Colonization of Lactobacillus rhamnosus GG in Cirrhinus molitorella (Mud Carp) Fingerling: Evidence for Improving Disease Resistance and Growth Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Feeding Trial
2.2. Preparation of Experimental Diets and Supplementation
2.3. Disease Resistance of Mud Carp against A. hydrophila
2.4. Verification of LGG Colonization at the Fish Gut Tissue
2.5. Statistical Analysis
3. Results
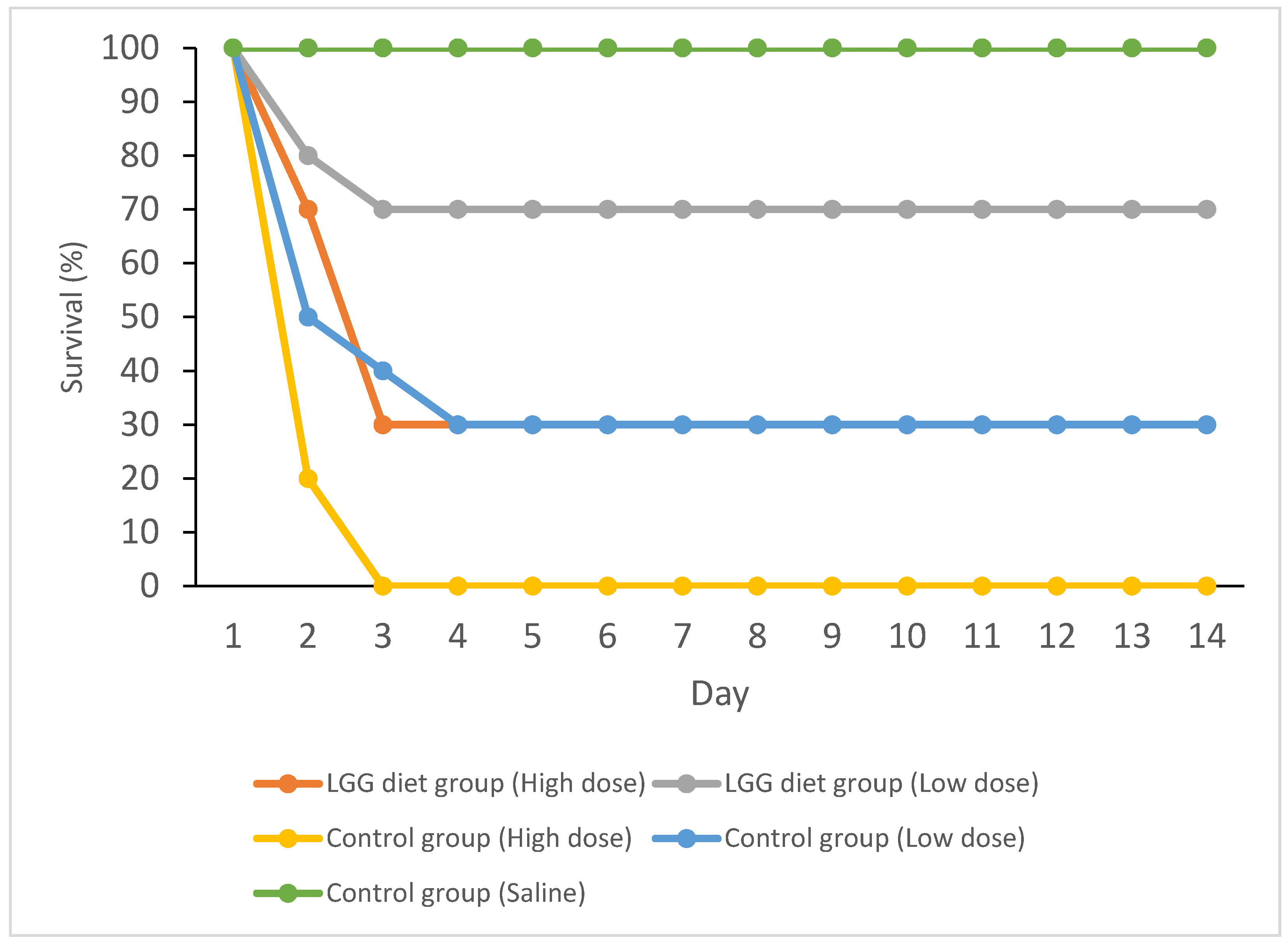
3.1. Disease Resistance of Mud Carp against A. hydrophila
3.2. Colonization of LGG in Gut of Mud Carp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Cirrhinus molitorella. In Cultured Aquatic Species Fact Sheets; FAO: Rome, Italy, 2009. [Google Scholar]
- Balcázar, J.L.; De Blas, I.; Ruiz-Zarzuela, I.; Cunningham, D.; Vendrell, D.; Múzquiz, J.L. The role of probiotics in aquaculture. Vet. Microbiol. 2006, 114, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Faruk, M.; Anka, I.; Azad, M. Investigation on fish health and diseases in rural pond aquaculture in three districts of Bangladesh. J. Bangladesh Agric. Univ. 2014, 11, 377–384. Available online: https://www.banglajol.info/index.php/JBAU/article/view/19944 (accessed on 31 December 2021). [CrossRef] [Green Version]
- Wei, Q. Social and economic impacts of aquatic animal health problems in aquaculture in China. In Primary Aquatic Animal Health Care in Rural, Small-Scale, Aquaculture Development; Arthur, J.R., Phillips, M.J., Subasinghe, R.P., Reantaso, M.B., MacRae, I.H., Eds.; FAO: Rome, Italy, 2002; pp. 55–61. [Google Scholar]
- Kaleeswaran, B.; Ilavenil, S.; Ravikumar, S. Dietary supplementation with Cynodon dactylon (L.) enhances innate immunity and disease resistance of Indian major carp, Catla catla (Ham.). Fish Shellfish Immunol. 2011, 31, 953–962. [Google Scholar] [CrossRef] [PubMed]
- CFS Finds Traces of Malachite Green in Canned Fried Dace Sample. Available online: https://www.info.gov.hk/gia/general/201909/19/P2019091900555.htm (accessed on 23 December 2021).
- Rico, A.; Satapornvanit, K.; Haque, M.M.; Min, J.; Nguyen, P.T.; Telfer, T.C.; van den Brink, P. Use of chemicals and biological products in Asian aquaculture and their potential environmental risks: A critical review. Rev. Aquac. 2012, 4, 75–93. [Google Scholar] [CrossRef]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Miller, R.; Harbottle, H. Antimicrobial drug resistance in fish pathogens. Microbiol. Spectr. 2018, 6, ARBA-0017-2017. [Google Scholar] [CrossRef]
- Jacobs, L.; Chenia, H.Y. Characterization of integrons and tetracycline resistance determinants in Aeromonas spp. isolated from South African aquaculture systems. Int. J. Food Microbiol. 2007, 114, 295–306. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Streptomyces bacteria as potential probiotics in aquaculture. Front. Microbiol. 2016, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Zuberi, A.; Ahmad, M.; Shah, A.B.; Younus, N.; Ullah, S.; Khattak, M.N.K. Dietary administration of the commercially available probiotics enhanced the survival, growth, and innate immune responses in Mori (Cirrhinus mrigala) in a natural earthen polyculture system. Fish Shellfish Immunol. 2018, 72, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.B. Probiotics, the other half of the antibiotic story. Anim. Nutr. Health 1974, 29, 4–8. [Google Scholar]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkea-aho, T.L.; Papadopoulou, A.; Heikkinen, J.; von Wright, A.; Adams, A.; Austin, B.; Thompson, K.D. Pseudomonas M162 confers protection against rainbow trout fry syndrome by stimulating immunity. J. Appl. Microbiol. 2012, 113, 24–35. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Adesiyun, A.A.; Mutani, A.; Ramsubhag, A.; Brunt, J.; Austin, B. Bacillus subtilis AB1 controls Aeromonas infection in rainbow trout (Oncorhynchus mykiss, Walbaum). J. Appl. Microbiol. 2007, 103, 1699–1706. [Google Scholar] [CrossRef]
- Ma, C.-W.; Cho, Y.-S.; Oh, K.-H. Removal of pathogenic bacteria and nitrogens by Lactobacillus spp. JK-8 and JK-11. Aquaculture 2009, 287, 266–270. [Google Scholar] [CrossRef]
- Abdou, A.M.; Hedia, R.H.; Omara, S.T.; Mahmoud, M.; Kandil, M.M.; Bakry, M.A. Interspecies comparison of probiotics isolated from different animals. Vet. World 2018, 11, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Yi, C.-C.; Liu, C.-H.; Chuang, K.-P.; Chang, Y.-T.; Hu, S.-Y. A potential probiotic Chromobacterium aquaticum with bacteriocin like activity enhances the expression of indicator genes associated with nutrient metabolism, growth performance and innate immunity against pathogen infections in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 93, 124–134. [Google Scholar] [CrossRef]
- Wuertz, S.; Schroeder, A.; Wanka, K.M. Probiotics in Fish Nutrition-Long-Standing Household Remedy or Native Nutraceuticals? Water 2021, 13, 1348. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Park, C.I.; Kim, D.H. Improved growth rate and disease resistance in olive flounder, Paralichthys olivaceus, by probiotic Lactococcus lactis WFLU12 isolated from wild marine fish. Aquaculture 2017, 471, 113–120. [Google Scholar] [CrossRef]
- Giri, S.S.; Sukumaran, V.; Oviya, M. Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol. 2013, 34, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahire, J.J.; Mokashe, N.U.; Chaudhari, B.L. Effect of Dietary Probiotic Lactobacillus helveticus on Growth Performance, Antioxidant Levels, and Absorption of Essential Trace Elements in Goldfish (Carassius auratus). Probiotics Antimicrob Proteins 2019, 11, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ringø, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618. [Google Scholar] [CrossRef]
- Endo, A.; Aakko, J.; Salminen, S. Evaluation of strain-specific primers for identification of Lactobacillus rhamnosus GG. FEMS Microbiol. Lett. 2012, 337, 120–125. [Google Scholar] [CrossRef]
- Lee, Y.K.; Puong, K.Y.; Ouwehand, A.C.; Salminen, S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 2003, 52, 925–930. [Google Scholar] [CrossRef]
- Fong, F.Y.L.; Kirjavainen, P.; Wong, V.H.Y.; El-Nezami, H. Immunomodulatory effects of Lactobacillus rhamnosus GG on dendritic cells, macrophages and monocytes from healthy donors. Sci. Rep. 2015, 13, 71–79. [Google Scholar] [CrossRef]
- He, S.; Ran, C.; Qin, C.; Li, S.; Zhang, H.; de Vos, W.M.; Ringø, E.; Zhou, Z. Anti-Infective Effect of Adhesive Probiotic Lactobacillus in Fish is Correlated with Their Spatial Distribution in the Intestinal Tissue. Sci. Rep. 2017, 7, 13195. [Google Scholar] [CrossRef]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2019, 86, 260–268. [Google Scholar] [CrossRef]
- Narasimhan, S.; Rajeevan, N.; Liu, L.; Zhao, Y.O.; Heisig, J.; Pan, J.; Eppler-Epstein, R.; DePonte, K.; Fish, D.; Fikrig, E. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe 2014, 15, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Bake, G.G.; Endo, M.; Akimoto, A.; Takeuchi, T. Growth Performance of Nile Tilapia Oreochromis niloticus Fingerlings fed Sweet Potato and Soy Sauce By-product Meal Diet in a Flow through System. Aquac. Sci. 2009, 57, 2, 193–199. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USAID Technical Bulletin #07: Feed Conversion Ratio (FCR): How to Calculate It and How It Is Used. Available online: https://pdf.usaid.gov/pdf_docs/PA00K8MQ.pdf (accessed on 15 July 2021).
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everad, A. Microbial regulation of organismal energy homeostasis. Nat Metabol. 2019, 1, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, W.; Liu, W.; Gatlin, D.M.; Zhang, Y.; Yao, B.; Ringø, E. Identification of highly-adhesive gut Lactobacillus strains in zebrafish (Danio rerio) by partial rpoB gene sequence analysis. Aquaculture 2012, 370–371, 150–157. [Google Scholar] [CrossRef]
- Pfeffer, E. Eintrag von Belastungen des Wassers durch die Fischfütterung (The pollution of water by fish feeding). Dtsch Tierarztl. Wochenschr. 1990, 97, 273–275. [Google Scholar] [PubMed]
- Boyd, C.E.; Tucker, C.S. Water Quality and Aquaculture: Preliminary Considerations. In Pond Aquaculture Water Quality Management; Springer: Boston, MA, USA, 1998. [Google Scholar] [CrossRef]
- Panigrahi, A.; Kiron, V.; Kobayashi, T.; Puangkaew, J.; Satoh, S.; Sugita, H. Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet. Immunol. Immunopathol. 2004, 102, 379–388. [Google Scholar] [CrossRef]
- Fong, F.; Kirjavainen, P.; El-Nezami, H. Immunomodulation of Lactobacillus rhamnosus GG (LGG)-derived soluble factors on antigen-presenting cells of healthy blood donors. Sci. Rep. 2016, 6, 2284. [Google Scholar] [CrossRef] [Green Version]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG Lysate Increases Re-Epithelialization of Keratinocyte Scratch Assays by Promoting Migration. Sci Rep. 2015, 5, 16147. [Google Scholar] [CrossRef] [Green Version]
- Archacka, M.; Celińska, E.; Białas, W. Techno-economic analysis for probiotics preparation production using optimized corn flour medium and spray-drying protective blends. Food Bioprod. Process. 2020, 123, 354–366. [Google Scholar] [CrossRef]
| Parameters | LGG Treatment Group | Control Group |
|---|---|---|
| Water temperature (°C) | 26.6 ± 0.4 | 26.8 ± 0.3 |
| Total ammonium nitrogen (mg/L) | <0.1 | <0.1 |
| Nitrite (mg/L) | 0.008 ± 0.003 | 0.014 ± 0.003 |
| Nitrate (mg/L) | 0.5 ± 0.1 | 0.7 ± 0.2 |
| Total Phosphorous (mg/L) | <0.1 | <0.1 |
| pH | 7.65 ± 0.20 | 7.87 ± 0.07 |
| Total dissolved solids (mg/L) | <0.1 | <0.1 |
| Parameters | LGG Treatment Group | Control Group |
|---|---|---|
| Average initial weight (g) | 2.69 ± 0.254 | 2.63 ± 0.218 |
| Average final weight (g) | 3.98 ± 0.288 * | 3.21 ± 0.276 |
| Relative weight gain (RWG) (%) | 47.6 ± 3.27 * | 21.9 ± 0.76 |
| Average feed intake (AFI) (g/fish per day) | 0.0378 ± 0.0073 | 0.0377 ± 0.0059 |
| Feed conversion ratio (FCR) | 1.80 ± 0.27 * | 3.99 ± 0.04 |
| Feed efficiency (FE) (%) | 55.7 ± 8.28 * | 25.1 ± 0.26 |
| Specific growth rate (SGR) (%/day) | 0.649 ± 0.016* | 0.332 ± 0.014 |
| Amount of A. hydrophila Injected | Accumulated Mortality Rate (%) | RPS (%) | |
|---|---|---|---|
| LGG Treatment Group | Control Group | ||
| 5 × 104 CFU (low dose) | 30 * | 70 | 57 |
| 1 × 105 CFU (high dose) | 70 * | 100 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-M.; Poon, P.M.-Y.; Sharma, A.A.; Chan, S.M.-N.; Lee, F.W.-F.; Mo, I.W.-Y.; Sze, E.T.-P. Colonization of Lactobacillus rhamnosus GG in Cirrhinus molitorella (Mud Carp) Fingerling: Evidence for Improving Disease Resistance and Growth Performance. Appl. Microbiol. 2022, 2, 175-184. https://doi.org/10.3390/applmicrobiol2010012
Yu Y-M, Poon PM-Y, Sharma AA, Chan SM-N, Lee FW-F, Mo IW-Y, Sze ET-P. Colonization of Lactobacillus rhamnosus GG in Cirrhinus molitorella (Mud Carp) Fingerling: Evidence for Improving Disease Resistance and Growth Performance. Applied Microbiology. 2022; 2(1):175-184. https://doi.org/10.3390/applmicrobiol2010012
Chicago/Turabian StyleYu, Yang-Mei, Peggy Miu-Yee Poon, Aayushi Ashok Sharma, Sidney Man-Ngai Chan, Fred Wang-Fat Lee, Ian Wing-Yin Mo, and Eric Tung-Po Sze. 2022. "Colonization of Lactobacillus rhamnosus GG in Cirrhinus molitorella (Mud Carp) Fingerling: Evidence for Improving Disease Resistance and Growth Performance" Applied Microbiology 2, no. 1: 175-184. https://doi.org/10.3390/applmicrobiol2010012
APA StyleYu, Y.-M., Poon, P. M.-Y., Sharma, A. A., Chan, S. M.-N., Lee, F. W.-F., Mo, I. W.-Y., & Sze, E. T.-P. (2022). Colonization of Lactobacillus rhamnosus GG in Cirrhinus molitorella (Mud Carp) Fingerling: Evidence for Improving Disease Resistance and Growth Performance. Applied Microbiology, 2(1), 175-184. https://doi.org/10.3390/applmicrobiol2010012









