1. Introduction
Infectious diseases are among the top causes of global mortality, morbidity, and disability [
1,
2]. The pathogens such as
Staphylococcus aureus form a serious burden on health due to related infections, including pneumonia, endocarditis, and sepsis [
3,
4]. The methicillin-resistant
Staphylococcus aureus (MRSA) is one of the most critical multidrug-resistant (MDR) pathogens mostly encountered in hospitals [
3,
5,
6]. The global incidence of MRSA infection has increased the burden on the health care system and overall mortality over the years [
5]. Additionally, the MRSA infection still extends to the community population [
3]. Due to the frequent emergence of antimicrobial resistance, limited options are available for treating MRSA-related infections [
7]. The ineffectiveness of the available antibacterial agents necessitates the search for new compounds for use against resistant bacteria [
8].
Plants have contributed to the general health and well-being of the populace for a long time [
2]. About 75% of the people globally depend on traditional medicine for their basic health requirements [
9]. Many therapeutic compounds employed in orthodox medicine originate from plants [
10]. Plants synthesize various secondary metabolites with different chemical diversity and biological actions [
11]. Plants and their secondary metabolites have been reported to serve as sources of antimicrobial agents [
6,
12,
13,
14]. For years, attention has been shifted to investigate phytochemical compounds with potential antibacterial activity, particularly against MDR bacteria [
13]. Given the roles of medicinal plants in curtailing infectious diseases, there is a need to continue the search for novel therapeutic agents to pave the way for the discovery of new therapeutic agents [
15,
16].
The plant
Senna alata (Linn) Roxb. synonymous with
Cassia alata (Leguminosae), is commonly distributed in Asia, Brazil, Australia, and many African nations, including Egypt, Somalia, and Nigeria [
16]. The plant is a tropically erect annual herb that grows up to 2–5 m high. It possesses big, leathery, and slippery compound bilateral leaves that usually fold at night [
17]. It has a pod fruit with small and square-shaped seeds [
18]. In English, this medicinally important plant is called candle bush, craw-craw, Acapulco or ringworm bush plant [
16]. The local names of the plant are
Asunwon oyinbo in Yoruba,
Nelkhi in Igbo, and
Hantsi in the Hausa languages of Nigeria [
17]. The plant is used as herbal preparation to manage hepatitis, gastroenteritis, constipation, dermatitis, eczema, jaundice, diarrhea [
18], and bacterial infections [
19]. The plant’s leaves, stems, and roots have been ingested as a decoction to treat wounds, the respiratory tract, and skin infections in Northern Nigeria [
18]. Additionally, in Cameroon, the leaves, stems, and bark of
S. alata were documented to be used to treat gastroenteritis and skin infections [
16]. Its leaves, bark, and stem are effective against intestinal parasitosis and syphilis as a decoction in China, the Philippines, and India [
20]. Keeping in view the ethnopharmacological indication and promising therapeutic properties of
S. alata for treating infectious ailments, this study was conducted to screen the leaves extracts of
S. alata for activity against MRSA, and identify the bioactive compounds likely responsible for the activity.
4. Discussion
Plant-derived products play a crucial role in exploring active biomolecules to treat infectious diseases, currently causing serious health challenges globally, especially in developing nations [
31]. Therefore, it is essential to intensify research on natural products to establish the secondary metabolites responsible for activity against infectious diseases [
1,
32]. The current study elucidates the antibacterial potentials of various extracts and bioactive components of the aqueous leaves extract of
Senna alata against Methicillin-resistant
Staphylococcus aureus (MRSA). A compound related to 9-octadecenoic acid methyl ester could be present and may have potent antibacterial activity against MRSA.
Based on the current investigation, the aqueous extract of the plant produced the highest extractive and could contain the highest amount of secondary metabolites present in the plant. Our findings are in line with the work of Saito et al. (2012) [
32], where the 100 g of milled
S. alata leaves produced 25.4% aqueous extract. However, the work of Faruq et al. (2010) [
33] has shown that the methanol extract produced the highest yield.
Interestingly, this investigation has shown that the crude aqueous extract of
S. alata exerted the highest antibacterial effects against the bacteria. Other studies by Adedayo et al. (2001), Modi et al. (2012), Saito et al. (2012), and Somchit et al. (2003) [
18,
19,
32,
34] demonstrated the inhibitory effect of aqueous extract of
S. alata against
S. aureus, which corroborates with the outcome of this study. On the contrary, a previous report by Faruq et al. (2010) [
33] has shown that the
S. aureus was resistant to aqueous leaf extract of
S. alata. In addition, the antibacterial activity elicited by the methanol extract of
S. alata against the MRSA in this work agrees with the findings of Hazni et al. (2008) [
35] and Sermakkani and Thangapandian (2012) [
36]. The difference in the antibacterial activities produced by the methanol and aqueous extracts of
S. alata leaves in the current work might be related to the secondary metabolites varying degrees of solubility in categories of solvents based on the polarity, which also corroborates with the higher extraction yield in the aqueous extract [
37].
Bioassay-guided fractionation is an effective procedure to discover novel therapeutic agents via obtaining active fractions or isolated bioactive agents. In this method, each fraction is usually investigated for biological activity, and subsequently, the most active portions are eventually fractionated for further evaluation [
38]. The chemical contents and potential mechanisms of activity of bioactive compounds usually vary in different parts of the plants and a difference in the solubility of the secondary metabolites in different solvents [
39]. In this study, the crude aqueous leaves extract of
S. alata afforded ethyl-acetate and diethyl ether fractions. However, only the diethyl ether fraction elicited an antibacterial activity related to the higher amount of the bioactive agents in the fraction, as shown by its higher extractive value than the ethyl-acetate fraction.
Phytochemical determination gives an overview of the possible category of the plants’ secondary metabolites and their quantity in a particular fraction which could guide the isolation strategy of the bioactive components [
40]. Based on this research, the aqueous extract of
S. alata leaves revealed saponins, alkaloids, emodin, tannins, steroids, anthraquinones, and flavonoids that could be associated with the antibacterial effects. The outcome agrees with the previous research by El-Mahmood and Doughari (2008) [
41] and Sule et al. (2011) [
42]. Generally, the anti-infective action of plants is related to the chemical compounds, including tannins, phenols, saponins, steroids, alkaloids, flavonoids, and many other compounds via various processes [
43]. Polyphenol agents such as tannins, flavonoids, and alkaloids possess antimicrobial effects [
44,
45]. Flavonoids, including quercetin and kaempferol, have antimicrobial actions by inhibiting the action of the bacterial enzymes [
42,
46,
47]. The antimicrobial action of flavonoids is also associated with their capability to produce a complex with soluble extracellular proteins and bacterial cell walls, whereas tannins inhibit microbial adhesions, transport proteins, and enzymes [
44,
48]. In addition, flavonoids destabilize microbial cell membranes [
48]. Therefore, these phytochemical compounds in
S. alata could be responsible for its antimicrobial activities.
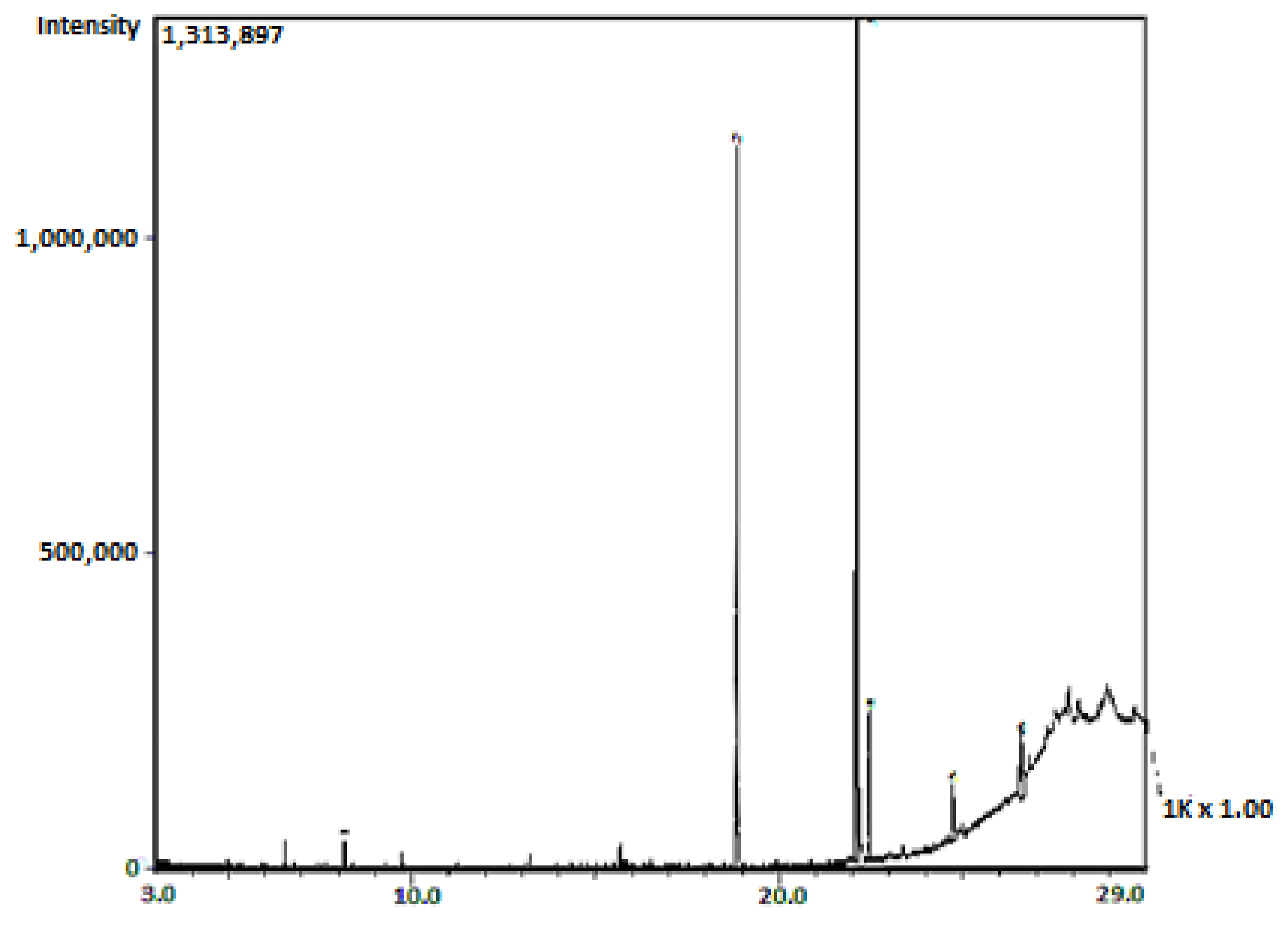
The observation in the current work that only sub-fraction 13 of the column fractions of the diethyl ether fraction of the crude aqueous
S. alata leaves extract showed activity concurs with the work of Faruq et al. (2010) [
33], in which only one of the column fractions elicited antibacterial activity. Furthermore, a single spot was observed to possess bioactivity following the active diethyl ether column fraction to silica gel TLC analysis. The bioactive spot (spot 2) of the sub-fraction 13 obtained from the diethyl ether fraction of the crude aqueous leaves extract of
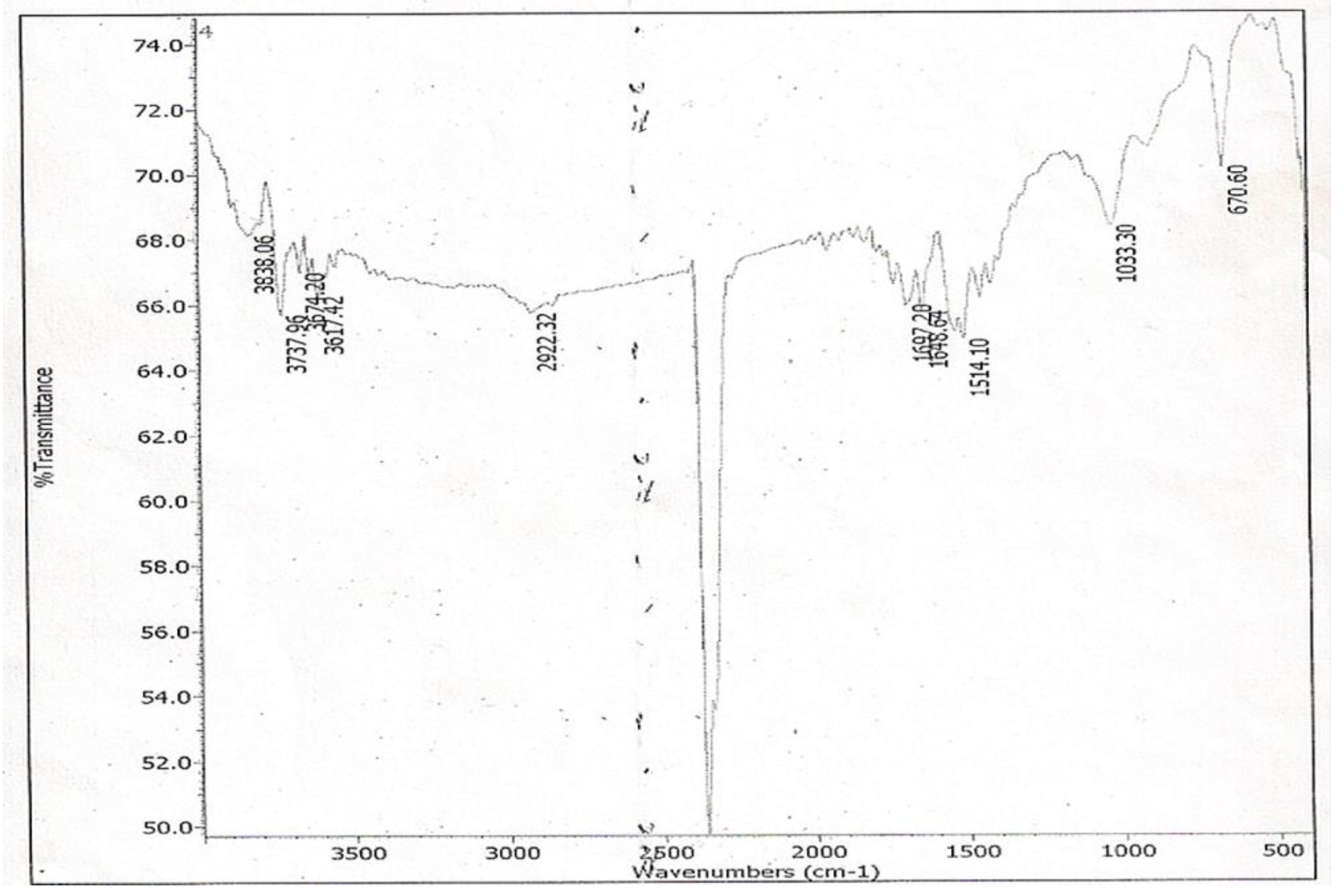
S. alata being the only spot effective against the test organism possesses a hydroxyl group (-OH) in its structure as shown in its IR spectrum assignable to the vibrational band at 3320 cm
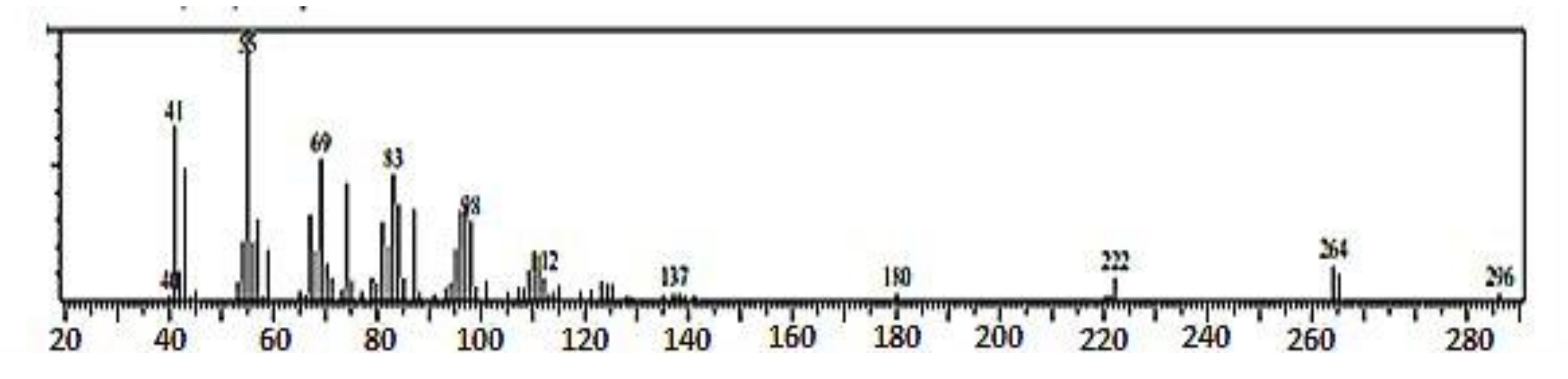
−1, which is broad and of low intensity. This is typical of the -OH group found in fatty acids, and it belongs to -OH bonded to a carboxyl group carbon atom. The presence of the carboxyl group in the structure of spot 2 of the sub-fraction 13 has been confirmed by the GC-MS spectral data. The loss of two oxygen atoms (2O) from the fragmentation of the molecular ion at m/z 296 to form the fragment ion peak at m/z 264 indicated that this loss of oxygen atom could have emanated from a carboxyl group, which is typical of fatty acids methyl ester [
49].
The FT-IR spectrum of spot 2 of sub-fraction 13 showed a carbon–carbon double bond (C=C) due to the stretching vibration band at 1684 cm
−1. The presence of this bond was confirmed in the GC-MS spectral data as the fragmentation of the fragment ion at m/z 137 is accompanied by a loss of carbon–carbon double bond (C=C) to produce the fragment ion at m/z 112. The orientation of the C=C bond is cis configuration. This is confirmed by the presence of C-H out-of-plane bending vibration (670 cm
−1) of an olefinic bond, as the bending vibration at 950–970 cm
−1 (characteristic of trans configuration) is absent in the IR spectrum of spot 2 of the sub-fraction 13. Furthermore, the C-H bending vibration at 1514 cm
−1 results from the presence of a terminal methyl (CH
3) group in the eluate. The GC-MS spectral data confirm this as the fragment ion at m/z 83 loses a methyl group and one hydrogen atom to form the fragment ion at m/z 69. This is a diagnostic of unsaturated fatty acid with a terminal methyl group [
49].
In this work, the IR spectra data of spot 2 of the sub-fraction 13 agrees with that of cis-Octadecenoic acid methyl ester [
50]. In addition, the entire fragment ions in the GC-MS spectra data of spot 2 of the active sub-fraction 13, including the molecular ion and base peak ion, are in line with the data reported for methyl 9-octadecenoate by Yayli et al. (2001) [
29]. Therefore, based on the data presented in this work and the literature, and by comparing with the library search result, spot 2 of the active sub-fraction 13 was determined to be 9-octadecenoic acid methyl ester (E). Furthermore, the molecular formula of the active spot 2 of the sub-fraction13 was deduced as (C
19H
35O
2) based on the information obtained from its molecular ion at m/z 296; this was supported by the findings of Igwe and Onwu (2015) [
51] that the oleic acid was 75.03 of total essential oil of
S. alata leaf extract using GC-MS. Rahman et al. (2006) [
52] have also shown that oleic acids are among the major fatty acids present in
S. alata, confirming the presence of fatty acids in the
S. alata leaves in the current work. Many researchers, including Ogunwande et al. (2010) [
53], Adiana and Mazura (2011) [
54], and Thenmozhi and Rajan (2015) [
55], have previously used FT-IR spectroscopy and GC-MS to analyze the various phytocompounds.
The antibacterial action of the active spot 2, which seems to be a fatty acid methyl ester based on the library search and spectral analysis, is in line with the findings of Igwe and Onwu (2015) [
51], who reported the antibacterial effects of essential oil from
S. alata leaves extract against
S. aureus. Similar reports by Kabara et al. (1972) [
56], Knapp and Melly (1986) [
57], Farrington et al. (1992) [
58], and Sun et al. (2003) [
59] have shown that long-chain unsaturated fatty acids, including oleic acids found naturally, are effective bactericidal against pathogens including MRSA. In addition, Zheng et al. (2005) [
60] in their work reported the effectiveness of unsaturated fatty acid esters and unsaturated fatty acids esters derivatives against
S. aureus and MRSA. However, the precise mechanism of antibacterial action is precisely unknown. These reports support the observation made on the antibacterial activity of the identified compound against MRSA in the present study, which could explain the traditional applications of
S. alata in treating bacterial infections.