Spring Wheat Breeding in Northern Kazakhstan: Drivers of Diversity and Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Spring Wheat Germplasm
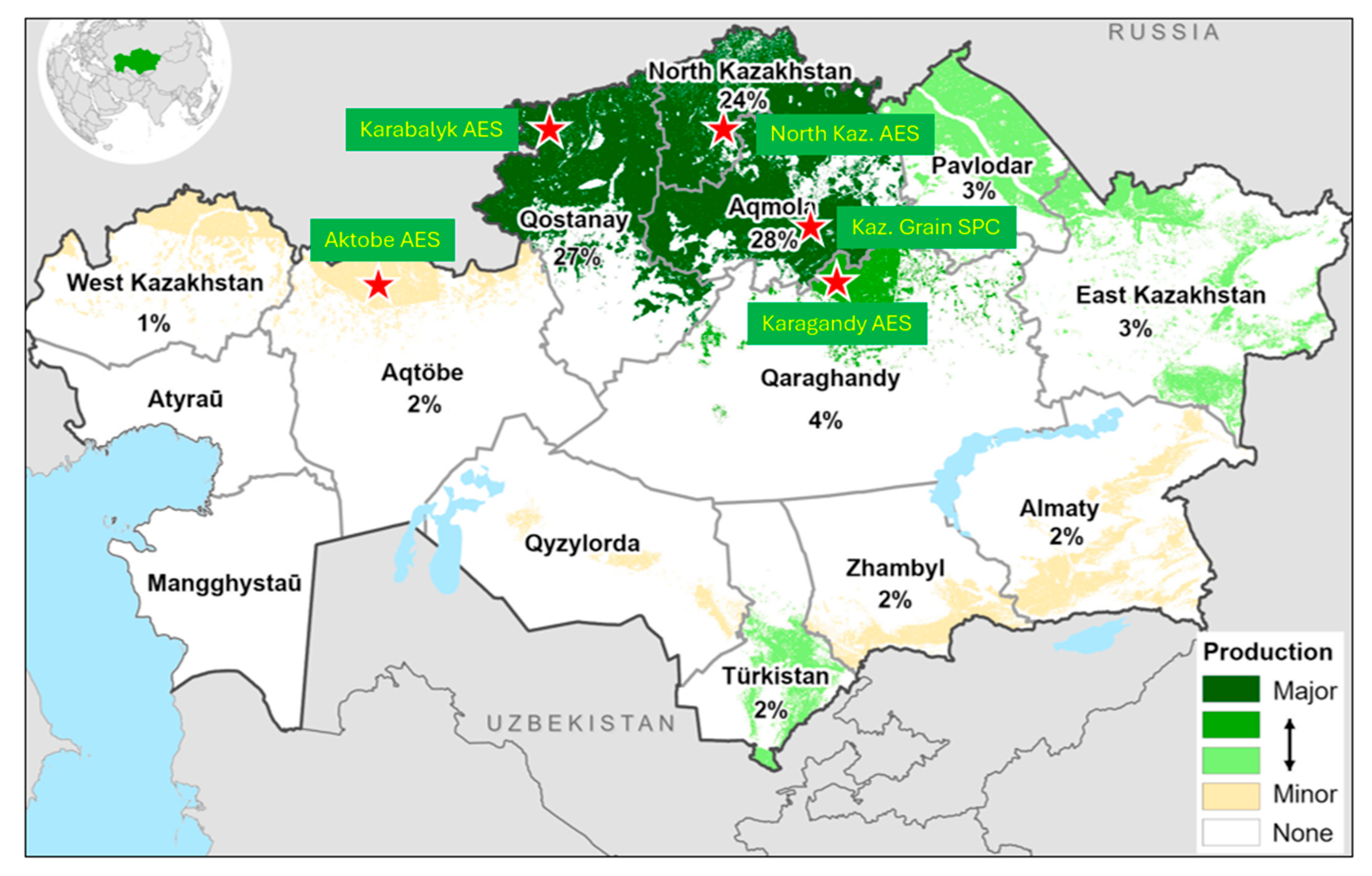
2.2. Experimental Sites and Trial Methodology
2.3. Genotyping and KASP Assay
2.4. Statistics
3. Results
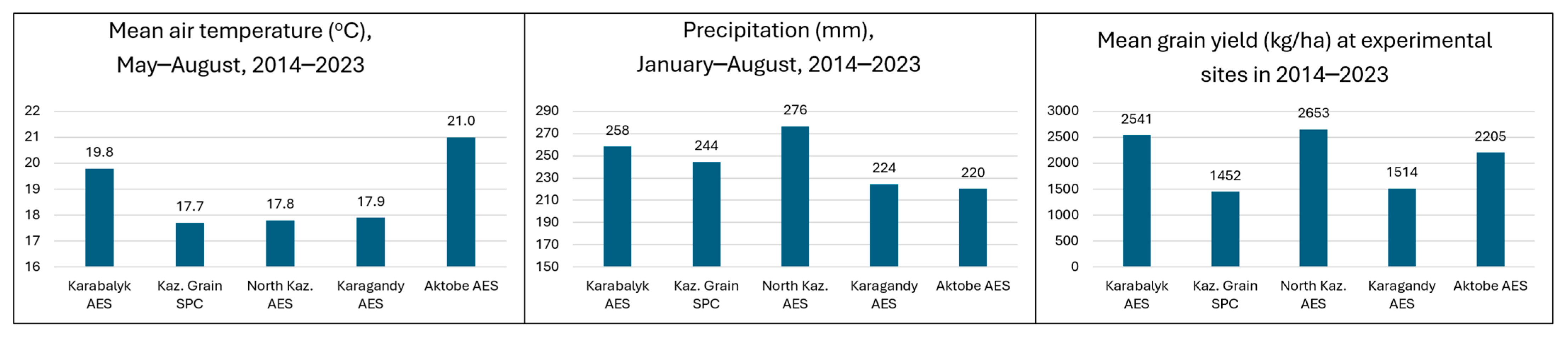
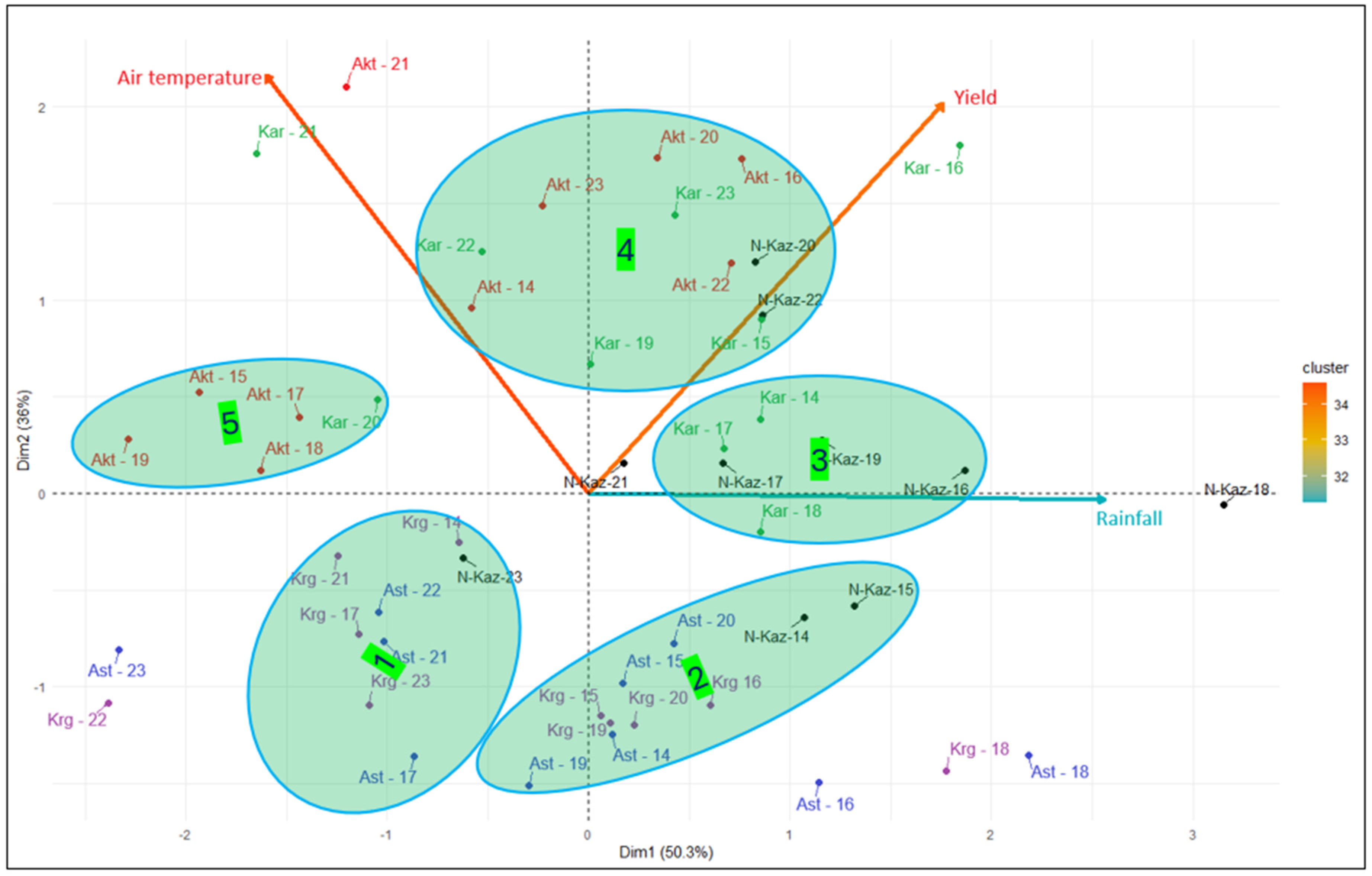
3.1. Variation of Breeding Sites for Weather Conditions and Grain Yield
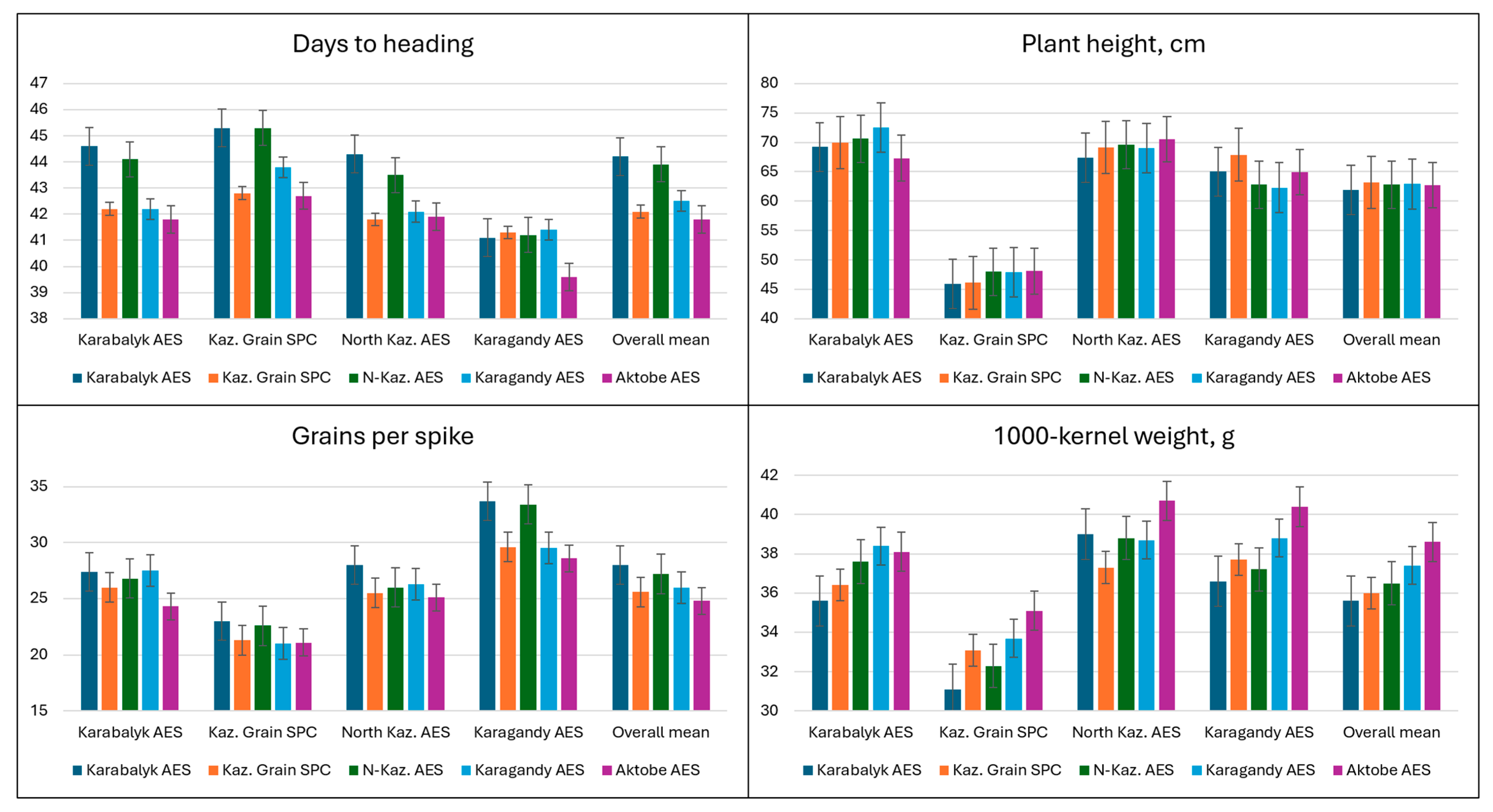
3.2. Variation for Agronomic Traits at Experimental Sites in 2022–2023
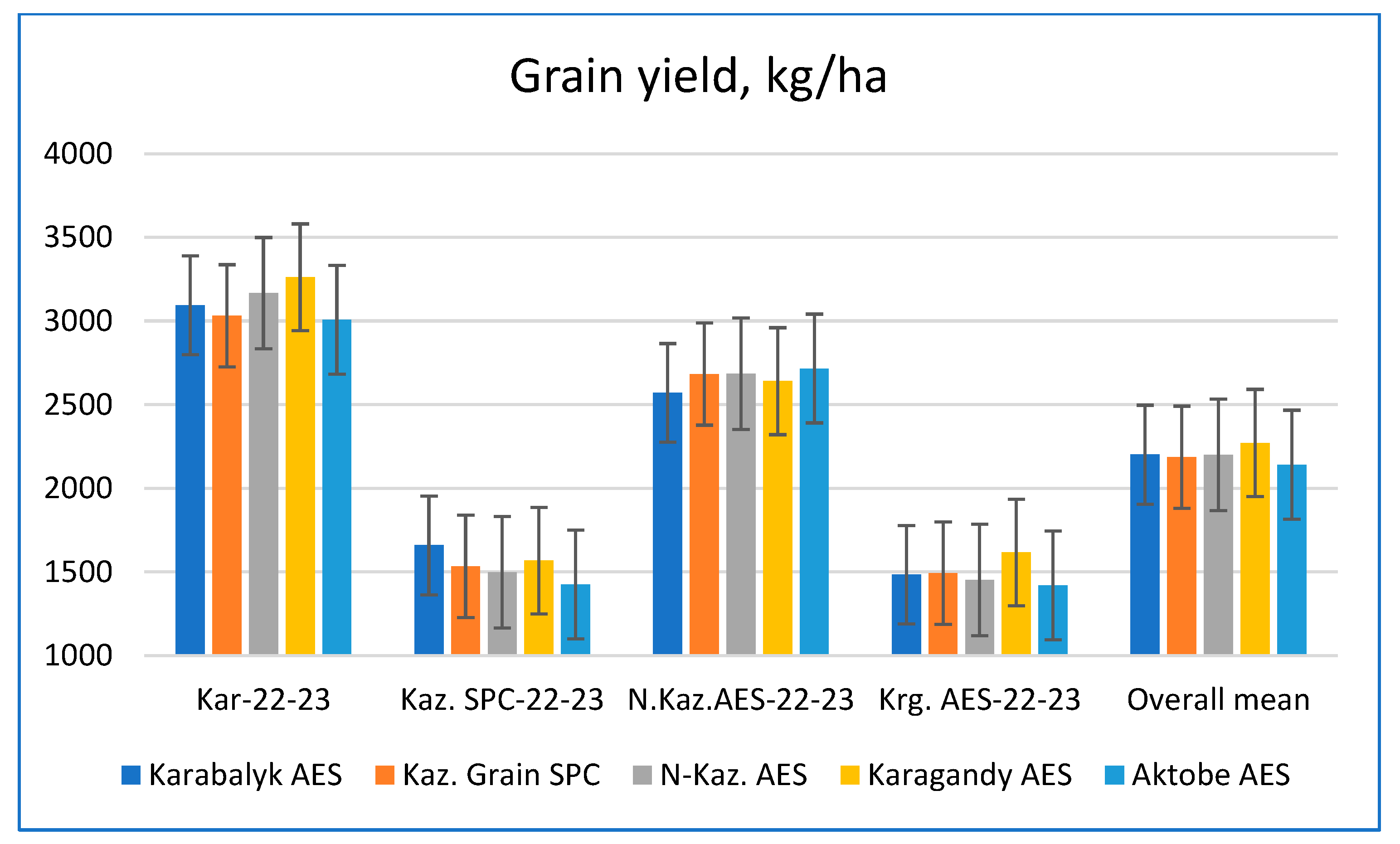
3.3. Relative Performance of Spring Wheat Germplasm Originating from Different Breeding Programs
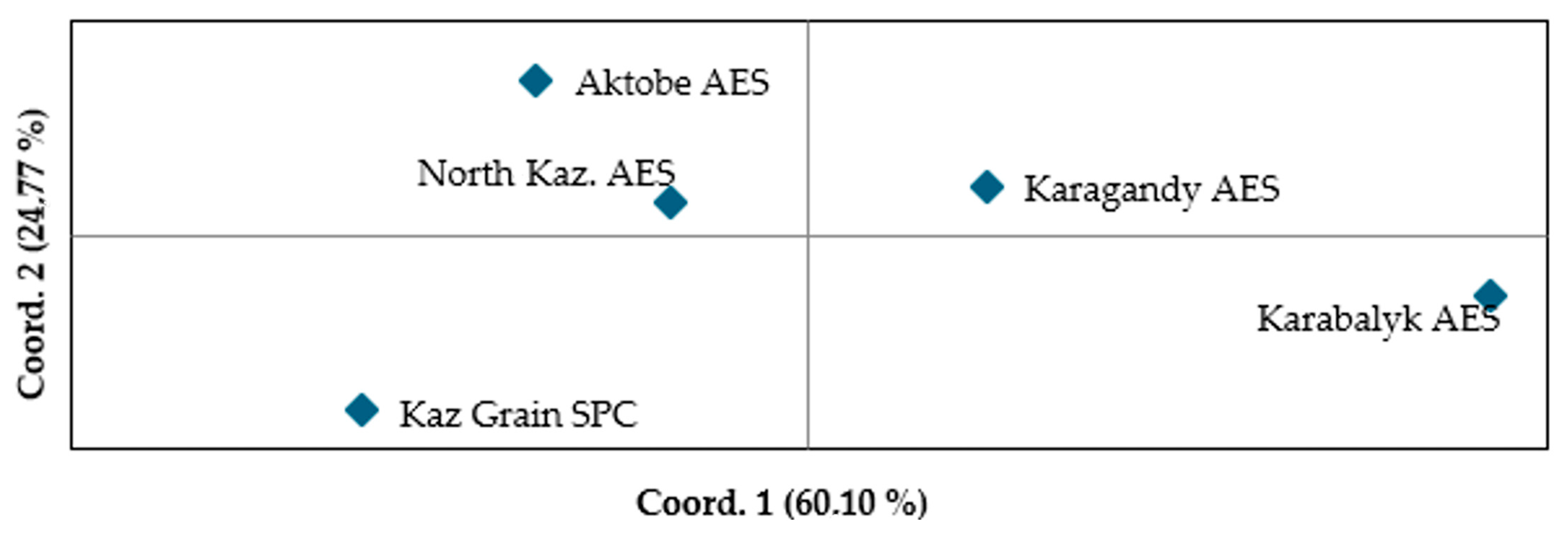
3.4. Genetic Diversity of Spring Wheat Germplasm Originating from Different Breeding Programs
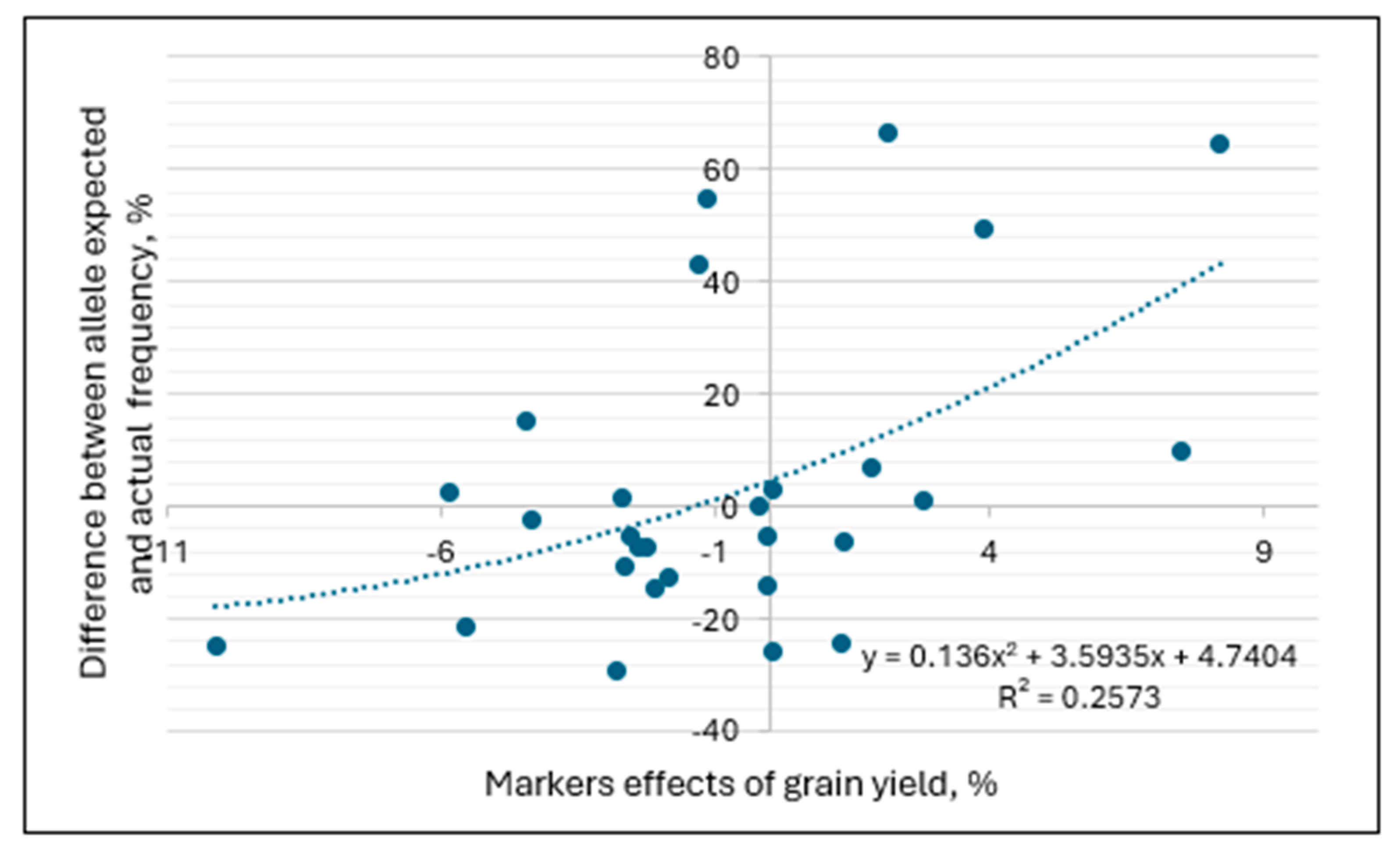
3.5. Molecular Marker Effects and Frequency of Alleles in Spring Wheat Germplasm Originating from Different Breeding Programs
3.6. High-Yielding Spring Wheat Genotypes Identified During the Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AES | Agricultural Experimental Station |
| SPC | Scientific Production Center |
References
- Anonymous. Eighth National Communication and Fifth Biennial Report of the Republic of Kazakhstan to the UN Framework Convention on Climate Change; Ministry of Ecology, Geology and Natural Resources: Astana, Kazakhstan, 2022; 473p. [Google Scholar]
- World Bank. Republic of Kazakhstan: Climate Adaptation Options and Opportunities in the Agriculture Sector; World Bank Group: Washington, DC, USA, 2024; 75p. [Google Scholar]
- Azhgaliev, T.B. State Register of Breeding Achievements Recommended for Use in the Republic of Kazakhstan; Ministry of Agriculture: Nursultan, Kazakhstan, 2022; pp. 7–12. [Google Scholar]
- Morgounov, A.; Babkenov, A.; Ben, C.; Chudinov, V.; Dolinny, Y.; Dreisigacker, S.; Fedorenko, E.; Gentzbittel, L.; Rasheed, A.; Savin, T. Molecular markers help with breeding for agronomic traits of spring wheat in Kazakhstan and Siberia. Genes 2024, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Tajibayev, D.; Mukin, K.; Babkenov, A.; Chudinov, V.; Dababat, A.A.; Jiyenbayeva, K.; Kenenbayev, S.; Savin, T.; Shamanin, V.; Tagayev, K. Exploring the agronomic performance and molecular characterization of diverse spring durum wheat germplasm in Kazakhstan. Agronomy 2023, 13, 1955. [Google Scholar] [CrossRef]
- Pask, A.J.D.; Pietragalla, J.; Mullan, D.M.; Reynolds, M.P. Physiological Breeding II: A Field Guide to Wheat Phenotyping; CIMMYT: Ciudad de México, Mexico, 2012; 140p. [Google Scholar]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Turuspekov, Y.; Baibulatova, A.; Yermekbayev, K.; Tokhetova, L.; Chudinov, V.; Sereda, G.; Ganal, M.W.; Griffiths, S.; Abugalieva, S. GWAS for plant growth stages and yield components in spring wheat (Triticum aestivum L.) harvested in three regions of Kazakhstan. BMC Plant. Biol. 2017, 17, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Amalova, A.; Abugalieva, S.; Chudinov, V.; Sereda, G.; Tokhetova, L.; Abdikhalyk, A.; Turuspekov, Y. QTL mapping of agronomic traits in wheat using the UK Avalon× Cadenza reference mapping population grown in Kazakhstan. PeerJ 2021, 9, 10733. [Google Scholar] [CrossRef] [PubMed]
- Amalova, A.; Abugalieva, S.; Babkenov, A.; Babkenova, S.; Turuspekov, Y. Genome-wide association study of yield components in spring wheat collection harvested under two water regimes in Northern Kazakhstan. PeerJ 2021, 9, 11857. [Google Scholar] [CrossRef] [PubMed]
- Amalova, A.; Yermekbayev, K.; Griffiths, S.; Abugalieva, S.; Babkenov, A.; Fedorenko, E.; Abugalieva, A.; Turuspekov, Y. Identification of quantitative trait loci of agronomic traits in bread wheat using a Pamyati Azieva × Paragon mapping population harvested in three regions of Kazakhstan. PeerJ 2022, 10, 14324. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2014, 33, 1–14. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; 245p. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Mukhametzhanov, A.; Zholaman, R. Economic analysis of spring soft wheat seed production in North Kazakhstan region. Sci. Horiz. 2023, 26, 92–100. [Google Scholar] [CrossRef]
- Kushenbekova, A.K.; Mukhomedyarova, A.S. Productivity of spring wheat varieties depending on climatic conditions of the dry steppe zone of western Kazakhstan. Sci. Edu. 2022, 66, 33–40. [Google Scholar]
- Goncharov, N.P. Scientific support to plant breeding and seed production in Siberia in the XXI century. Vavilov J. Genet. Breed. 2021, 25, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Kelbin, V.N.; Skolotneva, E.S.; Shamanin, V.P.; Salina, E.A. Diversity of stem rust resistance in modern Siberian bread wheat (Triticum aestivum) germplasm. Plant Breed. 2022, 141, 194–203. [Google Scholar] [CrossRef]
- Bazilova, D.S.; Dolinnyi, Y.Y.; Ivanova, G.N. Initial material for breeding of spring soft wheat in the conditions of northern Kazakhstan. KazNAU Res. Results 2022, 94, 37–46. [Google Scholar]
- Ren, J.; Sun, D.; Chen, L.; You, F.M.; Wang, J.; Peng, Y.; Nevo, E.; Sun, D.; Luo, M.-C.; Peng, J. Genetic diversity revealed by single nucleotide polymorphism markers in a worldwide germplasm collection of durum wheat. Int. J. Mol. Sci. 2013, 14, 7061–7088. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Iqbal, M.; Alachiotis, N.; N’Diaye, A.; Pozniak, C.; Spaner, D. Genetic diversity and selective sweeps in historical and modern Canadian spring wheat cultivars using the 90K SNP array. Sci. Rep. 2021, 11, 23773. [Google Scholar] [CrossRef] [PubMed]
- Genievskaya, Y.; Abugalieva, S.; Rsaliyev, A.; Yskakova, G.; Turuspekov, Y. QTL mapping for seedling and adult plant re-sistance to leaf and stem rusts in Pamyati Azieva × Paragon mapping population of bread wheat. Agronomy 2020, 10, 1285. [Google Scholar] [CrossRef]
- Boehm, J.D.; Masterson, S.; Palmer, N.; Cai, X.; Miguez, F. Genetic improvement of winter wheat (Triticum aestivum L.) grain yield in the Northern Great Plains of North America, 1959–2021. Crop Sci. 2023, 63, 3236–3249. [Google Scholar] [CrossRef]
- Bapela, T.; Shimelis, H.; Tsilo, T.J.; Mathew, I. Genetic improvement of wheat for drought tolerance: Progress, challenges and opportunities. Plants 2022, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Aidarbekova, T.J.; Syzdykova, G.T.; Malitskaya, N.V.; Nurgaziyev, R.E.; Husainov, A.T.; Zhabayeva, M.U.; Makhanova, S.K.; Shoykin, O.D. Comparative assessment of spring soft wheat lines (Triticum aestivum L.) in the steppe zone of the north Kazakhstan region. Agric. Biol. 2022, 57, 66–80. [Google Scholar] [CrossRef]
| Trait | KASP ID | Chromosome | Position, cM | Reference |
|---|---|---|---|---|
| Plant adaptation traits | ||||
| Flowering time | ipbb_ta_SH_223 | 7D | 84 | [10] |
| Heading date | ipbb_ta_PAxP_184 | 7B | 23.1 | [11] |
| Plant height | ipbb_ta_PAxP_180 | 7A | 101.4 | [11] |
| ipbb_ta_SH_155 | 6A | 123.5 | [10] | |
| Peduncle length | ipbb_ta_PAxP_190 | 7A | 55.4 | [11] |
| ipbb_ta_SH_161 | 6A | 99.1 | [10] | |
| ipbb_ta_SH_217 | 3B | 68.8 | [10] | |
| Yield components | ||||
| Number of productive spikes | ipbb_ta_106 | 5B | 51.3 | [8] |
| Grains per spike | ipbb_ta_AxC_297 | 2B | 84.00 | [9] |
| ipbb_ta_SH_222 | 6D | 1.12 | [10] | |
| Grain weight per spike | ipbb_ta_SH_165 | 3B | 32.9 | [10] |
| ipbb_ta_SH_162 | 2A | 106.3 | [10] | |
| 1000-kernel weight | ipbb_ta_AxC_302 | 5A | 17.00 | [9] |
| ipbb_ta_PAxP_290 | 2B | 94.34 | [11] | |
| Grain yield | ipbb_ta_AxC_301 | 4D | 69.60 | [9] |
| ipbb_ta_PAxP_291 | 5A | 20.04 | [11] | |
| ipbb_ta_SH_243 | 7B | 77.2 | [10] | |
| Number of productive spikes, grain yield | ipbb_ta_AxC_166 | 2D | 70.0 | [9] |
| Plant adaptation traits and yield components | ||||
| Plant height, grains per spike, spike length | ipbb_ta_PAxP_183 | 5A | 41.05 | [11] |
| Days to maturity, grain yield | ipbb_ta_PAxP_179 | 7B | 120.8 | [11] |
| Trait | Karabalyk AES | KazGrain SPC | North Kaz. AES | Karagandy AES |
|---|---|---|---|---|
| Days to heading | 43.0 ± 0.2 b | 44.1 ± 0.2 a | 42.8 ± 0.2 b | 41.0 ± 0.1 c |
| Plant height, cm | 70.1 ± 0.5 a | 47.1 ± 0.4 d | 68.9 ± 0.4 b | 64.7 ± 0.4 c |
| Spike length, cm | 8.64 ± 0.24 a | 7.19 ± 0.07 b | 6.87 ± 0.08 c | 8.76 ± 0.09 a |
| Spikelets/spike | 15.1 ± 0.17 a | 12.7 ± 0.13 c | 13.2 ± 0.13 b | 14.8 ± 0.13 a |
| Grains/spike | 26.6 ± 0.4 b | 21.8 ± 0.3 c | 26.3 ± 0.3 b | 31.1 ± 0.5 a |
| Grains/spikelet | 1.78 ± 0.02 c | 1.77 ± 0.05 c | 2.44 ± 0.02 a | 2.10 ± 0.02 b |
| 1000-kernel weight, g | 37.1 ± 0.26 c | 32.9 ± 0.40 d | 38.8 ± 0.27 a | 38.0 ± 0.38 b |
| Grain yield, kg/ha | 3118 ± 27 a | 1550 ± 21 c | 2652 ± 25 b | 1496 ± 19 d |
| Traits | Germplasm Group | ||
|---|---|---|---|
| Early (23 Genotypes) | Intermediate (36 Genotypes) | Late (25 Genotypes) | |
| Days to heading | 41.0 ± 0.1 c | 42.9 ± 0.1 b | 44.9 ± 0.2 a |
| Plant height, cm | 62.7 ± 0.4 | 62.4 ± 0.4 | 63.1 ± 0.6 |
| Spike length, cm | 7.23 ± 0.08 c | 7.95 ± 0.15 b | 8.33 ± 0.17 a |
| Spikelets/spike | 13.7 ± 0.2 b | 14.1 ± 0.1 a | 14.4 ± 0.2 a |
| Grains/spike | 25.1 ± 0.5 c | 26.6 ± 0.3 b | 27.4 ± 0.4 a |
| Grains/spikelet | 1.91 ± 0.03 b | 1.98 ± 0.03 a | 1.98 ± 0.03 a |
| 1000-kernel weight, g | 37.3 ± 0.5 a | 36.2 ± 0.4 b | 36.7 ± 0.4 a b |
| Grain yield, kg/ha | 2120 ± 29 b | 2245 ± 18 a | 2226 ± 34 a |
| Breeding Program | No of Lines | No. of Effective Alleles | Nei’s Unbiased Diversity Index | % of Polymorphic Loci |
|---|---|---|---|---|
| Karabalyk AES | 20 | 1.515 ± 0.068 | 0.329 ± 0.034 | 95.00% |
| North Kaz. AES | 15 | 1.602 ± 0.070 | 0.374 ± 0.035 | 95.00% |
| KazGrain SPC | 19 | 1.467 ± 0.072 | 0.301 ± 0.039 | 85.00% |
| Karagandy AES | 18 | 1.485 ± 0.070 | 0.312 ± 0.038 | 90.00% |
| Aktobe AES | 12 | 1.474 ± 0.078 | 0.309 ± 0.042 | 85.00% |
| Total | 84 | 1.509 ± 0.032 | 0.325 ± 0.017 | 90.00 ± 2.24% |
| Marker | SNP Allele | Effects of Markers (%) on Heading Date at | Effects of Markers (%) on Grain Yield at | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Karabalyk AES | North Kaz. AES | Kaz Grain SPC | Karagandy AES | Karabalyk AES | North Kaz. AES | Kaz Grain SPC | Karagandy AES | ||
| ipbb_ta_SH_162 | A | −2.03 | −2.30 | −2.59 * | +0.63 | +1.28 | −5.79 * | −4.51 | +7.46 * |
| ipbb_SH_223 | A | +4.44 *** | +4.50 *** | +3.96 *** | −0.12 | −1.16 | −2.11 | −9.47 ** | 0.00 |
| ipbb_SH_217 | A | +1.16 | +0.35 | +0.09 | −0.42 | −4.41 * | +0.11 | −1.24 | −2.77 |
| ipbb_ta_SH_222 | G | +0.14 | −0.28 | +0.43 | −1.90 ** | −1.89 | +1.39 | −2.24 | −2.66 |
| ipbb_AxC_302 | G | −2.71 * | −1.95 | −2.56 * | −0.54 | −2.42 | +1.85 | −5.93 | 0.00 |
| ipbb_PAxP_291 | A | +0.65 | +0.85 | +0.39 | −0.20 | +2.14 | +0.08 | +8.07 ** | +3.90 |
| ipbb_SH_155 | A | +1.00 | +0.89 | +0.11 | +0.20 | −0.16 | +2.73 * | −2.79 | −2.59 |
| Entry # | Entry Name | Origin Breeding Program | Mean Agronomic Traits Across Four Sites in 2022–2023 | ||||
|---|---|---|---|---|---|---|---|
| Days to Heading | Plant Height, cm | Grains Per Spike | 1000-KW, g | Yield, kg/ha | |||
| Early group | |||||||
| 55 | 233-10 | Kaz. Grain SPC | 41.7 | 66.7 | 29.6 | 36.3 | 2439 |
| 89 | Karagandinskaya 22 | Karagandy AES | 40.3 | 62.7 | 24.8 | 40.9 | 2271 |
| 92 | Lutescens 2231 | 41.4 | 60.2 | 23.2 | 42.1 | 2271 | |
| 82 | Lutescens 2203 | 41.0 | 59.3 | 25.0 | 42.0 | 2240 | |
| 87 | Lutescens 2216 | 41.4 | 63.4 | 27.4 | 35.2 | 2229 | |
| Intermediate group | |||||||
| 59 | 342-08 | Kaz. Grain SPC | 42.4 | 63.6 | 24.0 | 35.5 | 2453 |
| 103 | Line P-1413m | Aktobe AES | 43.1 | 62.7 | 26.0 | 36.4 | 2436 |
| 34 | Lutescens 54 190-09 | Karabalyk AES | 43.9 | 61.2 | 29.5 | 35.4 | 2436 |
| 33 | Lutescens 32 12-09 | 43.9 | 59.0 | 26.6 | 37.4 | 2401 | |
| 58 | 16-09 | Kaz. Grain SPC | 43.4 | 59.9 | 26.7 | 34.5 | 2377 |
| 93 | Lutescens 2240 | Karagandy AES | 43.3 | 60.1 | 28.1 | 35.6 | 2374 |
| 85 | Lutescens 2210 | 43.1 | 64.7 | 25.6 | 38.4 | 2364 | |
| Late group | |||||||
| 77 | Karagandinskaya 31 | Karagandy AES | 45.3 | 63.7 | 26.7 | 35.1 | 2617 |
| 95 | Bayterek 15 | 44.3 | 66.0 | 28.4 | 29.5 | 2419 | |
| 32 | Lutescens 57 4-09 | Karabalyk AES | 45.0 | 65.8 | 27.7 | 39.0 | 2398 |
| 40 | 486-Lut 22 | North Kaz. AES | 46.0 | 60.4 | 28.8 | 37.9 | 2382 |
| 61 | 55-08 | Kaz. Grain SPC | 44.4 | 64.1 | 29.1 | 36.4 | 2372 |
| LSD 0.05 | 0.32 | 1.5 | 0.7 | 0.6 | 59 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savin, T.; Turuspekov, Y.; Amalova, A.; Anuarbek, S.; Babkenov, A.; Chudinov, V.; Fedorenko, E.; Kairzhanov, Y.; Maulenbay, A.; Sereda, G.; et al. Spring Wheat Breeding in Northern Kazakhstan: Drivers of Diversity and Performance. Crops 2025, 5, 63. https://doi.org/10.3390/crops5050063
Savin T, Turuspekov Y, Amalova A, Anuarbek S, Babkenov A, Chudinov V, Fedorenko E, Kairzhanov Y, Maulenbay A, Sereda G, et al. Spring Wheat Breeding in Northern Kazakhstan: Drivers of Diversity and Performance. Crops. 2025; 5(5):63. https://doi.org/10.3390/crops5050063
Chicago/Turabian StyleSavin, Timur, Yerlan Turuspekov, Akerke Amalova, Shynar Anuarbek, Adylkhan Babkenov, Vladimir Chudinov, Elena Fedorenko, Yelzhas Kairzhanov, Akerke Maulenbay, Grigoriy Sereda, and et al. 2025. "Spring Wheat Breeding in Northern Kazakhstan: Drivers of Diversity and Performance" Crops 5, no. 5: 63. https://doi.org/10.3390/crops5050063
APA StyleSavin, T., Turuspekov, Y., Amalova, A., Anuarbek, S., Babkenov, A., Chudinov, V., Fedorenko, E., Kairzhanov, Y., Maulenbay, A., Sereda, G., Sereda, S., Tajibayev, D., Tsygankov, V., Tsygankov, A., Zotova, L., & Morgounov, A. (2025). Spring Wheat Breeding in Northern Kazakhstan: Drivers of Diversity and Performance. Crops, 5(5), 63. https://doi.org/10.3390/crops5050063






