Abstract
Brettanomyces bruxellensis is a wine spoilage concern in wineries around the world. In order to maintain wine quality during storage and ageing, it is imperative to control and monitor this yeast. Being a fastidious slow growing yeast, which requires 5 to 14 days of incubation for visible growth in agar plates, it is difficult to detect growth (colonies) by conventional agar plate count method. Yeast enumeration by impedance was investigated because previous research using other microorganisms has shown that it is potentially faster than plate counting. The relationship between plate counting and impedance detection times was investigated for Brettanomyces inoculated in red wine samples. A linear relationship between log plate count concentrations and impedance detection times was found. Incubation time was reduced from 120 h down to 0.9 and 57.7 h for samples with 6.7 × 107 and 1.8 × 102 cfu/mL, respectively, using the ‘indirect’ impedance method. The ‘direct’ method also reduced the incubation times to 9.5 and 81.9 h, for the same concentrations. The ‘indirect’ impedance method has the potential to be used by the wine industry to control and monitor the Brettanomyces numbers in wines.
1. Introduction
In 2015, the average global wine production was approximately 2.8 × 1010 L [1,2]. The yeast Brettanomyces bruxellensis (the anamorph Dekkera bruxellensis) is one of the major spoilage organisms faced by the wine industry, which leads to economic losses worldwide [3,4]. Concentrations as low as 103 cfu/mL of this spoilage yeast have been known to cause unpalatable off-odors and flavors [5]. Brettanomyces spoilage is most frequently associated with red wines due, in part, to the increased use and efficiency of sulfur dioxide preservative in low pH white wines [3]. Brettanomyces contamination has been detected in wines and wineries from all major wine producing countries around the world [3,4]. The development of fast and effective methods for their detection and enumeration in the wine industry is therefore vital.
General plating media, including yeast extract peptone dextrose (YPD) and Dekkera/Brettanomyces differential medium (DBDM), can be used with the addition of 20–100 mg/L of cycloheximide to detect Dekkera/Brettanomyces bruxellensis. Due to reliability and low cost, plate counting is still the predominant method for detecting this yeast in the industry [5,6,7]. B. bruxellensis typically has plate incubation times of more than 72 h (3 days) at 25–30 °C [3,5]. Costly and time-consuming gas chromatography mass spectrometry (GC-MS) can also be used to detect these yeasts by measuring 4-ethylphenol concentrations. Real-time polymerase chain reaction (PCR) method has been shown to detect as little as 10 cells/mL in under 3 h, with highly specific detection and enumeration of targeted microorganisms [5,7]. One disadvantage of PCR is that the wine matrix is complex, complicating DNA extraction and causing amplification problems [8]. Microscopic examination of the distinctive morphology of Brettanomyces is often used in conjunction with microscopic enumeration using Malassez or Thoma chambers [8,9]. Even though specific cell functions can be determined by staining cells, this is a time consuming and highly subjective method [8]. Malacrino et al. (2001) found that flow cytometry in combination with fluorescent dyes can be used to estimate yeast and bacteria counts in wines [10]. This is a rapid enumeration method, with high specificity and the ability to analyze the physiological state of cells [8].
Due to the lower growth rate of Brettanomyces compared to other yeasts, new technologies, including impedance, could have the potential to reduce detection and enumeration times. Impedance is the effective resistance of an electrical system to alternating electric current. The impedance enumeration method measures the change in impedance caused by microbial growth in a selective medium under an alternating current (AC) field. The lower molecular size charged compounds produced during microbial growth cause the change in impedance. The BacTracTM system utilizes impedance splitting technology to measure the change in the impedance of the electrode (E-value) and in the medium (M-value). The impedance splitting technology measures the change in the impedance of the electrochemical double layer of the electrode in addition to the standard impedance signal (media impedance = M-value). Impedance detection time is defined as the time from the start of the measurement period until the signal exceeds the specified M- or E-value threshold. For both methods, impedance is evaluated based on the growth of microbial cells added to the BacTrac measuring vials. Therefore, more cells result in more growth and shorter impedance detection times. A calibration curve is then used to convert the detection times to colony forming units (cfu) [11,12,13].
Impedance technology has been used to enumerate Dekkera bruxellensis, Saccharomyces cerevisiae, Candida stellata and Hanseniaspora uvarum in a model wine, Saccharomyces fragilis in fermented goat milk, Bifidobacterium lactis in milk powders and Escherichia coli in simulated milk ultra-filtrate and molluscs [14,15,16,17,18]. As wine is a complex mixture of interacting chemicals, it is important to investigate the viability of impedance technology using a real wine instead of a model wine. In this study, filter sterile red wine samples were inoculated with only B. bruxellensis with the main objectives: (i) to investigate the relationship between plate counting and impedance detection times in red wine; and (ii) to validate and compare the ‘direct’ and ‘indirect’ impedance methods using a different red wine.
2. Materials and Methods
2.1. Characteristics of Wines Used
For the calibration lines, Cabernet Sauvignon red wine (Australian, 2014 vintage, 13.5% v/v alcohol, 3.5 pH, 6.6 g/L total acidity, 30 mg/L free SO2, 73 mg/L total SO2) was used. Cabernet Sauvignon is the most popular table red wine [1,2]. As the focus was to solely enumerate Brettanomyces, the wine was filter sterilized (0.45 µm pore size) and the sterility of the wine was verified using plate counting. Then, inoculation of sterilized wine with different concentrations of B. bruxellensis was carried out. In addition, validation experiments were conducted using the 2015 vintage of the same wine.
2.2. Yeast Propagation and Inoculation
B. bruxellensis yeast strain AWRI 1499 (Australian Wine Research Institute, Adelaide, Australia), was used in this study as it was the most resistant to non-thermal high pressure inactivation of three strains investigated previously by van Wyk and Silva (2017) [19]. According to the method used by van Wyk and Silva (2017), the yeast was first streaked onto YPD agar (adjusted pH between 5 and 6, autoclaved at 121 °C for 15 min) and incubated for 5 days at 28 °C. One colony was then transferred to autoclaved YPD broth (adjusted pH between 5 and 6). After incubation (30 °C, 120 rpm for 4 days) and at the end of the microbial exponential growth phase, the cells were harvested and the sterilized wine inoculated. To ensure that the yeast remained fresh and active, it was transferred to fresh YPD agars on a monthly basis [19]. The Difco YPD broth and agar media were obtained from Fort Richard Laboratories, Auckland, New Zealand.
Regarding wine inoculation, dilutions of B. bruxellensis in sterile Ringer’s solution, with concentrations ranging between 102 and 108 cfu/mL were prepared. Preliminary results showed that the use of Ringer’s solution instead of wine for dilutions gave more consistent results, with less variability. The dilutions were then centrifuged (5000 rpm, 15 min) and the yeast transferred to 10 mL of filter sterilized wine samples.
2.3. Enumeration of B. bruxellensis
2.3.1. Plate Counting
The concentration of B. bruxellensis yeast in the wine samples were determined by spreading 100 µL of appropriately diluted samples onto duplicate YPD agar plates. Given this organism is a fastidious slow grower, 5 days at 28 °C were used for incubation. To ensure a uniform yeast suspension in the wine samples, a vortex mixer was used after each stage of dilution. Yeast colonies were then counted and B. bruxellensis concentration was expressed in colony forming units per milliliter (cfu/mL) of wine sample.
2.3.2. Impedance Technology
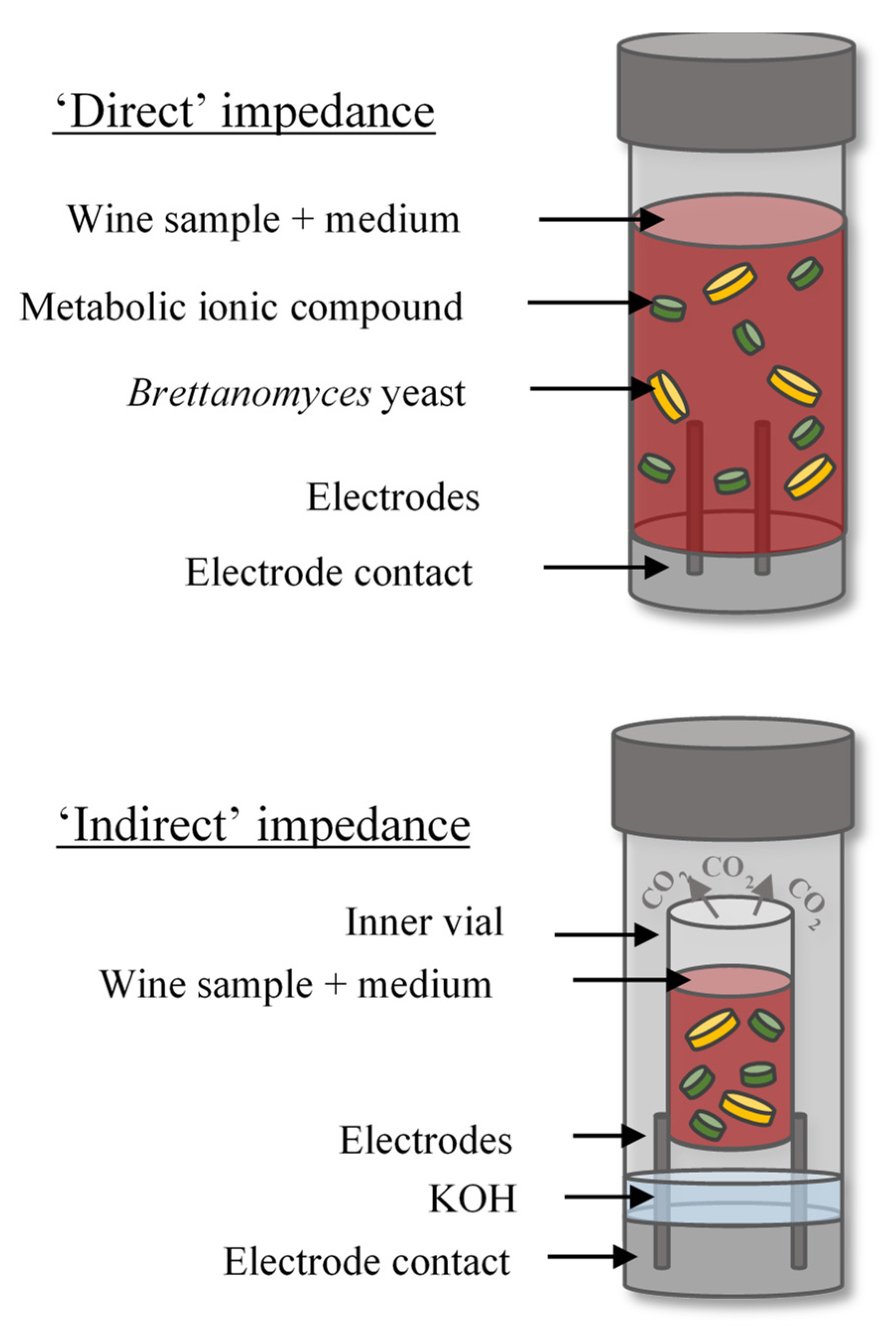
The BacTrac™ 4300 instrument (Sy-Lab, Neupurkersdorf, Austria), which consists of one incubator (64 measuring cells) and computer software, was used to perform impedance measurements. Figure 1 includes a detailed diagram of ‘direct’ and ‘indirect’ impedance methods. Selective media for yeast and mould BiMedia 510A and 501B nutrient broths were used for ‘direct’ and ‘indirect’ enumeration impedance methods, respectively. The ‘direct’ method measures the impedance of the nutrient broth-sample mixture in direct contact with the electrodes in the measurement cell. For the ‘indirect’ method, the nutrient broth-sample mixture is contained in an isolated smaller inner cell located inside the measurement cell. The bottom of the measurement cell contains a dilute solution of potassium hydroxide (KOH), which undergoes a change in impedance through the uptake of CO2 from the microorganisms in the inner cell during metabolism. Therefore, there is no direct contact between the sample mixture and the electrodes. The following detection parameters were used for the ‘direct’ method: delay time 1 h, evaluation type E and threshold E-value 12%. The parameters for the ‘indirect’ method were: delay time 1 h, evaluation type M2 and threshold M-value −25%. For the ‘direct’ method, 1 mL of homogenized wine sample was inoculated into 9 mL of BiMedia 510A in prefilled cells. For the ‘indirect’ method, 1 mL of 0.2% KOH solution was added to the outer cell and 1 mL of homogenized wine sample was added to 5 mL BiMedia 501B in the inner vial. The cells were sealed using plastic screw caps before being inserted into the BacTrac incubator. Next, the samples were incubated at 30 °C for up to 90 h, during which cell growth occurred and thus changing the total impedance, which was recorded in 10 min intervals. The method was adapted from protocol H51 and V7.50.1 (supplied by Sy-Lab) for the ‘direct’ and ‘indirect’ methods, respectively. Previous research demonstrated that BiMedia 510 A and B supports B. bruxellensis growth [13].
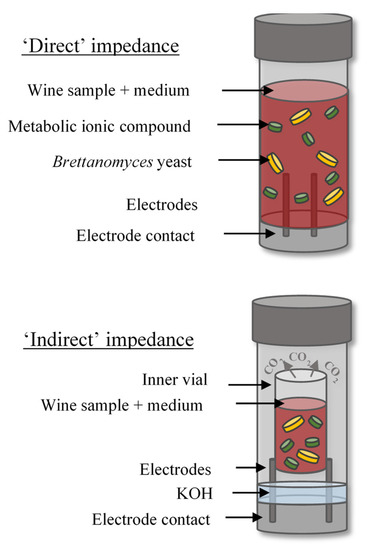
Figure 1.
Schematic of the ‘direct’ and ‘indirect’ impedance methods.
2.4. Calibration Lines of Impedance versus Plate Counting
Ten wine samples, with yeast concentrations, ranging between 102 and 108 cfu/mL were prepared as previously described. Each sample was then analyzed by transferring 1 mL to the ‘direct’ and ‘indirect’ measuring cells containing 9 mL and 5 mL of growth medium, respectively. Five repeat measurements of each sample were performed using both the ‘direct’ and ‘indirect’ methods according to the procedure described. In addition, each wine sample was plated in duplicate on YPD agar according to conventional plate counting. Colonies were counted and the Brettanomyces concentration (N, NPC) was calculated. Lastly, the log yeast concentration plate count results were plotted against the impedance detection times from the ‘direct’ and ‘indirect’ methods, generating two calibration lines. Linear regression analysis was performed using IBM SPSS Statistics (Version 23, Armonk, NY, USA).
2.5. Validation of Calibration Lines Using a Different Wine
To validate the calibration lines, a wine from a subsequent vintage was used. Wine samples with five different concentrations of Brettanomyces were prepared and analyzed using the impedance methods and reference plate counting. The wine samples were prepared according to the method described, with concentrations ranging between 103 and 108 cfu/mL. The B. bruxellensis concentration of each sample was then determined using the plate counting and the ‘direct’ and ‘indirect’ impedance methods. For plate counting, samples were analyzed in duplicate, whereas for the impedance methods, five repeat measurements were made.
2.6. Statistical Analysis
The mean and standard deviation of all Brettanomyces concentrations (N) by standard plate counting (NPC) and impedance detection (NI) times were calculated using IBM SPSS. The results were then plotted as mean ± standard deviation (SD). Percentage error was calculated using:
The yeast concentration for the ‘direct’ and ‘indirect’ impedance methods were calculated using the calibration equations (Equations (2) and (3), respectively). To validate the linear equations, the impedance and plate count results obtained were compared using Tukey tests followed by one-way analysis of variance (ANOVA), with a confidence level of 95% (p < 0.05).
3. Results and Discussion
3.1. Comparing the Relationship between B. bruxellensis Plate Counting and the ‘Direct’ and ‘Indirect’ Impedance Enumeration
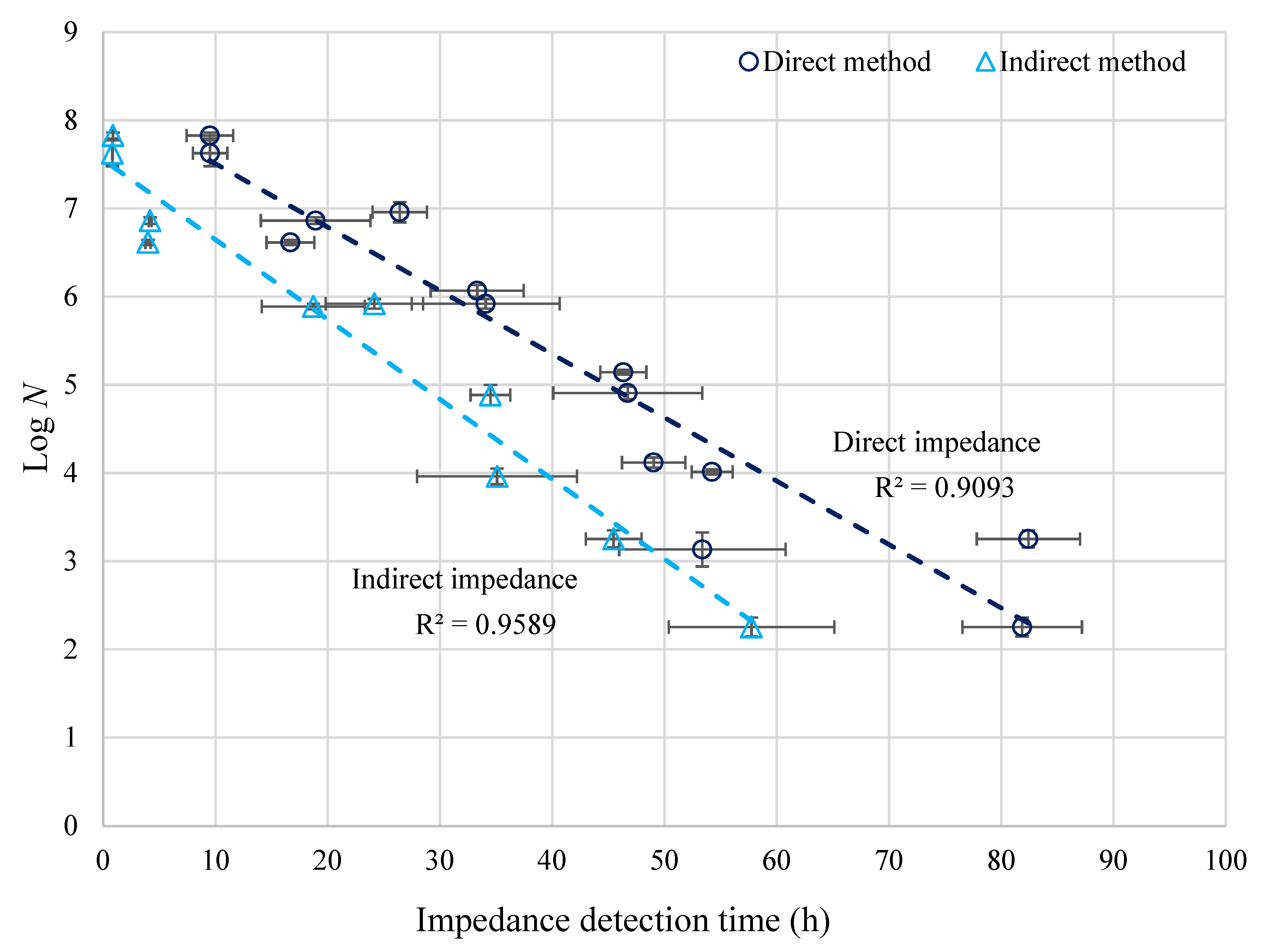
The calibration lines for the enumeration of Brettanomyces in wine using the ‘direct’ and ‘indirect’ impedance methods are shown in Figure 2. Clearly, as the B. bruxellensis concentration (N, cfu/mL by plate counting) increased, the impedance detection time t (hours) decreased. A strong linear relationship between Log N and detection time t was found, as shown by the R2 values:
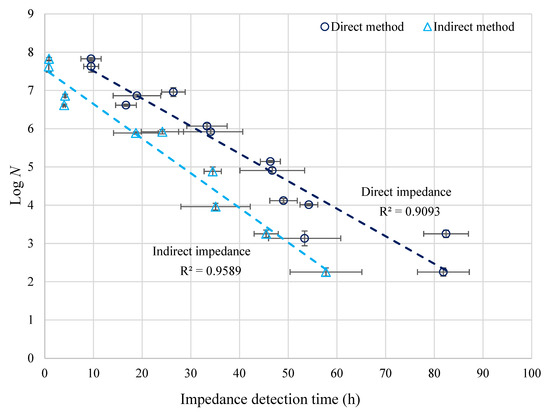
Figure 2.
Linear relationship between log numbers of B. bruxellensis in wine (N, cfu/mL) and ‘direct’ and ‘indirect’ impedance detection times (h); Error bars represent the standard deviations of the plate count and impedance results.
Overall, the detection times of the ‘indirect’ method were lower than the ‘direct’ method, especially at lower yeast concentrations. For the ‘direct’ and ‘indirect’ impedance, the detection times were 9.5 and 0.9 h for samples containing 6.7 × 107 cfu/mL (upper limit of detection). For a concentration of 1.8 × 102 cfu/mL (low limit of detection), the detection time for the ‘direct’ impedance was 81.9 h. This is significantly longer than the corresponding detection time for the ‘indirect’ impedance, which was 57.7 h. Indirect impedance thus decreased the incubation and the Brettanomyces detection time in wine compared to 5 days (120 h) taken for conventional plate counting.
No previous research has been conducted on using impedance to detect and enumerate B. bruxellensis contamination in wine. However, a study with B. bruxellensis in beer, also resulted in a log-linear relationship between plate count concentrations and impedance detection times, and ‘indirect’ impedance detection times of 1.9 and 50.0 h, respectively, for yeast concentrations of 106 and 104 cfu/mL [13]. The ‘indirect’ method was also found to give satisfactory results when working with yeasts, including Dekkera bruxellensis, S. cerevisiae, Candida stellata and Hanseniaspora uvarum [15,20]. B. lactis in milk powders also exhibited a log-linear relationship using the ‘direct’ impedance, with microbial concentrations between 106 and 109 cfu/mL, resulting in detection times of 15 and 3 h, respectively [17].
The standard deviations of the impedance detection times (horizontal error bars) were higher than for plate count results (vertical error bars). While the standard deviation of plate count results never exceeded 0.2, the standard deviations of the impedance detection times were 0.0 to 2.1 and 5.3 to 7.4 h for 6.7 × 107 and 1.8 × 102 cfu/mL yeast concentrations, respectively. Overall, the ‘indirect’ impedance is more suited for use in the wine industry for the enumeration of B. bruxellensis due to significantly faster detection times compared to the ‘direct’ impedance over the same detection range (Figure 2). The ‘indirect’ impedance is potentially less influenced by the type of wine samples analyzed, as they are not in direct contact with the electrodes. However, for the ‘direct’ impedance, the ionic composition of the growth medium and wine sample mixture could be of greater influence. Since no two wines have the same composition or properties, this potentially decreases the suitability of using the ‘direct’ method in the wine industry.
3.2. Validation, Advantages and Limitations of the ‘Direct’ and ‘Indirect’ Impedance
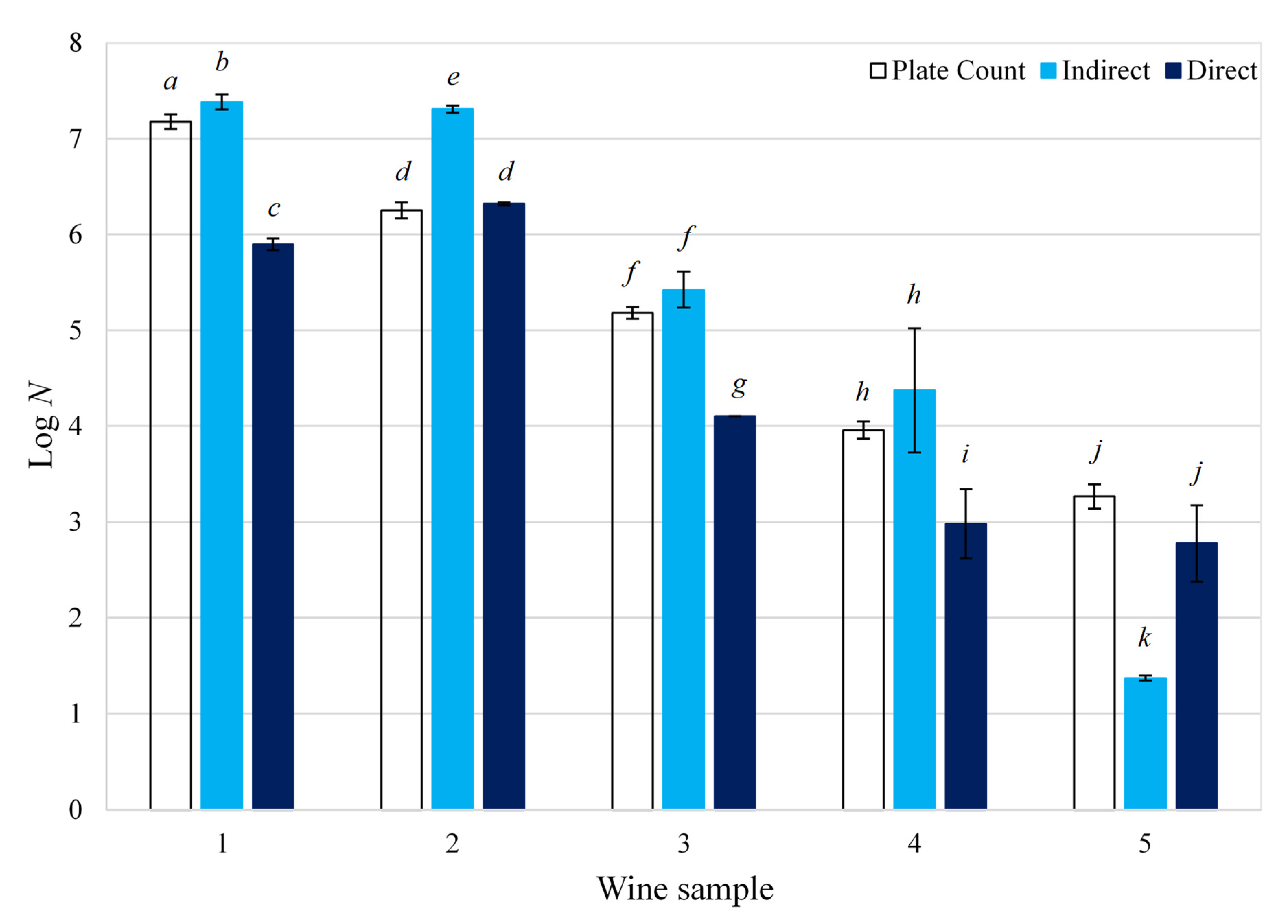
Five red wine samples of a different vintage, with concentrations ranging from 103 and 107 cfu/mL, were analyzed. The enumeration of Brettanomyces in these samples was conducted using plate counting as a reference. Equations (2) and (3) were then used to calculate the ‘direct’ and ‘indirect’ impedance yeast concentration, respectively. A comparison between the three B. bruxellensis enumeration methods is presented in Figure 3. The ‘direct’ method was slightly more accurate with an average percentage error of 15.9%, compared to that of the ‘indirect’ method, which was 18.6% (Equation (1)). For samples with a Brettanomyces concentration above 104 cfu/mL, the ‘indirect’ method generally proved to be more accurate, with an average percentage error of 8.2% compared to that of the ‘direct’ method (13.2%). Contrastingly, the ‘direct’ method was more accurate at concentrations below 104 cfu/mL, with an average percentage error of 19.8% compared to 34.2% for the ‘indirect’ method. As expected, samples with a low yeast concentration are more difficult to quantify and thus presented results with higher variability. These results are in agreement with the results described earlier.
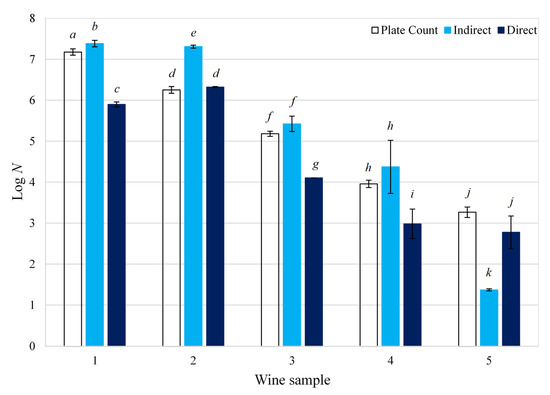
Figure 3.
Enumeration of Brettanomyces bruxellensis contamination in wine: a comparison between plate counting and impedance technology; N is the concentration of B. bruxellensis in red wine (cfu/mL); different letters indicate that the average Log N (logarithm of yeast concentration) are significantly different according to Tukey’s test (p < 0.05).
One drawback of plate counting is the prolonged incubation periods of 120 h (5 days) required for observing Brettanomyces colonies on the plates. Using the ‘indirect’ impedance, incubation times are reduced to 0.8 to 58 h. The reduced incubation time could be explained by the accelerated yeast proliferation rate in liquid broth compared to semi-solid agar medium, conventionally used for plate counting. Moreover, the principle of detection is different in the semi-solid agar medium contained in plates, where every cell is counted by itself, and in the liquid broth, where all cells grow together. Avoiding prolonged incubation times also decreases the chance of sample contamination during the enumeration process. Accelerated detection and enumeration times are especially important for Brettanomyces in connection with the wine industry. This would enable faster implementation of preventative actions in the wine industry, such as the addition of more SO2 and cleaning of equipment, to reduce the negative effects of Brettanomyces contamination and hence wine quality degradation. The risk of large-scale wine dumping due to Brettanomyces growth would also be reduced, in turn reducing economic losses.
One other important aspect is that standard plate counting is more labor intensive and possibly more time consuming, due to the multiple operations required for sample preparation. For the ‘direct’ and ‘indirect’ impedance methods, the sample preparation time can be significantly reduced from approximately 1 h (plate counting) to less than 5 min (impedance methods) per sample. Therefore, impedance technology has the potential to increase the throughput of enumeration analyses thereby providing faster responses to wine producers. Figure 3 and percentage error calculations (Equation (1)) demonstrated that the ‘indirect’ impedance method successfully (average error of 18.6% against plate counting as a reference) detected and enumerated the Brettanomyces yeast in wine. Lastly, even though the initial capital and material costs for the impedance technology is higher, this is offset by the reduction of economic risk, labor and turnaround time of samples analyzed.
This study is focused on wine samples inoculated only with B. bruxellensis. In addition, only exclusively fresh cultures of B. bruxellensis were used in the experiments. Since the age and viability of yeasts (and other microorganisms) are different in real wines, future research on the enumeration of B. bruxellensis in unfiltered wines should be conducted, including the identification of the impact of the age/condition of the yeast cells. Unfiltered wines contain the main fermenting organism and contaminant microorganisms (e.g., other yeasts and lactic acid bacteria) that can interfere with impedance detection. This could involve pre-treating the wine samples before analysis or adding antimicrobial agents to the detection media directly. Cycloheximide, to which Brettanomyces is known to be highly resistant, has been used to isolate this yeast from real wine samples, as S. cerevisiae’s (the predominant fermenting yeast) is susceptible to this antimicrobial additive [21].
4. Conclusions
‘Direct’ and ‘indirect’ impedance were able to detect B. bruxellensis concentrations as low as 1.8 × 102 cfu/mL. The ‘indirect’ impedance has a greater potential for use in the wine industry for the enumeration of Brettanomyces, being able to decrease the processing time of wine samples containing Brettanomyces by between 62 h and 119 h (at 1.8 × 102 cfu/mL and 6.7 × 107 cfu/mL, respectively), depending on concentration. For Brettanomyces concentration above 104 cfu/mL, the ‘indirect’ method was more accurate overall compared to the ‘direct’ impedance. The ‘indirect’ impedance, in addition to offering faster detection times, is potentially less influenced by the type or composition of the wine analyzed. Therefore, the same calibration lines can possibly be used for different vintage year red wines, saving time. In conclusion, the ‘indirect’ impedance has the potential to be an alternative option for the enumeration of yeasts in the wine industry because it offers faster preparation time, high throughput and has the potential to reduce economic losses. Further research is recommended using different wines and unfiltered wines that contain other microorganisms typically present in wines.
Author Contributions
Investigation and writing original draft, S.v.W.; Supervision and writing—revision and editing, F.V.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
University of Auckland doctoral grant, New Zealand. Morton Coutts scholarship from DB Breweries (Auckland), New Zealand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The University of Auckland Doctoral grant is greatly appreciated. The Morton Coutts scholarship from DB Breweries (Auckland) is also greatly valued. The support and collaboration from SY-Lab (Austria) and Manfred Schinkinger are acknowledged.
Conflicts of Interest
No conflict of interest.
References
- Jackson, R.S. Styles and Types of Wine. In Wine Tasting, 2nd ed.; Academic Press: San Diego, CA, USA, 2009; pp. 349–386. [Google Scholar]
- Han, G.; Ugliano, M.; Currie, B.; Vidal, S.; Diéval, J.-B.; Waterhouse, A.L. Influence of closure, phenolic levels and microoxygenation on Cabernet Sauvignon wine composition after 5 years’ bottle storage. J. Sci. Food Agric. 2014, 95, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Oelofse, A.; Pretorius, I.; Du Toit, M. Significance of Brettanomyces and Dekkera during Winemaking: A Synoptic Review. S. Afr. J. Enol. Vitic. 2016, 29, 128–144. [Google Scholar] [CrossRef] [Green Version]
- Wedral, D.; Shewfelt, R.; Frank, J. The challenge of Brettanomyces in wine. LWT Food Sci. Technol. 2010, 43, 1474–1479. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Dekkera/Brettanomyces spp. In Food Spoilage Microorganisms; Blackburn, C.W., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 354–398. [Google Scholar]
- Zuehlke, J.M.; Petrova, B.; Edwards, C.G. Advances in the control of wine spoilage by Zygosaccharomyces and Dekkera/Brettanomyces. Annu. Rev. Food Sci. Technol. 2013, 4, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Longin, C.; Degueurce, C.; Julliat, F.; Guilloux-Benatier, M.; Rousseaux, S.; Alexandre, H. Efficiency of population-dependent sulfite against Brettanomyces bruxellensis in red wine. Food Res. Int. 2016, 89, 620–630. [Google Scholar] [CrossRef]
- Cocolin, L.; Rantsiou, K.; Iacumin, L.; Zironi, R.; Comi, G. Molecular Detection and Identification of Brettanomyces/Dekkera bruxellensis and Brettanomyces/Dekkera anomalus in Spoiled Wines. Appl. Environ. Microbiol. 2004, 70, 1347–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malacrinò, P.; Zapparoli, G.; Torriani, S.; Dellaglio, F. Rapid detection of viable yeasts and bacteria in wine by flow cytometry. J. Microbiol. Methods 2001, 45, 127–134. [Google Scholar] [CrossRef]
- Dupont, J.; Dumont, F.; Menanteau, C.; Pommepuy, M. Calibration of the impedance method for rapid quantitative estimation of Escherichia coli in live marine bivalve molluscs. J. Appl. Microbiol. 2004, 96, 894–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Schnell, S.; Fischer, M. Rapid detection of Cronobacter spp. with a method combining impedance technology and rRNA based lateral flow assay. Int. J. Food Microbiol. 2012, 159, 54–58. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, S.; Silva, F.V.M. Impedance technology reduces the enumeration time of Brettanomyces yeast during beer fermentation. Biotechnol. J. 2016, 11, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, M.J.; Raposo, R.; Cayuela, J.M.; Zafrilla, P.; Piñeiro, Z.; Rojas, J.M.M.; Mulero, J.; Puertas, B.; Girón, F.; Guerrero, R.F.; et al. Valorization of grape stems. Ind. Crop. Prod. 2015, 63, 152–157. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Raposo, R.; Moreno-Rojas, J.M.; Zafrilla, P.; Cayuela, J.M.; Mulero, J.; Puertas, B.; Guerrero, R.F.; Piñeiro, Z.; Giron, F.; et al. Efficacy of olive oil mill extract in replacing sulfur dioxide in wine model. LWT Food Sci. Technol. 2015, 61, 117–123. [Google Scholar] [CrossRef]
- Agata, L.; Jan, P. Production of fermented goat beverage using a mixed starter culture of lactic acid bacteria and yeasts. Eng. Life Sci. 2012, 12, 486–493. [Google Scholar] [CrossRef]
- Walker, K.; Ripandelli, N.; Flint, S. Rapid enumeration of Bifidobacterium lactis in milk powders using impedance. Int. Dairy J. 2005, 15, 183–188. [Google Scholar] [CrossRef]
- Alkhafaji, S.R.; Farid, M. An investigation on pulsed electric fields technology using new treatment chamber design. Innov. Food Sci. Emerg. Technol. 2007, 8, 205–212. [Google Scholar] [CrossRef]
- van Wyk, S.; Silva, F.V.M. High pressure inactivation of Brettanomyces bruxellensis in red wine. Food Microbiol. 2017, 63, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Porrata, B.; Novo, M.; Guillamón, J.; Rozès, N.; Mas, A.; Otero, R.C. Vitality enhancement of the rehydrated active dry wine yeast. Int. J. Food Microbiol. 2008, 126, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).