Seed Morphometry and Germination of Four Edible Species of Passiflora spp. Conserved in a Gene Bank
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Samples
2.2. Morphometry
2.3. Seed Disinfection
2.4. Seed Germination Under Two Temperature Conditions
2.5. Seed Germination with Pre-Germination Treatments
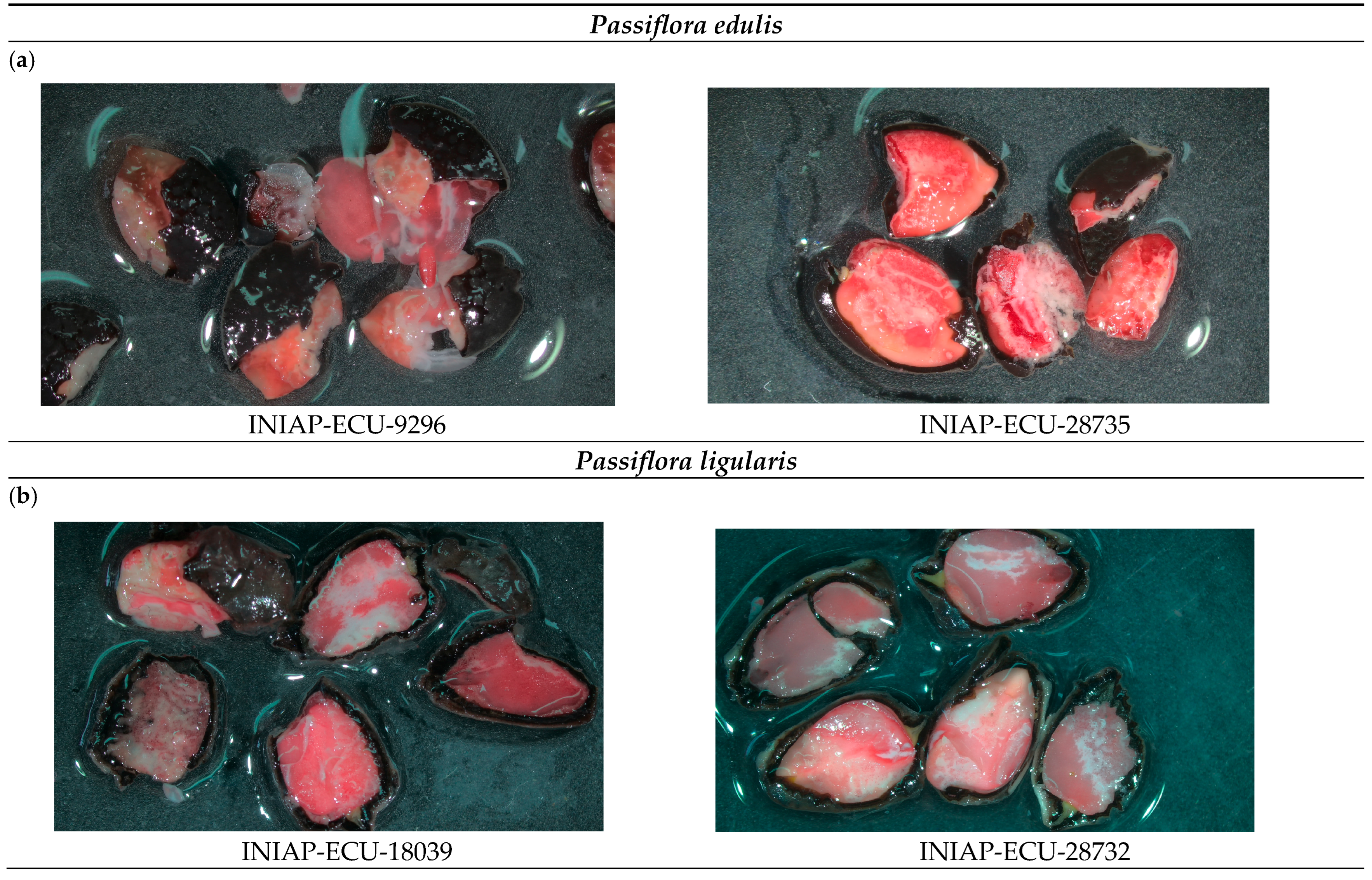
2.6. Viability Test with Tetrazolium
2.7. Statistical Analysis
3. Results
3.1. Seed Morphometry
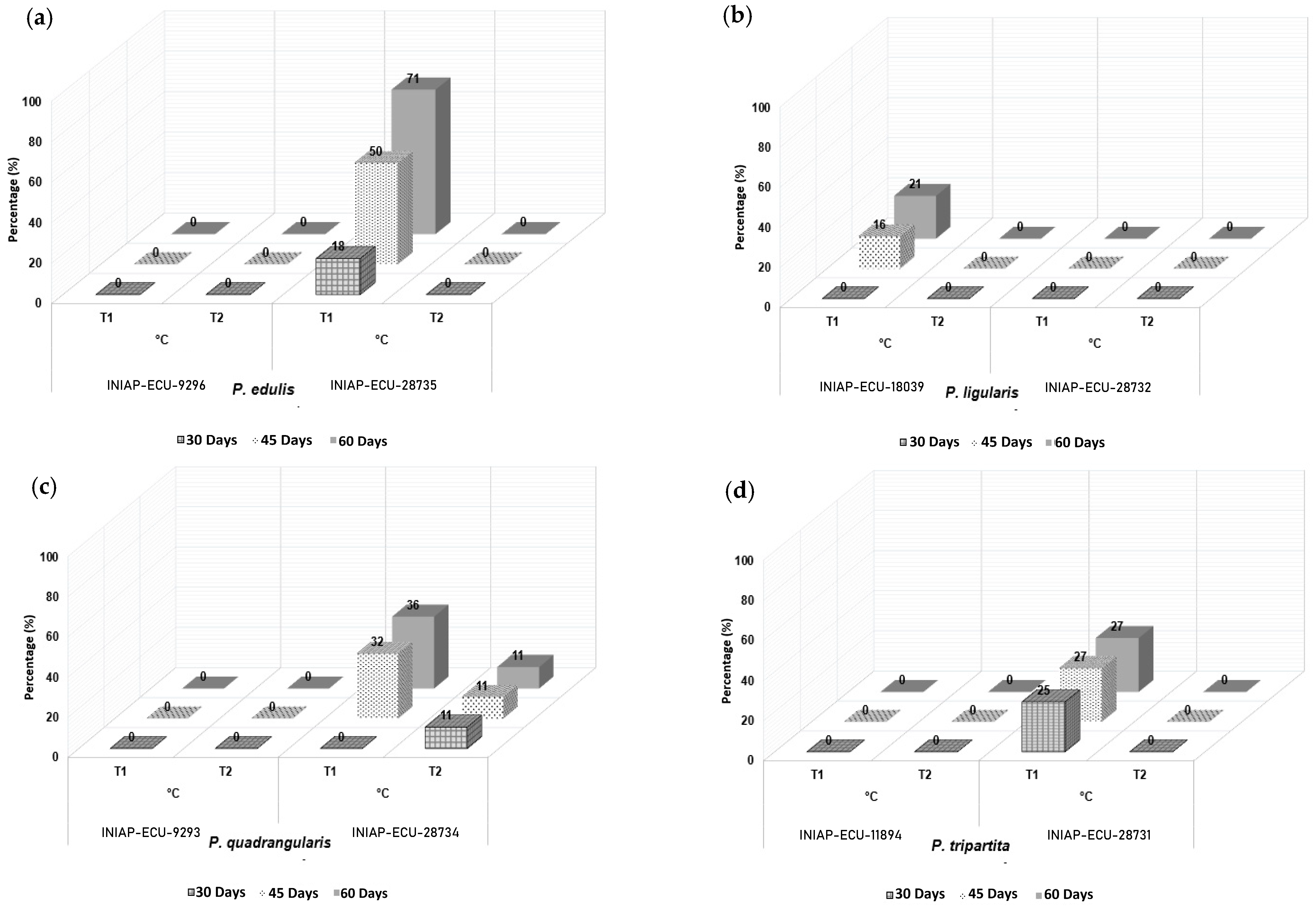
3.2. Seed Germination Under Two Temperature Conditions
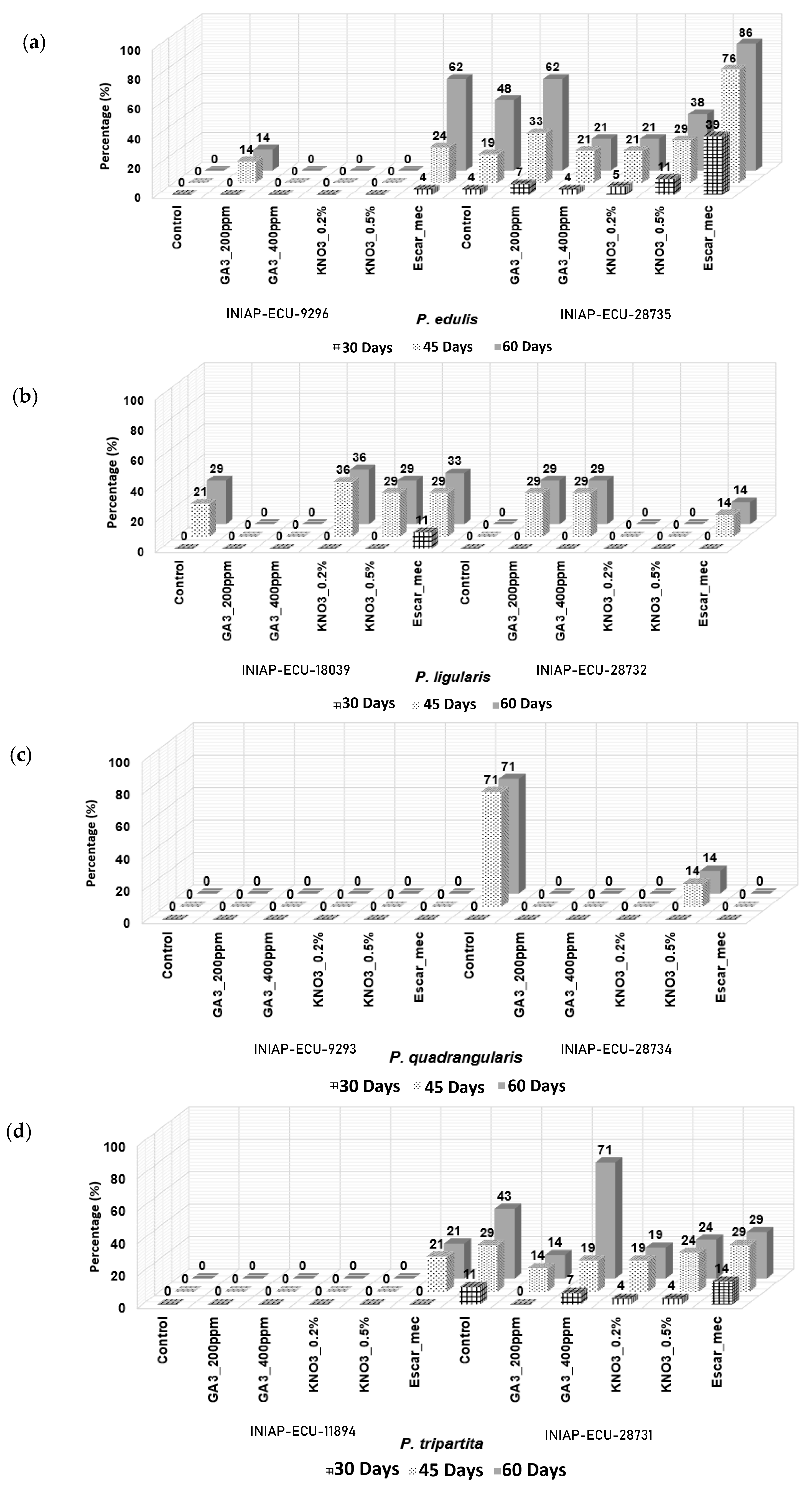
3.3. Seed Germination with Pre-Germination Treatments
3.4. Tetrazolium Test
4. Discussion
4.1. Seed Morphometry
4.2. Seed Germination Under Two Temperatures
4.3. Seed Germination with Pre-Germinative Treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GA3 | Gibberellic acid |
| KNO3 | Potassium nitrate |
| Escar_mec | Mechanical scarification |
Appendix A
Appendix A.1
| Variables | Parameters | Species | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P. edulis | P. ligularis | P. quadrangularis | P. tripartita | ||||||
| INIAP-ECU-9296 | INIAP-ECU-28735 | INIAP-ECU-18039 | INIAP-ECU-28732 | INIAP-ECU-9293 | INIAP-ECU-28734 | INIAP-ECU-11894 | INIAP-ECU-28731 | ||
| Thickness (mm) | Min. | 1.88 | 1.74 | 1.62 | 1.78 | 1.89 | 2.19 | 1.63 | 2.19 |
| Max. | 2.12 | 1.99 | 1.99 | 2.19 | 2.5 | 2.5 | 2.81 | 2.81 | |
| C.V. | 0.03 | 0.04 | 0.05 | 0.03 | 0.03 | 0.03 | 0.05 | 0.07 | |
| Media | 1.97 | 1.88 | 1.78 | 1.9 | 2.1 | 2.39 | 1.77 | 2.47 | |
| Length (mm) | Min. | 5.64 | 6.1 | 6.37 | 6.54 | 7.7 | 8.42 | 5.01 | 5.97 |
| Max. | 7.02 | 7.67 | 7.67 | 8.8 | 9.85 | 9.85 | 6.91 | 6.91 | |
| C.V. | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | |
| Media | 5.89 | 6.65 | 7.14 | 6.92 | 8.27 | 9.23 | 5.33 | 6.48 | |
| Width (mm) | Min. | 3.70 | 3.94 | 3.48 | 3.91 | 6.47 | 7.09 | 2.95 | 3.18 |
| Max. | 4.45 | 4.45 | 4.22 | 7.32 | 7.90 | 7.9 | 4.54 | 4.54 | |
| C.V. | 3.10 | 2.92 | 4.22 | 1.95 | 3.39 | 2.95 | 4.51 | 7.78 | |
| Media | 4.03 | 4.25 | 3.98 | 4.05 | 7.00 | 7.55 | 3.24 | 3.99 | |
| Area (mm2) | Min. | 15.52 | 17.58 | 16.58 | 19.29 | 39.66 | 48.51 | 11.18 | 13.01 |
| Max. | 22.56 | 23.56 | 23.56 | 48.62 | 59.68 | 59.68 | 20.29 | 20.29 | |
| C.V. | 5.61 | 6.7 | 7.67 | 3.75 | 5.57 | 5.44 | 6.34 | 9.07 | |
| Media | 17.59 | 20.59 | 20.83 | 20.66 | 45.22 | 53.98 | 12.65 | 17.7 | |
| Weight (g) | Total | 0.53 | 0.62 | 0.63 | 0.82 | 1.12 | 1.37 | 0.42 | 0.80 |
Appendix A.2
| Species | Accessions | Samples | Shape | Apex | Margin | Base | Ornamentation |
|---|---|---|---|---|---|---|---|
| P. edulis | INIAP-ECU-9296 | n = 20 | Obovate | Sharp apex with raised area | Part | Truncated sharp | Alveolate reticulum |
| P. edulis | INIAP ECU-28735 | n = 20 | Obovate | Sharp apex with raised area | Complete | Truncated sharp | Alveolate reticulum |
| P. ligularis | INIAP ECU-18039 | n = 20 | Elliptical | Prominent sharp | Dentate | Truncated sharp | Foveated Reticulum |
| P. ligularis | INIAP ECU-28732 | n = 20 | Elliptical | Prominent sharp | Crestated | Truncated | Foveated Reticulum |
| P. quadrangularis | INIAP ECU-9293 | n = 20 | Elliptical | Concaved Horn | Part | Truncated | Reticulated Tosca |
| P. quadrangularis | INIAP ECU-28734 | n = 20 | Elliptical | Concaved Horn | Part | Cordulated | Reticulated Tosca |
| P. tripartita | INIAP ECU-11894 | n = 20 | Obovate | Prominent sharp | Complete | Orbed | Reticulated |
| P. tripartita | INIAP ECU-28731 | n = 20 | Elliptical | Prominent sharp | Complete | Acute | Reticulated |
Appendix A.3
| Description | Media | |||
|---|---|---|---|---|
| Days | Trat. | Temperature (°C) | Accession | Germination Percentage (%) |
| 45 | 1 | T1 | INIAP-ECU-9296 | 0.00 d * |
| 2 | T1 | INIAP-ECU-28735 | 47.62 a | |
| 3 | T1 | INIAP-ECU-18039 | 21.43 b | |
| 4 | T1 | INIAP-ECU-28732 | 0.00 d | |
| 5 | T1 | INIAP-ECU-9293 | 0.00 d | |
| 6 | T1 | INIAP-ECU-28734 | 42.87 a | |
| 7 | T1 | INIAP-ECU-11894 | 0.00 d | |
| 8 | T1 | INIAP-ECU-28731 | 35.72 a | |
| 9 | T2 | INIAP-ECU-9296 | 0.00 d | |
| 10 | T2 | INIAP-ECU-28735 | 0.00 d | |
| 11 | T2 | INIAP-ECU-18039 | 0.00 d | |
| 12 | T2 | INIAP-ECU-28732 | 0.00 d | |
| 13 | T2 | INIAP-ECU-9293 | 0.00 d | |
| 14 | T2 | INIAP-ECU-28734 | 14.29 b | |
| 15 | T2 | INIAP-ECU-11894 | 0.00 d | |
| 16 | T2 | INIAP-ECU-28731 | 0.00 d | |
| 60 | 1 | T1 | INIAP-ECU-9296 | 0.00 d |
| 2 | T1 | INIAP-ECU-28735 | 71.44 a | |
| 3 | T1 | INIAP-ECU-18039 | 28.57 c | |
| 4 | T1 | INIAP-ECU-28732 | 0.00 d | |
| 5 | T1 | INIAP-ECU-9293 | 0.00 d | |
| 6 | T1 | INIAP-ECU-28734 | 47.62 b | |
| 7 | T1 | INIAP-ECU-11894 | 0.00 d | |
| 8 | T1 | INIAP-ECU-28731 | 35.72 b | |
| 9 | T2 | INIAP-ECU-9296 | 0.00 d | |
| 10 | T2 | INIAP-ECU-28735 | 0.00 d | |
| 11 | T2 | INIAP-ECU-18039 | 0.00 d | |
| 12 | T2 | INIAP-ECU-28732 | 0.00 d | |
| 13 | T2 | INIAP-ECU-9293 | 0.00 d | |
| 14 | T2 | INIAP-ECU-28734 | 14.29 c | |
| 15 | T2 | INIAP-ECU-11894 | 0.00 d | |
| 16 | T2 | INIAP-ECU-28731 | 0.00 d | |
Appendix A.4
| Description | Media | |||
|---|---|---|---|---|
| Trat. | Pre-Germinative Treatment | Accession | Germination Percentage (%) | |
| 45 Days | 60 Days | |||
| 1 | Control | INIAP-ECU-9296 | 0.0 c | 0.0 c * |
| 2 | Control | INIAP-ECU-28735 | 19.05 b | 47.62 a |
| 3 | Control | INIAP-ECU-18039 | 21.43 b | 28.57 b |
| 4 | Control | INIAP-ECU-28732 | 0.0 c | 0.0 c |
| 5 | Control | INIAP-ECU-9293 | 0.0 c | 0.0 c |
| 6 | Control | INIAP-ECU-28734 | 71.10 a | 71.10 a |
| 7 | Control | INIAP-ECU-11894 | 0.0 c | 0.0 c |
| 8 | Control | INIAP-ECU-28731 | 28.57 b | 42.86 a |
| 9 | GA3 200 ppm | INIAP-ECU-9296 | 14.29 b | 14.29 b |
| 10 | GA3 200 ppm | INIAP-ECU-28735 | 33.33 b | 61.90 a |
| 11 | GA3 200 ppm | INIAP-ECU-18039 | 0.0 c | 0.0 c |
| 12 | GA3 200 ppm | INIAP-ECU-28732 | 28.57 b | 28.57 b |
| 13 | GA3 200 ppm | INIAP-ECU-9293 | 0.0 c | 0.0 c |
| 14 | GA3 200 ppm | INIAP-ECU-28734 | 0.0 c | 0.0 c |
| 15 | GA3 200 ppm | INIAP-ECU-11894 | 0.0 c | 0.0 c |
| 16 | GA3 200 ppm | INIAP-ECU-28731 | 14.29 b | 14.29 b |
| 17 | GA3 400 ppm | INIAP-ECU-9296 | 0.0 c | 0.0 c |
| 18 | GA3 400 ppm | INIAP-ECU-28735 | 21.43 b | 21.43 b |
| 19 | GA3 400 ppm | INIAP-ECU-18039 | 0.0 c | 0.0 c |
| 20 | GA3 400 ppm | INIAP-ECU-28732 | 28.57 b | 28.57 b |
| 21 | GA3 400 ppm | INIAP-ECU-9293 | 0.0 c | 0.0 c |
| 22 | GA3 400 ppm | INIAP-ECU-28734 | 0.0 c | 0.0 c |
| 23 | GA3 400 ppm | INIAP-ECU-11894 | 0.0 c | 0.0 c |
| 24 | GA3 400 ppm | INIAP-ECU-28731 | 19.05 b | 71.43 a |
| 25 | KNO3 (0.2%) | INIAP-ECU-9296 | 0.0 c | 0.0 c |
| 26 | KNO3 (0.2%) | INIAP-ECU-28735 | 21.43 b | 21.43 b |
| 27 | KNO3 (0.2%) | INIAP-ECU-18039 | 35.72 b | 35.72 b |
| 28 | KNO3 (0.2%) | INIAP-ECU-28732 | 0.0 c | 0.0 c |
| 29 | KNO3 (0.2%) | INIAP-ECU-9293 | 0.0 c | 0.0 c |
| 30 | KNO3 (0.2%) | INIAP-ECU-28734 | 0.0 c | 0.0 c |
| 31 | KNO3 (0.2%) | INIAP-ECU-11894 | 0.0 c | 0.0 c |
| 32 | KNO3 (0.2%) | INIAP-ECU-28731 | 19.05 b | 19.05 b |
| 33 | KNO3 (0.5%) | INIAP-ECU-9296 | 0.0 c | 0.0 c |
| 34 | KNO3 (0.5%) | INIAP-ECU-28735 | 28.58 b | 38.10 a |
| 35 | KNO3 (0.5%) | INIAP-ECU-18039 | 28.57 b | 28.57 b |
| 36 | KNO3 (0.5%) | INIAP-ECU-28732 | 0.0 c | 0.0 c |
| 37 | KNO3 (0.5%) | INIAP-ECU-9293 | 0.0 c | 0.0 c |
| 38 | KNO3 (0.5%) | INIAP-ECU-28734 | 14.29 b | 14.29 b |
| 39 | KNO3 (0.5%) | INIAP-ECU-11894 | 0.0 c | 0.0 c |
| 40 | KNO3 (0.5%) | INIAP-ECU-28731 | 23.81 b | 23.81 b |
| 41 | Escar_mec | INIAP-ECU-9296 | 23.81 b | 61.90 a |
| 42 | Escar_mec | INIAP-ECU-28735 | 76.19 a | 85.71 a |
| 43 | Escar_mec | INIAP-ECU-18039 | 28.57 b | 33.34 b |
| 44 | Escar_mec | INIAP-ECU-28732 | 14.29 b | 14.29 b |
| 45 | Escar_mec | INIAP-ECU-9293 | 0.0 c | 0.0 c |
| 46 | Escar_mec | INIAP-ECU-28734 | 0.0 c | 0.0 c |
| 47 | Escar_mec | INIAP-ECU-11894 | 21.43 b | 21.43 b |
| 48 | Escar_mec | INIAP-ECU-28731 | 23.81 b | 28.60 b |
Appendix A.5

References
- Muschner, V.; Zamberlan, P.; Bonatto, S.; Freitas, L. Phylogeny, biogeography and divergence times in Passiflora (Passifloraceae). Genet. Mol. Biol. 2012, 35, 1036–1043. [Google Scholar] [CrossRef]
- Ibáñez, B.; Idrogo, C.; Quiroz, S.; Paredes, G.E. El género Passiflora L. (Passifloraceae) en el Departamento de Lambayeque, Perú. Acta Botanica Malacitana 2014, 39, 55–70. [Google Scholar] [CrossRef]
- Coppens D’Eeckenbrugge, G. Diversité Génétique et Domestication des Fruitiers Néotropicaux. Ph.D. Thesis, Université de Montpellier 2, Montpellier, France, 2013. [Google Scholar]
- Fischer, G.; Miranda, D. Review on the ecophysiology of important Andean fruits: Passiflora L. Rev. Fac. Nac. Agron. Medellín 2021, 74, 9471–9481. [Google Scholar] [CrossRef]
- Miranda, D.; Fischer, G.; Carranza, C.; Magnitskiy, S.; Casierra, F.; Piedrahíta, W.; Flórez, L.E. Cultivo, Poscosecha y Comercialización de las Pasifloráceas en Colombia: Maracuyá, Granadilla, Gulupa y Curuba; Sociedad Colombiana de Ciencias Hortícolas: Bogotá, Colombia, 2009. [Google Scholar]
- INEC. Encuesta de Superficie y Producción Agropecuaria Continua 2025. ecuadorencifras.gob.ec2025. Available online: https://www.ecuadorencifras.gob.ec/encuesta-de-superficie-y-produccion-agropecuaria-continua-bbd/ (accessed on 14 August 2025).
- SIPA. Ficha del Cultivo de Maracuyá. agricultura.gob.ec2025. Available online: http://sipa.agricultura.gob.ec/index.php/maracuya (accessed on 17 August 2025).
- Ramos, M.M.; Redin, E.; Júnior, A.R.L. Panorama de la producción de maracuyá en Brasil, Minas Gerais y Unaí. Rev. Fac. Agron. 2023, 122, 127. [Google Scholar] [CrossRef]
- Cerqueira-Silva, C.B.M.; Faleiro, F.G.; de Jesus, O.N.; dos Santos, E.S.L.; de Souza, A.P. The genetic diversity, conservation, and use of passion fruit (Passiflora spp.). In Genetic Diversity and Erosion in Plants: Case Histories; Springer: Berlin/Heidelberg, Germany, 2015; pp. 215–231. [Google Scholar]
- Aribi, M.M. Plant gene banks: Conservation of genetic resources. In Sustainable Utilization and Conservation of Plant Genetic Diversity; Springer: Berlin/Heidelberg, Germany, 2024; pp. 753–775. [Google Scholar]
- FAO. Practical Guide for the Application of the Genebank Standards for Plant Genetic Resources for Food and Agriculture: Conservation Via In Vitro Culture; FAO: Rome, Italy, 2022. [Google Scholar]
- García, J.; González, S. Germinación de semillas de tomate (Solanum lycopersicum), papaya (Carica papaya L.) y maracuyá (Passiflora edulis) utilizando sustratos orgánicos. Rev. Sist. Prod. Agroecol. 2018, 9, 18–35. [Google Scholar] [CrossRef]
- Torres, A. Seed dormancy and germination of two cultivated species of Passifloraceae. Boletín Científico Cent. Mus. Mus. Hist. Nat. 2018, 22, 15–27. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology Fisiologia Vegetal; Editora Artemed: Porto Alegre, Brazil, 2013. [Google Scholar]
- Gutiérrez, M.I.; Miranda, D.; Cárdenas, J. Efecto de tratamientos pregerminativos sobre la germinación de semillas de gulupa (Passiflora edulis Sims.), granadilla (Passiflora ligularis Juss.) y cholupa (Passiflora maliformis L.). Rev. Colomb. Cienc. Hortícolas 2011, 5, 209–219. [Google Scholar] [CrossRef]
- Posada, P.; Ocampo, J.; Santos, L.G. Estudio del comportamiento fisiológico de la semilla de tres especies cultivadas de Passiflora L. (Passifloraceae) como una contribución para la conservación ex situ. Rev. Colomb. Cienc. Hortícolas 2014, 8, 9–19. [Google Scholar] [CrossRef]
- Carranza, C.; Castellanos, G.; Deaza, D.; Miranda, D. Effect of growth regulator application on the germination of badea (Passiflora quadrangularis L.) seeds under greenhouse conditions. Rev. Colomb. Cienc. Hortícolas 2016, 10, 284–291. [Google Scholar] [CrossRef]
- Cárdenas Hernández, J.F. Morfología y Tratamientos Pregerminativos de Semillas de Granadilla (Passiflora ligularis Juss). Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2011. [Google Scholar]
- Mezzonato, A.; Mendonça, C.; De Azevedo, M.; Gonçalves, V. The taxonomic significance of seed morphology in the Passiflora subgenus Astrophea (Passifloraceae). Acta Bot. Bras. 2017, 31, 68–83. [Google Scholar] [CrossRef][Green Version]
- Mabundza, R.M.; Wahome, P.K.; Masarirambi, M.T. Effects of different pre-germination treatment methods on the germination of passion (Passiflora edulis) seeds. J. Agric. Soc. Sci. 2010, 6, 57–60. [Google Scholar][Green Version]
- Gurung, N.; Swamy, G.; Sarkar, S.; Ubale, N. Effect of chemicals and growth regulators on germination, vigour and growth of passion fruit (Passiflora edulis Sims.). Bioscan 2014, 9, 155–157. [Google Scholar][Green Version]
- Ghosh, A.; Dey, K.; Bauri, F.K.; Dey, A.N. Effects of different pre-germination treatment methods on the germination and seedling growth of yellow passion fruit (Passiflora edulis var. flavicarpa). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 630–636. [Google Scholar][Green Version]
- Viera, W.; Shinohara, T.; Sanada, A.; Terada, N.; Ron, L.; Koshio, K. Seed germination and seedling growth of yellow and purple passion fruit genotypes cultivated in Ecuador. Horticulturae 2022, 8, 754. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Stafne, E. Comparison of seed treatments on the germination of seven passion fruit species. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3074–3083. [Google Scholar] [CrossRef]
- Marostega, T.N.; Araujo, L.M.; Luz, P.B.D.; Neves, L.G.; Barelli, M.A.A. Genetic diversity of Passiflora accessions based on morphophysiological seed descriptors. Rev. Bras. Frutic. 2017, 39, e-365. [Google Scholar] [CrossRef]
- Boboc Oros, P.; Cantor, M.; Cordea, M.I.; Cătană, C. Plant Regeneration Protocol for Recalcitrant Passionflower (Passiflora quadrangularis L.). Horticulturae 2022, 8, 337. [Google Scholar] [CrossRef]
- Delanoy, M.; Van Damme, P.; Scheldeman, X.; Beltran, J. Germination of Passiflora mollissima (Kunth) LH Bailey, Passiflora tricuspis Mast. and Passiflora nov sp. seeds. Sci. Hortic. 2006, 110, 198–203. [Google Scholar] [CrossRef]
- Romero-Murcia, J. Ex situ conservation of seeds of four Andean species of Pasiflora. Agroproductividad 2018, 11, 75–81. [Google Scholar]
- Cadorin, D.A.; Villa, F.; Dalastra, G.M.; Heberle, K.; Rotili, M.C.C. Pre-germination treatment in granadilha (Passiflora ligularis) seeds. 2017. Available online: https://revistas.udesc.br/index.php/agroveterinaria/article/view/223811711632017256 (accessed on 15 September 2025).
- Cárdenas, J.; Carranza, C.; Miranda, D.; Magnitskiy, S. Efeito do GA3, KNO3 e o desponte basal nas sementes em germinação de granadilla (Passiflora ligularis Juss) e o maracujá comum (Passiflora edulis f. flavicarpa). Rev. Bras. Frutic. 2013, 35, 853–859. [Google Scholar] [CrossRef]
- Aguacía, L.M.; Miranda, D.; Carranza, C. Effect of fruit maturity stage and fermentation period on the germination of passion fruit (Passiflora edulis f. flavicarpa Deg.) and sweet granadilla seeds (Passiflora ligularis Juss.). Agron. Colomb. 2015, 33, 305–314. [Google Scholar] [CrossRef]
- Monteros, A.; Tacán, M.; Peña Monserrate, G.R.; Tapia, C.; Paredes Andrade, N.; Lima, L. Guía Para el Manejo y Conservación de recursos Fitogenéticos en Ecuador: Protocolos; Publicación Miscelánea No. 432; INIAP: Quito, Ecuador, 2018. [Google Scholar]
- FAO. Guía Práctica Para la Aplicación de las Normas para Bancos de Germoplasma de Recursos Fitogenéticos para la Alimentación y la Agricultura: Conservación en Bancos de Germoplasma de Campo; FAO, Comisión de Recursos Genéticos para la Alimentación y la Agricultura: Rome, Italy, 2023. [Google Scholar]
- Pérez, S.; Tillett, S.; Escala, M. Estudio morfológico de la semilla de 51 especies del género Passiflora L. Acta Botánica Venez. 2002, 25, 67–96. [Google Scholar]
- Morillo, E.M.S. Manual de Técnicas y Procedimientos del Laboratorio de Cultivo de Tejidos del INIAP; EESC, EPD: Brussels, Belgium, 2022. [Google Scholar]
- Angelini, L.G.; Clemente, C.; Tavarini, S. Pre-germination treatments, temperature, and light conditions improved seed germination of Passiflora incarnata L. Agriculture 2021, 11, 937. [Google Scholar] [CrossRef]
- Rao, N.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Nowell, D.; Larinde, M. Manual Para el Manejo de Semillas en Bancos de Germoplasma; Bioversity International: Rome, Italy, 2007. [Google Scholar]
- ISTA. Reglas Internacionales Para el Análisis de las Semillas 2016. Available online: https://vri.umayor.cl/images/ISTA_Rules_2016_Spanish.pdf (accessed on 4 April 2025).
- Roa Nieto, C.E. Efecto de la Estratificación Sobre la Germinación de SEMILLAS de badea (Passiflora quadrangularis L.). Bachelor´s Thesis, Universidad Nacional Abierta y a Distancia UNAD, Bogotá, Colombia, 2017. [Google Scholar]
- Peralta, J. Evaluación de dos Tipos de Escarificación Para Incrementar el Porcentaje de Germinación de Gulupa (Passiflora edulis sims F. edulis), Badea (Passiflora quadrangularis) y Taxo (Passiflora tarminiana) Bajo Invernadero. Bachelor’s Thesis, Universidad San Francisco de Quito Repositorio Digital, Quito, Ecuador, 2021. [Google Scholar]
- INFOSTAT; Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. Programa Estadístico para el Análisis de Datos; Córdoba, Argentina. 2017. Available online: https://repositorio.catie.ac.cr/handle/11554/10346?locale-attribute=es (accessed on 17 July 2025).
- Ruiz Bolívar, C. Mediación de estrategias metacognitivas en tareas divergentes y transferencia recíproca. Investig. Postgrado 2002, 17, 53–82. [Google Scholar]
- Martín, P.; de Pascual, A.; Lezama, E.; Olmos, E. Una aplicación del análisis de componentes principales en el área educativa. Economía 1994, 19, 55–72. [Google Scholar]
- de Jesus Silva, J.; Souza, F.V.D.; Junghans, T.G.; da Silva Ledo, C.A.; Rossi, M.L.; de Souza, E.H. Seed morphoanatomy of the genus Passiflora L. (Passifloraceae) by scanning electron microscopy and light microscopy. Microsc. Res. Tech. 2023, 86, 28–40. [Google Scholar] [CrossRef]
- Castillo, N.R.; Melgarejo, L.M.; Blair, M.W. Seed structural variability and germination capacity in Passiflora edulis Sims f. edulis. Front. Plant Sci. 2020, 11, 498. [Google Scholar] [CrossRef]
- Meneses, B. El Análisis de Conglomerados en los Estudios de Mercado. Rev. Cienc. Adm. 2000. Available online: https://www.uv.mx/iiesca/files/2013/01/conglomerados2000.pdf (accessed on 12 May 2025).
- de Assis, J.P.; de Sousa, R.P.; Linhares, P.C.F.; Cardoso, E.d.A.; Rodrigues, W.M.; Pereira, J.O.; de Sousa, R.P.; Pereira, B.B.M.; da Silva, N.V.; Alves, L.d.S.; et al. Biometric evaluation of Passiflora cincinnata seeds obtained from the herbaceous extract of the caatinga biome. Int. J. Adv. Eng. Res. Sci. 2020, 7, 57–67. [Google Scholar] [CrossRef]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.; Dias, D. Germinação e vigor de sementes de maracujá-amarelo. (Passiflora edulis Sims. f. flaviacarpa Deg.) submetidas a diferentes métodos de remoção da mucilagem. Rev. Bras. Sementes 2000, 22, 288–291. [Google Scholar]
- Gutiérrez, R.H.C. Efecto de la escarificación y la dosis del ácido giberélico (AG3) en la germinación de semilla de curuba (Passiflora mollisima). Acta Biológica Colomb. 1988, 1, 127–132. [Google Scholar]
- Ayala, C.E.C.; Tatis, H.A.; Robles, J.; López, V.; Ubarnes, J. Efecto de diferentes ambientes y empaques sobre la viabiilidad de semillas de maracuya (Passiflora edulis var. Flavicarpa degener). Temas Agrar. 2005, 10, 15–25. [Google Scholar] [CrossRef][Green Version]
- Agüero, C.; Pereyra, G.; Rolando, R. Método alternativo de germinación para determinar la calidad de semillas en Buffel Grass (Cenchrus ciliaris L.). Agriscientia 2017, 34, 47–58. [Google Scholar] [CrossRef]
- Martín, I.; Sánchez, S.; Guerrero, M.; Andrés Fd Guasch, L. Viabilidad de semillas después de 40 años. In Resultados del Banco de Germoplasma del CRF-INIA-CSIC, Proceedings of the XI Congreso Nacional de Mejora Genética de Plantas, Cáceres, España, 24–26 September 2024; Centro de Recursos Fitogenéticos y Agricultura Sostenible (CRF): Madrid, Spain, 2024; Acta de Horticultura. [Google Scholar]
- Gutiérrez, B.; Magni, C.; Gutiérrez, P. Almacenamiento de Colecciones de Germoplasma Ex Situ; Conservación de Recursos Genéticos Forestales Instituto Forestal: Santiago, Spain, 2015; pp. 197–213. [Google Scholar]
- Ramírez Gil, J.G.; Agudelo, M.M.; Bedoya, L.O.; Osorio, N.W.; Osorio, J.G.M. Germination and growth of purple passion fruit seedlings under pre-germination treatments and mycorrhizal inoculation. Agric. Res. Trop. Pesqui. Agropecuária Trop. 2015, 45, 257–265. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Ribeiro-Oliveira, J.P.; Ranal, M.A. Sample size in studies on the germination process. Botany 2016, 94, 103–115. [Google Scholar] [CrossRef]
- Miño Domínguez, J.E. Identificación molecular del género Passiflora (Passifloraceae), de la región norte del Ecuador por medio del método DNA Barcoding. Bachelor´s Thesis, Universidad Politécnica Salesiana, Quito, Ecuador, 2018. Available online: https://dspace.ups.edu.ec/bitstream/123456789/16148/1/UPS-QT13326.pdf (accessed on 28 April 2025).
- Barrios Arango, L. Estudios de la Diversidad de Passifloraceae en los Departamentos de Caldas, Chocó, Nariño, Quindío, Risaralda y Valle del Cauca (Colombia), Apoyado en los Análisis Ecogeográficos, Palinológicos y Citogenéticos. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2005. [Google Scholar]
- Chen, Y.; Zhang, L.; Lu, X.; Lan, X.; Shen, M.; Lu, C. Role of mucilage during achene germination and sprout growth of the endangered Tibetan medicinal herb Mirabilis himalaica (Nyctaginaceae) exposed to abiotic stresses. J. Plant Ecol. 2018, 11, 328–337. [Google Scholar] [CrossRef]
- Ortega, A.; Estudios en fisiología de semillas de Passiflora rubra L en dos épocas de colecta en el Jardín Botánico del Quindío. Bachelor´s Thesis. 2006. Available online: https://www.scribd.com/document/919690985/COLECTA (accessed on 12 February 2025).
- Vega, E.; Campos, V.; Monge, A.A.; Bertsch, S.; Vargas, E. Morfología y optimización de prueba de viabilidad en semillas de Passiflora spp. de Costa Rica. Agron. Mesoam. 2022, 33, 51567. [Google Scholar] [CrossRef]
- Pramanik, K.; Sahoo, J.P.; Mohapatra, P.P.; Acharya, L.K.; Jena, C. Insights into the embryo rescue—A modern in-vitro crop improvement approach in horticulture. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 20–33. [Google Scholar]
- Salazar Mercado, S.A.; Botello Delgado, E.A. Viabilidad de semillas de Glycine max (L.) utilizando la prueba de tetrazolio. Rev. Investig. Agrar. Ambient. 2018, 9, 89–98. [Google Scholar]


| INIAP-ECU-28735 | INIAP-ECU-9296 | INIAP-ECU-18039 | INIAP-ECU-28732 | INIAP-ECU-9293 | INIAP-ECU-28734 | INIAP-ECU-11894 | INIAP-ECU-28731 | |
| INIAP-ECU-28735 | 0.00 | |||||||
| INIAP-ECU-9296 | 0.33 | 0.00 | ||||||
| INIAP-ECU-18039 | 0.26 | 0.40 | 0.00 | |||||
| INIAP-ECU-28732 | 0.26 | 0.39 | 0.30 | 0.00 | ||||
| INIAP-ECU-9293 | 0.71 | 0.75 | 0.72 | 0.67 | 0.00 | |||
| INIAP-ECU-28734 | 0.87 | 0.90 | 0.87 | 0.84 | 0.50 | 0.00 | ||
| INIAP-ECU-11894 | 0.48 | 0.41 | 0.46 | 0.53 | 0.85 | 0.99 | 0.00 | |
| INIAP-ECU-28731 | 0.49 | 0.48 | 0.53 | 0.45 | 0.73 | 0.79 | 0.63 | 0.00 |
| Correlation | ||||||
|---|---|---|---|---|---|---|
| Control Variables | Weight | Thickness | Length | Broad | Area | |
| Weight | Correlation | 1.000 | ||||
| Significance (unilateral) | ||||||
| Thickness | Correlation | - | 1.000 | |||
| Significance (unilateral) | ||||||
| Length | Correlation | 0.867 | - | 1.000 | ||
| Significance (unilateral) | 0.003 * | |||||
| Broad | Correlation | 0.959 | - | 0.899 | 1.000 | |
| Significance (unilateral) | 0.000 * | 0.001 * | ||||
| Area | Correlation | 0.961 | - | 0.914 | 0.996 | 1.000 |
| Significance (unilateral) | 0.000 * | 0.001 * | 0.000 * | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdezoto-Merino, F.; Monteros-Altamirano, Á.; Roura, A.; Andrade-Bolaños, H. Seed Morphometry and Germination of Four Edible Species of Passiflora spp. Conserved in a Gene Bank. Crops 2025, 5, 64. https://doi.org/10.3390/crops5050064
Verdezoto-Merino F, Monteros-Altamirano Á, Roura A, Andrade-Bolaños H. Seed Morphometry and Germination of Four Edible Species of Passiflora spp. Conserved in a Gene Bank. Crops. 2025; 5(5):64. https://doi.org/10.3390/crops5050064
Chicago/Turabian StyleVerdezoto-Merino, Fabricio, Álvaro Monteros-Altamirano, Alberto Roura, and Héctor Andrade-Bolaños. 2025. "Seed Morphometry and Germination of Four Edible Species of Passiflora spp. Conserved in a Gene Bank" Crops 5, no. 5: 64. https://doi.org/10.3390/crops5050064
APA StyleVerdezoto-Merino, F., Monteros-Altamirano, Á., Roura, A., & Andrade-Bolaños, H. (2025). Seed Morphometry and Germination of Four Edible Species of Passiflora spp. Conserved in a Gene Bank. Crops, 5(5), 64. https://doi.org/10.3390/crops5050064







