | 16 | HPV16 | YES | 6 | 3.3 | CI | |||
| 103 | 29 | LSIL | 56 | Nonα9 | 7 | 4.1 | 3.6 | CI | |||
| 104 | 43 | NILM | 52 | α9 | 3.7 | 3.9 | 3.8 | 3.2 | NCI | ||
| 105 | 28 | NILM | 16 | HPV16 | YES | 4.2 | 3.8 | 0 | NCI | ||
| 106 | 29 | NILM | 56 | Nonα9 | 5.9 | 5 | 0 | NCI | |||
| 107 | 58 | NILM | 31/53 | α9 | 4.7 | 4.3 | 5.2 | CI | |||
| 108 | 31 | NILM | 39 | Nonα9 | 6.2 | 4.6 | 5 | 0 | NCI | ||
| 109 | 49 | NILM | 16 | HPV16 | NO | 5.1 | 4.7 | CI | |||
| 110 | 33 | ASCUS | 56 | Nonα9 | 7.1 | 5.6 | 3.6 | 4.8 | CI | ||
| 111 | 46 | LSIL | 31/51 | α9 | 3.6 | 6.7 | 6.1 | 6.1 | CI | ||
| 112 | 38 | NILM | 16 | HPV16 | NO | 5.4 | 3.6 | 4.3 | CI | ||
| 113 | 28 | HSIL | 16/31 | HPV16/α9 | YES | 7.9 | 7.8 | 3.4 | CI | ||
| 114 | 27 | NILM | 16 | HPV16 | NO | 2.1 | 4.3 | 3.4 | 3.2 | 0 | NCI |
| 115 | 61 | NILM | 45 | Nonα9 | 3.2 | 4.4 | 4.5 | 0 | NCI | ||
| 116 | 53 | NILM | 66 | Nonα9 | 6 | 3.3 | 4.3 | CI | |||
| 117 | 48 | NILM | 16 | HPV16 | YES | 4.9 | 3.2 | 5.3 | CI | ||
| 118 | 49 | LSIL | 16/52 | HPV16/α9 | NO | 8.8 | 9.3 | 9.6 | CI | ||
| 119 | 30 | LSIL | 51 | Nonα9 | 5.8 | 7.1 | 4.3 | 5.4 | 5.2 | CI | |
| 120 | 26 | NILM | 31 | α9 | 5.6 | 5.2 | 5.6 | CI |
| Age CI Patients | Age NCI Patients | p | |||||
|---|---|---|---|---|---|---|---|
| n | x ± σ (Range) | CI95 | n | x ± σ (Range) | CI95 | ||
| Total | 78 | 41.4 ± 11.49 (22–68) | 38.8–43.9 | 42 | 37.7 ± 12.13 (23–65) | 33.9–41.4 | 0.104 |
| Single | 62 | 40.66 ± 11.34(22–67) | 37.9–43.7 | 34 | 39.41 ± 12.63 (23–65) | 34.5–43.6 | 0.52 |
| Mixed | 16 | 44.56 ± 11.93 (28–68) | 38.2–50.9 | 8 | 30.5 ± 6 (23–42) | 25.4–35.5 | 0.005 |
| HPV16 | 30 | 41.03 ± 10.72 (22–68) | 37.02–45.03 | 11 | 37.82 ± 15.55 (24–63) | 27.3–48.2 | 0.53 |
| HPVα9non16 | 34 | 43.82 ± 13.32 (24–68) | 39.1–48.4 | 20 | 34.50 ± 10.19 (23–60) | 29.7–39.2 | 0.019 |
| HPVnonα9 | 21 | 39.1 ± 9.42 (27–57) | 34.8–43.3 | 13 | 38.77 ± 12.05 (28–65) | 31.4–46.05 | 0.934 |
| Initial Test (0) | Follow-Up 1 | Follow-Up 2 | Follow-Up 3 | Follow-Up 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | VL | n | VL | n | VL | n | VL | n | VL | p | ||
| NCI | ||||||||||||
| VL | 42 | 4.66 ± 1.55 (2.1–11.2) | 42 | 4.71 ± 1.53 (2.2–10.5) | 41 | 3.45 ± 2.37 (0.0–8.7) | 32 | 1.61 ± 2.17 (0.0–8.1) | 8 | 1.58 ± 2.21 (0.0–4.6) | <0.0001 | |
| CI95% | 4.17/5.14 | 4.23/5.18 | 2.7/4.19 | 0.82/2.39 | −0.26/3.4 | |||||||
| Undetectable (number and %) | 0 | 1 | 2.3% | 10 | 23.8% | 23 | 54.7% | |||||
| CI | ||||||||||||
| VL | 78 | 4.96 ± 1.57 (2.1–10.9) | 78 | 4.83 ± 1.79 (2.0–10.8) | 54 | 4.9 ± 1.65 (2.1–11.5) | 21 | 4.98 ± 1.76 (2.7–9.6) | 5 ** | 5.02 ± 1.13 (4.1–6.9) | 0.989 | |
| CI95% | 4.6/5.31 | 4.42/5.23 | 4.44/5.35 | 4.15/5.8 | 3.61/6.42 | |||||||
| Surgery * | 0 | 24 | 30.7% | 33 | 42.3% | 16 | 20.5% | |||||
| p | 0.318 | 0.713 | 0.0007 | <0.0001 | 0.0086 | |||||||
| Follow-Up 1 | Follow-Up 2 | Follow-Up 3 | Follow-Up 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | x ± σ (Range) | CI95 | n | x ± σ (Range) | CI95 | n | x ± σ (Range) | CI95 | n | x ± σ (Range) | CI95 | |
| Total | ||||||||||||
| CI | 78 | −0.13 ± 1.44 (−3.3/4.3) | −0.4/0.19 | 54 | −0.11 ± 1.53 (−4.5/3) | −0.52/0.30 | 20 | −0.43 ± 1.5 (−4.1/2.5) | −1.13/0.27 | 5 | −0.3 ± 0.62 (−1/0.4) | −1.28/0.68 |
| NCI | 42 | 0.05 ± 1.29 (−3.3/3.2) | −0.35/0.62 | 41 | −1.22 ± 2.55 (−6.5/3.9) | −2.02/0.41 | 32 | −3.26 ± 2.42 (−6.7/2.2) | −4.07/−2.29 | 8 | −3.35 ± 2.22 (−6.6/0.5) | −5.05/−1.64 |
| p | 0.46 | 0.017 | 0.0000016 | 0.02 | ||||||||
| HPV16 | ||||||||||||
| CI | 30 | −0.17 ± 1.27 (−3.1/2.5) | −0.64/0.30 | 19 | 0 ± 1 (−1.9/2.9) | −0.48/0.48 | 8 | −0.1 ± 1.42 (−1.9/2.5) | −1.28/1.08 | 1 | 0.4 | |
| NCI | 11 | 0.09 ± 0.91 (−1.4/2.2) | −0.52/0.70 | 11 | −1.66 ± 2.44 (−4.9/1.9) | −3.29/−0.02 | 9 | −2.37 ± 2.29 (−5.3/1.1) | −4.1/−0.60 | 2 | −1.2 | |
| p | 0.469 | 0.052 | 0.026 | |||||||||
| HPVα9non16 | ||||||||||||
| CI | 34 | −0.11 ± 1.05 (−2.4/3.1) | −0.47/0.25 | 24 | 0.24 ± 1.30 (−2.7/2.6) | −0.30/0.78 | 5 | −0.24 ± 2.49 (−4.1/2.5) | −3.33/2.85 | 0 | ||
| NCI | 20 | −0.21 ± 1.35 (−3.3/2.2) | −0.84/0.42 | 19 | −1.12 ± 2 (−5.4/2.9) | −2.09/−0.16 | 18 | −3.71 ± 2.5 (−6.7/2.2) | −4.96/−2.47 | 3 | −2.8 (−4.7–0.5) | |
| p | 0.77 | 0.014 | 0.03 | |||||||||
| HPVnonα9 | ||||||||||||
| CI | 21 | −0.20 ± 1.75 (−3.3/4) | −0.99/0.59 | 17 | −0.31 ± 1.76 (−3.5/3) | −1.21/0.59 | 9 | −0.34 ± 1.16 (−2.3/1.4) | −1.23/0.55 | 3 | −0.53 (−1–0) | |
| NCI | 13 | 0.47 ± 0.99 (−0.9/2.6) | −0.1/1.06 | 13 | −1.42 ± 3.05 (−6.5/3.3) | −3.26/0.42 | 10 | −3.52 ± 2.27 (−6.5–/0.6) | −5.14/−1.89 | 1 | −3.8 | |
| p | 0.155 | 0.25 | 0.0017 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo-Alba, S.; Álvarez-Argüelles, M.E.; Ruano, Y.; Pérez-Martinez, Z.; Boga, J.A.; De Oña, M.; Palacio, A.; Solares, M.C.; Melón, S. Meaning of the Decreased HPV Normalized Viral Load Marker in Clinical Evolution of Women with HPV Infection. Appl. Microbiol. 2022, 2, 651-661. https://doi.org/10.3390/applmicrobiol2030050
Rojo-Alba S, Álvarez-Argüelles ME, Ruano Y, Pérez-Martinez Z, Boga JA, De Oña M, Palacio A, Solares MC, Melón S. Meaning of the Decreased HPV Normalized Viral Load Marker in Clinical Evolution of Women with HPV Infection. Applied Microbiology. 2022; 2(3):651-661. https://doi.org/10.3390/applmicrobiol2030050
Chicago/Turabian StyleRojo-Alba, Susana, Marta Elena Álvarez-Argüelles, Yolanda Ruano, Zulema Pérez-Martinez, Jose Antonio Boga, María De Oña, Ana Palacio, María Concepción Solares, and Santiago Melón. 2022. "Meaning of the Decreased HPV Normalized Viral Load Marker in Clinical Evolution of Women with HPV Infection" Applied Microbiology 2, no. 3: 651-661. https://doi.org/10.3390/applmicrobiol2030050
APA StyleRojo-Alba, S., Álvarez-Argüelles, M. E., Ruano, Y., Pérez-Martinez, Z., Boga, J. A., De Oña, M., Palacio, A., Solares, M. C., & Melón, S. (2022). Meaning of the Decreased HPV Normalized Viral Load Marker in Clinical Evolution of Women with HPV Infection. Applied Microbiology, 2(3), 651-661. https://doi.org/10.3390/applmicrobiol2030050






