Abstract
Biological control is an environmentally friendly approach that holds promise to complement or replace chemicals to effectively protect crop plants against pests and pathogens. Environmental samples with highly diverse and competitive microbiomes that harbor antagonistic microbes with diverse modes-of-action can provide a rich source of microbial biopesticides. In the current study, bacteria isolated from rhizosphere soil and food spoilage samples were subsequently screened against various plant fungal and oomycete pathogens in growth inhibition assays. These included the new potential biocontrol bacteria Corynebacterium flavescens, Sporosarcina aquimarina and Sporosarcina saromensis with anti-fungal and antioomycete activities. Potential candidates selected by preliminary screening in plant assays were then applied to tomato, cabbage and chickpea plants to control bacterial (Pseudomonas syringae pv. tomato), fungal (Alternaria brassicicola) and oomycete (Phytophtora medicaginis) phytopathogens. Ten potential microbial biopesticides were demonstrated to be effective against these diseases, and led to significant (p < 0.05) reductions in symptoms and/or pathogen DNA compared to mock-treated diseased plants. We conclude that new and effective microbial biopesticides to control crop pathogens can be rapidly isolated from biodiverse microbiomes, where bacteria may employ these features to effectively compete against each other.
1. Introduction
Pests and pathogens use plants as sources of energy and pose significant threats to agricultural production and food security [1]. Food production must increase by 60% by 2050 to support human demands; however, 10 to 16% of crop production is estimated to be destroyed by pests and pathogens [2]. These include the five most important crops–wheat, rice, maize, potato and soybean–with estimated global crop losses caused by pests and pathogens of 21.5, 30.0, 22.6, 17.2 and 21.4%, respectively [3]. Agrochemicals are frequently applied to control pests and pathogens, but they often harm beneficial microbes and their residues remain in almost 40% of foods, according to FAO-WHO reports [4]. Furthermore, pesticides pose significant health risks to humans and their environment, and provoked or induced the development of resistance both in plant pathogens and pests [5,6]. Many beneficial microorganisms isolated from plants or other environments can promote plant growth and/or suppress plant pathogens [7]. These microbes can be applied as biocontrol agents called biopesticides [8]. These biopesticides are considered much more environmentally friendly alternatives to protect crops against pathogens in the future. There are currently only about 25 microbial products that are in regular use [9,10]. This contrasts with the thousands of chemical products that are in the market, and there is thus a need to develop more biopesticides that have diverse modes-of-action [11]. The present study focused on bespoke treatments of three important vegetable crops (tomato, cabbage and chickpea) and three types of economically significant pathogens with different nutrient uptake mechanisms, including the hemibiotrophic bacterium Pseudomonas syringae, the necrotrophic fungus Alternaria brassicicola and the hemibiotrophic oomycete Phytophthora medicaginis, respectively.
Currently, only a few bacterial genera, Bacillus, Pseudomonas, Agrobacterium and Streptomyces, are used as biopesticides [12]. According to the Dunham Trimmer estimation, the biopesticide market is around 5–6% of the total global pesticide, valuing US$3–4 billion [10]. Looking into highly diverse microbial environments, such as organic carbon-rich soil and food spoilage, may enhance new biopesticide discovery that could potentially improve food production. Soils are highly diverse environments constructed from billions of individual organisms, including various bacteria [13]. While it had previously been thought that most bacteria are unculturable, it has recently been shown that up to 70% of plant-associated microbes may be culturable using diverse media (Known Media Database; KOMODO; [14,15]). We hypothesised that the plant rhizosphere presents a highly diverse, carbon-rich environment where bacteria with interesting biotechnological activities can be isolated. Plant rhizosphere bacteria compete with other microbes, including plant pathogens, for nutrition and resources such as root exudates via antibiosis or hyperparasitism. These include various strategies such as nutrient competition and modulating pathogen growth conditions [16,17]. Environmental samples with highly diverse and competitive microbiomes can provide a rich source of anti-microbial compounds [18,19], and microbiomes with an abundance of organic nutrients (rhizosphere soils and food spoilage samples) were chosen for the current study.
Both in vitro and in vivo tests can be used to identify effective antagonistic bacteria that possibly have not been considered as biocontrol agents [20]. Many vegetables are severely affected by bacterial, fungal and oomycete pathogens [21,22]. In the current study, we have focused on 68 previously identified bacteria antagonistic against bacterial pathogens obtained from rhizosphere soil and food spoilage [23], and developed ten potential new microbial biopesticides against bacterial, fungal and oomycete phytopathogens.
2. Materials and Methods
2.1. Anti-Fungal and Antioomycete Assays
A total of 68 rhizosphere soil and food spoilage bacteria were previously found to be active against bacterial pathogens Pseudomonas syringae pv. tomato DC3000 (Pst) and Clavibacter michiganensis [23]. These came from clay-rich soil collected in Tennyson, Queensland, Australia (27°31′37.0″ S 152°59′51.7″ E) and food spoilage samples (mixed vegetable and fruit scraps from a compost bin), and were identified by 16S rRNA sequencing. In the present study, these bacteria were further evaluated for their potential as biocontrol agents against various fungal and oomycete plant pathogens. Bacterial isolates were grown on various liquid media including Yeast Extract Peptone (YEP), Liquid Medium (LM), Luria Bertani (LB) and De Man, Rogosa and Sharpe (MRS) at 28 °C in a shaker incubator at 120 rpm (Supplementary Table S1). All growth inhibition assays were then performed on Petri dishes using PDA (Supplementary Table S1) [24]. The potential antagonizing effects of bacterial isolates were examined against fungal and oomycete plant pathogens, including Fusarium oxysporum, Alternaria brassicicola, Alternaria solani, Phytophthora capsici, Phytophthora medicaginis and Phytophthora cinnamomi. Potential biocontrol bacteria were cultured overnight at 28 °C in a shaker incubator. The pure fungal inoculum was placed in the center of a new PDA plate with two lines of streaked biocontrol bacteria, including Staphylococcus saprophyticus for comparison in three replicate plates for each isolate (Supplementary Figure S1). Staphylococcus saprophyticus was chosen as a reference microbe because its antagonistic activity against the pathogens tested was low, it came from the same soil samples and can be incubated under identical conditions. Plates were incubated at 28 °C until the control plate for each pathogen (without biocontrol bacteria) reached ~80% fungal coverage of the plate (1–2 weeks). Anti-fungal/oomycete effects were quantified by the following formula [24]:
(Average control plate colony diameter − Average bacteria treated plate colony diameter)/Average control plate colony diameter × 100.
A minimum of 50% in vitro pathogen inhibition was applied as a threshold for further experimentation with these microbial isolates. These were then used in preliminary plant assays where they were sprayed onto tomato and cabbage or watering to chickpea seedlings (one plant per isolate) to select four isolates for subsequent in plant biocontrol assays.
2.2. Biopesticide and Plant Bacterial Disease Suppression Assays
Solanum lycopersicum (tomato) plants (cv Moneymaker) were grown in 55 mm pots in UQ23 potting mix for 4 weeks in growth cabinets with 16 h light at 26 °C and 8 h night at 21 °C, with a light intensity of 100 μmol photons m−2 s−1 (white fluorescent lamps). Eight-week-old tomato plants, in three biological replicates containing 10 pooled plants per replicate, were sprayed with potential beneficial biocontrol bacteria (33YE, 46YE, 14th, 28MC and a mixture of all isolates) that also previously showed antibacterial activity against Pst [23], 1 day before and 1 day after bacterial pathogen treatment. Briefly, the four bacterial isolates were grown overnight in 10 mL cultures that were upscaled to five 100 mL cultures each in 500 mL flasks. The cultures were centrifuged at 4170× g for 10 min, the supernatants were discarded and bacterial pellets were resuspended in 10 mM MgCl2 containing 0.02% Silwet L-77. The OD600 for all bacterial cultures was adjusted to 1.57. The same procedure was repeated for Pst, and the OD600 was adjusted to 0.2 based on previous studies [25]. Bacterial suspensions were then sprayed onto plant leaves by applying both sides of each leaflet of all leaves for each plant, and mock-treatments comprised application with MgCl2 solution only. Six days after pathogen inoculation, all plant leaves were collected. Disease symptom scores were based on the percentage of leaflet area displaying chlorosis, brown spots or necrosis (1 = 0–20%; 2 = 20–40%; 3 = 40–60%; 4 = 60–80%; 5 = 80–100%). Total DNA from the same leaflets was extracted with the CTAB method [26]. For pathogen quantification using real-time quantitative PCR (qPCR), 40 ng of genomic DNA was mixed with 6 µL of FastStart Essential DNA Green Master mix (Bioline, UK) containing 3 µM each of the corresponding primers (Supplementary Table S2). The copy number of a specific Pst gene (gyrA) was determined for each sample using qPCR (Roche LightCycler® 96, Basel, Switzerland) and normalized to tomato ACTIN [27,28].
2.3. Biopesticide and Plant Fungal Disease Suppression Assays
Golden Acre cabbage plants (Brassica oleracea var. capitata) were grown in a growth cabinet under 16 h light at 26 °C and 8 h night at 21 °C with a light intensity of 100 μmol photons m−2 s−1 (white fluorescent lamps). 4-week-old plants in three biological replicates containing 10 plants per replicate were inoculated with potential biocontrol isolates 46YE, 28M, 4YE, 44LGS and a mixture of all isolates 1 day before and 1 day after fungal pathogen treatment. Cultivations of bacterial strains and preparations for mock treatments are explained in Section 2.2. Cabbage plants were inoculated with Alternaria brassicicola 24 h after initial treatment, with biocontrol bacteria based on the established protocol for A. solani [29]. Briefly, spores from 2-week-old A. brassicicola cultures on PDA plates were harvested using sterile water containing 0.005% (v/v) Tween 40. The spore concentration was then measured using a hemocytometer, and adjusted to make up 500 mL of a 1.8 × 105 spores/mL suspension, which was sprayed on cabbage plants (application on both sides of the leaves). Five days after the initial inoculation, plants were symptom-scored based on the percentage of leaf area displaying necrosis (1 = 0–20%; 2 = 20–40%; 3 = 40–60%; 4 = 60–80%; 5 = 80–100%). The same leaves were harvested for qPCR (Roche LightCycler® 96, Basel, Switzerland) using primers listed in Table S2, Supplementary File. Pathogen amplicons were normalized to cabbage DLH [30,31].
2.4. Biopesticide and Plant Oomycete Disease Suppression Assays
Chickpea (Cicer arietinum var genesis 090) plants (14–17 plants per treatment) were grown in UQ23 potting mix in growth cabinets with 16 h light at 26 °C and 8 h night at 21 °C and a light intensity of 100 μmol photons m−2 s−1 (white fluorescent lamps) for 3 weeks. Cultivations of bacterial strains and preparations for mock treatments are explained in Section 2.2. Each pot with a 3-week-old plant in the treatment was watered with 10 mL biocontrol bacteria (44LGS, 43M, 30LM, 14TH and a mix of all four) 1 day before oomycete inoculation. The P. medicaginis infection assay was conducted based on the method described by Ozgonen et al. [32]. Briefly, P. medicaginis was grown on PDA plates at 28 °C in a dark incubator for 2 weeks. Then, 1 cm2 agar plugs from plates were transferred into a 1 L flask containing 300 g of autoclaved wheat seeds, and the oomycete was grown for 2 weeks at 28 °C under continuous light. Eight infected wheat seeds were placed on the soil surface for each pot, which contained a 3-week-old chickpea plant after the biocontrol isolate treatments. An additional 20 infected wheat seeds were randomly distributed in the chickpea water tray where pots were kept. Biocontrol treatments continued every week until the end of the experiment. At 4 weeks post-inoculation with P. medicaginis, plants were uprooted, dried and weighed.
2.5. Statistical Analyses
Results of fungal plate assays were assessed for significant differences (p < 0.05) using Microsoft Office Excel Student’s t-test compared to S. saprophyticus assays. Student’s t-test was performed on biopesticide raw data to determine significant differences (p < 0.05) between mock-treated control and biocontrol treatment plant samples.
3. Results
3.1. Anti-Fungal and Antioomycete Assays
Anti-fungal and anti-oomycete plate inhibition assays showed activity against three fungi and three oomycetes for 38 out of the 68 selected bacterial isolates (Supplementary Figure S1; Table S3). Bacillus amyloliquefaciens (isolate 1, 2), Bacillus licheniformis, Brevibacillus laterosporus, Bacillus megaterium (2, 3), Bacillus methylotrophicus (1, 2), Bacillus mojavensis, Bacillus pumilus (9), Bacillus safensis (3), Bacillus subtilis (1), Corynebacterium flavescens, Klebsiella pneumoniae, Sporosarcina aquimarina (3) and Pseudochrobactrum kiredjianiae (3) were all able to inhibit the growth of all fungal and oomycete pathogens by more than 50% (Figure 1). However, other bacterial isolates were able to also inhibit at least one fungus or oomycete.
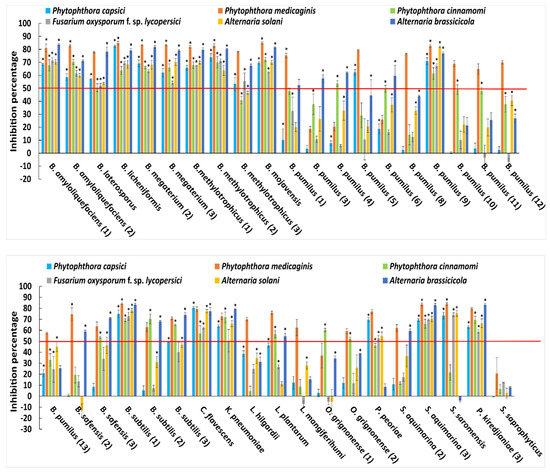
Figure 1.
Growth inhibition assay of 38 bacterial isolates out of 68 isolates tested in comparison to S. saprophyticus. Shown are mean values ± SEs from three biological replicates of percentage growth inhibition relative to bacteria-free control plates with P. capsici, P. medicaginis, F. oxysporium f.sp. lycopersici, A. solani and A. brassicicola. The red line indicates a 50% inhibition cut-off. Asterisks indicate statistically significant differences (p < 0.05 in Student’s t-test) to the S. saprophyticus control. This bacterium was chosen for comparisons, as it showed low in vitro bioactivity against plant pathogens.
3.2. Use of Biocontrol Bacteria as Biopesticides to Control Bacterial Plant Pathogens in Tomato
The four best biocontrol bacteria, which inhibited Pst on pathogen growth inhibition plate assays, were applied on tomato plants as potential biopesticides agents against Pst (Figure 2A,B). After symptom analysis and qPCR quantification, disease symptoms and the number of pathogens per plant cell significantly (p < 0.05) declined for plants treated with isolates Paenibacillus peoriae (14TH), Comamonas jiangduensis (28MC), B. methylotrophicus (46YE), Bacillus amyloliquefaciens (33YE) and the mixture of all four compared to mock (MgCl2) treatments (Figure 2C,D).
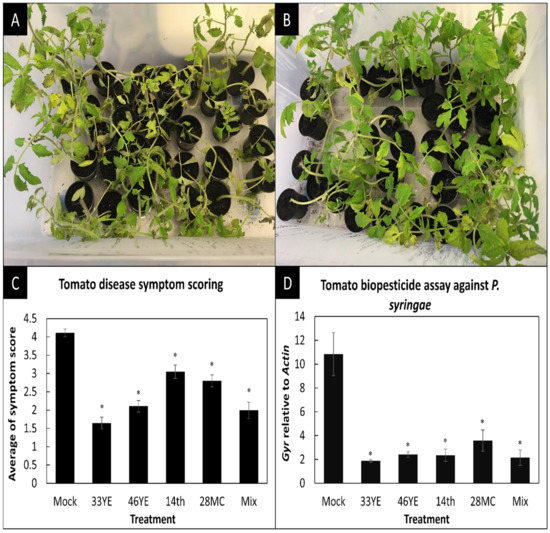
Figure 2.
Biopesticide assay to control bacterial speck in tomato at 5 days post-infection. (A) Tomato mock treatment after Pst infection. (B) Tomato treated with B. amyloliquefaciens (33YE) after Pst infection. (C) Disease symptom scores of Pst by four biocontrol bacteria and their mixture compared to mock-treated control plants (shown are mean values ± SEs; n = 30). (D) qPCR results showing the amount of Pst DNA (GYRASE A) relative to tomato DNA (ACTIN). Shown are mean values ± SEs of three biological replicates, each containing DNA of ten pooled plants. Asterisks indicate values that were significantly different (p < 0.05 in Student’s t-test) compared to mock (MgCl2)-treated control plants.
3.3. Use of Biocontrol Bacteria as Biopesticides to Control Fungal Plant Pathogens in Cabbage
Based on preliminary screening in plant assays, the four most promising A. brassicicola antagonists, B. methylotrophicus (46YE), Brevibacillus laterosporus (4YE), B. licheniformis (28M), Bacillus megaterium (44LGS) and a mixture of these four bacterial isolates were examined for their potential as biopesticides to control dark leaf spot in cabbage (Figure 3A,B). The symptom scoring suggests that individual isolates significantly (p < 0.05) reduced disease symptoms on cabbage plants, but not the mixture of isolates (Figure 3C). However, qPCR data show that all treatments, including the mixture of isolates, significantly decreased the amount of pathogen DNA (p < 0.05; Figure 3D).
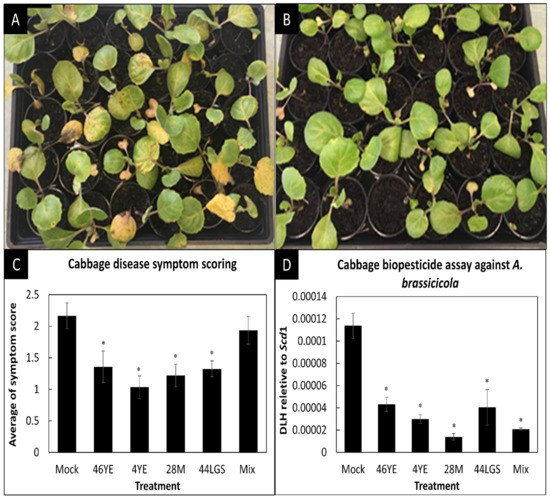
Figure 3.
Biopesticide assay to control leaf spot in cabbage at 5 days post-infection. (A) Cabbage mock (MgCl2) treatment post-infection with A. brassicicola. (B) Cabbage treated with B. laterosporus (4YE) treatment post-infection with A. brassicicola. (C) A. brassicicola symptom scores, including chlorosis and dark leaf spot (mean values ± SEs; n = 30). (D) qPCR results showing the amount of A. brassicicola DNA (Scd1) relative to cabbage DNA (DLH). Shown are mean values ± SEs of three biological replicates, each containing DNA of ten pooled plants. Asterisks indicate values that were significantly different (p < 0.05 in Student’s t-test) compared to the mock (MgCl2)-treated control plants.
3.4. Use of Biocontrol Bacteria as Biopesticides to Control Oomycete Plant Pathogens in Chickpea
Based on preliminary screening in plant assays, the four most promising biocontrol isolates B. megaterium (44LGS), C. flavescens (43M), Bacillus mojavensis (30LM), Paenibacillus peoriae (14TH) and a mixture of all four were used as pretreatment to the soil of chickpea plants before they were challenged with P. medicaginis. No leaf symptoms were observed at 4 weeks post-infection, but upon inspection of the roots, it was evident that all bacterial pretreatments significantly (p < 0.05) prevented root biomass decline compared to mock-treated (MgCl2) P. medicaginis-infected control plants (Figure 4). This suggests that in the presence of biocontrol bacteria, P. medicaginis was not able to infect plants effectively and significantly prevented root damage. The efforts to quantify P. medicaginis using molecular techniques were unsuccessful due to the lack of specific primers for P. medicaginis quantification.
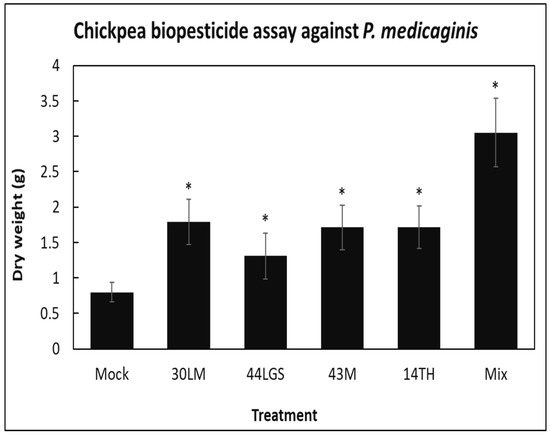
Figure 4.
Biopesticide assay to control Phytophthora root rot in chickpea at 4 weeks post-infection. Average root dry weight (g) per treatment of chickpea plants 4 weeks post-infection with P. medicaginis (mean values ± SEs, n = 14–17). Asterisks indicate values that were significantly different (p < 0.05 in Student’s t-test) compared to the mock (MgCl2)-treated P. medicaginis-infected control plants.
4. Discussion
In this study, previously identified bacteria active against bacterial phytopathogens and food pathogens [23] were tested in the current study against fungal and oomycete pathogens (Figure 1 and Supplementary Figure S1). Several reviews summarised reports on anti-fungal activities of Bacillus, Brevibacillus, Lactobacillus and Paenibacillus species, as they produce a wide range of anti-microbial compounds [33,34,35,36]. There are a few reports that show anti-fungal activity of Ochrobactrum and Klebsiella [37,38,39], and a recent study has shown the potential anti-fungal activity of Pseudochrobactrum kiredjianiae [40], which was also confirmed in our study. To our knowledge, however, there is currently no scientific report on C. flavescens, S. aquimarina and Sporosarcina saromensis anti-fungal or oomycete activities. To evaluate the potential application of biocontrol bacteria as biopesticides, we have developed three different assays against bacterial, fungal and oomycete pathogens.
Bacterial speck caused by Pst is an important economical bacterial phytopathogen in tomato plants [41]. In our biopesticide assay, we have applied four potential biocontrol bacteria as biopesticides, and B. amyloliquefaciens (33YE) was the best biopesticide among other treatments to suppress Pst symptoms (Figure 2). B. amyloliquefaciens is a well-known plant growth-promoting bacterium; for example, it can alleviate the symptoms of Pst in sugar beet plants [42].
Alternaria leaf spot causes severe infection and significant loss to brassica plants, such as canola [43]. Effects of Bacillus sp. on Alternaria alternata and Paenibacillus sp. on A. solani have been investigated before on tomato plants [44,45]; however, there is no evidence to demonstrate the effectiveness of Bacillus sp. and Brevibacillus sp. as biocontrol agents for A. brassicicola in cabbage. In our in vivo study, we found that B. laterosporus (4YE) supplied the best biocontrol performance in reducing the symptom and number of pathogens compared to other treatments (Figure 3). Although B. laterosporus has insecticidal effects, it has not been used as a biopesticide to control fungal pathogens or insects on plants [46]. The treatment with bacterial mixture also reduced the number of pathogens in cabbage leaf cells (as shown by qPCR), but the disease symptoms were exacerbated. This may have occurred because pathogen DNA in necrotic tissue could be degraded. A mixture of several biocontrol bacteria may also increase the likelihood for the activation of defense signaling pathways and plant hypersensitive response [47,48].
Phytophthora root rot is an important soil-borne disease of chickpea in Australia caused by P. medicaginis with up to $8.2 million annual yield losses [49]. Anti-fungal activity of Mesorhizobium ciceri and its growth promotion on chickpeas against P. medicaginis has been evaluated, and it has been shown that this bacterium can increase the biomass, improve nodulation and enhance disease resistance [50]. We chose four potential biocontrol bacteria and tested them in vivo against this phytopathogen. The mixture of the four bacterial isolates was by far the most effective treatment to prevent chickpea root biomass loss from P. medicaginis infection, although each individual bacterial treatment decreased root infection (Figure 4). A possible explanation could be a synergistic effect [51] from the mixture of bacteria that complement each other, and further studies may focus on the mechanisms on how this occurs. This could be done by direct anti-oomycete activity from all four isolates used. In addition, some of these isolates may also prime the plants to trigger defense responses, and induce systemic resistance or provide direct root growth-promoting effects.
In the future, it may also be possible to directly use the bioactive compounds of the biocontrol bacteria used as biopesticides. For this purpose, it is important to further elucidate the modes-of-action. Apart from the anti-fungal and oomycete activity shown in the current study (Figure 1 and Figure 3), we have previously shown that B. laterosporus isolate 4YE has activity against the Gram-positive tomato pathogen Clavibacter michiganensis, and that during this process, several anti-microbial metabolites (AMMs) and peptides (AMPs) were produced [23]. These were the diketopiperazines (DKPs) 3-isobutylhexahydropyrrolo [1,2-a]pyrazine-1,4-dione, 3-butyl-6-methylpiperazine-2,5-dione, 2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione, as well as 9-octadecenamide, DMSO and acetic acid. Potential AMPs were derived from 50S ribosomal protein L7/L12 and cold-shock protein [23]. Other studies for the invertebrate pathogen, B. laterosporus, also showed the biosynthesis of a wide range of anti-microbials, including tauramamide [52,53,54].
B. amyloliquefaciens is one of the best-studied biocontrol bacteria, which produces a wide range of anti-microbial compounds, including amylocyclicin [55,56,57,58]. Our B. amyloliquefaciens isolate 33YE possesses AMM activity against the tomato pathogen C. michiganensis and AMP activity against the human pathogen Listeria monocytogenes [23]. Potential AMPs were derived from thioredoxin, septation protein SpoVG, 50S ribosomal protein L7/L12, phosphocarrier protein HPr, non-specific DNA-binding protein Hbs, a chaperonin and other hypothetical and cold shock proteins.
Apart from the anti-bacterial, -fungal and -oomycete activity shown in the current study (Figure 1 and Figure 2), P. peoriae (14TH) also showed AMM activity against food-pathogenic E. coli. AMMs included the DKP 3-isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione, as well as DMSO and eicosanol [23]. P. peoriae also produces glycopeptides, 10 kDa peptides and polymyxin [33,59]. B. methylotrophicus also produces a wide range of anti-microbials, including lipopeptides and volatile compounds [60,61], and B. methylotrophicus (46YE) also showed AMP activity against C. michiganensis from a number of proteins [23].
Further anti-microbial activity has been reported for B. mojavensis (30LM) against L. monocytogenes (AMP activity) [23]. B. mojavensis anti-microbial agents include mojavensin and the heat-stable toxin amylosin [62,63,64]. C. jiangduensis, a biosurfactant-producing bacterium [65], also produces AMPs and AMMs against P. syringae for the isolate (28MC) tested in the current study [23], which may explain the observed resistance against this pathogen in tomato (Figure 2). Apart from the observed activity against P. medicaginis (Figure 2), C. flavescens (43M) also had AMM activity against C. michiganensis and P. syringae [23]. C. flavescens is a beneficial bacterium applied in food industries to make cheese pigmentation, and its application as a biocontrol agent needs to be investigated further [66]. Apart from the observed activity against A. brassicicola (Figure 3), B. licheniformis (28M) also possessed AMP against L. monocytogenes [23]. B. licheniformis produces bacteriocin-like molecule lichenin [67,68]. B. megaterium (44LGS) was active against all pathogens tested in the present study, and previous studies found that B. megaterium produces anti-microbial metabolites and a cyclic polypeptide [69,70].
Symptom scoring and molecular analysis have shown that biopesticide treatments significantly lowered disease symptoms and the number of pathogens in tomato, cabbage and chickpea. This could happen due to direct interactions between biocontrol bacteria and their anti-microbial compounds and pathogens. In the current study ten potential biopesticides have been developed from Bacillus, Brevibacillus, Paenibacillus, Comamonas and Corynebacterium species that should be further tested under field conditions. The application of Bacillus species as biopesticides has been widely explored [71], but Brevibacillus, Paenibacillus and Comamonas species, while known for their anti-microbial activity, have not yet been tested as potential biopesticides. Several reports have suggested the potential application of Paenibacillus species as biopesticide agents. However, this is the first report that shows the potential application of P. peoriae as a biopesticide [45,72,73]. In addition, this study has shown potential uses of Brevibacillus, or Comamonas and Corynebacterium species as biopesticides against bacterial, fungal and oomycete pathogens.
Regulatory and technical barriers have limited biopesticide applications in agricultural industries [74]. Compared to conventional pesticides, biopesticides typically have a lower efficacy and stability [75]. Further studies will be required to determine if these bacteria when applied to plants would remain dominant in the soil and phyllosphere microbiomes or require formulation (e.g., preferred carbon source) to improve their stability. Biopesticides may have potential side effects on the environment, which need to be well studied. It has been shown that a fungus-based bioinsecticide could impact insect pollinators’ functioning [76]. Therefore, possible environmental tests are needed before industrial applications of biopesticides are implemented.
5. Conclusions
Biopesticide application is an environmentally-friendly alternative for chemical pesticides. However, the current lack of diversity of available microbial biopesticides and their limited adaptability to different climate and farming conditions need to be investigated and addressed [11,77,78]. The current study took a biodiscovery approach by accessing diverse microbial taxa from environmental samples with the aim to rapidly develop microbial biopesticides to control the most devastating plant pathogens. The technology to rapidly develop new biopesticides is relevant for both agriculture and natural ecosystems where fungal threats to plant and ecosystem health increased 5-fold within 15 years [79]. The current study reports the application of soil bacteria against bacterial, fungal and oomycete plant pathogens as biocontrol agents. Our work suggests that environmental microbiomes, where bacteria compete against each other, such as rhizosphere soil, are a plentiful source for biocontrol agents against a wide range of diseases. Among these, three new potential biocontrol bacteria, Corynebacterium flavescens, Sporosarcina aquimarina and Sporosarcina saromensis, have been isolated that displayed anti-fungal and antioomycete activities against vegetable crop diseases that have not been reported previously. In total, we developed ten potential microbial biopesticides that significantly reduced the number of pathogens and disease symptoms in tomato, cabbage and chickpea plants.
Supplementary Materials
The following supplemental information is available online https://www.mdpi.com/article/10.3390/applmicrobiol2010021/s1. Table S1: Media used in this study, Table S2: Primers and qPCR conditions, Table S3: Media used in this study, Figure S1: Screening microbial isolates against fungal and oomycete pathogens.
Author Contributions
P.M.S. secured funding; P.M.S. and H.M. conceived the study; H.M. and J.B. performed the experiments; H.M. and J.B. analysed data; and H.M. and P.M.S. wrote the manuscript, with input from all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
H.M. gratefully acknowledges a University of Queensland International Scholarship. We thank Dean Beasley from Department of Agriculture and Fisheries at the Ecosciences Precinct Dutton Park for Alternaria solani BRIP23347, Elizabeth Aitken from the University of Queensland for Fusarium oxysporum f. sp. lycopersici UQFOL1070, Christopher O’Brien for Phytophthora cinnamomi and André Drenth for Phytophthora capsici UQ6139 from Queensland Alliance for Agriculture and Food Innovation and the Plant and Mycology Herbarium, New South Wales (Phytophthora medicaginis DAR65042).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Pst, Pseudomonas syringae; Silwet LL-77, 3-(8-methoxyoctoxy)propyl-methyl-bis(trimethylsilyloxy)silane; qPCR, Quantitative Polymerase Chain Reaction, AMMs, anti-microbial metabolites and AMPs, anti-microbial peptides.
References
- Wirthmueller, L.; Maqbool, A.; Banfield, M.J. On the front line: Structural insights into plant-pathogen interactions. Nat. Rev. Micro. 2013, 11, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Pathol. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.V.; Abizgil’dina, R.R.; Pusenkova, L.I. Plant growth promoting rhizobacteria as alternative to chemical crop protectors from pathogens (review). Appl. Biochem. Microbiol. 2011, 47, 333–345. [Google Scholar] [CrossRef]
- Søgaard Jørgensen, P.; Aktipis, A.; Brown, Z.; Carriere, Y.; Downes, S.; Dunn, R.R.; Epstein, G.; Frisvold, G.B.; Hawthorne, D.; Grohn, Y.T. Antibiotic and pesticide susceptibility and the Anthropocene operating space. Nat. Sustain. 2018, 1, 632–641. [Google Scholar]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101. [Google Scholar]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Ruiu, L. Microbial biopesticides in agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products–status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
- Thakur, N.; Kaur, S.; Tomar, P.; Thakur, S.; Yadav, A.N. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–282. [Google Scholar]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Oberhardt, M.A.; Zarecki, R.; Gronow, S.; Lang, E.; Klenk, H.-P.; Gophna, U.; Ruppin, E. Harnessing the landscape of microbial culture media to predict new organism–media pairings. Nat. Commun. 2015, 6, 1–4. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Wintzingerode, F.V.; Göbel, U.B.; Stackebrandt, E. Determination of microbial diversity in environmental samples: Pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 1997, 21, 213–229. [Google Scholar] [CrossRef]
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998, 5, R245–R249. [Google Scholar] [CrossRef]
- Besset-Manzoni, Y.; Joly, P.; Brutel, A.; Gerin, F.; Soudière, O.; Langin, T.; Prigent-Combaret, C. Does in vitro selection of biocontrol agents guarantee success in planta? A study case of wheat protection against Fusarium seedling blight by soil bacteria. PLoS ONE 2019, 14, e0225655. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Raizada, M.N. Bacterial seed endophytes of domesticated cucurbits antagonize fungal and oomycete pathogens including powdery mildew. Front. Microbiol. 2018, 9, 42. [Google Scholar] [CrossRef]
- Rajesh Kannan, V.; Bastas, K.; Rajendran, S. Scientific and economic impact of plant pathogenic bacteria. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Mirzaee, H.; Ariens, E.; Blaskovich, M.A.; Clark, R.J.; Schenk, P.M. Biostimulation of Bacteria in Liquid Culture for Identification of New Antimicrobial Compounds. Pharmaceuticals 2021, 14, 1232. [Google Scholar] [CrossRef] [PubMed]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.; Xiao, Y.; Wass, T.J.; Schenk, P.M. Identification of soil bacterial isolates suppressing different Phytophthora spp. and promoting plant growth. Front. Plant Sci. 2018, 9, 1502. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, S.R.; Ishiga, Y.; Wangdi, T.; Kunkel, B.N.; Anand, A.; Mysore, K.S.; Bender, C.L. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 2007, 20, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.C.; Mayers, P.; Henry, R. A simplified method for the preparation of fungal genomic DNA for PCR and RAPD analysis. Biotechniques 1994, 16, 48. [Google Scholar] [PubMed]
- Prada-Ramírez, H.A.; Pérez-Mendoza, D.; Felipe, A.; Martínez-Granero, F.; Rivilla, R.; Sanjuán, J.; Gallegos, M.T. AmrZ regulates cellulose production in Pseudomonas syringae pv. tomato DC 3000. Mol. Microbiol. 2016, 99, 960–977. [Google Scholar]
- Mascia, T.; Santovito, E.; Gallitelli, D.; Cillo, F. Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol. Plant Pathol. 2010, 11, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant. Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Su’udi, M.; Park, J.-M.; Park, S.-R.; Hwang, D.-J.; Bae, S.-C.; Kim, S.; Ahn, I.-P. Quantification of Alternaria brassicicola infection in the Arabidopsis thaliana and Brassica rapa subsp. pekinensis. Microbiology 2013, 159, 1946–1955. [Google Scholar] [CrossRef]
- Berg, T.; Tesoriero, L.; Hailstones, D. A multiplex real-time PCR assay for detection of Xanthomonas campestris from brassicas. Lett. Appl. Microbiol. 2006, 42, 624–630. [Google Scholar] [CrossRef]
- Ozgonen, H.; Erkilic, A. Growth enhancement and Phytophthora blight (Phytophthora capsici Leonian) control by arbuscular mycorrhizal fungal inoculation in pepper. J. Crop Prot. 2007, 26, 1682–1688. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.-C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell. Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Panda, A.K.; Bisht, S.S.; DeMondal, S.; Kumar, N.S.; Gurusubramanian, G.; Panigrahi, A.K. Brevibacillus as a biological tool: A short review. Anton. Leeuw. 2014, 105, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Messens, W.; De Vuyst, L. Inhibitory substances produced by Lactobacilli isolated from sourdoughs—A review. Int. J. Food Microbiol. 2002, 72, 31–43. [Google Scholar] [CrossRef]
- Zhao, L.; Teng, S.; Liu, Y. Characterization of a versatile rhizospheric organism from cucumber identified as Ochrobactrum haematophilum. J. Basic. Microbiol. 2012, 52, 232–244. [Google Scholar] [CrossRef]
- Nogueira, M.F.; Pereira, L.; Jenull, S.; Kuchler, K.; Lion, T. Klebsiella pneumoniae prevents spore germination and hyphal development of Aspergillus species. Sci. Rep. 2019, 9, 218. [Google Scholar] [CrossRef]
- Al-Rubaye, A.F.; Kadhim, M.J.; Hameed, I.H. Characterization of antifungal secondary metabolites produced by Klebsiella pneumoniae and screening of its chemical compounds using GC-MS. J. Curr. Pharm. Res. 2017, 8, 141–148. [Google Scholar] [CrossRef][Green Version]
- Fu, Y.; Gao, H.; Li, H.; Qin, Y.; Tang, W.; Lu, J.; Li, M.; Shao, L.; Liu, H. Change of growth promotion and disease resistant of wheat seedling by application of biocontrol bacterium Pseudochrobactrum kiredjianiae A4 under simulated microgravity. Acta Astronaut. 2017, 139, 222–227. [Google Scholar] [CrossRef]
- Cai, R.; Lewis, J.; Yan, S.; Liu, H.; Clarke, C.R.; Campanile, F.; Almeida, N.F.; Studholme, D.J.; Lindeberg, M.; Schneider, D. The plant pathogen Pseudomonas syringae pv. tomato is genetically monomorphic and under strong selection to evade tomato immunity. PLoS Pathog. 2011, 7, e1002130. [Google Scholar]
- Nikolić, I.; Berić, T.; Dimkić, I.; Popović, T.; Lozo, J.; Fira, D.; Stanković, S. Biological control of Pseudomonas syringae pv. aptata on sugar beet with Bacillus pumilus SS-10.7 and Bacillus amyloliquefaciens (SS-12.6 and SS-38.4) strains. J. Appl. Microbiol. 2019, 126, 165–176. [Google Scholar]
- Al-Lami, H.; You, M.; Barbetti, M. Role of foliage component and host age on severity of Alternaria leaf spot (caused by Alternaria japonica and A. brassicae) in canola (Brassica napus) and mustard (B. juncea) and yield loss in canola. Crop. Pasture Sci. 2019, 70, 969–980. [Google Scholar] [CrossRef]
- Pane, C.; Zaccardelli, M. Evaluation of Bacillus strains isolated from solanaceous phylloplane for biocontrol of Alternaria early blight of tomato. Biol. Control 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Khan, N.; Mishra, A.; Nautiyal, C.S. Paenibacillus lentimorbus B-30488r controls early blight disease in tomato by inducing host resistance associated gene expression and inhibiting Alternaria solani. Biol. Control 2012, 62, 65–74. [Google Scholar] [CrossRef]
- Barbieri, G.; Ferrari, C.; Mamberti, S.; Gabrieli, P.; Castelli, M.; Sassera, D.; Ursino, E.; Scoffone, V.C.; Radaelli, G.; Clementi, E. Identification of a Novel Brevibacillus laterosporus Strain With Insecticidal Activity Against Aedes albopictus Larvae. Front. Microbiol. 2021, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, A.; Pieterse, C.M. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef]
- Amalraj, A.; Taylor, J.; Bithell, S.; Li, Y.; Moore, K.; Hobson, K.; Sutton, T. Mapping resistance to Phytophthora root rot identifies independent loci from cultivated (Cicer arietinum L.) and wild (Cicer echinospermum PH Davis) chickpea. Theor. Appl. Genet. 2019, 132, 1017–1033. [Google Scholar] [CrossRef]
- Jahan, M.; Shazad, U.; Naqvi, S.; Tahir, I.; Abbas, T.; Iqbal, M. Effects of Mesorhizobium ciceri and Biochar on the Growth, Nodulation and Antifungal Activity Against Root Pathogenic Fungi in Chickpea (Cicer arietinum L.). J. Plant. Pathol. Microbiol. 2020, 11, 520. [Google Scholar]
- Kannan, V.; Sureendar, R. Synergistic effect of beneficial rhizosphere microflora in biocontrol and plant growth promotion. J. Basic Microbiol. 2009, 49, 158–164. [Google Scholar] [CrossRef]
- Ruiu, L. Brevibacillus laterosporus, a pathogen of invertebrates and a broad-spectrum antimicrobial species. Insects 2013, 4, 476–492. [Google Scholar] [CrossRef]
- Desjardine, K.; Pereira, A.; Wright, H.; Matainaho, T.; Kelly, M.; Andersen, R.J. Tauramamide, a lipopeptide antibiotic produced in culture by Brevibacillus laterosporus isolated from a marine habitat: Structure elucidation and synthesis. J. Nat. Prod. 2007, 70, 1850–1853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, L.; Zeng, H.; Yang, X.; Yuan, J.; Shi, H.; Xiong, Y.; Chen, M.; Han, L.; Qiu, D. Purification and characterization of a novel antimicrobial peptide from Brevibacillus laterosporus strain A60. Peptides 2012, 33, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.E.; An, F.Y.; Dholpe, V.; Ramamoorthy, A.; Lopatin, D.E.; Lantz, M.S. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J. Antimicrob. Chemother. 2007, 59, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.; Vater, J.; Budiharjo, A.; Wang, Z.; He, Y.; Dietel, K.; Schwecke, T.; Herfort, S.; Lasch, P.; Borriss, R. Amylocyclicin, a Novel Circular Bacteriocin Produced by Bacillus amyloliquefaciens FZB42. J. Bacteriol. 2014, 196, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiradate, S.; Tsukamoto, T.; Hatakeda, K.; Shirata, A. Antimicrobial activity of culture filtrate of Bacillus amyloliquefaciens RC-2 isolated from mulberry leaves. Phytopathology 2001, 91, 181–187. [Google Scholar] [CrossRef]
- Von der Weid, I.; Alviano, D.S.; Santos, A.L.S.; Soares, R.M.A.; Alviano, C.S.; Seldin, L. Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J. Appl. Microbiol. 2003, 95, 1143–1151. [Google Scholar] [CrossRef]
- Frikha-Gargouri, O.; Ben Abdallah, D.; Ghorbel, I.; Charfeddine, I.; Jlaiel, L.; Triki, M.A.; Tounsi, S. Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress Agrobacterium crown gall tumours on tomato plants. Pest Manag. Sci. 2017, 73, 568–574. [Google Scholar] [CrossRef]
- He, C.-N.; Ye, W.-Q.; Zhu, Y.-Y.; Zhou, W.-W. Antifungal activity of volatile organic compounds produced by Bacillus methylotrophicus and Bacillus thuringiensis against five common spoilage fungi on loquats. Molecules 2020, 25, 3360. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; Hu, J.; Wang, S. Isolation and characterization of a new iturinic lipopeptide, mojavensin A produced by a marine-derived bacterium Bacillus mojavensis B0621A. J. Antibiot. 2012, 65, 317. [Google Scholar] [CrossRef]
- Jasim, B.; Sreelakshmi, S.; Mathew, J.; Radhakrishnan, E.K. Identification of endophytic Bacillus mojavensis with highly specialized broad spectrum antibacterial activity. 3 Biotech 2016, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Apetroaie-Constantin, C.; Mikkola, R.; Andersson, M.A.; Teplova, V.; Suominen, I.; Johansson, T.; Salkinoja-Salonen, M. Bacillus subtilis and B. mojavensis strains connected to food poisoning produce the heat stable toxin amylosin. J. Appl. Microbiol. 2009, 106, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-N.; Zhang, J.; Chen, Q.; He, J.; Li, Q.-F.; Li, S.-P. Comamonas jiangduensis sp. nov., a biosurfactant-producing bacterium isolated from agricultural soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 2168–2173. [Google Scholar] [CrossRef] [PubMed]
- Masoud, W.; Jakobsen, M. Surface ripened cheeses: The effects of Debaryomyces hansenii, NaCl and pH on the intensity of pigmentation produced by Brevibacterium linens and Corynebacterium flavescens. Int. Dairy J. 2003, 13, 231–237. [Google Scholar] [CrossRef]
- Pattnaik, P.; Kaushik, J.; Grover, S.; Batish, V. Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J. Appl. Microbiol. 2001, 91, 636–645. [Google Scholar] [CrossRef]
- Kayalvizhi, N.; Gunasekaran, P. Production and characterization of a low-molecular-weight bacteriocin from Bacillus licheniformis MKU3. Lett. Appl. Microbiol. 2008, 47, 600–607. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, Q.; Ji, Y.; Xie, A.; Yang, L.; Mu, S.; Li, Z.; He, T.; Xiao, Y.; Zhao, J. Isolation and identification of antibacterial bioactive compounds from Bacillus megaterium L2. Front. Microbiol. 2021, 12, 645484. [Google Scholar] [CrossRef]
- Al-Thubiani, A.S.; Maher, Y.A.; Fathi, A.; Abourehab, M.A.; Alarjah, M.; Khan, M.S.; Al-Ghamdi, S.B. Identification and characterization of a novel antimicrobial peptide compound produced by Bacillus megaterium strain isolated from oral microflora. Saudi Pharm. J. 2018, 26, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef]
- Kim, S.G.; Khan, Z.; Jeon, Y.H.; Kim, Y.H. Inhibitory effect of Paenibacillus polymyxa GBR-462 on Phytophthora capsici causing phytophthora blight in chili pepper. J. Phytopathol. 2009, 157, 329–337. [Google Scholar] [CrossRef]
- Timmusk, S.; Van West, P.; Gow, N.; Paul Huffstutler, R. Paenibacillus polymyxa antagonizes oomycete plant pathogens Phytophthora palmivora and Pythium aphanidermatum. J. Appl. Microbiol. 2009, 106, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.K.; Boyetchko, S.M. Research initiatives in the art and science of biopesticide formulations. Soil Biol. Biochem. 2006, 38, 845–849. [Google Scholar] [CrossRef]
- Almeida, F.C.R.; Magalhães, D.M.; Favaris, A.P.; Rodríguez, J.; Azevedo, K.E.X.; Bento, J.M.S.; Alves, D.A. Side effects of a fungus-based biopesticide on stingless bee guarding behaviour. Chemosphere 2022, 287, 132147. [Google Scholar] [CrossRef]
- Trivedi, P.; Schenk, P.M.; Wallenstein, M.D.; Singh, B.K. Tiny microbes, big yields: Enhancing food crop production with biological solutions. Microb. Biotechnol. 2017, 10, 999–1003. [Google Scholar] [CrossRef]
- Sudakin, D.L. Biopesticides. Toxicol. Rev. 2003, 22, 83–90. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).