Abstract
The novel coronavirus disease 2019 (COVID-19) pandemic has brought to light the role of environmental hygiene in controlling disease transmission. Healthcare facilities are hot spots for infectious pathogens where physical distancing and personal protective equipment (PPE) are not always sufficient to prevent disease transmission. Healthcare facilities need to consider adjunct strategies to prevent transmission of infectious pathogens. In combination with current infection control procedures, many healthcare facilities are incorporating ultraviolet (UV) disinfection into their routines. This review considers how pathogens are transmitted in healthcare facilities, the mechanism of UV microbial inactivation and the documented activity of UV against clinical pathogens. Emphasis is placed on the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) as well as multidrug resistant organisms (MDROs) that are commonly transmitted in healthcare facilities. The potential benefits and limitations of UV technologies are discussed to help inform healthcare workers, including clinical studies where UV technology is used in healthcare facilities.
1. Introduction
Nosocomial, or healthcare-acquired infections (HAIs), refer to infections that originate within a healthcare setting. These infections are largely preventable and encompass significant morbidity and mortality for patients [1,2]. HAIs also hold a significant financial burden for healthcare systems, with cost estimates exceeding billions of dollars [3]. The global spread of coronavirus disease 2019 (COVID-19) has accelerated this burden and is overwhelming healthcare capacities worldwide. The most common nosocomial pathogens are bacteria and viruses. While there is a plethora of antibiotic therapies available resistance towards antibiotics and the inability of these compounds to treat viral infections, limits effective treatment options [4]. These pathogens can survive on surfaces for days, even years, where they can be transmitted between people and surfaces throughout healthcare facilities [5]. Diseases that are difficult to treat (e.g., COVID-19 and multidrug-resistant organisms (MDROs)) present the highest risk to healthcare systems [4]. Thus, prevention of disease, rather than treatment, is paramount.
Effective disinfection of clinical surfaces is therefore essential, as gaps in infection control procedures facilitate the transmission of these infectious pathogens [6]. However, studies have shown that less than 50% of surfaces in patient rooms are cleaned effectively [7,8] and that these rooms become environmental reservoirs, that can infect subsequent room occupants [9]. Enhanced environmental cleaning protocols result in lower overall contamination [7,8,10], and a decreased bioburden results in reduced infection rates [11,12]. Therefore, it is essential to consider enhanced environmental cleaning to prevent the transmission of healthcare-associated infections. While essential, manual cleaning alone is not sufficient to control the transmission of infectious pathogens. Novel strategies must focus on the disinfection of pathogens for all modes of disease transmission. Particularly pathogens that are the most difficult to treat, including viral and MDR infections.
1.1. Mechanisms of Viral Mutation
Viral mutation is an important driver of the spread of disease. Mutant strains may not be recognized by the immune system and treatment options may be limited. Antigenic drift and antigenic shift can diminish the efficacy of vaccines (Figure 1). When a shift occurs, the mutant virus is more likely to cause a pandemic as the population has no immunity towards it [13]. For example, as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spreads around the world it is slowly mutating [14]. Most mutations do not significantly affect virility, however, gain-of-function mutations offer an evolutionary advantage and spread quickly through populations. For example, both SARS-CoV-1 and SARS-CoV-2 use the angiotensin-converting enzyme 2 (ACE2) receptor for entry into host cells [15]. Yet the SARS-CoV-2 virus is more efficient for cell entry [16,17]. The delta variant of SARS-CoV-2 spreads up to 3× faster than the original strain [18]. This variant replicates rapidly in human hosts, is more effective to enter cells, and may suppress the host immune system [19]. Researchers have identified mutations in the spike protein D614G, which increases the density of this protein on the surface, facilitating greater entry into cells [20,21]. Currently, the available vaccines for SARS-CoV-2 are effective against the delta variant [22]. However, it is evolutionarily unavoidable that a vaccine-resistant strain will emerge and leave us unprotected [23]. Thus, non-vaccine strategies must be included to prevent the spread of disease. To replicate and spread, viruses need a living host, and so the most effective way to reduce the spread of mutant strains is to eradicate them before they can infect a host.
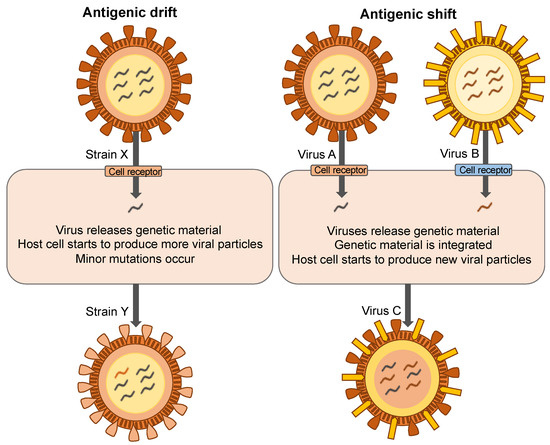
Figure 1.
Mechanisms of viral mutation.
1.2. Mechanisms of Antibiotic Resistance
It’s important to note that while the world has focused on the COVID-19 pandemic, antimicrobial resistance is another pandemic in the making [24,25]. Many believe that we are already amid this “silent pandemic” [26,27,28]. The consequences of widespread antimicrobial resistance are almost unimaginable. Many aspects of medicine rely on effective antimicrobial treatment and several everyday procedures would no longer be as safe. These include elective surgery, joint replacement, open heart surgery, cesarean births, cancer chemotherapy, organ transplants, diabetes management, and dental procedures [29,30,31].
Despite their simple unicellular composition, bacteria have continued to survive for over three billion years. One reason for this is their remarkable genetic plasticity and adaptability to changing environments [32]. In the same way that bacteria can acquire resistance to antibiotics, they can also develop resistance to detergents and disinfectants [33,34]. Sub-optimal concentrations and/or contact times can result in the survival of microbial populations [35]. Gram-negative bacteria, bacterial spores, biofilms, and enveloped viruses are more resistant to disinfectants due to their outer membranes [36]. These bacteria may persist in the healthcare environment where they may be easily transmitted to vulnerable patients [37]. Thus, research into infection control strategies distinct from current strategies (e.g., antibiotics, detergents, disinfectants) is needed to prevent MDRO infections.
1.3. Disease Transmission in Healthcare Facilities
Infections in healthcare facilities have an enormous socioeconomic burden with severe mortality and morbidity for patients [38]. With the rapid spread of COVID-19 and the increasing emergence of MDROs, it is therefore essential to understand routes of disease transmission to prevent future infections. The epidemiological triad reflects the three key factors of pathogen transmission: a susceptible host, a virulent pathogen, and a favorable environment (Figure 2). Host factors are the most difficult for healthcare facilities to regulate. Many patients are admitted with comorbidities or age-related diseases [39]. Vaccination status is particularly relevant in the context of COVID-19, but hospitals cannot discriminate against unvaccinated patients and not all infectious pathogens have an available vaccine. The most relevant factors for infection control are the pathogen and the environment.
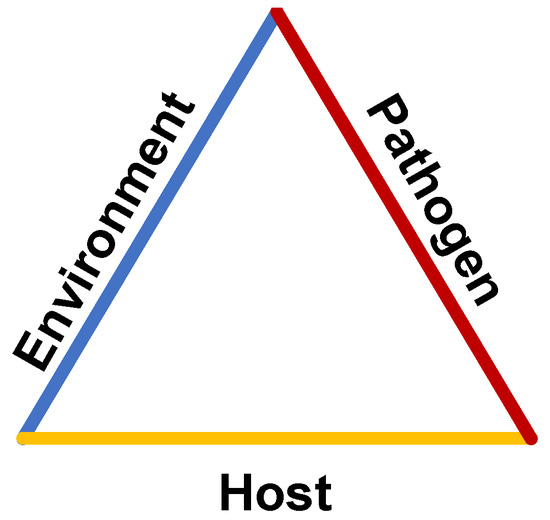
Figure 2.
The epidemiological triad of disease transmission.
The four main routes of disease transmission are contact, vehicle, airborne, and vector transmission. Contact transmission involves physical contact and the direct transfer of the organism from one individual to another. Appropriate hand hygiene protocols are essential to prevent direct contact transmission [40]. Vehicle transmission is the indirect transfer from an infectious agent from a reservoir to a host. The most common vehicles in healthcare facilities are via fomites, where microbes are transmitted by contaminated surfaces in the patient’s environment. These could be contaminated catheters, surgical instruments, or objects in patient rooms. Regular disinfection of surfaces is needed to prevent cross-contamination in healthcare facilities [41]. Vehicle transmission also encompasses oral ingestion of contaminated foods such as norovirus and rotavirus. Airborne transmission occurs when microorganisms are suspended in the air and enter the host through the respiratory passages. This is often the most difficult mode of transmission to regulate in healthcare facilities and is largely dependent on correct room ventilation [42]. Vector transmission is spread via an animal, most commonly arthropods biting the host to facilitate entry into the body. This is most relevant to the transmission of malaria, Dengue fever, and Lyme disease [43].
Fomite and airborne transmission are underappreciated environmental causes of HAIs [44]. While most infection control procedures focus on handwashing compliance, recent evidence continues to reveal that contaminated hospital surfaces and air play a large role in the transmission of disease [45,46]. Cohen and colleagues most recently provided evidence indicating that patients allocated to wardrooms of previously infected occupants had a 5.8× increased risk of acquiring the same infection [9]. This suggests current terminal room cleaning standards are not effective to remove pathogens. Another study demonstrated that prosthetic joint infection rates decreased when a supplemental ultraclean air decontamination system was introduced into the operating room, indicating that airborne transmission of pathogens may play an unprecedented role in the transmission of surgical site infections [47].
While there are many routes of transmission to consider in healthcare facilities the COVID-19 pandemic has highlighted the critical role of environmental disinfection. Personal protective equipment (PPE) is considered the most paramount infection control strategy, followed by physical distancing and hand hygiene protocols [48]. While these are essential in limiting transmission of COVID-19, these alone are not effective in controlling the spread of disease [49,50,51]. In a remarkable feat of medicine, multiple efficacious and safe vaccines were developed and have dramatically reduced the transmission of COVID-19 across the world. However, we must consider a multi-strategy approach to prevent the transmission of all infectious pathogens, particularly vaccine-resistant and drug-resistant pathogens.
1.4. Current Approach to Infection Control
The key focus areas for infection control stem from the relevant modes of disease transmission [52,53]. To prevent contact transmission of infectious pathogens the primary focus is on correct hand hygiene procedures. This includes following the ‘‘5 moments of hand hygiene”, where hand hygiene must be performed before and after touching a patient, before and after the procedure, and after touching a patient’s surroundings [54]. For routine medical procedures, gloves are considered as single-use items and must be changed between patients and procedures. It is recommended that visibly soiled hands are cleaned with soap and water, and for routine disinfection, an alcohol-based hand rub containing 60–80% v/v ethanol is used. Hand hygiene practices have decreased the incidence of infections associated with MDROs [55]. However, hand hygiene practices alone are not sufficient to prevent all HAIs. Hands may become rapidly re-contaminated after hand hygiene procedures [56]. Incorrect hand washing techniques may reduce the efficacy of microbial elimination. It is also notoriously difficult to maintain compliance after a hand hygiene intervention [57]. Many healthcare workers have documented skin irritation after the prolonged use of ethanol gels and therefore may reduce or eliminate the use of these products [58]. Similar limitations apply to the use of PPE. While gloves protect healthcare workers’ hands from infectious pathogens, they can become quickly contaminated and result in cross-transmission to the patient and the healthcare environment [59].
To prevent fomite transmission of infectious pathogens the primary focus is on environmental cleaning. This includes medical equipment, clinical and patient rooms. A detergent solution is used to clean most clinical surfaces (e.g., handrails, benches) and a disinfectant is added to higher risk surfaces (e.g., operating theatres, bathrooms) [45]. While manual cleaning is essential there are multiple limitations to manual disinfection. First of all, effective cleaning requires time and resources, which are often stretched in healthcare facilities [60]. The suitability of cleaning products must be determined for each surface or setting [61]. Manufacturers’ instructions and effectiveness of the product against particular organisms, including microbiological activity and contact time required to kill pathogens, should be considered.
To prevent airborne transmission of infectious pathogens, the primary focus is on air filtration. This includes face coverings, high-efficiency particulate absorbing (HEPA) air filtration, and ventilation procedures. The use of facemasks has become commonplace not only in healthcare facilities but in the community, to prevent the transmission of COVID-19. However, their effectiveness depends on correct supply and use. Shortages of PPE often meant that healthcare workers needed to reuse PPE, particularly N95 respirators [62]. Additionally, HEPA air filters and increased air exchanges were introduced into heating, ventilation, and air conditioning (HVAC) systems. While effective, these systems cannot be implemented in all areas.
There is a clear need for adjunct disinfection strategies to further reduce the transmission of infectious pathogens. Where human health is concerned, prevention is certainly better than cure. This is especially relevant when we consider the increasing rates of infections caused by COVID-19 and MDROs. Several studies have assessed the cost/benefit analysis of infection prevention strategies. Economically, it is more cost-effective to fund infection prevention strategies as opposed to treating established infections [63]. Throughout the COVID-19 pandemic, UVC disinfection for air and surfaces has gained popularity in healthcare facilities across the world. UVC disinfection is not a new discovery, rather in the last 10 years has been developed for specific healthcare applications. The following sections of this review discuss how UVC technology may be used as an adjunct disinfection strategy in healthcare facilities.
2. Ultraviolet Germicidal Irradiation
Ultraviolet germicidal irradiation uses UV wavelengths of light in the germicidal range (320–200 nm). The electromagnetic spectrum is presented in Figure 3. Radiation below 320 nm is acintic, causing photochemical reactions when exposed [64]. UVA (320–400 nm) lies outside this range and is not considered germicidal [64]. As the name suggests, vacuum UV only propagates in a vacuum due to its rapid absorption by oxygen in the air. Due to the predominant germicidal action of UVC light and its dominant use in commercial systems, other forms of UV are not discussed in detail unless required.
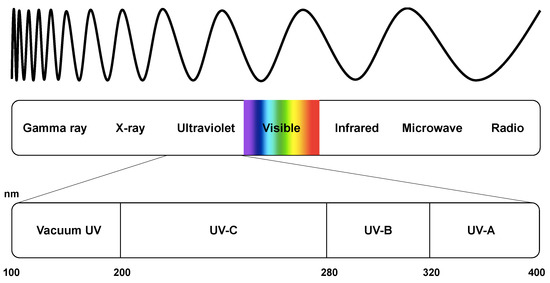
Figure 3.
The spectrum of electromagnetic radiation.
2.1. Ultraviolet Disinfection History
Today, ultraviolet light is used routinely in many industries for its germicidal properties. The first known report of light having germicidal properties was published in 1877 by Downes and Blunt. They observed that solutions exposed to sunlight remained sterile, whereas covered solutions became contaminated with bacteria [65]. In 1892, Marshal Ward went on to show that it was the violet end of the light spectrum responsible for bacterial inactivation [66]. This discovery sparked global interest in the germicidal properties of ultraviolet (UV) light (Figure 3). In 1903 Niels Finsen received the Nobel Prize in Physiology or Medicine for his contribution to the treatment of tuberculosis-related diseases with concentrated light radiation. The premise of this work was that concentrated UV light could be used to treat lupus vulgaris-affected patients. In 1930, Gates developed the bactericidal spectrum, where germicidal effectiveness was compared to the wavelength of UV light. Peak germicidal activity was seen at 265 nm and that UV-light was most effective at the 260 nm wavelength [67]. Gates suggested that UV absorbance by nucleic acids may be responsible for cell death—not proteins, as was a common belief at the time [68]. Hollaender and Emmons continued this notion and suggested that UV-induced damage correlated to the absorption of nucleic acids at 265 nm [69]. It was at this time that the discovery of penicillin prompted a boom in antibiotic research. As more affordable antibiotics came onto the market interest in UV light diminished. However, in 1988 Bolton demonstrated that UV light acted as a broad-spectrum disinfectant capable of inactivating almost all bacteria, viruses, and protozoa [70]. Following this research UVC light has been used widely as an effective tool for water disinfection. This chemical-free process eliminates stubborn pathogens, including cryptosporidia, which are extremely resistant to chemical disinfectants [71]
2.2. Mechanism of UV Microbial Inactivation
UV light is a form of electromagnetic radiation that causes photochemical changes in nucleic acids and proteins, resulting in the cessation of cell replication [64]. As the wavelength of light decreases, the energy increases (Figure 3). UVC light is most damaging to cells as UV is most strongly absorbed by nucleic acids at 260 nm [64]. UV light is strongly absorbed by thymine-cysteine double bonds in pyrimidine bases. This reaction results in the breaking of this hydrogen bond, allowing the pyrimidine bases to react with neighboring molecules. An adjacent pyrimidine base can therefore form a covalent bond, resulting in a tight four-membered ring (Figure 4). UV can also induce cross-linking between non-adjacent thymines, or between cytosine and guanine, but this requires increased energy for these photochemical reactions, and thymine dimers predominate [64]. In RNA, uracil replaces thymine and in a similar mechanism, uracil dimers are formed when exposed to UV. Alternatively, carbon atoms on the ring structures may form a single bond resulting in a 6-4-photoproduct.
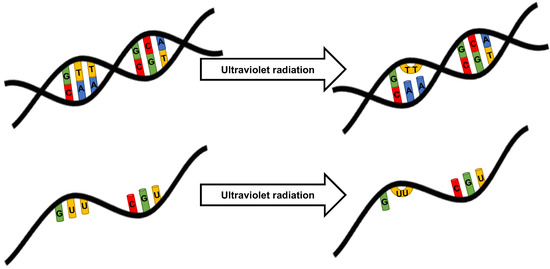
Figure 4.
Mechanism of ultraviolet damage to nucleic acids.
UV light rapidly targets DNA. In one second of sunlight, a UVB dose of 4.5 J/m2 causes 50–100 double-stranded breaks in each cell [72]. Comparatively, UVC light has a much higher energy and at the same dose causes 50,000 pyrimidine dimers per cell [73]. However, these genetic lesions can be repaired by DNA repair mechanisms of the cell [74]. Endogenous proteins identify and remove damaged sections of DNA, and then DNA repair machinery restores the DNA to its correct sequence. If the damage is not repaired the organism may be permanently mutated and inhibit essential metabolic functions thus inhibiting replication [64]. The amount of cross-linking required to inactivate is surprisingly little. When mengovirus was exposed to UV, a 6-log reduction in viral survival was related to a 9% cross-linking of uracil bonds [75].
UV light may also damage the protein capsid of some viruses. Damage to proteins occurs slower than nucleic acids due to the peak absorption at 280 nm [64,76]. UV light is known to alter protein secondary structures, expose hydrophobic regions and cause protein unfolding or aggregation [77]. Viral RNA in close proximity to proteins may also undergo covalent linkage when exposed to UV radiation, however, this is responsible for less than 2% of the total protein capsid [75].
2.3. Clinical Applicability of Ultraviolet Disinfection
While detergents are commonly used to clean surfaces, they do not inactivate antibiotic-resistant genomic material. Unless a second sterilization process occurs, these resistant genes may linger in the environment and spread to neighboring bacteria via the process of horizontal gene transfer. Detergents and disinfectants have no measurable impact on airborne pathogens, which highlights the need for novel air and surface disinfection strategies. UV light has been used for microbial inactivation for decades in industries such as water sterilization, wastewater treatment, HVAC, food processing, beverage production, and laboratory sterilization [78,79,80]. It is regarded as an efficacious and cost-effective antimicrobial strategy [81].
The delay for implementation and widespread use in hospitals relates to the engineering of suitable devices and clinical studies to prove efficacy. Exposed UVC is not suitable for some healthcare environments (e.g., emergency rooms where patients and staff cannot vacate). The most suitable application of UV disinfection is for terminal cleaning of high-risk areas, such as operating theatres and patient isolation rooms. Upper room UV systems can be used in occupied rooms if appropriately designed to limit UV exposure to the lower room. Enclosed UVC air filtrations systems can be used anywhere, but must be designed for sufficient UVC exposure time before filtered air is released back into the environment. Exposure time is calculated using the inverse square law, and standards to calculate the required dose for particular pathogens (Figure 5). As UVC needs to penetrate the outer cell layers, spores require more time than bacteria and viruses due to their biological composition (Figure 6). UV dose is often reported in mJ/cm2 where 1 mJ/cm2 = 1000 μW·s/cm2.
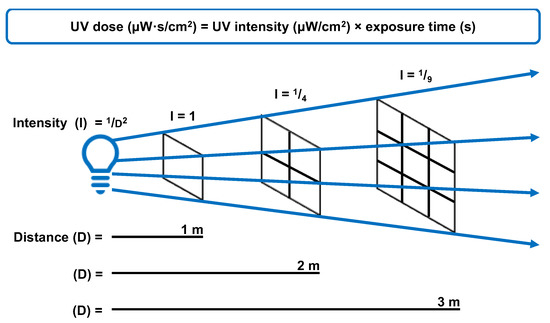
Figure 5.
The relationship between UV intensity, distance from the source, and time required to achieve germicidal dose for effective microbial inactivation.
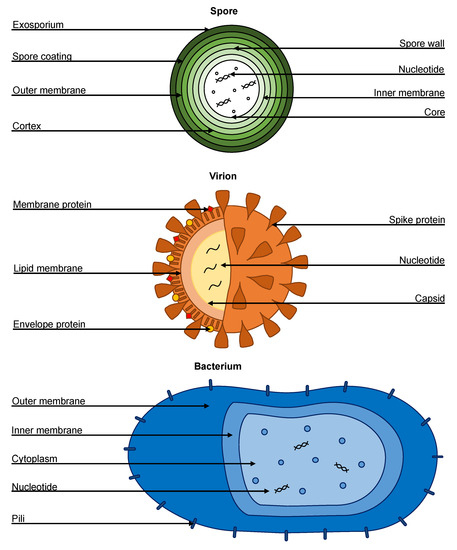
Figure 6.
Biological compositions of a spore, virion, and bacterium.
Automated UV disinfection robots can be used in most areas of the hospital environment including patient rooms, operating theatres, bathrooms, supply rooms, ambulances, etc. However, due to the safety considerations of UVC, it is not a viable option for high-traffic departments such as the emergency department. It is important to note that UV disinfection is an adjunct cleaning strategy and that manual cleaning to remove physical debris is still required. At this stage, there is no product on the market able to replace manual cleaning. UV light can also be incorporated into HVAC air filtration units for the enhanced elimination of airborne pathogens.
When choosing a UV surface disinfection system to suit the hospital environment it is important to consider the various factors. Price is ultimately an important consideration for purchasing equipment. Some devices may be purchased via a consignment rental model which decreases the cost of an outright capital purchase. When factoring in the price of a UV-disinfection system it is important to create a cost-benefit analysis factoring in a 20% infection reduction model [82]. The chosen UV device must be easy to use and transport around the hospital. Many UV systems are mobile and adaptable to their surroundings. Many devices have scanning technology to detect their surroundings. They may include height sensors, room mapping technology, and sensors to detect reflected UV dose. These parameters are often generated into user reports which may be helpful to monitor compliance. As UVC may be harmful to humans in high concentrations, each device must be fitted with multiple safety features—including motion-sensing technology and emergency stop buttons. UV lights built into air filtration systems should also comply with safety measures to prevent leakage of light into the surroundings, and be monitored and cleaned regularly to prevent dust accumulation.
2.4. Activity of UVC against Clinical Pathogens
The benefit of UV disinfection is the action on evolutionarily conserved targets (e.g., nucleotides). These non-specific targets are found in bacteria, viruses, fungi, and spores; making UV disinfection a promising strategy to prevent a variety of clinical pathogens. In theory, UVC disinfection presents an efficient method to prevent environmental transmission of COVID-19. Kowalski highlights that while there are varying dosages required to inactivate different viruses, that all microbes are susceptible to UVC inactivation [64]. In a somewhat foreshadowing prediction in 2009, they suggested that UV had “a definite future in the control of contagious disease and if applied on a widespread basis it may be the key to controlling epidemics and pandemics” [64].
The COVID-19 pandemic has indeed seen an influx of UV technologies being used clinically for disinfection of clinical rooms, but there is limited data on the efficacy of UV against the SARS-CoV-2 virus. Due to the similarities between SARS-CoV-1 and SARS-CoV-2, early hypotheses were generated based on evidence of UV inactivation of SARS-CoV-1. Several reports demonstrated the ability of UVC to completely inactivate high viral loads of SARS-CoV-1 [83]. In a laboratory setting, SARS-CoV-1 was killed rapidly after 6 min, and viable virus particles were not detected after 15 min of exposure to corresponding to a UVC (254 nm) dose of 1446 mJ/cm2. Since then, the ability of UVC to inactivate SARS-CoV-2 has been confirmed [84]. High viral loads of SARS-CoV-2 were completely inactivated after 9 min of UVC (254 nm) exposure, corresponding to 1048 mJ/cm2. The data suggest that both SARS-CoV-1 and SARS-CoV-2 are highly susceptible to UVC inactivation and show promise as a chemical-free disinfection tool for clinical use. However, it is important to note that while UVC is highly effective under laboratory conditions, the true clinical benefit of UVC will depend on a variety of real-world factors.
While studies are limited on the ability of UVC disinfection to reduce SARS-CoV-2 transmission, many studies have looked at the ability of UVC to inactivate other clinically relevant pathogens. Of particular concern are the number of increasing bacteria that are resistant to antibiotics. Antibiotic-resistant bacterial infections are becoming increasingly more difficult to treat, with pan-resistant strains (resistant to all available antibiotics) found across the world. As UVC has a distinct mechanism compared to antimicrobial chemotherapy, it does not discriminate between antibiotic-sensitive and antibiotic-resistant strains. Many studies have shown the ability of UVC to inactivate MDROs. Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE) are common gram-positive bacteria causing outbreaks that are difficult to contain in healthcare facilities. When compared to their antibiotic-susceptible counterparts, similar UVC dosages were required for inactivation [85].
3. Airborne Transmission of Pathogens
Correctly identifying the routes of SARS-CoV-2 transmission, has profound implications for the spread of COVID-19 throughout healthcare facilities and communities. Thus, assumptions about routes of transmission can have devastating public health implications. It is clear that detection of viable viral particles in the air are difficult to recover accurately, and depends on many environmental factors such as humidity, sunlight, and ventilation [86]. The limitation of current sampling methodologies and cultures has led to studies that incorrectly dismiss the role of airborne transmission [87,88]. While originally not considered important, the evidence is now clear that SARS-CoV-2 is primarily transmitted in the air [89,90,91]. Early 2020 infection control procedures focused on disinfection of surfaces, PPE, and physical distancing [92]. After an outcry from many clinicians and epidemiological experts, it was confirmed that SARS-CoV-2 is an airborne pathogen, and we should consider this the principal mode by which people are infected [92].
Empirical evidence in the early stages of the COVID-19 pandemic supports this mode of transmission [93,94]. Pandemics are often potentiated in communities by "super-spreader" events, where a single person infects multiple others in a single event. Analyzing these highly infectious events shows patterns of transmission that are only consistent with the airborne transmission. An outbreak in Washington, USA saw 53 of 61 choir attendees contract COVID-19, with no evidence of fomite or droplet transmission [95]. At the time there were no identified cases in the area. Precautions were taken to limit contact between people, physical distancing during rehearsal, and adherence to hand hygiene protocols. Airborne transmission has also been widely documented in hotel quarantine facilities, where SARS-CoV-2 is transmitted to people in the absence of physical contact or shared facilities. Viable SARS-CoV-2 particles have even been collected from uninhabited apartments in buildings with known COVID-19 positive cases [94]. This transmission was linked to the spread of fecal aerosols through dried-out baths and floor drains.
3.1. UV Disinfection of Air
UV robots can disinfect both air and surfaces, however, due to their mutagenic effects on organic cells they cannot be used in occupied rooms. For high-traffic environments such as waiting rooms and operating theatres, new sources of contamination are introduced throughout the day [96]. Airborne microbial populations are important, especially in the flu season. It is known that the main source of airborne particles in the operating theatre is from human occupants. It has been estimated that people shed up to 1 billion skin cells per day [97]. Moreover, that 10% of these cells are laden with bacteria [98]. The most common bacteria in prosthetic joint infections are the commensal bacteria Staphylococcus aureus [99].
Traditional air decontamination systems use HEPA filtration to trap particles and limit their recirculation into the room air [100]. However, there is a limit to the efficacy of this technology. Frequent replacement of HEPA filters is essential for their mechanism of microbial clearance, yet most filters are not replaced frequently enough [101]. HEPA filters cannot trap particles smaller than 0.3 µm, which includes viruses, volatile organic compounds (carcinogenic), and some proteins. The diameter of SARS-CoV-2 is approximately 60 to 140 nm which is far below the size that HEPA filters can reliably trap [102]. Additionally, any viable pathogens (bacteria and mold spores) trapped in the HEPA matrix can feed off other trapped particles [103]. These pathogens can then multiply and be re-circulated into the room air. Live bacteria also produce toxins which like volatile organic compounds are too small to be trapped in the HEPA matrix [104]. These toxins can be released back into the air and have been shown to promote an inflammatory response in asthmatic people [105].
The latest air filtration systems utilize a hybrid of physical and biological systems to reduce the airborne microbial burden [106,107]. These new technologies not only HEPA filter the air, but also use UVC to disinfect the filtered particles. In many systems, contaminated room air is drawn in through a vent where it is passed through a pre-filter to remove any large particles, for maximum efficacy this pre-filter should be changed daily. Air is then circulated through a solid-state germicidal UVC chamber. This chamber consists of a crystalline matrix that slows and traps airborne particles, where they are rapidly inactivated by UVC light. The air is then passed through a HEPA filter before being released back into circulation. As the air coming from the UVC chamber is 99.999% sterile, the final HEPA filters receive little contamination and therefore are recommended to be replaced yearly for maximum efficacy.
3.2. Upper Room UV Systems
Upper room UV systems are engineered to create a germicidal zone of UVC light, confined to the uppermost portion of the room (Figure 7). The basic principle is to maximize the UVC exposure of upper-room air while minimizing exposure to room occupants below. This requires sufficient room air circulation and the exchange of ‘‘clean’’ and ‘‘contaminated’’ air between the two zones. As air enters the germicidal zone, it is disinfected. These upper room UV systems can run continuously, which is useful for high-risk environments such as operating theatres or waiting rooms. As UVC is particularly hazardous to the human cornea, the maximum eye-level irradiance dose is set to 0.2 µW/cm2 in the lower portion of the room [108]. Many studies have shown the benefits of upper room UV systems, particularly reducing transmission of tuberculosis and measles [109,110,111,112].
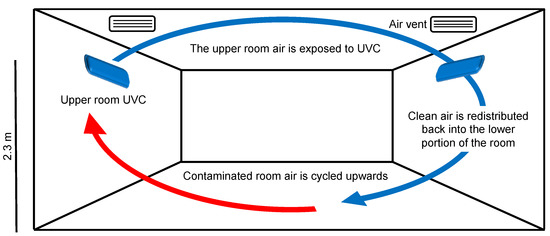
Figure 7.
Upper room UVC air disinfection system.
Essential considerations for upper room UV systems include air exchange rates, UVC dose, UVC exposure time, ceiling height, humidity, temperature, exposure to medical equipment, and lamp maintenance [64]. To effectively inactivate microbes in the air, the exposure time of air to UVC must be balanced with the number of air exchanges per hour required. A calculated rate of six air changes per hour has a relative microbial reduction equivalent to 12 air changes without UVC [64]. The dose required to inactivate different microbes varies, the guidelines suggest using one 30 W UVC (254 nm) lamp per 60 m2 [113]. These units are presented as guidelines and require correct installation and ventilation to provide adequate results. Ceiling height is a limiting factor for the use of upper room UV systems. Ceiling heights less than 2.3 m risk exposure of UVC at hazardous levels in the lower portion of the room [64]. Room conditions such as humidity and temperature also affect microbial clearance. Relative humidity should be lower than 75%, and temperature below 25 °C to minimize microbial self-protective mechanisms [64]. Another consideration is the potential degradation of medical equipment exposed to continuous UVC. Fading, bleaching, and photodegradation of certain materials (e.g., plastics) have been observed after prolonged exposure to UVC—even at safe levels [114]. While this photodegradation is often limited to the superficial material surface and does not affect the structural properties of medical equipment, it should be monitored [115]. Finally, for sufficient UVC output, the UVC lamps and systems should be regularly maintained. It is recommended that lamps are wiped with a 70% ethanol solution to remove dust, and bulbs replaced yearly.
4. Surface Transmission of Pathogens
The healthcare environment is predisposed to harbor infectious pathogens due to the high density of infected patients. Pathogens are shed daily from the skin of healthcare workers and patients, or respiratory droplets and aerosols may settle on clinical surfaces, generating fomites. These pathogens can survive on surfaces for days, to years [116]. Recently, the SARS-CoV-2 virus was viable on non-porous surfaces for at least 28 days, and 21 days on the N95 mask material [117]. A clinical isolate of VRE was viable for over 3 years when dried onto glass surfaces [118]. C. difficile persisted on a hospital floor for 5 months, although it was not clear if this room was subject to regular cleaning [119].
Pathogens persist in the environment due to the inherent limitations of manual cleaning. First of all, surface disinfection is highly dependent on the correct chemical choice and application. Many chemicals are toxic, either to the user, or the environment, and are therefore not suitable for clinical use. Chemicals such as bleach are corrosive for some medical equipment and can be hazardous to use [120]. Disinfectants are often used at insufficient wet-contact times, which are required for complete pathogen inactivation. For example, bleach solutions require 10 min of wet contact time, often requiring multiple applications, which is difficult to maintain in the healthcare setting [121]. Because of the specific chemical and contact requirements for bleach, clinical surfaces are often left contaminated. After bleach disinfection, 44% of clinical surfaces were contaminated with C. difficile and 71% with VRE [122]. The physical size of the room and the time required for manual cleaning is also problematic for cleaning staff. Moreover, the cleaning materials (e.g., cloths, mops) are often contaminated and result in cross-contamination between rooms [123].
The risk of transmission of SARS-CoV-2 via surfaces is relatively low compared to airborne and direct contact transmission. Nonetheless, it is important to consider the role environmental surfaces play in disease transmission. Respiratory droplets and airborne particles that carry SARS-CoV-2 can settle on surfaces, where they remain viable for up to 7 days [117]. Hand hygiene is the most effective strategy to prevent fomite transmission, however, compliance rates are low [70]. Within households of infected and non-infected people, surface disinfection effectively prevents secondary transmission of SARS-CoV-2, but this is difficult to study in cases of nosocomial and community transmission [124]. While the surface transmission is difficult to study in healthcare facilities, it is known that routine cleaning can substantially reduce virus levels on surfaces [125].
4.1. UV Disinfection of Non-Porous Surfaces
There are several devices currently on the market that use UV technology for use in healthcare facilities (Figure 8). The two main classes are pulsed xenon UV and steady-state UVC emitting devices. The first UV light devices developed were pulsed-xenon UV units. They emit a broad spectrum of UV light (100–280 nm) as well as visible light (400–700 nm). These devices often involve repositioning of the device in multiple areas of the room [126]. This adds time as well as manual input into the disinfection process. Contrastingly, continuous-wave UVC devices emit UV light at the 253.7 nm wavelength. This wavelength of light is highly absorbed by nucleic acids and therefore is accountable for the mechanism of kill [127]. Nerandzic and colleagues compared the efficacy of a pulsed xenon UV device and continuous wave UVC [126]. They concluded that following a 10 min exposure period continuous UVC decreased the recovery of C. difficile and MRSA two times greater, and VRE six times greater than pulsed xenon UV-disinfection. C. difficile spores are notoriously difficult to eradicate in hospitals and have caused outbreaks that last for months to years [128]. This can be attributed to their multi-layered biological composition, and the difficulty of disinfectants to completely destroy environmental reservoirs of this pathogen [129].

Figure 8.
Portable UVC disinfection robot in an unoccupied operating theatre.
Automated UVC disinfection technologies effectively reduce the environmental bioburden, including MRDOs [130,131,132,133,134]. As environmental bioburden is linked to transmission of HAIs, a study on the ability of UV disinfection to reduce infection rates was conducted. The results of a multicenter, randomized clinical trial were published that assessed the relationship between terminal room disinfection and acquisition of HAIs [135,136]. This is the first clinical trial to publish outcomes directly related to environmental UVC disinfection. The target organisms identified were C. difficile, MRSA, VRE, and MDR-Acinetobacter spp. Four terminal disinfection strategies were assessed: (1) quaternary ammonium compound (QAC) disinfectant, (2) QAC + UVC, (3) bleach, and (4) bleach + UVC. In rooms of patients with C. difficile infection, the control disinfection protocol also contained bleach as per clinical standards.
The authors present that compared to standard cleaning measures, after UVC disinfection there was a non-significant, hospital-wide decrease in the risk of HAIs for all pathogens listed [136]. p-values are often arbitrarily set to p = 0.05, meaning there is a 5% chance that the null hypothesis is true. The p-value for the hospital-wide reduction in HAI risk was 0.052. Looking closer at the relative risk (RR) value and confidence interval (CI), RR (0.89, 95% CI 0.79–1.00) the upper CI is exactly 1. If the significance threshold had been 0.002 higher it would be considered statistically significant. Interpretations of this must be taken with caution and are discussed below.
There are several factors not considered that may hide the true effect of UVC disinfection. There is a phenomenon called the Hawthorne effect, that simply studying something can affect the results. In this study, the compliance of terminal cleaning in the control group was 90%, but many other studies have shown much lower compliance rates, below 50% [8,137]. Cleaning compliance also increases with regular training sessions and monitoring [138], which were incorporated into this study. Many studies have linked improved cleaning to a reduction in bioburden and reduced HAI rates [11,139]. Thus, the control group could be enhanced cleaning in itself—and dampen the treatment group results.
Using a p-value of 0.05, there were statistically significant reductions in HAI rates for C. difficile (RR 0.89, 95% CI 0.80–0.99, p = 0.031) and VRE (RR 0.56, 95% CI 0.31–0.996, p = 0.048) when UVC disinfection was compared to control. This data highlights the importance of environmental contamination and the transmission of infectious pathogens throughout healthcare facilities. Overall, this study starts the conversation on the clinical outcomes and benefits of UV disinfection. It demonstrates the relationship between enhanced room disinfection, environmental bioburden, and the incidence of hospital-wide HAI rates.
In a separate study that year, it was found that UVC disinfection in a pediatric long-term care facility reduced viral infections by 44% (95% CI 0.37–0.84, p = 0.003) [140]. Most importantly, when incorporated into routine cleaning, the benefit of UVC disinfection increased each month. This may be due to the elimination of environmental reservoirs and/or improved user training. While much of the research around UVC surface disinfection focuses on the reduction of bacterial bioburden, this study highlights the importance of viral transmission from surfaces.
4.2. UV Disinfection of Porous Materials
Another consequence of the COVID-19 pandemic was the global shortage of medical-grade PPE [141]. With the supply system strained, single-use PPE such as surgical masks, N95 respirators, and disposable gowns were reused for days at a time [142]. A recent study showed that healthcare workers who reused PPE had an increased risk of COVID-19 transmission [49]. Moreover, this risk was comparable to inadequate PPE; leaving frontline healthcare workers extremely vulnerable to infection [49]. UVC disinfection of single-use PPE was implemented during this supply shortage. Many studies have evaluated the ability of UVC to inactivate SARS-CoV2 virions trapped in porous materials, particularly face coverings.
A collection of disinfection studies showed that UVC light was able to significantly reduce the microbial burden of soiled masks [62,143,144,145]. The time required for disinfection ranged from 1 min to 10 min. All studies noted that the mask material integrity was maintained after multiple rounds of disinfection. UVC disinfection of these materials does have some limitations. To effectively disinfect materials, UVC must have direct contact with the material. Any shadowing, or coverage by other materials (glass, plastic) would reduce the amount of UVC available [134]. The efficacy of UVC to disinfect porous materials, such as fabrics, is lower than non-porous surfaces, and often increases the time required to disinfect these materials [146]. This must be considered when designing and implementing UVC disinfection technologies. Overall, the ability to effectively decontaminate PPE would not only reduce the risk of infection but also decrease the costs associated with single-use PPE and the sheer volume of medical waste.
5. Conclusions
This review explores disease transmission in healthcare facilities and how environmental disinfection is key to minimizing the transmission of infectious pathogens. UV disinfection holds promise as an adjunct disinfection strategy to reduce the microbial burden, and thus reduce transmission of disease. It is imperative that UV disinfection does not replace manual cleaning of surfaces and is integrated into the existing regime. Different UV applications may be suitable for each mode of disease transmission and should be evaluated by trained infection control personnel to determine the most effective use of equipment. Supplemental air-filtration units that utilize UVC may reduce the circulation of infectious airborne pathogens, such as SARS-CoV-2, and may provide benefits when used in high-risk areas. Further research into the long-term benefits of UV disinfection and reduction of HAI rates will further promote this technology for use in clinical settings.
Funding
UNSW Scientia PhD Scholarship.
Acknowledgments
The author would like to acknowledge the many workers around the world who are on the frontline of the COVID-19 pandemic. Your tireless dedication inspires us all.
Conflicts of Interest
The author declares no conflict of interest.
References
- Russo, P.L.; Stewardson, A.J.; Cheng, A.C.; Bucknall, T.; Mitchell, B.G. The Prevalence of Healthcare Associated Infections among Adult Inpatients at Nineteen Large Australian Acute-Care Public Hospitals: A Point Prevalence Survey. Antimicrob. Resist. Infect. Control 2019, 8, 114. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care-Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Douglas, S. The Direct Medical Costs of Healthcare-Associated Infections in U.S. Hospitals and the Benefits of Prevention; National Center for Preparedness, Detection, and Control of Infectious Diseases: Atlanta, GA, USA, 2009.
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How Long Do Nosocomial Pathogens Persist on Inanimate Surfaces? A Systematic Review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Blanco, N.; O’Hara, L.M.; Harris, A.D. Transmission Pathways of Multidrug-Resistant Organisms in the Hospital Setting: A Scoping Review. Infect. Control Hosp. Epidemiol. 2019, 40, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Carling, P.C.; Parry, M.F.; Bruno-Murtha, L.A.; Dick, B. Improving Environmental Hygiene in 27 Intensive Care Units to Decrease Multidrug-Resistant Bacterial Transmission. Crit. Care Med. 2010, 38, 1054–1059. [Google Scholar] [CrossRef]
- Carling, P.C.; Parry, M.M.; Rupp, M.E.; Po, J.L.; Dick, B.; Von Beheren, S. Healthcare Environmental Hygiene Study Group Improving Cleaning of the Environment Surrounding Patients in 36 Acute Care Hospitals. Infect. Control Hosp. Epidemiol. 2008, 29, 1035–1041. [Google Scholar] [CrossRef]
- Cohen, B.; Liu, J.; Cohen, A.R.; Larson, E. Association Between Healthcare-Associated Infection and Exposure to Hospital Roommates and Previous Bed Occupants with the Same Organism. Infect. Control Hosp. Epidemiol. 2018, 39, 541–546. [Google Scholar] [CrossRef]
- Goodman, E.R.; Platt, R.; Bass, R.; Onderdonk, A.B.; Yokoe, D.S.; Huang, S.S. Impact of an Environmental Cleaning Intervention on the Presence of Methicillin-Resistant Staphylococcus Aureus and Vancomycin-Resistant Enterococci on Surfaces in Intensive Care Unit Rooms. Infect. Control Hosp. Epidemiol. 2008, 29, 593–599. [Google Scholar] [CrossRef]
- Hayden, M.K.; Bonten, M.J.M.; Blom, D.W.; Lyle, E.A.; van de Vijver, D.A.M.C.; Weinstein, R.A. Reduction in Acquisition of Vancomycin-Resistant Enterococcus after Enforcement of Routine Environmental Cleaning Measures. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 42, 1552–1560. [Google Scholar] [CrossRef]
- Everett, B.R.; Sitton, J.T.; Wilson, M. Efficacy and Cost-Benefit Analysis of a Global Environmental Cleaning Algorithm on Hospital-Acquired Infection Rates. J. Patient Saf. 2017, 13, 207–210. [Google Scholar] [CrossRef]
- Honigsbaum, M. Pandemic. Lancet 2009, 373, 1939. [Google Scholar] [CrossRef]
- Mercatelli, D.; Giorgi, F.M. Geographic and Genomic Distribution of SARS-CoV-2 Mutations. Front. Microbiol. 2020, 11, 1800. [Google Scholar] [CrossRef]
- Anand, S.; Chen, Y.; Prévost, J.; Gasser, R.; Beaudoin-Bussières, G.; Abrams, C.; Pazgier, M.; Finzi, A. Interaction of Human ACE2 to Membrane-Bound SARS-CoV-1 and SARS-CoV-2 S Glycoproteins. Viruses 2020, 12, 1104. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef]
- Peng, R.; Wu, L.-A.; Wang, Q.; Qi, J.; Gao, G.F. Cell Entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Alizon, S.; Haim-Boukobza, S.; Foulongne, V.; Verdurme, L.; Trombert-Paolantoni, S.; Lecorche, E.; Roquebert, B.; Sofonea, M.T. Rapid Spread of the SARS-CoV-2 Delta Variant in Some French Regions, June 2021. Eurosurveillance 2021, 26, 2100573. [Google Scholar] [CrossRef]
- Khateeb, J.; Li, Y.; Zhang, H. Emerging SARS-CoV-2 Variants of Concern and Potential Intervention Approaches. Crit. Care 2021, 25, 244. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Niu, Z.; Zhang, B.; Wang, C.; Yao, X.; Peng, H.; Franca, D.N.; Wang, Y.; Zhu, Y.; et al. SARS-CoV-2 Spike Protein Dictates Syncytium-Mediated Lymphocyte Elimination. Cell Death Differ. 2021, 28, 2765–2777. [Google Scholar] [CrossRef]
- Ozono, S.; Zhang, Y.; Ode, H.; Sano, K.; Tan, T.S.; Imai, K.; Miyoshi, K.; Kishigami, S.; Ueno, T.; Iwatani, Y.; et al. SARS-CoV-2 D614G Spike Mutation Increases Entry Efficiency with Enhanced ACE2-Binding Affinity. Nat. Commun. 2021, 12, 848. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Rella, S.A.; Kulikova, Y.A.; Dermitzakis, E.T.; Kondrashov, F.A. Rates of SARS-CoV-2 Transmission and Vaccination Impact the Fate of Vaccine-Resistant Strains. Sci. Rep. 2021, 11, 15729. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Yadegar, A.; Hatami, B.; Asadzadeh Aghdaei, H.; Zali, M.R. Antimicrobial Resistance as a Hidden Menace Lurking Behind the COVID-19 Outbreak: The Global Impacts of Too Much Hygiene on AMR. Front. Microbiol. 2020, 11, 590683. [Google Scholar] [CrossRef]
- Founou, R.C.; Blocker, A.J.; Noubom, M.; Tsayem, C.; Choukem, S.P.; Dongen, M.V.; Founou, L.L. The COVID-19 Pandemic: A Threat to Antimicrobial Resistance Containment. Future Sci. OA 2021, 7, FSO736. [Google Scholar] [CrossRef] [PubMed]
- Manesh, A.; Varghese, G.M. Rising Antimicrobial Resistance: An Evolving Epidemic in a Pandemic. Lancet Microbe 2021, 2, e419–e420. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial Resistance and COVID-19: Intersections and Implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The Silent Pandemic: Emergent Antibiotic Resistances Following the Global Response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef]
- Teillant, A.; Gandra, S.; Barter, D.; Morgan, D.J.; Laxminarayan, R. Potential Burden of Antibiotic Resistance on Surgery and Cancer Chemotherapy Antibiotic Prophylaxis in the USA: A Literature Review and Modelling Study. Lancet Infect. Dis. 2015, 15, 1429–1437. [Google Scholar] [CrossRef]
- Smith, R.; Coast, J. The True Cost of Antimicrobial Resistance. BMJ 2013, 346, f1493. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Health Care (ACSQHC). AURA 2019: Third Australian Report on Antimicrobial Use and Resistance in Human Health; Australian Commission on Safety and Quality in Health Care: Sydney, Australia, 2019; ISBN 978-1-925948-00-4.
- Kussell, E.; Kishony, R.; Balaban, N.Q.; Leibler, S. Bacterial Persistence. Genetics 2005, 169, 1807–1814. [Google Scholar] [CrossRef]
- Langsrud, S.; Sundheim, G.; Borgmann-Strahsen, R. Intrinsic and Acquired Resistance to Quaternary Ammonium Compounds in Food-Related Pseudomonas Spp. J. Appl. Microbiol. 2003, 95, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.S. Disinfectant Resistance Mechanisms, Cross-Resistance, and Co-Resistance. Int. Biodeterior. Biodegrad. 2003, 51, 271–276. [Google Scholar] [CrossRef]
- Hong, Y.; Teska, P.J.; Oliver, H.F. Effects of Contact Time and Concentration on Bactericidal Efficacy of 3 Disinfectants on Hard Nonporous Surfaces. Am. J. Infect. Control 2017, 45, 1284–1285. [Google Scholar] [CrossRef]
- Russell, A.D. Bacterial Resistance to Disinfectants: Present Knowledge and Future Problems. J. Hosp. Infect. 1999, 43, S57–S68. [Google Scholar] [CrossRef]
- Han, J.H.; Sullivan, N.; Leas, B.F.; Pegues, D.A.; Kaczmarek, J.L.; Umscheid, C.A. Cleaning Hospital Room Surfaces to Prevent Health Care—Associated Infections. Ann. Intern. Med. 2015, 163, 598–607. [Google Scholar] [CrossRef]
- Plowman, R. The Socioeconomic Burden of Hospital Acquired Infection. Eurosurveillance 2000, 5, 49–50. [Google Scholar] [CrossRef]
- McGregor, J.C.; Perencevich, E.N.; Furuno, J.P.; Langenberg, P.; Flannery, K.; Zhu, J.; Fink, J.C.; Bradham, D.D.; Harris, A.D. Comorbidity Risk-Adjustment Measures Were Developed and Validated for Studies of Antibiotic-Resistant Infections. J. Clin. Epidemiol. 2006, 59, 1266–1273. [Google Scholar] [CrossRef]
- Allegranzi, B.; Pittet, D. Role of Hand Hygiene in Healthcare-Associated Infection Prevention. J. Hosp. Infect. 2009, 73, 305–315. [Google Scholar] [CrossRef]
- Assadian, O.; Harbarth, S.; Vos, M.; Knobloch, J.K.; Asensio, A.; Widmer, A.F. Practical Recommendations for Routine Cleaning and Disinfection Procedures in Healthcare Institutions: A Narrative Review. J. Hosp. Infect. 2021, 113, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Todi, S.; Myatra, S.N.; Samaddar, D.P. Guidelines for Prevention of Hospital Acquired Infections. Indian J. Crit. Care Med. 2014, 18, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Schorderet-Weber, S.; Noack, S.; Selzer, P.M.; Kaminsky, R. Blocking Transmission of Vector-Borne Diseases. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, G.; Alangaden, G.; Bardossy, A.C. The Role of Environmental Contamination in the Transmission of Nosocomial Pathogens and Healthcare-Associated Infections. Curr. Infect. Dis. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Dancer, S.J. Controlling Hospital-Acquired Infection: Focus on the Role of the Environment and New Technologies for Decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef]
- Doll, M.; Stevens, M.; Bearman, G. Environmental Cleaning and Disinfection of Patient Areas. Int. J. Infect. Dis. 2018, 67, 52–57. [Google Scholar] [CrossRef]
- Cook, T.M.; Piatt, C.J.; Barnes, S.; Edmiston, C.E. The Impact of Supplemental Intraoperative Air Decontamination on the Outcome of Total Joint Arthroplasty: A Pilot Analysis. J. Arthroplasty 2019, 34, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Coronavirus Disease 2019 (COVID-19): Protecting Hospitals From the Invisible. Ann. Intern. Med. 2020, 172, 619–620. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H.; et al. Risk of COVID-19 among Front-Line Health-Care Workers and the General Community: A Prospective Cohort Study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Quigley, A.L.; Stone, H.; Nguyen, P.Y.; Chughtai, A.A.; MacIntyre, C.R. Estimating the Burden of COVID-19 on the Australian Healthcare Workers and Health System during the First Six Months of the Pandemic. Int. J. Nurs. Stud. 2021, 114, 103811. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H.; Kawaoka, Y. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. mSphere 2020, 5, e00637-20. [Google Scholar] [CrossRef]
- World Health Organization, Regional Office for Europe. Infection Prevention and Control: Guidance to Action Tools; World Health Organization, Regional Office for Europe: København, Denmark, 2021; ISBN 978-92-890-5543-7. [Google Scholar]
- Jefferson, T.; Del Mar, C.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; Bawazeer, G.A.; van Driel, M.L.; Foxlee, R.; Rivetti, A. Physical Interventions to Interrupt or Reduce the Spread of Respiratory Viruses: Systematic Review. BMJ 2009, 339, b3675. [Google Scholar] [CrossRef]
- Chou, D.T.S.; Achan, P.; Ramachandran, M. The World Health Organization ‘5 Moments of Hand Hygiene’. J. Bone Joint Surg. Br. 2012, 94-B, 441–445. [Google Scholar] [CrossRef]
- Gordin, F.M.; Schultz, M.E.; Huber, R.A.; Gill, J.A. Reduction in Nosocomial Transmission of Drug-Resistant Bacteria After Introduction of an Alcohol-Based Handrub. Infect. Control Hosp. Epidemiol. 2005, 26, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Kampiatu, P.; Cozean, J. A Controlled, Crossover Study of a Persistent Antiseptic to Reduce Hospital-Acquired Infection. Afr. J. Infect. Dis. 2015, 9, 6–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Son, C.; Chuck, T.; Childers, T.; Usiak, S.; Dowling, M.; Andiel, C.; Backer, R.; Eagan, J.; Sepkowitz, K. Practically Speaking: Rethinking Hand Hygiene Improvement Programs in Health Care Settings. Am. J. Infect. Control 2011, 39, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D. Improving Adherence to Hand Hygiene Practice: A Multidisciplinary Approach. Emerg. Infect. Dis. 2001, 7, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Duckro, A.N.; Blom, D.W.; Lyle, E.A.; Weinstein, R.A.; Hayden, M.K. Transfer of Vancomycin-Resistant Enterococci via Health Care Worker Hands. Arch. Intern. Med. 2005, 165, 302–307. [Google Scholar] [CrossRef]
- Dancer, S.J. Hospital Cleaning in the 21st Century. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1473–1481. [Google Scholar] [CrossRef]
- WHO. Use of Disinfectants: Alcohol and Bleach; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Fischer, R.J.; Morris, D.H.; van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N.; et al. Effectiveness of N95 Respirator Decontamination and Reuse against SARS-CoV-2 Virus. Emerg. Infect. Dis. 2020, 26, 2253–2255. [Google Scholar] [CrossRef]
- Dick, A.W.; Perencevich, E.N.; Pogorzelska-Maziarz, M.; Zwanziger, J.; Larson, E.L.; Stone, P.W. A Decade of Investment in Infection Prevention: A Cost-Effectiveness Analysis. Am. J. Infect. Control 2015, 43, 4–9. [Google Scholar] [CrossRef]
- Kowalski, W.J. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection; Springer: New York, NY, USA, 2009; ISBN 978-3-642-01998-2. [Google Scholar]
- Downes, A.; Blunt, T.P. The Influence of Light upon the Development of Bacteria 1. Nature 1877, 16, 218. [Google Scholar] [CrossRef]
- Ward, H.M.V. Experiments on the Action of Light on Bacillus Anthracis. Proc. R. Soc. Lond. 1893, 52, 393–400. [Google Scholar] [CrossRef]
- Gates, F.L. A study of the bactericidal action of ultra violet light. J. Gen. Physiol. 1930, 14, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Reed, N.G. The History of Ultraviolet Germicidal Irradiation for Air Disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Hollaender, A.; Emmons, C.W. Wavelength dependence of mutation production in the ultraviolet with special emphasis on fungi. Cold Spring Harb. Symp. Quant. Biol. 1941, 9, 179–186. [Google Scholar] [CrossRef]
- Bolton, J.; Bertrand, D.; Zia, B.; Hargey, T.; Clancy, J. Inactivation of Cryptosporidium Parvum by Medium-Pressure Ultraviolet Light in Finished Drinking Water. In Proceedings of the AWWA 1998 Annual Conference, Dallas, TX, USA, 21–24 June 1998; Volume A, pp. 389–403. [Google Scholar]
- Clancy, J.L.; Bukhari, Z.; Hargy, T.M.; Bolton, J.R.; Dussert, B.W.; Marshall, M.M. Using UV to Inactivate Cryptosporidium. J. Am. Water Works Assoc. 2000, 92, 97–104. [Google Scholar] [CrossRef]
- Zhao, H.; Traganos, F.; Darzynkiewicz, Z. Kinetics of the UV-Induced DNA Damage Response in Relation to Cell Cycle Phase. Correlation with DNA Replication. Cytom. Part J. Int. Soc. Anal. Cytol. 2010, 77, 285–293. [Google Scholar] [CrossRef]
- Rothman, R.H.; Setlow, R.B. An action spectrum for cell killing and pyrimidine dimer formation in chinese hamster V-79 cells. Photochem. Photobiol. 1979, 29, 57–61. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-Induced DNA Damage and Repair: A Review. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Plagemann, P.G.W. Effect of Ultraviolet Light on Mengovirus: Formation of Uracil Dimers, Instability and Degradation of Capsid, and Covalent Linkage of Protein to Viral RNA. J. Virol. 1974, 13, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.E.; Hull, N.M.; Poepping, C.; Linden, K.G. Wavelength-Dependent Damage to Adenoviral Proteins Across the Germicidal UV Spectrum. Environ. Sci. Technol. 2018, 52, 223–229. [Google Scholar] [CrossRef]
- Espinoza, J.H.; Mercado-Uribe, H. Visible Light Neutralizes the Effect Produced by Ultraviolet Radiation in Proteins. J. Photochem. Photobiol. B 2017, 167, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Bandla, S. Ultraviolet Pasteurization for Food Industry. Int. J. Food Sci. Nutr. Eng. 2012, 2, 12–15. [Google Scholar] [CrossRef]
- Chatterley, C.; Linden, K. Demonstration and Evaluation of Germicidal UV-LEDs for Point-of-Use Water Disinfection. J. Water Health 2010, 8, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kujundzic, E.; Hernandez, M.; Miller, S.L. Ultraviolet Germicidal Irradiation Inactivation of Airborne Fungal Spores and Bacteria in Upper-Room Air and HVAC in-Duct Configurations. J. Environ. Eng. Sci. 2007, 6, 1–9. [Google Scholar] [CrossRef]
- Koutchma, T. Advances in Ultraviolet Light Technology for Non-Thermal Processing of Liquid Foods. Food Bioprocess Technol. 2009, 2, 138–155. [Google Scholar] [CrossRef]
- Spencer, M.; Vignari, M.; Bryce, E.; Johnson, H.B.; Fauerbach, L.; Graham, D. A Model for Choosing an Automated Ultraviolet-C Disinfection System and Building a Case for the C-Suite: Two Case Reports. Am. J. Infect. Control 2017, 45, 288–292. [Google Scholar] [CrossRef]
- Darnell, M.E.R.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the Coronavirus That Induces Severe Acute Respiratory Syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV Irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Conner-Kerr, T.A.; Sullivan, P.K.; Gaillard, J.; Franklin, M.E.; Jones, R.M. The Effects of Ultraviolet Radiation on Antibiotic-Resistant Bacteria in Vitro. Ostomy. Wound Manag. 1998, 44, 50–56. [Google Scholar]
- Pan, M.; Lednicky, J.A.; Wu, C.-Y. Collection, Particle Sizing and Detection of Airborne Viruses. J. Appl. Microbiol. 2019, 127, 1596–1611. [Google Scholar] [CrossRef]
- Heneghan, C.J.; Spencer, E.A.; Brassey, J.; Plüddemann, A.; Onakpoya, I.J.; Evans, D.H.; Conly, J.M.; Jefferson, T. SARS-CoV-2 and the Role of Airborne Transmission: A Systematic Review. F1000Research 2021, 10, 232. [Google Scholar] [CrossRef]
- Tang, J.W.; Bahnfleth, W.P.; Bluyssen, P.M.; Buonanno, G.; Jimenez, J.L.; Kurnitski, J.; Li, Y.; Miller, S.; Sekhar, C.; Morawska, L.; et al. Dismantling Myths on the Airborne Transmission of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). J. Hosp. Infect. 2021, 110, 89–96. [Google Scholar] [CrossRef]
- Wilson, N.; Corbett, S.; Tovey, E. Airborne Transmission of COVID-19. BMJ 2020, 370, m3206. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Jaafarzadeh, N.; Martínez, S.S.; Mirzaee, S.A. A Systematic Review of Possible Airborne Transmission of the COVID-19 Virus (SARS-CoV-2) in the Indoor Air Environment. Environ. Res. 2021, 193, 110612. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, C. COVID-19: What Do We Know about Airborne Transmission of SARS-CoV-2? BMJ 2021, 373, n1030. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten Scientific Reasons in Support of Airborne Transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Hwang, S.E.; Chang, J.H.; Oh, B.; Heo, J. Possible Aerosol Transmission of COVID-19 Associated with an Outbreak in an Apartment in Seoul, South Korea, 2020. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 104, 73–76. [Google Scholar] [CrossRef]
- Kang, M.; Wei, J.; Yuan, J.; Guo, J.; Zhang, Y.; Hang, J.; Qu, Y.; Qian, H.; Zhuang, Y.; Chen, X.; et al. Probable Evidence of Fecal Aerosol Transmission of SARS-CoV-2 in a High-Rise Building. Ann. Intern. Med. 2020, 173, M20-0928. [Google Scholar] [CrossRef]
- Miller, S.L.; Nazaroff, W.W.; Jimenez, J.L.; Boerstra, A.; Buonanno, G.; Dancer, S.J.; Kurnitski, J.; Marr, L.C.; Morawska, L.; Noakes, C. Transmission of SARS-CoV-2 by Inhalation of Respiratory Aerosol in the Skagit Valley Chorale Superspreading Event. Indoor Air 2021, 31, 314–323. [Google Scholar] [CrossRef]
- Spagnolo, A.M.; Ottria, G.; Amicizia, D.; Perdelli, F.; Cristina, M.L. Operating Theatre Quality and Prevention of Surgical Site Infections. J. Prev. Med. Hyg. 2013, 54, 131–137. [Google Scholar]
- Milstone, L.M.; Hu, R.-H.; Dziura, J.D.; Zhou, J. Impact of Epidermal Desquamation on Tissue Stores of Iron. J. Dermatol. Sci. 2012, 67, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Whyte, W.; Hodgson, R.; Tinkler, J.; Graham, J. The Isolation of Bacteria of Low Pathogenicity from Faulty Orthopaedic Implants. J. Hosp. Infect. 1981, 2, 219–230. [Google Scholar] [CrossRef]
- Benito, N.; Mur, I.; Ribera, A.; Soriano, A.; Rodríguez-Pardo, D.; Sorlí, L.; Cobo, J.; Fernández-Sampedro, M.; del Toro, M.D.; Guío, L.; et al. The Different Microbial Etiology of Prosthetic Joint Infections According to Route of Acquisition and Time after Prosthesis Implantation, Including the Role of Multidrug-Resistant Organisms. J. Clin. Med. 2019, 8, 673. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W.J.; Bahnfleth, W.P. Airborne Respiratory Diseases and Mechanical Systems for Control of Microbes. HPAC Heat. Pip. Air Cond. 1998, 70, 7. [Google Scholar]
- Abraham, G.; Smith, P.M.L.B.; McCabe, P. Hepa Filter Replacement Experience in a Biological Laboratory. J. Am. Biol. Saf. Assoc. 1998, 3, 134–142. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Guo, J.; Xiong, Y.; Kang, T.; Xiang, Z.; Qin, C. Bacterial Community Analysis of Floor Dust and HEPA Filters in Air Purifiers Used in Office Rooms in ILAS, Beijing. Sci. Rep. 2020, 10, 6417. [Google Scholar] [CrossRef]
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological Treatment of Indoor Air for VOC Removal: Potential and Challenges. Biotechnol. Adv. 2008, 26, 398–410. [Google Scholar] [CrossRef]
- Rumchev, K.; Spickett, J.; Bulsara, M.; Phillips, M.; Stick, S. Association of Domestic Exposure to Volatile Organic Compounds with Asthma in Young Children. Thorax 2004, 59, 746–751. [Google Scholar] [CrossRef]
- Barnewall, R.E.; Bischoff, W.E. Removal of SARS-CoV-2 Bioaerosols Using Ultraviolet Air Filtration. Infect. Control Hosp. Epidemiol. 2021, 42, 1014–1015. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, M.; Marabella, I.A.; McGee, D.A.J.; Aboubakr, H.; Goyal, S.; Hogan, C.J., Jr.; Olson, B.A.; Torremorell, M. Greater than 3-Log Reduction in Viable Coronavirus Aerosol Concentration in Ducted Ultraviolet-C (UV–C) Systems. Environ. Sci. Technol. 2021, 55, 4174–4182. [Google Scholar] [CrossRef]
- First, M.; Rudnick, S.N.; Banahan, K.F.; Vincent, R.L.; Brickner, P.W. Fundamental Factors Affecting Upper-Room Ultraviolet Germicidal Irradiation—Part I. Experimental. J. Occup. Environ. Hyg. 2007, 4, 321–331. [Google Scholar] [CrossRef]
- Escombe, A.R.; Moore, D.A.J.; Gilman, R.H.; Navincopa, M.; Ticona, E.; Mitchell, B.; Noakes, C.; Martínez, C.; Sheen, P.; Ramirez, R.; et al. Upper-Room Ultraviolet Light and Negative Air Ionization to Prevent Tuberculosis Transmission. PLoS Med. 2009, 6, e1000043. [Google Scholar] [CrossRef] [PubMed]
- Wells, W.F.; Wells, M.W.; Wilder, T.S. The environmental control of epidemic contagion. Am. J. Epidemiol. 1942, 35, 97–121. [Google Scholar] [CrossRef]
- Kane, D.W.; Finley, C.; Brown, D. UV-C Light and Infection Rate in a Long Term Care Ventilator Unit. Can. J. Infect. Control 2018, 33, 5. [Google Scholar]
- Ethington, T.; Newsome, S.; Waugh, J.; Lee, L.D. Cleaning the Air with Ultraviolet Germicidal Irradiation Lessened Contact Infections in a Long-Term Acute Care Hospital. Am. J. Infect. Control 2018, 46, 482–486. [Google Scholar] [CrossRef]
- Miller, S.L. Upper Room Germicidal Ultraviolet Systems for Air Disinfection Are Ready for Wide Implementation. Am. J. Respir. Crit. Care Med. 2015, 192, 407–409. [Google Scholar] [CrossRef]
- Nardell, E.A.; Bucher, S.J.; Brickner, P.W.; Wang, C.; Vincent, R.L.; Becan-McBride, K.; James, M.A.; Michael, M.; Wright, J.D. Safety of Upper-Room Ultraviolet Germicidal Air Disinfection for Room Occupants: Results from the Tuberculosis Ultraviolet Shelter Study. Public Health Rep. 2008, 123, 52–60. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Wiles, D.M. Surface Changes during the Photo-Oxidation of Polypropylene. J. Polym. Sci. B 1970, 8, 419–424. [Google Scholar] [CrossRef]
- Kramer, A.; Assadian, O. Survival of Microorganisms on Inanimate Surfaces. In Use of Biocidal Surfaces for Reduction of Healthcare Acquired Infections; Borkow, G., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 7–26. ISBN 978-3-319-08057-4. [Google Scholar]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The Effect of Temperature on Persistence of SARS-CoV-2 on Common Surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Wagenvoort, J.H.T.; De Brauwer, E.I.G.B.; Penders, R.J.R.; Willems, R.J.; Top, J.; Bonten, M.J. Environmental Survival of Vancomycin-Resistant Enterococcus Faecium. J. Hosp. Infect. 2011, 77, 282–283. [Google Scholar] [CrossRef]
- Kim, K.-H.; Fekety, R.; Batts, D.H.; Brown, D.; Cudmore, M.; Silva, J.; Waters, D. Isolation of Clostridium Difficile from the Environment and Contacts of Patients with Antibiotic-Associated Colitis. J. Infect. Dis. 1981, 143, 42–50. [Google Scholar] [CrossRef]
- Woodside, J.; Weaver, T. Guide to Infection Prevention in Emergency Medical Services; Association for Professionals in Infection Control and Epidemiology: Washington, DC, USA, 2013; ISBN 978-1-933013-54-1. [Google Scholar]
- Alfa, M.J.; Olson, N.; Murray, B.-L. Adenosine Tri-Phosphate (ATP)-Based Cleaning Monitoring in Health Care: How Rapidly Does Environmental ATP Deteriorate? J. Hosp. Infect. 2015, 90, 59–65. [Google Scholar] [CrossRef]
- Eckstein, B.C.; Adams, D.A.; Eckstein, E.C.; Rao, A.; Sethi, A.K.; Yadavalli, G.K.; Donskey, C.J. Reduction of Clostridium Difficile and Vancomycin-Resistant Enterococcus Contamination of Environmental Surfaces after an Intervention to Improve Cleaning Methods. BMC Infect. Dis. 2007, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Engelhart, S.; Krizek, L.; Glasmacher, A.; Fischnalle, E.; Marklein, G.; Exner, M. Pseudomonas Aeruginosa outbreak Ina Haematology-Oncology Unit Associated Withcontaminated Surface Cleaning Equipment. J. Hosp. Infect. 2002, 52, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, H.; Zhang, L.; Zhang, M.; Guo, D.; Wu, W.; Zhang, X.; Kan, G.L.; Jia, L.; Huo, D.; et al. Reduction of Secondary Transmission of SARS-CoV-2 in Households by Face Mask Use, Disinfection and Social Distancing: A Cohort Study in Beijing, China. BMJ Glob. Health 2020, 5, e002794. [Google Scholar] [CrossRef] [PubMed]
- National Center for Immunization and Respiratory Diseases (NCIRD). Division of Viral Diseases Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. In CDC COVID-19 Science Briefs; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. [Google Scholar]
- Nerandzic, M.M.; Thota, P.; Sankar, C.T.; Jencson, A.; Cadnum, J.L.; Ray, A.J.; Salata, R.A.; Watkins, R.R.; Donskey, C.J. Evaluation of a Pulsed Xenon Ultraviolet Disinfection System for Reduction of Healthcare-Associated Pathogens in Hospital Rooms. Infect. Control Hosp. Epidemiol. 2015, 36, 192–197. [Google Scholar] [CrossRef]
- Brunk, C.F. Distribution of Dimers in Ultraviolet-Irradiated DNA. Nat. New Biol. 1973, 241, 74–76. [Google Scholar] [CrossRef]
- Eggertson, L.; Sibbald, B. Hospitals Battling Outbreaks of C. Difficile. CMAJ Can. Med. Assoc. J. 2004, 171, 19–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bryce, E.; Zurberg, T.; Zurberg, M.; Shajari, S.; Roscoe, D. Identifying Environmental Reservoirs of Clostridium Difficile with a Scent Detection Dog: Preliminary Evaluation. J. Hosp. Infect. 2017, 97, 140–145. [Google Scholar] [CrossRef]
- Browne, K.; Wood, D.; Clezy, K.; Lehm, J.; Walsh, W.R. Reduction of Bacterial Load with the Addition of Ultraviolet-C Disinfection inside the Hyperbaric Chamber. Diving Hyperb. Med. J. 2020, 50, 332–337. [Google Scholar] [CrossRef]
- Browne, K.L.; Crowley, J.D.; Tan, C.J.; O’Sullivan, C.B.; Walsh, W.R. Effect of Ultraviolet-C Light on the Environmental Bacterial Bioburden in Various Veterinary Facilities. Am. J. Vet. Res. 2021, 82, 582–588. [Google Scholar] [CrossRef]
- Anderson, D.J.; Gergen, M.F.; Smathers, E.; Sexton, D.J.; Chen, L.F.; Weber, D.J.; Rutala, W.A. Decontamination of Targeted Pathogens from Patient Rooms Using an Automated Ultraviolet-C-Emitting Device. Infect. Control Hosp. Epidemiol. 2013, 34, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Havill, N.L.; Moore, B.A. Terminal Decontamination of Patient Rooms Using an Automated Mobile UV Light Unit. Infect. Control Hosp. Epidemiol. 2011, 32, 737–742. [Google Scholar] [CrossRef]
- Yang, J.-H.; Wu, U.-I.; Tai, H.-M.; Sheng, W.-H. Effectiveness of an Ultraviolet-C Disinfection System for Reduction of Healthcare-Associated Pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef]
- Anderson, D.J.; Chen, L.F.; Weber, D.J.; Moehring, R.W.; Lewis, S.S.; Triplett, P.F.; Blocker, M.; Becherer, P.; Schwab, J.C.; Knelson, L.P.; et al. Enhanced Terminal Room Disinfection and Acquisition and Infection Caused by Multidrug-Resistant Organisms and Clostridium Difficile (the Benefits of Enhanced Terminal Room Disinfection Study): A Cluster-Randomised, Multicentre, Crossover Study. Lancet Lond. Engl. 2017, 389, 805–814. [Google Scholar] [CrossRef]
- Anderson, D.J.; Moehring, R.W.; Weber, D.J.; Lewis, S.S.; Chen, L.F.; Schwab, J.C.; Becherer, P.; Blocker, M.; Triplett, P.F.; Knelson, L.P.; et al. Effectiveness of Targeted Enhanced Terminal Room Disinfection on Hospital-Wide Acquisition and Infection with Multidrug-Resistant Organisms and Clostridium Difficile: A Secondary Analysis of a Multicentre Cluster Randomised Controlled Trial with Crossover Design (BETR Disinfection). Lancet Infect. Dis. 2018, 18, 845–853. [Google Scholar] [CrossRef]
- Blue, J.; O’Neill, C.; Speziale, P.; Revill, J.; Ramage, L.; Ballantyne, L. Use of a Fluorescent Chemical as a Quality Indicator for a Hospital Cleaning Program. Can. J. Infect. Control Off. J. Community Hosp. Infect. Control Assoc.-Can. Rev. Can. Prev. Infect. 2008, 23, 216–219. [Google Scholar]
- Pittet, D.; Hugonnet, S.; Harbarth, S.; Mourouga, P.; Sauvan, V.; Touveneau, S.; Perneger, T.V. Effectiveness of a Hospital-Wide Programme to Improve Compliance with Hand Hygiene. Lancet 2000, 356, 1307–1312. [Google Scholar] [CrossRef]
- Datta, R.; Platt, R.; Yokoe, D.S.; Huang, S.S. Environmental Cleaning Intervention and Risk of Acquiring Multidrug-Resistant Organisms From Prior Room Occupants. Arch. Intern. Med. 2011, 171, 491–494. [Google Scholar] [CrossRef]
- Pavia, M.; Simpser, E.; Becker, M.; Mainquist, W.K.; Velez, K.A. The Effect of Ultraviolet-C Technology on Viral Infection Incidence in a Pediatric Long-Term Care Facility. Am. J. Infect. Control 2018, 46, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.M.; Donzanti, M.J.; Minahan, D.J.; Shirazi, J.; Hatem, C.L.; Hayward-Piatkovskyi, B.; Dang, A.M.; Nelson, K.M.; Bothi, K.L.; Gleghorn, J.P. Mask Reuse in the COVID-19 Pandemic: Creating an Inexpensive and Scalable Ultraviolet System for Filtering Facepiece Respirator Decontamination. Glob. Health Sci. Pract. 2020, 8, 582–595. [Google Scholar] [CrossRef]
- Kea, B.; Johnson, A.; Lin, A.; Lapidus, J.; Cook, J.N.; Choi, C.; Chang, B.P.; Probst, M.A.; Park, J.; Atzema, C.; et al. An International Survey of Healthcare Workers Use of Personal Protective Equipment during the Early Stages of the COVID-19 Pandemic. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12392. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Wielick, C.; Jolois, O.; Dams, L.; Razafimahefa, R.M.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Thiry, E.; et al. “Don, Doff, Discard” to “Don, Doff, Decontaminate”—FFR and Mask Integrity and Inactivation of a SARS-CoV-2 Surrogate and a Norovirus Following Multiple Vaporised Hydrogen Peroxide-, Ultraviolet Germicidal Irradiation-, and Dry Heat Decontaminations. PLoS ONE 2021, 16, e0251872. [Google Scholar] [CrossRef]
- Kayani, B.J.; Weaver, D.T.; Gopalakrishnan, V.; King, E.S.; Dolson, E.; Krishnan, N.; Pelesko, J.; Scott, M.J.; Hitomi, M.; Cadnum, J.L.; et al. UV-C Tower for Point-of-Care Decontamination of Filtering Facepiece Respirators. Am. J. Infect. Control 2021, 49, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Wielick, C.; Dams, L.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Haubruge, E.; Thiry, E. The Use of Germicidal Ultraviolet Light, Vaporized Hydrogen Peroxide and Dry Heat to Decontaminate Face Masks and Filtering Respirators Contaminated with a SARS-CoV-2 Surrogate Virus. J. Hosp. Infect. 2020, 106, 577–584. [Google Scholar] [CrossRef]
- Tande, B.M.; Carson, P.; Rutala, W.; Guerrero, D.M. Designing Healthcare Facilities to Maximize the Effectiveness of UV Disinfection. Am. J. Infect. Control 2014, 42, S42–S43. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).