Isolation, Identification, Optimization of Baker’s Yeast from Natural Sources, Scale-Up Production Using Molasses as a Cheap Carbohydrate Source, and Evaluation for Bread Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Enrichment of the Samples
2.3. Selection of Yeast Isolates
2.4. Culture Purification
- The purified isolates were sub-cultured into two sets of YMA slants in test tubes and incubated at 30 °C for 48 h. Then one set was taken to apply sterile 60% glycerol above the cells grown in YMA slant to save the cells from dehydration. Then both sets were preserved at 4 °C as stock culture.
- The purified isolates to be frozen were grown for about 24 h in 1 mL PDB (Potato Dextrose Broth) in a 5 mL vial before adding 400 µL of 60% solution of glycerol in water. Then these vials were shaken well to mix and then kept at −20 °C.
2.5. Identification of Yeast Isolates
Cultural Characterization
2.6. Growth in Liquid Media
2.7. Morphological Characterization
2.8. Staining
2.9. Microscopy
2.10. Biochemical Characterization
2.11. Carbohydrate Fermentation
2.12. Ascospore Production
2.13. Urease Test
2.14. Nitrate Reduction Test
2.15. Utilization of Ethanol as a Sole Carbon Source
2.16. Flocculation
2.17. Assessment of the Potency of Identified S. cerevisiae for Bread Production
2.17.1. Production of H2S
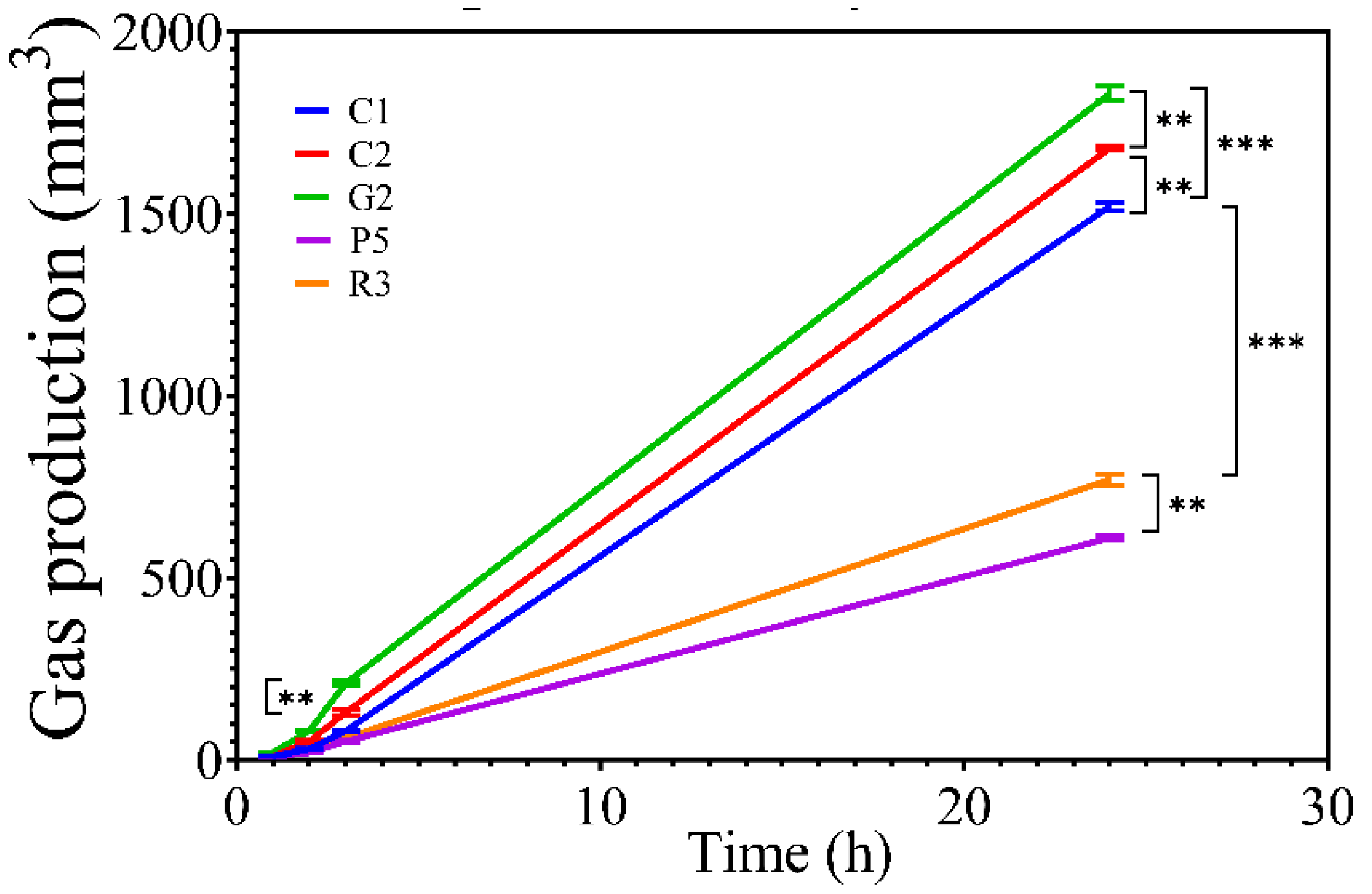
2.17.2. Measurement of Gas Production
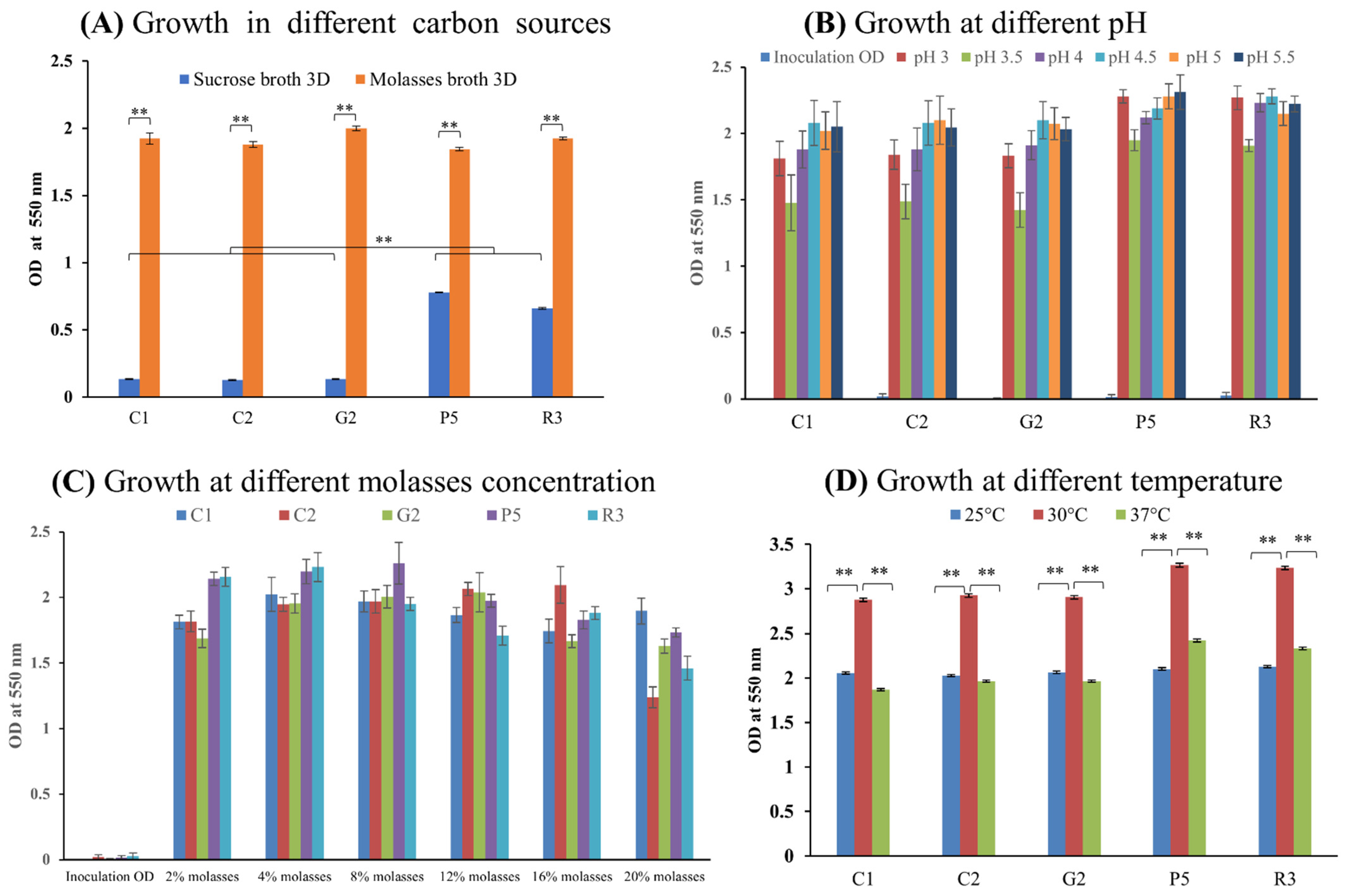
2.18. Optimization of Physicochemical Parameters for Maximum Growth of Isolates
2.18.1. Optimum Carbon Sources
2.18.2. Optimum pH
2.18.3. Optimum Molasses Concentration
2.18.4. Optimum Temperature
2.19. Incubation Period
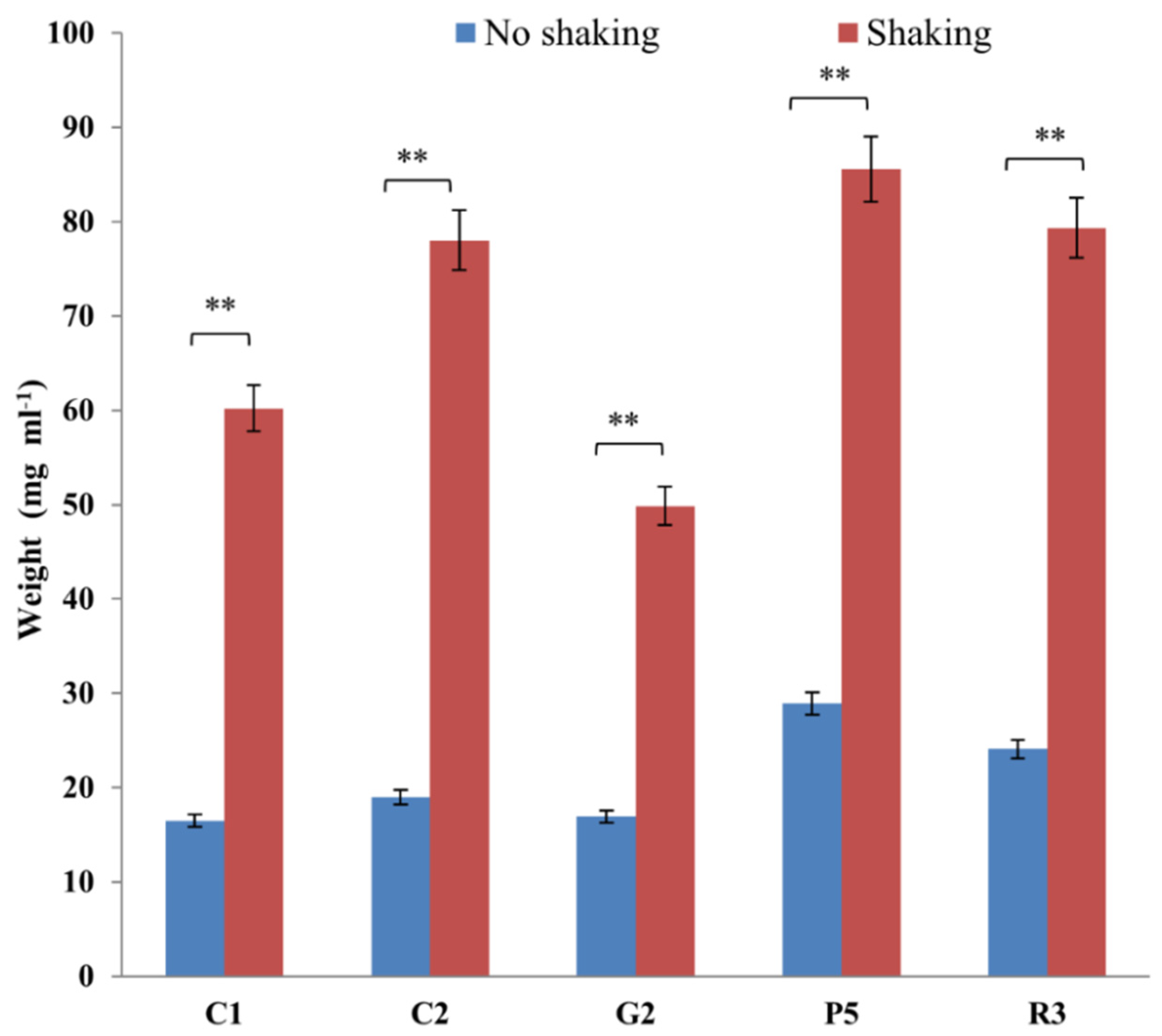
2.20. Effect of Agitation
2.21. Preparation of Bread from Composite Flour
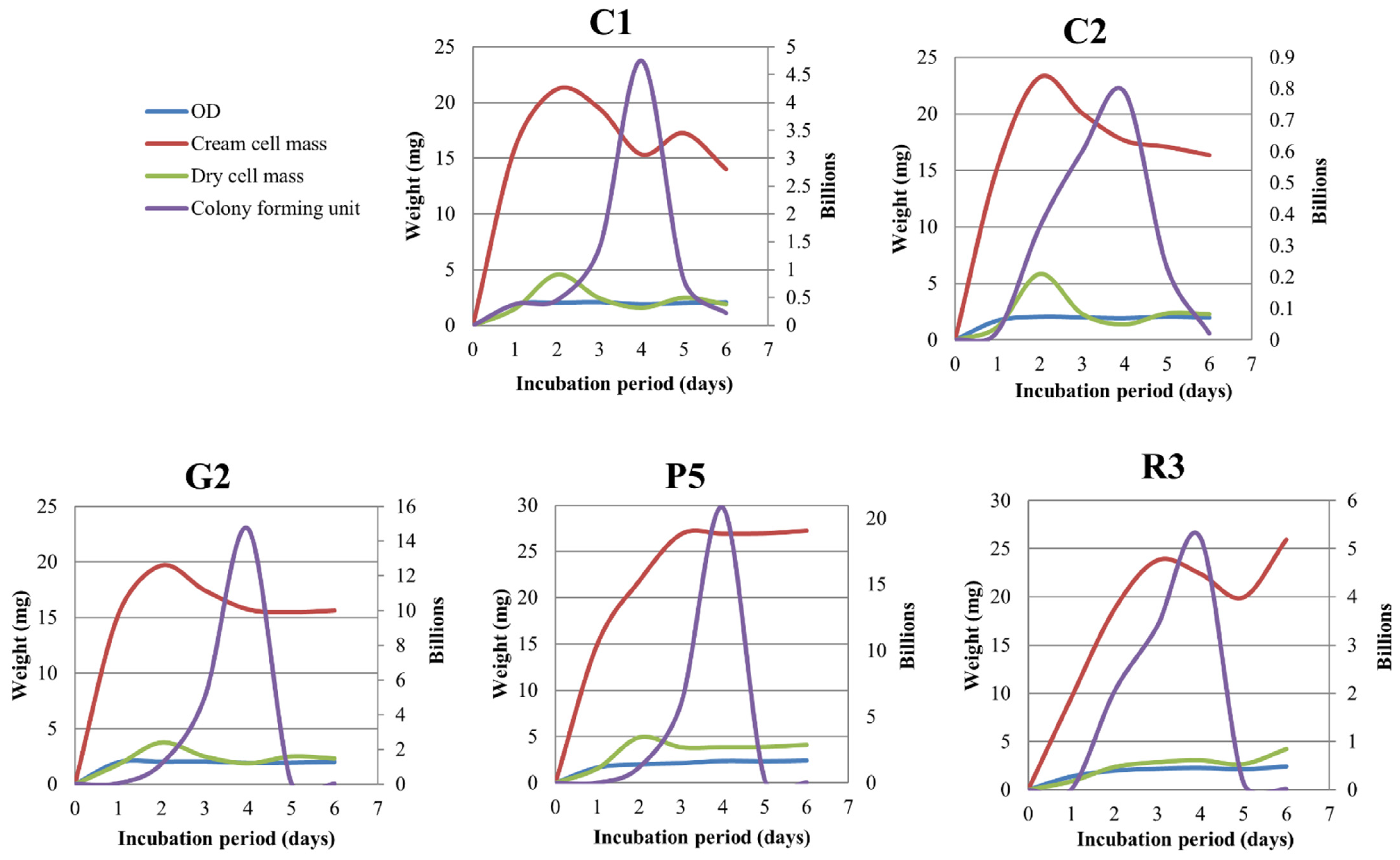
2.22. Measurement of Dry Cell Mass and CFU after Different Incubation Period
3. Results and Discussion
3.1. Selection of Yeast Strains with Desired Characteristics for Bread Production
3.2. Characterization of Isolated Yeast Strains
3.3. Assessment on the Potency of Identified S. cerevisiae for Bread Production
3.4. Production of H2S
3.5. Measurement of Gas Production
3.6. Optimization
3.7. Incubation Period
3.8. Relationship between OD, Cream Cell Mass, Dry Cell Mass, CFU mL−1, and Incubation Period
3.9. Effect of Shaking
3.10. Preparation of Bread
4. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Zhou, N.; Semumu, T.; Gamero, A. Non-conventional yeasts as alternatives in modern baking for improved performance and aroma enhancement. Fermentation 2021, 7, 102. [Google Scholar] [CrossRef]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410.e16. [Google Scholar] [CrossRef] [PubMed]
- Landis, E.A.; Oliverio, A.M.; McKenney, E.A.; Nichols, L.M.; Kfoury, N.; Biango-Daniels, M.; Shell, L.K.; Madden, A.A.; Shapiro, L.; Sakunala, S.; et al. The diversity and function of sourdough starter microbiomes. Elife 2021, 10, e61644. [Google Scholar] [CrossRef] [PubMed]
- Madden, A.A.; Lahue, C.; Gordy, C.L.; Little, J.L.; Nichols, L.M.; Calvert, M.D.; Dunn, R.R.; Smukowski Heil, C. Sugar-seeking insects as a source of diverse bread-making yeasts with enhanced attributes. Yeast 2021, 39, 108–127. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, H.; Lu, J.; Chen, S.C.A.; Kong, F.; Ma, X.J.; Xu, Y.C. Three clustered cases of candidemia caused by Candida quercitrusa and mycological characteristics of this novel species. J. Clin. Microbiol. 2014, 52, 3044–3048. [Google Scholar] [CrossRef][Green Version]
- Bell, P.J.L.; Higgins, V.J.; Attfield, P.V. Comparison of fermentative capacities of industrial baking and wild-type yeasts of the species Saccharomyces cerevisiae in different sugar media. Lett. Appl. Microbiol. 2001, 32, 224–229. [Google Scholar] [CrossRef]
- Hino, A.; Mihara, K.; Nakashima, K.; Takano, H. Trehalose levels and survival ratio of freeze-tolerant versus freeze-sensitive yeasts. Appl. Environ. Microbiol. 1990, 56, 1386–1391. [Google Scholar] [CrossRef]
- Birch, A.N.; Petersen, M.A.; Arneborg, N.; Hansen, Å.S. Influence of commercial baker’s yeasts on bread aroma profiles. Food Res. Int. 2013, 52, 160–166. [Google Scholar] [CrossRef]
- Gelinas, P.; Gélinas, P. Inventions on baker’s yeast storage and activation at the bakery plant. Recent Pat. Food. Nutr. Agric. 2010, 2, 1–11. [Google Scholar] [CrossRef]
- Johansen, P.G.; Owusu-Kwarteng, J.; Parkouda, C.; Padonou, S.W.; Jespersen, L. Occurrence and Importance of Yeasts in Indigenous Fermented Food and Beverages Produced in Sub-Saharan Africa. Front. Microbiol. 2019, 10, 1789. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.X.; Opulente, D.A.; Kominek, J.; Zhou, X.; Steenwyk, J.L.; Buh, K.V.; Haase, M.A.B.; Wisecaver, J.H.; Wang, M.; Doering, D.T.; et al. Tempo and Mode of Genome Evolution in the Budding Yeast Subphylum. Cell 2018, 175, 1533–1545.e20. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.I.; Lachance, M.A.; Herrera, C.M. Nectar yeasts of two southern Spanish plants: The roles of immigration and physiological traits in community assembly. FEMS Microbiol. Ecol. 2012, 80, 281–293. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Nyanga, L.K.; Nout, M.J.R.; Gadaga, T.H.; Theelen, B.; Boekhout, T.; Zwietering, M.H. Yeasts and lactic acid bacteria microbiota from masau (Ziziphus mauritiana) fruits and their fermented fruit pulp in Zimbabwe. Int. J. Food Microbiol. 2007, 120, 159–166. [Google Scholar] [CrossRef]
- Spencer, J.F.T.; Spencer, D.M. Yeasts in Natural and Artificial Habitats; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997; Volume 381, pp. 11–32. [Google Scholar]
- Hernandez-Lopez, M.J.; Prieto, J.A.; Randez-Gil, F. Osmotolerance and leavening ability in sweet and frozen sweet dough. Comparative analysis between Torulaspora delbrueckii and Saccharomyces cerevisiae baker’s yeast strains. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2003, 84, 125–134. [Google Scholar] [CrossRef]
- Tamang, J.P.; Fleet, G.H. Yeasts diversity in fermented foods and beverages. In Yeast Biotechnology: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2009; pp. 169–198. [Google Scholar]
- Azmuda, N.; Jahan, N.; Khan, A.R. Production and Comparison of Indigenous and Commercial Baker’s Yeasts. Bangladesh J. Microbiol. 2006, 23, 89–92. [Google Scholar] [CrossRef]
- Reale, A.; Reale, A.; Di Renzo, T.; Succi, M.; Tremonte, P.; Coppola, R.; Sorrentino, E. Microbiological and Fermentative Properties of Baker’s Yeast Starter Used in Breadmaking. J. Food Sci. 2013, 78, M1224–M1231. [Google Scholar] [CrossRef]
- Alfonzo, A.; Gaglio, R.; Barbera, M.; Francesca, N.; Moschetti, G.; Settanni, L. Evaluation of the Fermentation Dynamics of Commercial Baker’s Yeast in Presence of Pistachio Powder to Produce Lysine-Enriched Breads. Fermentation 2019, 6, 2. [Google Scholar] [CrossRef]
- Meroth, C.B.; Hammes, W.P.; Hertel, C. Identification and Population Dynamics of Yeasts in Sourdough Fermentation Processes by PCR-Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2003, 69, 7453–7461. [Google Scholar] [CrossRef] [PubMed]
- Mamun-Or-Rashid, A.N.M.; Dash, B.K.; Chowdhury, M.N.A.; Waheed, M.F.; Pramanik, M.K. Exploration of potential baker’s yeast from sugarcane juice: Optimization and evaluation. Pakistan J. Biol. Sci. 2013, 16, 617–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Akyüz, G.; Mazı, B.G. Physicochemical and sensory characterization of bread produced from different dough formulations by Kluyveromyces lactis. J. Food Process. Preserv. 2020, 44, e14498. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Herrera-Malaver, B.; Rezaei, M.N.; Steensels, J.; Courtin, C.M.; Verstrepen, K.J. Non-conventional yeast strains increase the aroma complexity of bread. PLoS ONE 2016, 11, e0165126. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; House, J.F.; Joseph, C.M.L.; Bisson, L.F.; Bamforth, C.W. Lachancea thermotolerans as an alternative yeast for the production of beer. J. Inst. Brew. 2016, 122, 599–604. [Google Scholar] [CrossRef]
- Urien, C.; Legrand, J.; Montalent, P.; Casaregola, S.; Sicard, D. Fungal species diversity in French bread sourdoughs made of organic wheat flour. Front. Microbiol. 2019, 10, 201. [Google Scholar] [CrossRef]
- Singh Nehra, K.; Jangra, M.R.; Pooja, S.; Minakshi, A.; Pooja, M.; Rama, B.; Hitesh, S.; Pardeep, P.; Sumit, J. Production of Bioethanol from Sugarcane Juice, Molasses and Paddy Straw using Saccharomyces cerevisiae. Biosci. Biotechnol. Res. Commun. 2021, 14, 581–586. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Concas, S.; Bengtsson, S.; Reis, M.A.M. Mixed culture polyhydroxyalkanoates production from sugar molasses: The use of a 2-stage CSTR system for culture selection. Bioresour. Technol. 2010, 101, 7112–7122. [Google Scholar] [CrossRef]
- Rattanapan, A.; Limtong, S.; Phisalaphong, M. Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. Appl. Energy 2011, 88, 4400–4404. [Google Scholar] [CrossRef]
- Cáceres-Farfán, M.; Lappe, P.; Larqué-Saavedra, A.; Magdub-Méndez, A.; Barahona-Pérez, L. Ethanol production from henequen (Agave fourcroydes Lem.) juice and molasses by a mixture of two yeasts. Bioresour. Technol. 2008, 99, 9036–9039. [Google Scholar] [CrossRef] [PubMed]
- Rández-Gil, F.; Ballester-Tomás, L.; Prieto, J.A. Yeast. In Bakery Products Science and Technology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 153–174. ISBN 9781119967156. [Google Scholar]
- Satyanarayana, T.; Kunze, G. Yeast Biotechnology: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-90-481-7833-9. [Google Scholar]
- Masneuf-Pomarede, I.; Bely, M.; Marullo, P.; Albertin, W. The genetics of non-conventional wine yeasts: Current knowledge and future challenges. Front. Microbiol. 2016, 6, 1563. [Google Scholar] [CrossRef] [PubMed]
- Naseeb, S.; Visinoni, F.; Hu, Y.; Hinks Roberts, A.J.; Maslowska, A.; Walsh, T.; Smart, K.A.; Louis, E.J.; Delneri, D. Restoring fertility in yeast hybrids: Breeding and quantitative genetics of beneficial traits. Proc. Natl. Acad. Sci. USA 2021, 118, e2101242118. [Google Scholar] [CrossRef] [PubMed]
- Ceccato-Antonini, S.R.; Covre, E.A. From baker’s yeast to genetically modified budding yeasts: The scientific evolution of bioethanol industry from sugarcane. FEMS Yeast Res. 2020, 20, foaa065. [Google Scholar] [CrossRef] [PubMed]
- Codón, A.C.; Rincón, A.; Rincon, A.M.; Moreno-Mateos, M.A.; Delgado-Jarana, J.; Rey, M.; Limon, C.; Rosado, I.V.; Cubero, B.; Peñate, X.; et al. New Saccharomyces cerevisiae baker’s yeast displaying enhanced resistance to freezing. J. Agric. Food Chem. 2003, 51, 483–491. [Google Scholar] [CrossRef]
- Davis, T.S.; Boundy-Mills, K.; Landolt, P.J. Volatile Emissions from an Epiphytic Fungus are Semiochemicals for Eusocial Wasps. Microb. Ecol. 2012, 64, 1056–1063. [Google Scholar] [CrossRef]
- Bellut, K.; Krogerus, K.; Arendt, E.K. Lachancea fermentati Strains Isolated From Kombucha: Fundamental Insights, and Practical Application in Low Alcohol Beer Brewing. Front. Microbiol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Salomé, S.; Pais, M.; Chaves Das Neves, H.J. Sugar Content of the Nectary Exudate of Epipactis Atropurpurea Rafin. Apidologie 1980, 11, 39–45. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Branco, P.; Almeida, M.G.; Caldeira, J.; Albergaria, H.; Arneborg, N. Cell-to-cell contact and antimicrobial peptides play a combined role in the death of Lachanchea thermotolerans during mixed-culture alcoholic fermentation with Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2015, 362, fnv103. [Google Scholar] [CrossRef]
- Zhou, W.; Hui, Y.H.; De Leyn, I.; Pagani, M.A.; Rosell, C.M.; Selman, J.D.; Therdthai, N. Bakery Products Science and Technology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 9781119967156. [Google Scholar]
- De Araújo Vicente, M.; Fietto, L.G.; De Miranda Castro, I.; Gonçalves Dos Santos, A.N.; Coutrim, M.X.; Brandão, R.L. Isolation of Saccharomyces cerevisiae strains producing higher levels of flavoring compounds for production of “cachaça” the Brazilian sugarcane spirit. Int. J. Food Microbiol. 2006, 108, 51–59. [Google Scholar] [CrossRef]
- Sanni, A.I.; Lönner, C. Identification of yeasts isolated from Nigerian traditional alcoholic beverages. Food Microbiol. 1993, 10, 517–523. [Google Scholar] [CrossRef]
- Barnett, J.A.; James, A.; Payne, R.W.; Yarrow, D. Yeasts: Characteristics and Identification; Cambridge University Press: Cambridge, UK, 1983; p. 811. [Google Scholar]
- Boekhout, T.; Robert, V. Yeasts in Food; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–488. [Google Scholar]
- Harrigan, W.F.; McCance, M.E. Laboratory Methods in Microbiology; Academic Press: Cambridge, MA, USA, 1982; ISBN 1483274349. [Google Scholar]
- Palla, M.; Blandino, M.; Grassi, A.; Giordano, D.; Sgherri, C.; Quartacci, M.F.; Reyneri, A.; Agnolucci, M.; Giovannetti, M. Characterization and selection of functional yeast strains during sourdough fermentation of different cereal wholegrain flours. Sci. Rep. 2020, 10, 12856. [Google Scholar] [CrossRef]
- Camargo, J.Z.; Nascimento, V.M.; Stefanello, I.; de Andrade Silva, C.A.; Gonçalves, F.A.; Perdomo, I.C.; Vilela, D.M.; Simionatto, S.; Pereira, R.M.; da Paz, M.F.; et al. Biochemical evaluation, molecular characterization and identification of novel yeast strains isolated from Brazilian savannah fruits, chicken litter and a sugar and alcohol mill with biotechnological potential for biofuel and food industries. Biocatal. Agric. Biotechnol. 2018, 16, 390–399. [Google Scholar] [CrossRef]
- Murali, N.; Srinivas, K.; Ahring, B.K. Biochemical production and separation of carboxylic acids for biorefinery applications. Fermentation 2017, 3, 22. [Google Scholar] [CrossRef]
- Krogerus, K.; Magalhães, F.; Castillo, S.; Peddinti, G.; Vidgren, V.; De Chiara, M.; Yue, J.-X.; Liti, G.; Gibson, B. Lager Yeast Design Through Meiotic Segregation of a Saccharomyces cerevisiae × Saccharomyces eubayanus Hybrid. Front. Fungal Biol. 2021, 2, 733655. [Google Scholar] [CrossRef]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter cultures for sparkling wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Bigey, F.; Segond, D.; Friedrich, A.; Guezenec, S.; Bourgais, A.; Huyghe, L.; Agier, N.; Nidelet, T.; Sicard, D. Evidence for Two Main Domestication Trajectories in Saccharomyces cerevisiae Linked to Distinct Bread-Making Processes. Curr. Biol. 2021, 31, 722–732.e5. [Google Scholar] [CrossRef]
- Hamelman, J. Bread: A Baker’s Book of Techniques and Recipes; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 415. [Google Scholar]
- Bailey, C.H.; Sherwood, R.C. The Carbohydrate Sequence. Biochemistry of Bread Making. Ind. Eng. Chem. 1935, 27, 1426–1430. [Google Scholar] [CrossRef]
- Jansen, M.L.A.; Bracher, J.M.; Papapetridis, I.; Verhoeven, M.D.; de Bruijn, H.; de Waal, P.P.; van Maris, A.J.A.; Klaassen, P.; Pronk, J.T. Saccharomyces cerevisiae strains for second-generation ethanol production: From academic exploration to industrial implementation. FEMS Yeast Res. 2017, 17, fox044. [Google Scholar] [CrossRef]
- Naumova, E.S.; Sadykova, A.Z.; Martynenko, N.N.; Naumov, G.I. Molecular genetic characteristics of Saccharomyces cerevisiae distillers’ yeasts. Microbiol. Russian Fed. 2013, 82, 175–185. [Google Scholar] [CrossRef]
- Naumov, G.I.; Naumova, E.S.; Michels, C.A. Genetic variation of the repeated MAL loci in natural populations of Saccharomyces cerevisiae and Saccharomyces paradoxus. Genetics 1994, 136, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Baranwal, R. Yeast genetics and biotechnological applications. In Yeast Biotechnology: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2009; pp. 323–355. [Google Scholar]
- Hyma, K.E.; Saerens, S.M.; Verstrepen, K.J.; Fay, J.C. Divergence in wine characteristics produced by wild and domesticated strains of Saccharomyces cerevisiae. FEMS Yeast Res. 2011, 11, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.J.; Legras, J.L.; Saliba, R.; Gaillardin, C. Application of Multi Locus Sequence Typing to the analysis of the biodiversity of indigenous Saccharomyces cerevisiae wine yeasts from Lebanon. J. Appl. Microbiol. 2006, 100, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Azumi, M.; Goto-Yamamoto, N. AFLP analysis of type strains and laboratory and industrial strains of Saccharomyces sensu stricto and its application to phenetic clustering. Yeast 2001, 18, 1145–1154. [Google Scholar] [CrossRef]
- Legras, J.L.; Merdinoglu, D.; Cornuet, J.M.; Karst, F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 2007, 16, 2091–2102. [Google Scholar] [CrossRef]
- Alves-Araújo, C.; Almeida, M.J.; Sousa, M.J.; Leão, C. Freeze tolerance of the yeast Torulaspora delbrueckii: Cellular and biochemical basis. FEMS Microbiol. Lett. 2004, 240, 7–14. [Google Scholar] [CrossRef]
- Donalies, U.E.B.; Nguyen, H.T.T.; Stahl, U.; Nevoigt, E. Improvement of Saccharomyces yeast strains used in brewing, wine making and baking. Adv. Biochem. Eng. Biotechnol. 2008, 111, 67–98. [Google Scholar]
- Timouma, S.; Balarezo-Cisneros, L.N.; Pinto, J.; De La Cerda, R.; Bond, U.; Schwartz, J.M.; Delneri, D. Transcriptional Profile of the Industrial Hybrid Saccharomyces pastorianus Reveals Temperature-Dependent Allele Expression Bias and Preferential Orthologous Protein Assemblies. Mol. Biol. Evol. 2021, 38, 5437–5452. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Cook, D.J. Food, Fermentation, and Micro-Organisms, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–245. [Google Scholar]
- Compagno, C.; Porro, D.; Smeraldi, C.; Ranzi, B.M. Fermentation of whey and starch by transformed Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 1995, 43, 822–825. [Google Scholar] [CrossRef]
- Moyad, M.A. Brewer’s/baker’s yeast (Saccharomyces cerevisiae) and preventive medicine: Part I. Urol. Nurs. 2007, 27, 560–561. [Google Scholar]
- Lopez, C.L.F.; Beaufort, S.; Brandam, C.; Taillandier, P. Interactions between Kluyveromyces marxianus and Saccharomyces cerevisiae in tequila must type medium fermentation. World J. Microbiol. Biotechnol. 2014, 30, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montaño, D.M.; De Jesús Ramírez Córdova, J. The fermentative and aromatic ability of Kloeckera and Hanseniaspora yeasts. In Yeast Biotechnology: Diversity and Applications; Springer: Dordrecht, The Netherlands, 2009; pp. 281–305. [Google Scholar]
- Picazo, C.; Gamero-Sandemetrio, E.; Orozco, H.; Albertin, W.; Marullo, P.; Matallana, E.; Aranda, A. Mitochondria inheritance is a key factor for tolerance to dehydration in wine yeast production. Lett. Appl. Microbiol. 2015, 60, 217–222. [Google Scholar] [CrossRef] [PubMed]

| Isolates | C1 | C2 | G1 | G2 | P1 | P2 | P3 | P4 | P5 | R1 | R2 | R3 | R4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultural characteristics | Shape | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular | Circular |
| Colony color | Creamy White | Creamy White | Creamy | Creamy White | Creamy | Creamy | Creamy | Creamy | Creamy White | White | White | White | Creamy | |
| Opacity | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque | Opaque | |
| Elevation | Convex | Convex | Umbonate | Convex | Raised | Convex | Convex | Raised | Convex | Convex | Convex | Convex | Umbonate | |
| Surface | Smooth | Smooth | Smooth | Smooth | Rough | Smooth | Smooth | Rough | Smooth | Smooth | Smooth | Smooth | Smooth | |
| Edge | Entire | Entire | Entire | Entire | Entire | Entire | Entire | Entire | Entire | Dentate | Dentate | Dentate | Entire | |
| Consistency | Viscid | Viscid | Viscid | Viscid | Viscid | Viscid | Viscid | Viscid | Viscid | Viscid | Viscid | Viscid | Viscid | |
| Growth in liquid media | SG | SG | ALG | SG | ALG | ALG | ALG | ALG | SG | SG | SG | SG | ALG | |
| Morphological | Cell shape | Spherical | Spherical | Oval | Spherical | Elongated | Elongated | Elongated | Elongated | Spherical | Oval | Oval | Spherical | Elongated |
| Bud | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | |
| Ascospore | Present | Present | Absent | Present | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Present | |
| Biochemical characteristics | Glucose | + | + | + | + | w | w | w | w | + | + | + | + | + |
| Fructose | w | w | w | + | − | − | − | − | + | + | w | w | w | |
| Sucrose | + | + | − | + | − | − | − | − | + | + | + | + | − | |
| Maltose | w | + | w | w | − | − | w | − | + | + | + | + | w | |
| Galactose | + | + | − | + | − | − | − | − | + | + | + | + | − | |
| Lactose | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| KNO3 | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| (NH4)2SO4 | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Urease | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Ascospore | + | + | − | + | − | − | − | − | − | − | − | − | + | |
| Ethanol as sole carbon source | w | w | + | w | + | + | + | + | + | + | + | + | + | |
| Identity | S. cerevisiae | S. cerevisiae | S. rouxii | S. cerevisiae | S. bisporus | S. bisporus | S. rouxii | S. bisporus | S. cerevisiae | S. exigus | S. exigus | S. cerevisiae | S. rouxii | |
| Isolates | H2S Production | ||
|---|---|---|---|
| Bismuth Sulfite Agar | Kligler Iron Agar (KIA) | Sulfide Indole Motility (SIM) | |
| C1 | − | − | − |
| C2 | − | − | − |
| G2 | − | − | − |
| P5 | − | − | − |
| R3 | − | − | − |
| Samples | Granular Yeast, C1 (DBL, China) | Fresh C1 | Fresh G2 | |
|---|---|---|---|---|
| Amount (gm) | Yeast | 2.4 | 2.4 | 2.4 |
| Dough | 356 | 356 | 356 | |
| Incubation | Temperature | Room | Room | Room |
| Time | 30 min | 30 min | 30 min | |
| Increased dough volume (%) | 114 | 108 | 108 | |
| Baking | Temperature | 180 °C | 180 °C | 180 °C |
| Period | 1.5 h | 1.5 h | 1.5 h | |
| Increased bread volume (%) | 171 | 170 | 170 | |
| Color | Characteristics | Characteristics | Characteristics | |
| Texture | Best | Good | Better | |
| Taste | Best | Good | Better | |
| Flavor | Pleasant | Pleasant | Better | |
| Mouth feeling | Good | Medium | Best | |
| Remarks | Longer incubation and baking period required | Longer incubation and baking period required | Longer incubation and baking period required | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun-Or-Rashid, A.N.M.; Lucy, T.T.; Pramanik, M.K. Isolation, Identification, Optimization of Baker’s Yeast from Natural Sources, Scale-Up Production Using Molasses as a Cheap Carbohydrate Source, and Evaluation for Bread Production. Appl. Microbiol. 2022, 2, 516-533. https://doi.org/10.3390/applmicrobiol2030040
Mamun-Or-Rashid ANM, Lucy TT, Pramanik MK. Isolation, Identification, Optimization of Baker’s Yeast from Natural Sources, Scale-Up Production Using Molasses as a Cheap Carbohydrate Source, and Evaluation for Bread Production. Applied Microbiology. 2022; 2(3):516-533. https://doi.org/10.3390/applmicrobiol2030040
Chicago/Turabian StyleMamun-Or-Rashid, A. N. M., Tanzima Tarannum Lucy, and Md. Kamruzzaman Pramanik. 2022. "Isolation, Identification, Optimization of Baker’s Yeast from Natural Sources, Scale-Up Production Using Molasses as a Cheap Carbohydrate Source, and Evaluation for Bread Production" Applied Microbiology 2, no. 3: 516-533. https://doi.org/10.3390/applmicrobiol2030040
APA StyleMamun-Or-Rashid, A. N. M., Lucy, T. T., & Pramanik, M. K. (2022). Isolation, Identification, Optimization of Baker’s Yeast from Natural Sources, Scale-Up Production Using Molasses as a Cheap Carbohydrate Source, and Evaluation for Bread Production. Applied Microbiology, 2(3), 516-533. https://doi.org/10.3390/applmicrobiol2030040







