Enhanced Anti-Melanogenic Effect of Adlay Bran Fermented with Lactobacillus brevis MJM60390
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Adlay Bran Extracts
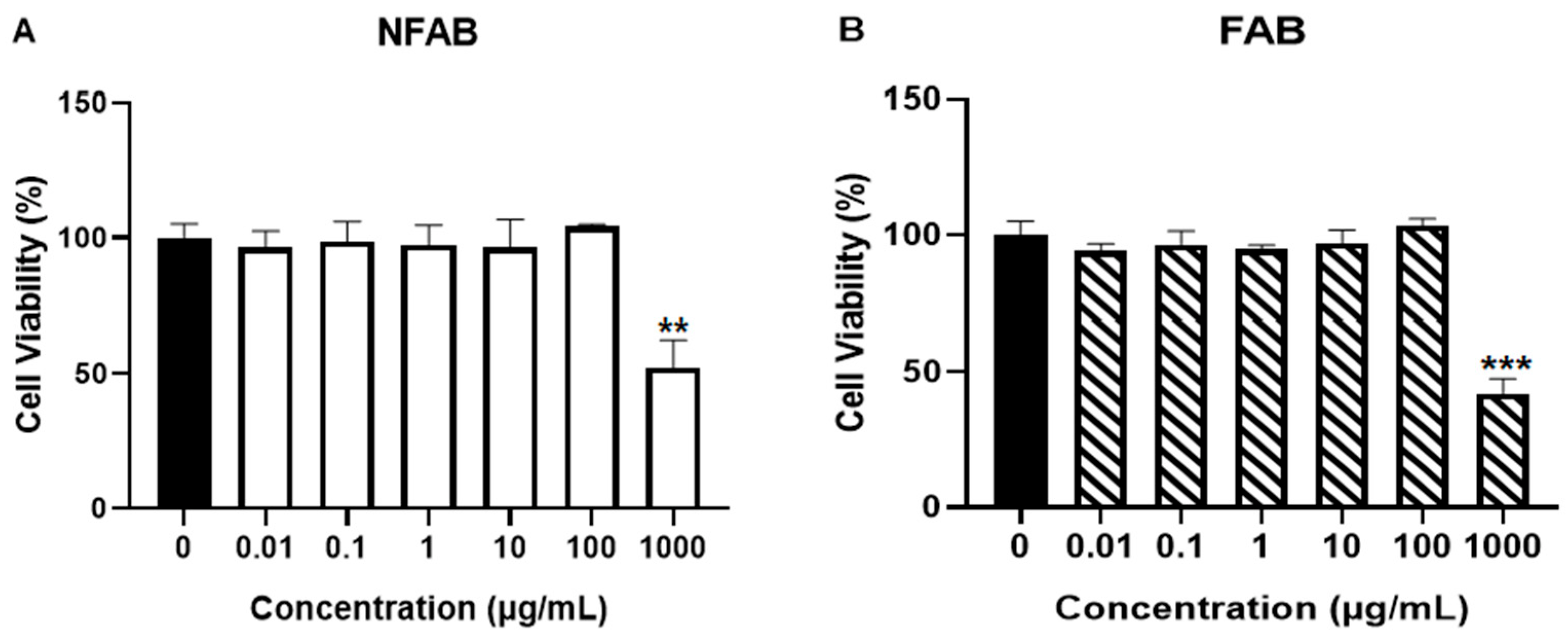
2.3. Cell Viability Assay
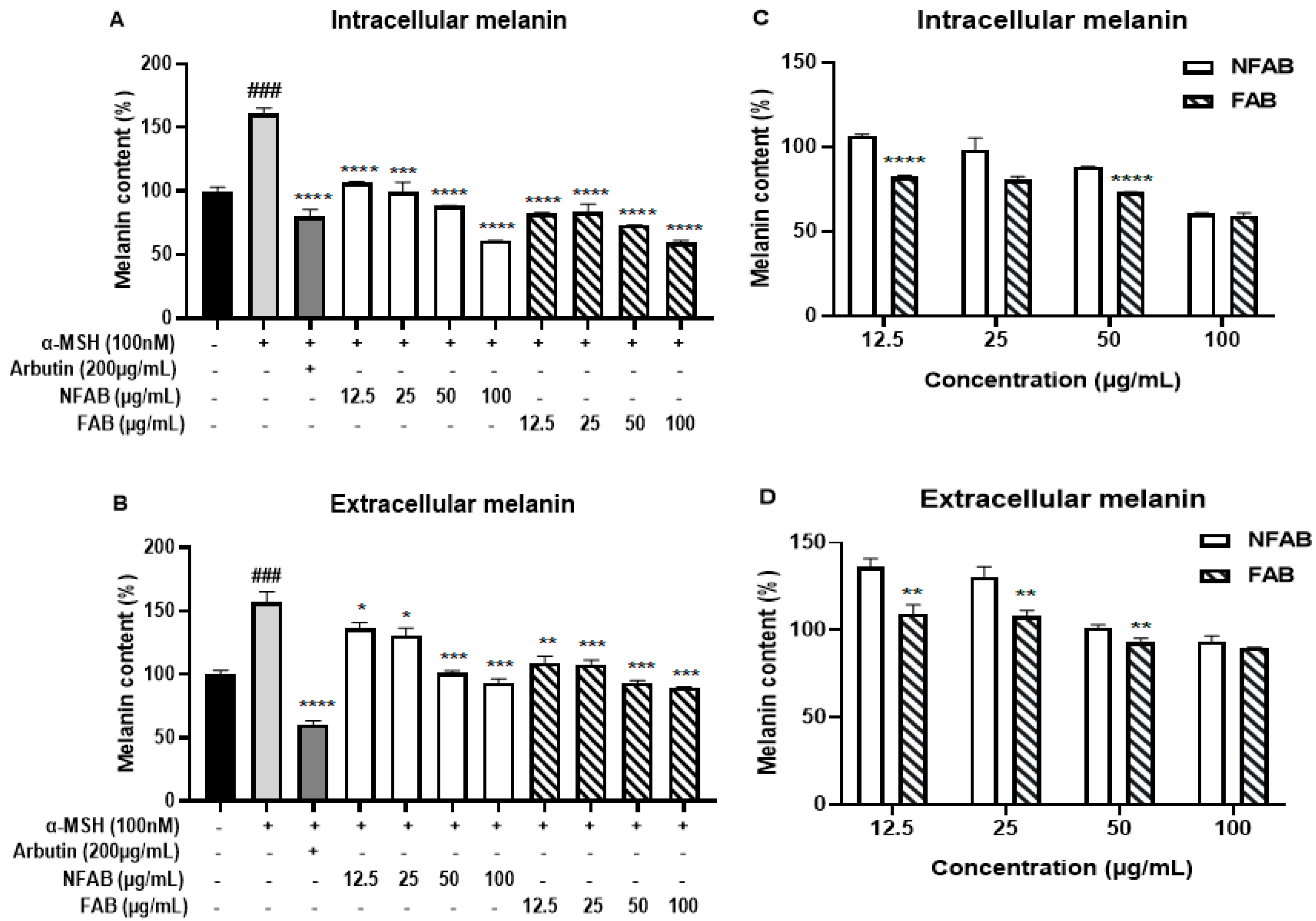
2.4. Determination of Melanin Content
2.5. Intracellular Tyrosinase Activity Assay
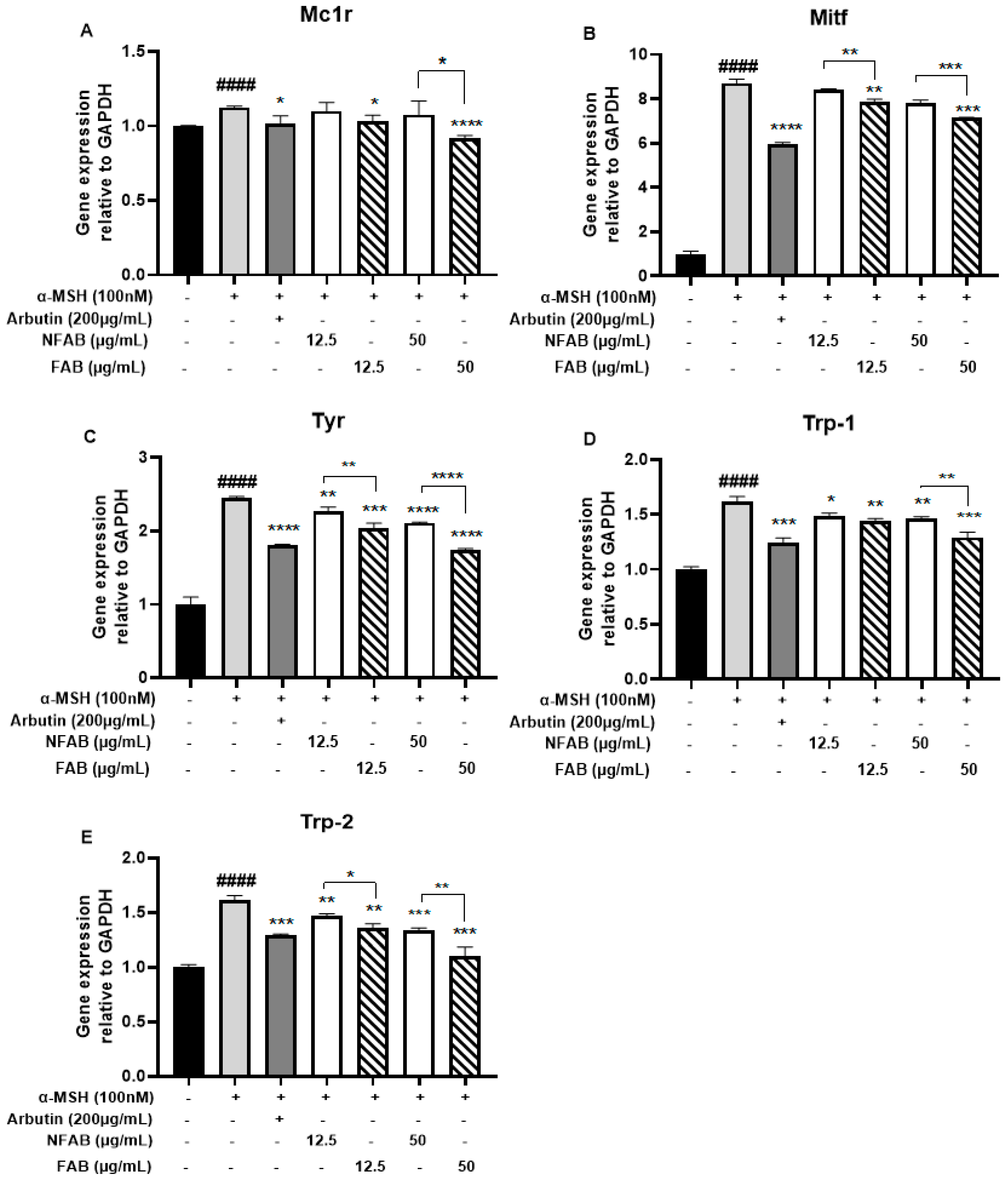
2.6. RNA Isolation and RT-qPCR
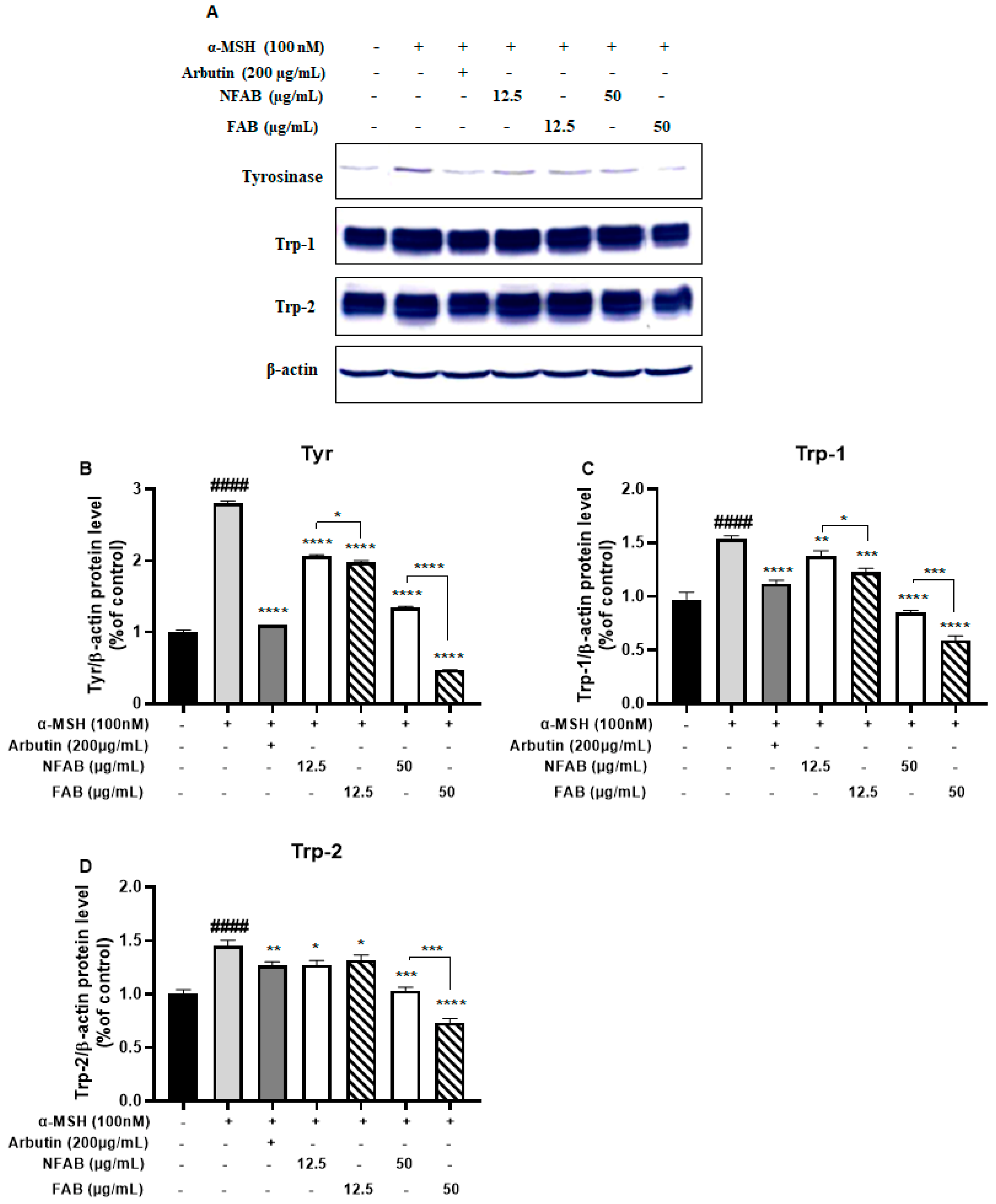
2.7. Western Blot Analysis
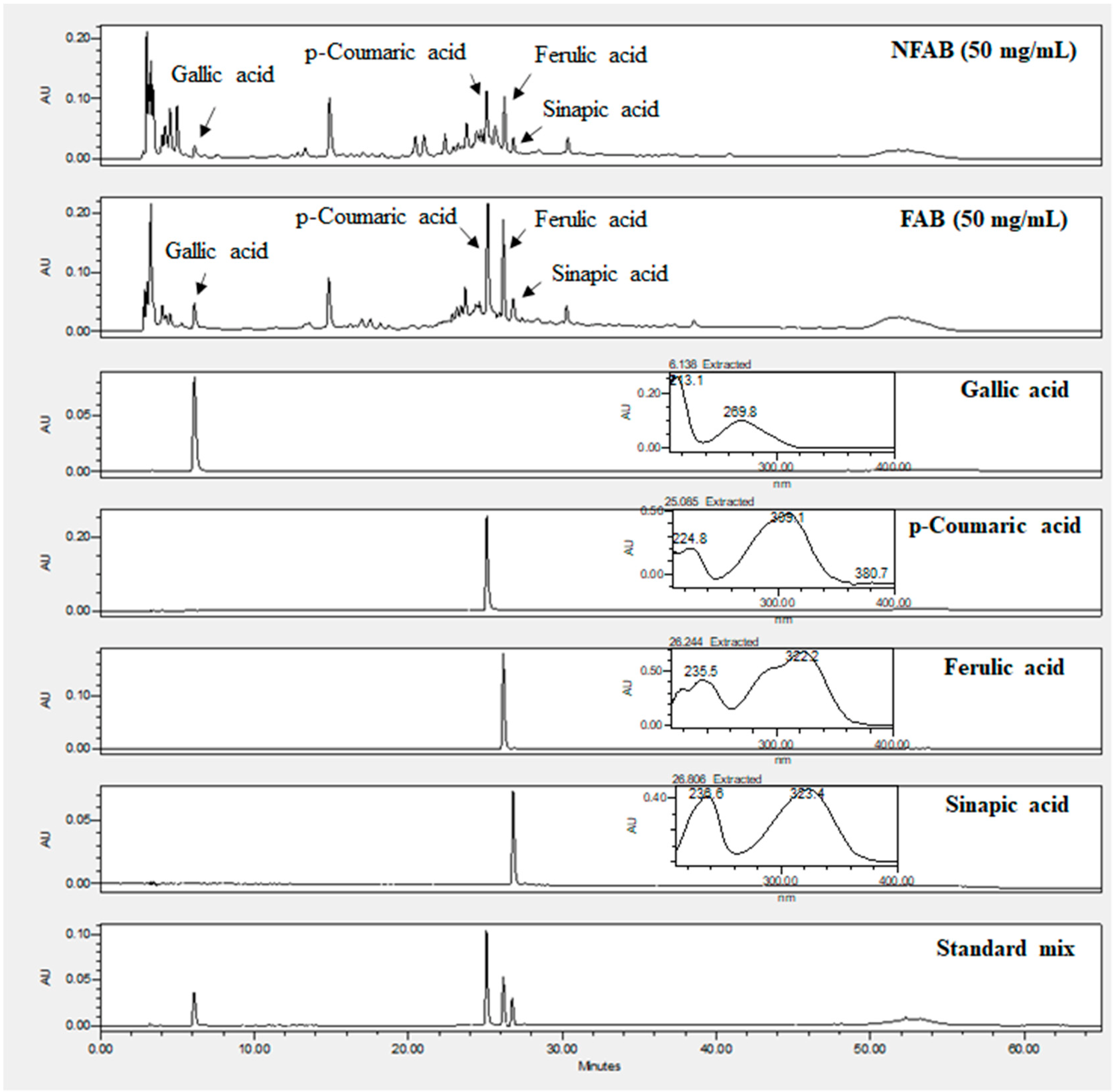
2.8. Analysis of Some Major Free Phenolic Compounds in Adlay Bran Extracts
3. Results
3.1. Effect of Adlay Bran Extracts on Melanocyte Proliferation
3.2. Effect of Adlay Bran Extracts on Melanin Content
3.3. Effect of Adlay Bran Extracts on Tyrosinase Activity
3.4. Effect of Adlay Bran Extracts on the Gene Expression of Mc1r, Mitf, Tyr, Trp-1, and Trp-2 in B16F10 Cells
3.5. Effect of Adlay Bran Extracts on Melanogenic Enzyme Protein Expression in B16F10 Cells
3.6. Changes in Phenolic Compounds in Adlay Bran Extracts after Fermentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortonne, J. Normal and abnormal skin color. In Annales de Dermatologie et de Vénéréologie; Elsevier: Amsterdam, The Netherlands, 2012; pp. S125–S129. [Google Scholar]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Choi, C.W.; Lee, J.Y.; Ahn, E.-K.; Oh, J.S.; Hong, S.S. Phytochemical constituents of Coix lachryma-jobi var. ma-yuen roots and their tyrosinase inhibitory activity. J. Appl. Biol. Chem. 2017, 60, 49–54. [Google Scholar] [CrossRef]
- Braun-Falco, O.; Plewig, G.; Wolff, H.H.; Burgdorf, W.H. Disorders of Melanin Pigmentation. In Dermatology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1013–1042. [Google Scholar]
- Hearing, V.J. Determination of melanin synthetic pathways. J. Investig. Dermatol. 2011, 131, E8. [Google Scholar] [CrossRef] [Green Version]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, T.; Hearing, V.J. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev. Dermatol. 2011, 6, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Busca, R.; Ballotti, R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000, 13, 60–69. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020. [Google Scholar] [CrossRef] [Green Version]
- De Jager, T.; Cockrell, A.; Du Plessis, S. Ultraviolet light induced generation of reactive oxygen species. Ultrav. Light Hum. Health Dis. Environ. 2017, 996, 15–23. [Google Scholar]
- Otsuka, H.; Hirai, Y.; Nagao, T.; Yamasaki, K. Anti-inflammatory activity of benzoxazinoids from roots of Coix lachryma-jobi var. ma-yuen. J. Nat. Prod. 1988, 51, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Gao, J.; Zeng, Y.; Liu, C.X. Effects of adlay seed oil on blood lipids and antioxidant capacity in hyperlipidemic rats. J. Sci. Food Agric. 2011, 91, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, Y.; Okamoto, K.; Sakamoto, H.; Sonoda, Y. Antimicrobial substances coixindens A and B in etiolated seedlings of adlay [Coix lachryma-jobi]. J. Agric. Chem. Soc. Jpn. 1993, 67, 1405–1410. [Google Scholar]
- Taddese, S.; Weiss, A.S.; Neubert, R.H.; Schmelzer, C.E. Mapping of macrophage elastase cleavage sites in insoluble human skin elastin. Matrix Biol. 2008, 27, 420–428. [Google Scholar] [CrossRef]
- Yao, H.-T.; Lin, J.-H.; Chiang, M.-T.; Chiang, W.; Luo, M.-N.; Lii, C.-K. Suppressive effect of the ethanolic extract of adlay bran on cytochrome P-450 enzymes in rat liver and lungs. J. Agric. Food Chem. 2011, 59, 4306–4314. [Google Scholar] [CrossRef]
- Huang, S.-H.; Ng, L.-T. Quantification of polyphenolic content and bioactive constituents of some commercial rice varieties in Taiwan. J. Food Compos. Anal. 2012, 26, 122–127. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Yi, Q.; Wang, X.; Ju, X. Protective effect of polyphenols extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) on hypercholesterolemia-induced oxidative stress in rats. Molecules 2012, 17, 8886–8897. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-C.; Chen, H.-H.; Chiang, W. Adlay (薏苡 yì yĭ; “soft-shelled Job’s tears”; the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) is a Potential Cancer Chemopreventive Agent toward Multistage Carcinogenesis Processes. J. Tradit. Complement. Med. 2012, 2, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-J.; Chung, C.-P.; Chiang, W.; Lin, Y.-L. Anti-inflammatory effects and chemical study of a flavonoid-enriched fraction from adlay bran. Food Chem. 2011, 126, 1741–1748. [Google Scholar] [CrossRef]
- Huang, D.-W.; Wu, C.-H.; Shih, C.-K.; Liu, C.-Y.; Shih, P.-H.; Shieh, T.-M.; Lin, C.-I.; Chiang, W.; Hsia, S.-M. Application of the solvent extraction technique to investigation of the anti-inflammatory activity of adlay bran. Food Chem. 2014, 145, 445–453. [Google Scholar] [CrossRef]
- Wu, T.-T.; Charles, A.L.; Huang, T.-C. Determination of the contents of the main biochemical compounds of Adlay (Coxi lachrymal-jobi). Food Chem. 2007, 104, 1509–1515. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of fermentation in improving nutritional quality of soybean meal—A review. Asian Australas. J. Anim. Sci. 2016, 29, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galati, A.; Oguntoyinbo, F.A.; Moschetti, G.; Crescimanno, M.; Settanni, L. The cereal market and the role of fermentation in cereal-based food production in Africa. Food Rev. Int. 2014, 30, 317–337. [Google Scholar] [CrossRef]
- Taskila, S.; Ojamo, H. The Current Status and Future Expectations in Industrial Production of Lactic Acid by Lactic Acid Bacteria; IntechOpen: Vienna, Austria, 2013. [Google Scholar]
- Rhee, S.J.; Lee, J.-E.; Lee, C.-H. Importance of Lactic Acid Bacteria in Asian Fermented Foods. In Microbial Cell Factories; BioMed Central: London, UK, 2011; pp. 1–13. [Google Scholar]
- Hole, A.S.; Rud, I.; Grimmer, S.; Sigl, S.; Narvhus, J.; Sahlstrøm, S. Improved bioavailability of dietary phenolic acids in whole grain barley and oat groat following fermentation with probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 2012, 60, 6369–6375. [Google Scholar] [CrossRef] [PubMed]
- Ngan, N.T.; Dao, D. Investigating The Effect of Ratio Lactobacilus acidophillus to The Antioxidant of Fermented Eggplant (Solanum melongena). J. Food Nutr. Sci. 2015, 3, 140–142. [Google Scholar]
- Chung, S.-Y.; Seo, Y.-K.; Park, J.-M.; Seo, M.-J.; Park, J.-K.; Kim, J.-W.; Park, C.-S. Fermented rice bran downregulates MITF expression and leads to inhibition of α-MSH-induced melanogenesis in B16F1 melanoma. Biosci. Biotechnol. Biochem. 2009, 73, 1704–1710. [Google Scholar] [CrossRef]
- Rashid, N.; Jamaluddin, A.; Ghani, A.; Razak, D.; Jonit, J.; Mansor, A.; Manan, M. Quantification of phenolic compounds changes by Aspergillus oryzae on rice bran fermentation. Food Res. 2018, 3, 133–137. [Google Scholar] [CrossRef]
- Huang, H.-C.; Hsieh, W.-Y.; Niu, Y.-L.; Chang, T.-M. Inhibitory effects of adlay extract on melanin production and cellular oxygen stress in B16F10 melanoma cells. Int. J. Mol. Sci. 2014, 15, 16665–16679. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kim, N.; Werlinger, P.; Suh, D.-A.; Lee, H.; Cho, J.-H.; Cheng, J. Probiotic Characterization of Lactobacillus brevis MJM60390 and In Vivo Assessment of Its Antihyperuricemic Activity. J. Med. Food 2022, 25, 367–380. [Google Scholar] [CrossRef]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B.A. Skin aging: Postulated mechanisms and consequent changes in structure and function. Clin. Geriatr. Med. 2001, 17, 617–630. [Google Scholar] [CrossRef]
- Barsh, G.S. What controls variation in human skin color? PLoS Biol. 2003, 1, e27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.; Yin, L.; Smuda, C.; Batzer, J.; Hearing, V.J.; Kolbe, L. Molecular and histological characterization of age spots. Exp. Dermatol. 2017, 26, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Ito, S. A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Chang, N.-F.; Chen, Y.-S.; Lin, Y.-J.; Tai, T.-H.; Chen, A.-N.; Huang, C.-H.; Lin, C.-C. Study of hydroquinone mediated cytotoxicity and hypopigmentation effects from UVB-irradiated arbutin and deoxyarbutin. Int. J. Mol. Sci. 2017, 18, 969. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Charlín, R.; Barcaui, C.B.; Kac, B.K.; Soares, D.B.; Rabello-Fonseca, R.; Azulay-Abulafia, L. Hydroquinone-induced exogenous ochronosis: A report of four cases and usefulness of dermoscopy. Int. J. Dermatol. 2008, 47, 19–23. [Google Scholar] [CrossRef]
- Spínola, V.; Mendes, B.; Câmara, J.S.; Castilho, P.C. Effect of time and temperature on vitamin C stability in horticultural extracts. UHPLC-PDA vs iodometric titration as analytical methods. LWT—Food Sci. Technol. 2013, 50, 489–495. [Google Scholar] [CrossRef]
- Ookubo, N.; Michiue, H.; Kitamatsu, M.; Kamamura, M.; Nishiki, T.-i.; Ohmori, I.; Matsui, H. The transdermal inhibition of melanogenesis by a cell-membrane-permeable peptide delivery system based on poly-arginine. Biomaterials 2014, 35, 4508–4516. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, S.; Wang, M.; Feng, X.-Y. Characterization of free, conjugated, and bound phenolic acids in seven commonly consumed vegetables. Molecules 2017, 22, 1878. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.H.; Celeghini, R.M.; Chang, Y.K. Effect of the fermentation of whole soybean flour on the conversion of isoflavones from glycosides to aglycones. Food Chem. 2011, 128, 640–644. [Google Scholar] [CrossRef]
- Cho, K.M.; Hong, S.Y.; Math, R.K.; Lee, J.H.; Kambiranda, D.M.; Kim, J.M.; Islam, S.M.A.; Yun, M.G.; Cho, J.J.; Lim, W.J. Biotransformation of phenolics (isoflavones, flavanols and phenolic acids) during the fermentation of cheonggukjang by Bacillus pumilus HY1. Food Chem. 2009, 114, 413–419. [Google Scholar] [CrossRef]
- Kim, Y.-J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar] [CrossRef] [Green Version]
- Su, T.-R.; Lin, J.-J.; Tsai, C.-C.; Huang, T.-K.; Yang, Z.-Y.; Wu, M.-O.; Zheng, Y.-Q.; Su, C.-C.; Wu, Y.-J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [Green Version]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, H.; Kawakami, F.; Lwin, T.-T.; Imai, M.; Shamsa, F. Biochemical characterization of ferulic acid and caffeic acid which effectively inhibit melanin synthesis via different mechanisms in B16 melanoma cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.S.; Lee, N.-H.; Hyun, C.-G.; Shin, D.-B. Differential effects of methoxylated p-coumaric acids on melanoma in B16/F10 cells. Prev. Nutr. Food Sci. 2015, 20, 73. [Google Scholar] [CrossRef] [Green Version]

| Name | Primer | Sequence (5′ to 3′) |
|---|---|---|
| Tyr | Forward | AAGAATGCTGCCCACCATGG |
| Reverse | CACGGTCATCCACCCCTTTG | |
| Trp-1 | Forward | CAGTGCAGCGTCTTCCTGAG |
| Reverse | TTCCCGTGGGAGCACTGTAA | |
| Trp-2 | Forward | CGTGCTGAACAAGGAATGC |
| Reverse | CGAAGGATATAAGGGCCACTC | |
| Mitf | Forward | ATCCCATCCACCGGTCTCTG |
| Reverse | CCGTCCGTGAGATCCAGAGT | |
| Mc1r | Forward | TCATCGTCCTCTGCCCTCAG |
| Reverse | GCAGCACCTCCTTGAGTGTC | |
| Gapdh | Forward | GGTTGTCTCCTGCGACTTCA |
| Reverse | TGGTCCAGGGTTTCTTACTCC |
| Free Phenolic Compounds (mg/100 g Extract) | Samples | Linearity and Linear Range for Standard | |
|---|---|---|---|
| NFAB | FAB | ||
| Gallic acid | 29.81 ± 1.77 | 51.11 ± 0.97 **** | 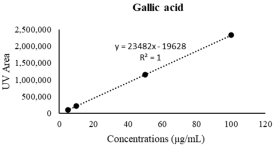 |
| p-Coumaric acid | 23.15 ± 0.34 | 41.01 ± 0.98 **** | 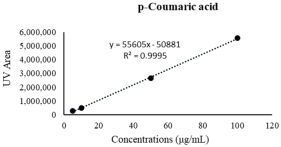 |
| Ferulic acid | 83.37 ± 1.59 | 155.07 ± 2.36 **** | 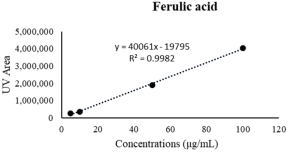 |
| Sinapic acid | 24.35 ± 1.94 | 43.75 ± 1.76 *** | 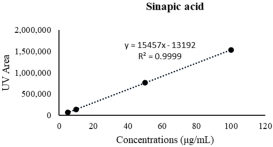 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Gu, M.; Choi, C.W.; Choi, Y.-H.; Suh, J.-W.; Cheng, J. Enhanced Anti-Melanogenic Effect of Adlay Bran Fermented with Lactobacillus brevis MJM60390. Appl. Microbiol. 2022, 2, 502-515. https://doi.org/10.3390/applmicrobiol2030039
Nguyen HT, Gu M, Choi CW, Choi Y-H, Suh J-W, Cheng J. Enhanced Anti-Melanogenic Effect of Adlay Bran Fermented with Lactobacillus brevis MJM60390. Applied Microbiology. 2022; 2(3):502-515. https://doi.org/10.3390/applmicrobiol2030039
Chicago/Turabian StyleNguyen, Huong T., Mingkun Gu, Chun Whan Choi, Yun-Hyeok Choi, Joo-Won Suh, and Jinhua Cheng. 2022. "Enhanced Anti-Melanogenic Effect of Adlay Bran Fermented with Lactobacillus brevis MJM60390" Applied Microbiology 2, no. 3: 502-515. https://doi.org/10.3390/applmicrobiol2030039
APA StyleNguyen, H. T., Gu, M., Choi, C. W., Choi, Y.-H., Suh, J.-W., & Cheng, J. (2022). Enhanced Anti-Melanogenic Effect of Adlay Bran Fermented with Lactobacillus brevis MJM60390. Applied Microbiology, 2(3), 502-515. https://doi.org/10.3390/applmicrobiol2030039






