Abstract
Water-use efficiency (WUE) plays a crucial role in sustainable crop production, particularly in water-limited environments where maximizing natural resource use is essential. This study evaluated the physiological and agronomic performance of two Piper nigrum cultivars, Clonada and Uthirankotta, grown under different soil water potential conditions. The trial was conducted in a 1930 m2 field using a randomized block design and drip irrigation system, calibrated to 3.55 L h−1 with a uniformity of 97%. Soil water availability was managed based on daily tensiometer readings at 20 and 30 cm depths, triggering irrigation at defined tensions (10–55 kPa). Clonada exhibited higher net CO2 assimilation rates (A) and stomatal conductance (gs), but these responses did not lead to higher yields. In contrast, Uthirankotta consistently maintained superior water-use efficiency and yield across all soil moisture conditions by favoring water conservation and targeted biomass allocation over maximized gas exchange. Both cultivars performed optimally at a soil water potential range of 25–35 kPa, with declines in yield and gas exchange parameters at higher tensions (45–55 kPa). Under such conditions, Uthirankotta was 51.3% more water-use efficient and 40.8% more productive than Clonada. Based on this, a Principal Component Analysis (PCA) further demonstrated distinct physiological profiles, underscoring trade-offs between yield and water-use strategies. These results highlight the significance of cultivar selection for optimizing WUE and provide valuable insights into irrigation management and breeding programs aimed at boosting black pepper performance under water-limited conditions.
1. Introduction
Water-use efficiency (WUE) is a key factor in sustainable agriculture, especially in water-restricted environments where efficient resource utilization is essential for sustaining productivity and ensuring environmental resilience [1,2,3]. Increasing WUE has been a primary goal in agronomic research, as it contributes to improved crop performance under fluctuating water availability while minimizing excessive water consumption [1,2]. While previous studies have demonstrated that certain cultivars exhibit enhanced WUE under specific conditions, most research has focused on instantaneous WUE measurements rather than long-term yield sustainability [4]. Understanding the physiological and hydraulic mechanisms underlying WUE at different time scales remains a critical aspect of improving water management strategies in crop production [1,2,5].
Water-use efficiency is often quantified through multiple physiological parameters, including intrinsic WUE (WUEi), which accounts for the ratio of carbon assimilation to stomatal conductance, and instantaneous WUE (WUEE), which reflects immediate responses to environmental conditions [5,6,7]. These parameters provide insights into how plants regulate water loss and carbon gain under dynamic stress conditions [6,7,8]. However, the extent to which short-term physiological adjustments correlate with long-term agronomic performance remains uncertain [5,6,7,9]. Recent studies suggest that WUE responses are highly cultivar-dependent, with trade-offs occurring between gas exchange efficiency, hydraulic traits, and yield stability under varying water conditions [10].
Black pepper (Piper nigrum) is a high-value crop cultivated in tropical and subtropical regions, where water availability significantly influences its productivity and quality [10,11,12]. Given the increasing challenges posed by climate variability, optimizing WUE in P. nigrum is essential for maintaining stable yields under diverse environmental conditions [9,10]. While studies have reported variability in WUE among black pepper cultivars, there is limited knowledge on how different cultivars balance physiological performance and yield potential under varying soil water tensions and over seasons. Investigating these responses at both physiological and agronomic levels is necessary to develop improved management strategies for sustainable pepper cultivation [10,11,13].
Despite extensive research on WUE, most studies rely on instantaneous measurements without fully integrating the long-term consequences of water availability on yield [14,15,16]. On the other hand, there is a lack of understanding regarding the cultivar-specific hydraulic and physiological responses that contribute to WUE differences over time. Moreover, how and whether the hydraulic bases underpinning the performance of different P. nigrum cultivars explain their responses to varying levels of water availability, and temporal dynamics remain largely unexplored. Therefore, identifying the physiological and structural shifts that drive such differences can help refine irrigation strategies and breeding programs aimed at improving WUE without compromising yield.
In this sense, by analyzing cultivar-specific physiological parameters, including net CO2 assimilation rate (A), stomatal conductance (gs) regulation, and water potential (Ψw), this study seeks to elucidate the fundamental drivers of WUE across different time frames and soil moisture conditions. We hypothesize that the studied Piper nigrum cultivars with diverse physiological and growth patterns would differ in yield levels and water-use efficiency under graded water availability. These contrasting responses are expected to be reflected in cultivar-specific traits such as A, gs, leaf area maintenance, biomass allocation, and yield, which together determine their capacity to optimize growth and yield under varying water availability. This study, therefore, examined the physiological and agronomic responses of two black pepper (Piper nigrum) cultivars, Clonada and Uthirankotta, across different soil water potential levels and throughout the growing period.
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
The experiment was conducted in the fields of the Tropical Products Company of Castanhal (Tropoc), located in the municipality of Castanhal, Pará, Brazil (01°17′50″ S, 47°55′20″ W, 40 m above sea level). The trial was developed in partnership with the Water and Soil Engineering in the Amazon Study Group (GEEASA) at the Universidade Federal Rural da Amazônia (UFRA). The experimental area, covering 1930 m2, was planted with two-year-old seedlings of two Piper nigrum L. cultivars—Clonada and Uthirankotta. A randomized block design was employed, with each plot comprising four plants per cultivar, arranged in a double-row system. Plant spacing was set at 4.0 m between rows and 2.20 × 2.20 m between individual plants.
The soil is classified as a dystrophic Yellow Argisol (medium texture), with predominating secondary vegetation [17]. The soil chemical and acidity corrections were carried out by applying liming (3.7 t ha−1) and both organic and mineral planting fertilizers as previously described by Cardoso Júnior et al. [18]. All data are presented in Table S1. The plants were grown under a global radiation (GR) level of 1303 W/m2 (approximately 700 µmol photons m−2 s−1), with daytime temperatures ranging from 25.3 to 26.2 ± 2 °C (day/night) and relative humidity varying between 60.5% and 86%. According to the Köppen classification, the region exhibits an Af-type climate (humid equatorial), characterized by an average annual rainfall of 2432 mm and a mean temperature of 26.5 °C. In particular, the rainfall regime in this Amazon region concentrates from December to May and presents the lowest volumes during the dry period, corresponding to June to November. Climatological data from the Castanhal automatic weather station A202, operated by the National Institute of Meteorology (INMET), were analyzed using the methodology described by Thornthwaite and Mather [19]. All data covering the period from January 2022 to December 2022 are presented in Figure S1. Overall, the climatological water balance for Castanhal presented the greatest water oversupply between February and May, with a maximum in March (186 mm) and April (190 mm) (Figure S2).
2.2. Irrigation System Set Up and Management
Soil water availability was managed by a drip irrigation system, set up to a flow rate of 3.55 L h−1, with emitters spaced 30 cm apart. The drip tubing was arranged superficially with a self-compensating drip-tech PC/AS flat emitter for irrigation, with DN 16 mm, set up to a working pressure of 1 bar at the end of the hose. The dripping tubes were positioned within the plot in a double line to meet the plants’ double spacing (7 drippers per plant). The automation consisted of a 24-volt time/sector programmer and a standard box with five solenoids. Moreover, 1250 m of 8 × 4 mm linear polyethylene control and necessary connections were used. Five water tanks, 10,000 L each; a 1 hp electric pump (flow of 8.44 m 3 h−1); and a disk filter were used to supply the irrigation system. The irrigation system was tested for efficiency by applying a Distribution Uniformity Coefficient (DUC). The uniformity analysis was carried out by 250 mL collection containers under eight emitters, which collected the drip water for 1 min. This procedure was performed with three replications. The system demonstrated an efficiency rate of 97%, which is considered excellent according to the classification postulated by Vieira et al. [20].
To determine the critical soil tensions (or soil water potential), a set of three puncture tensiometers was installed: two installed at a depth of 20 cm, to indicate the time of irrigation; and one at a depth of 30 cm to check whether water percolation is occurring. The tensiometers were positioned in line with the plant set, about 15 cm from the drippers. Daily readings of tensions were carried out around 8:30 am, which was followed by the activation of the irrigation system. Irrigation was performed when the tensiometers (at the same depth) presented the critical tension for each treatment, seeking to raise the soil to its respective soil moisture at field capacity (10, 25, 35, 45, and 55 kPa). For puncture tensiometer outputs, the value of matric potential (Ψm) was determined using the method described by Franco [21].
Finally, the irrigation regime and management were based on the soil water characteristic curve obtained in the 0–20 cm deep layer. The outputs of soil water retention were achieved using the Richards pressure chamber at potentials of 0, 6, 10, 30, 100, 500, and 1500 kPa [22]. The data were modeled using the Van Genuchten equation in R software v. 4.3.3 [23,24]. The outputs allowed the determination of soil water retention curve parameters (Figure S2). Irrigation water volumes and system operating times were calculated based on the methodology described by Franco, considering an effective root system depth of 20 cm [21,25]. The summary of irrigation regime and management, such as irrigation levels, the total amount of water applied, number of irrigations, irrigation frequency, and daily water demand, is available in Figure S2 and Table S2.
2.3. Green and Black Pepper Yield Coupled to Water-Use Efficiency
Fruits were harvested during the respective harvest periods for each cultivar: from July to October for Clonada, and from August to November for Uthirankotta. Green pepper yield (GPY) was calculated using the fresh weight of pepper fruits, following the formula GPY = P/A, where Y represents yield (kg ha−1), P is total production (kg), and A is the cultivated area (ha). Water balance was monitored throughout the experimental period and expressed in millimeters (mm). Based on this data, water-use efficiency (WUE) was determined for each cultivar using the ratio between fruit yield (kg ha−1) and total water consumption (mm), according to Doorenbos and Kassam [26]. WUE = Y/w, where WUE is water-use efficiency (kg ha−1 mm−1), Y is total yield (kg h−1), and w is the amount of water applied (mm). The black pepper yield (BPY) was calculated using the percentage of yield related to the production of black pepper with the production of harvested green pepper, calculated by the equation BPY = (BPP/GPP) × 100%, where BPY, black pepper yield, %; BPP, black pepper production, kg plant−1; GPP, green pepper production, kg plant−1. Yield was measured after the harvest season (36 months), as flower production gradually increases and typically peaks around 3–4 years [27].
2.4. Gas Exchange
Gas exchange parameters were measured using an infrared gas analyzer (LCpro-SD, ADC BioScientific Ltd., Hoddesdon, United Kingdom). Assessments were conducted on the third to fourth fully expanded leaf of the twelfth branch, counted from the base to the apex of the plant. Measurements included net CO2 assimilation rate (A), stomatal conductance (gs), transpiration rate (E), and intercellular CO2 concentration (Ci), taken during the daytime under a photosynthetically active radiation (PAR) of 1000 µmol photons m−2 s−1 and ambient CO2 concentration of 400 ppm at the leaf level. Instantaneous water-use efficiency (WUEE) and intrinsic water-use efficiency (WUEᵢ) were calculated as the ratios A/E and A/gs, respectively. These measurements were conducted at 40, 80, 120, and 160 days after the start of the experiment.
2.5. Water Status
Leaf water potential was measured at both predawn (Ψpd) and midday (Ψmd) using a Scholander-type pressure chamber (Model M 1505D, PMS Instrument Company, Albany, NY, USA) as described by Scholander et al. [28]. Measurements were taken on fully expanded, healthy leaves from the 12th plagiotropic branch of two plants per cultivar in each replicate. Predawn measurements were conducted between 2:30 am and 5:30 am, while midday measurements were taken between 12:00 pm and 3:00 pm. The Ψpd was assessed at 40, 80, 120, and 160 days after the experiment was initiated, and the Ψmd was only assessed on the 80th and 120th days.
2.6. Data Analysis
The data were obtained from the experiments using a randomized blocks design, in a factorial scheme in split plots of 5 × 2, using 10 treatments and three blocks. The treatments consisted of five soil water tensions (15, 25, 35, 45, and 55 kPa), which were used to identify both the critical tension and optimal irrigation period, and two black pepper cultivars (Clonada and Uthirankotta). Data were tested for homoscedasticity and normality to ensure compliance with the assumptions of ANOVA. When significant effects were detected by ANOVA, Tukey’s test (p < 0.05) was applied to identify differences between cultivars over time or across water availability levels (see Tables S2–S4). Furthermore, a Principal Component Analysis (PCA) was conducted considering both cultivars and variables. Statistical procedures were performed using R version 4.3.3. [24].
3. Results
3.1. Soil Water Potential Influences Yield and Water-Use Efficiency in Black Pepper Cultivars
Green pepper yield (GPY), black pepper yield (BPY), and water-use efficiency (WUE) were all significantly affected by soil water potentials (Figure 1A–C). For both Piper nigrum cultivars, Clonada and Uthirankotta, GPY exhibited a clear response to soil water potentials (Figure 1A). In Clonada, GPY increased from 3500 kg ha−1 at 15 kPa to a peak of 4500 kg ha−1 at 35 kPa. This value was significantly higher than GPY observed at 15, 25, and 45 kPa. However, beyond 35 kPa, GPY declined sharply, reaching a minimum of 3000 kg ha−1 at 55 kPa, which was significantly lower than all other soil water potentials. A similar trend was observed in Uthirankotta, where GPY also peaked at 35 kPa (4500 kg ha−1). At both 15 and 55 kPa, Uthirankotta presented lower GPY (3000 kg ha−1), with no significant differences observed among 15, 45, and 55 kPa regimes. When comparing cultivars, Clonada showed GPY higher than Uthirankotta at 15 kPa. At 35 kPa, both cultivars achieved their highest and statistically equivalent GPY. Under severe water deficit (55 kPa), both cultivars performed similarly, with no significant differences in GPY.
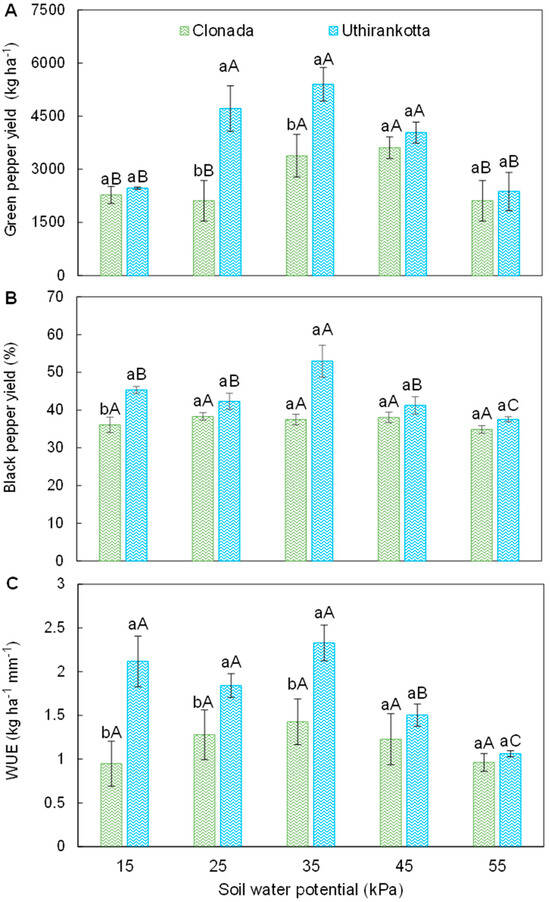
Figure 1.
Green pepper yield (A), black pepper yield (B), and water-use efficiency (C) of two black pepper cultivars (Clonada and Uthirankotta) cultivated under five soil water potentials. Different capital letters indicate statistical differences among soil water potentials within the same cultivar. Different lowercase letters denote statistical differences between cultivars at the same soil water potential. Means comparison was accomplished by the Tukey test (p > 0.05).
The BPY exhibited significant cultivar- and soil water potential-dependent responses (Figure 1B). In Clonada, BPY remained statistically stable across all tested soil water potentials (15–55 kPa), with values ranging from approximately 36% to 42%, and no significant differences among treatments. In contrast, Uthirankotta displayed a more pronounced response to soil water availability. BPY peaked at 35 kPa, reaching ~56%, which was significantly higher than at 15 kPa (~44%) and 55 kPa (~41%). At 25 and 45 kPa, intermediate values (~45–46%) were observed, statistically similar to both the highest and lowest observed BPY. When comparing cultivars at each soil water potential, differential performance was evident. At 15 kPa, Uthirankotta presented BPY significantly higher than Clonada. At 25, 35, and 45 kPa, Uthirankotta also outperformed Clonada, but the differences were not statistically significant. At 55 kPa, however, Uthirankotta experienced a significant decline in BPY relative to its performance at 35 and 45 kPa, and its BPY did not differ from Clonada at this level of water stress.
The WUE displayed distinct trends across soil water potentials and between the two cultivars (Figure 1C). In Clonada, WUE increased from 15 kPa (1.5 kg ha−1 mm−1) to its maximum at 35 kPa (2.5 kg ha−1 mm−1). At 45 kPa (2.0 kg ha−1 mm−1), WUE decreased significantly compared to 35 kPa. The lowest WUE was observed at 55 kPa (1.0 kg ha−1 mm−1), which was significantly lower than all other soil tensions. In Uthirankotta, WUE was consistently higher than Clonada at all soil water potentials. It increased from 15 kPa (2.0 kg ha−1 mm−1) to its maximum at 35 kPa (2.6 kg ha−1 mm−1). Beyond 35 kPa, WUE declined, reaching its lowest value at 55 kPa (1.2 kg ha−1 mm−1). At all soil tensions, Uthirankotta exhibited WUE statistically higher as compared to Clonada. For example, at 15 kPa, Uthirankotta recorded 2.0 kg ha−1 mm−1 compared to Clonada’s 1.5 kg ha−1 mm−1. Both cultivars reached their maximum WUE at 35 kPa, with Uthirankotta significantly surpassing Clonada.
3.2. Temporal Variation in Gas Exchange and Water-Use Efficiency Between Black Pepper Cultivars
The net CO2 assimilation rate (A) (Figure 2A) varied significantly between measurement days (40, 80, 120, and 160 days) and between both cultivars, Clonada and Uthirankotta. In Clonada, the highest A was observed at 40 days (approximately 25 µmol CO2 m−2 s−1) and 80 days (25 µmol CO2 m−2 s−1), with no statistical difference between these two time points. The A declined significantly after 80 days, reaching its lowest value at 160 days (10 µmol CO2 m−2 s−1). In Uthirankotta, a similar trend was observed, with maximum rates at 40 and 80 days (20 µmol CO2 m−2 s−1) and a significant decline by 160 days (8 µmol CO2 m−2 s−1). The A for Uthirankotta was consistently lower than observed in Clonada at all time points, with statistical differences at 40, 80, and 160 days.
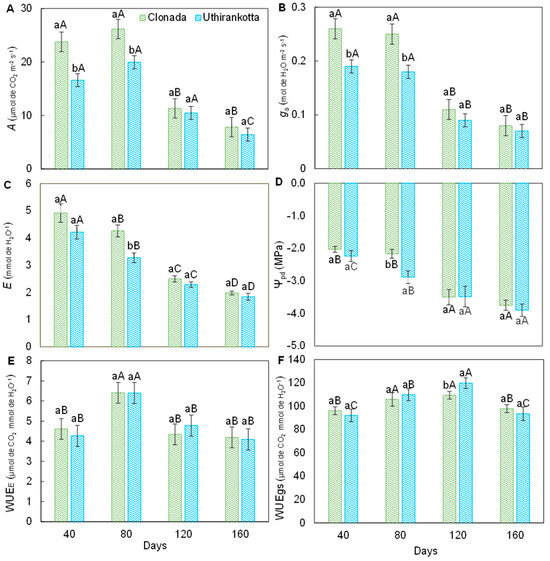
Figure 2.
Net CO2 assimilation rate (A), stomatal conductance (B), transpiration (C), predawn water potential (D), instantaneous water-use efficiency (E), and intrinsic water-use efficiency (F) of two black pepper cultivars (Clonada and Uthirankotta) throughout the experiment (days). Different capital letters indicate statistical differences among days within the same cultivar. Different lowercase letters denote statistical differences between cultivars on the day. Means comparison was accomplished by the Tukey test (p > 0.05).
The stomatal conductance (gs) (Figure 2B) followed a pattern similar to A, with significant differences between days and cultivars. In Clonada, the highest gs was recorded at 40 and 80 days (both approximately 0.25 mol H2O m−2 s−1), followed by a decline at 120 days (0.15 mol H2O m−2 s−1) and 160 days (0.10 mol H2O m−2 s−1). In Uthirankotta, gs was slightly lower than Clonada at all time points, with maximum values at 40 and 80 days (approximately 0.20 mol H2O m−2 s−1) and the lowest at 160 days (0.08 mol H2O m−2 s−1). Statistical differences were observed between cultivars at 40, 80, and 160 days.
The transpiration rate (E) (Figure 2C) displayed significant variation over time and between cultivars. In Clonada, E was highest at 40 days (6 mmol H2O m−2 s−1) and decreased significantly over time, reaching its lowest value at 160 days (2 mmol H2O m−2 s−1). Intermediate values in E were observed at 80 and 120 days, with statistical differences between these time points. In Uthirankotta, a similar declining trend was observed, with the highest value at 40 days (4 mmol H2O m−2 s−1) and the lowest at 160 days (1.5 mmol H2O m−2 s−1). Uthirankotta consistently exhibited lower E than Clonada, with significant differences at 40 and 80 days.
Predawn leaf water potential (Ψpd) (Figure 2D) became progressively more negative with time for both cultivars. In Clonada, the highest Ψpd was recorded at 40 days (−1.5 MPa), which became increasingly negative, reaching its lowest value at 160 days (−3.5 MPa). In Uthirankotta, a similar trend was observed, with Ψpd ranging from −2.0 MPa at 40 days to −4.0 MPa at 160 days. Uthirankotta consistently displayed more negative values compared to Clonada, with significant differences at 40, 80, and 120 days.
Instantaneous water use efficiency (WUEE) (Figure 2E) demonstrated variations over time and between cultivars. In Clonada, WUEE was highest at 80 days (4.5 µmol CO2 mmol−1 H2O) and remained stable between 40 and 80 days. However, a significant decline in WUEE was observed at 120 and 160 days, reaching 3.0 µmol CO2 mmol−1 H2O. In Uthirankotta, WUEE followed a similar trend, peaking at 80 days (4.0 µmol CO2 mmol−1 H2O) and declining thereafter to 2.8 µmol CO2 mmol−1 H2O at 160 days. Statistical differences between cultivars were observed at 40 and 80 days, with Uthirankotta showing lower WUEE as compared to its counterpart.
Intrinsic water-use efficiency (WUEi) (Figure 2F) increased over time for both cultivars. In Clonada, WUEi was highest at 160 days (100 µmol CO2 mmol−1 H2O) and lowest at 40 days (80 µmol CO2 mmol−1 H2O). A significant increase was observed over the period of evaluation. In Uthirankotta, WUEi followed a similar pattern, increasing from 40 days (70 µmol CO2 mmol−1 H2O) to 160 days (90 µmol CO2 mmol−1 H2O). Uthirankotta showed consistently lower WUEi as compared to Clonada, with statistical differences at all time points except 160 days.
3.3. Gas Exchange and Water Potential Responses to Soil Potential in Black Pepper Cultivars
The net CO2 assimilation rate (A) (Figure 3A) showed significant variations with soil water potential (15, 25, 35, 45, and 55 kPa) and between the two cultivars, Clonada and Uthirankotta. In Clonada, the highest A was observed at 15 kPa (approximately 30 µmol CO2 m−2 s−1) and remained high at 25 and 35 kPa (27–28 µmol CO2 m−2 s−1). A significant decline occurred at 45 and 55 kPa, reaching a minimum at 55 kPa (approximately 12 µmol CO2 m−2 s−1). In Uthirankotta, the highest A was also at 15 kPa (approximately 25 µmol CO2 m−2 s−1) and decreased progressively with increasing soil water potential, reaching a minimum at 55 kPa (10 µmol CO2 m−2 s−1). At all soil water potentials, Uthirankotta showed statistically lower A as compared to Clonada, except at 55 kPa, where differences were not significant.
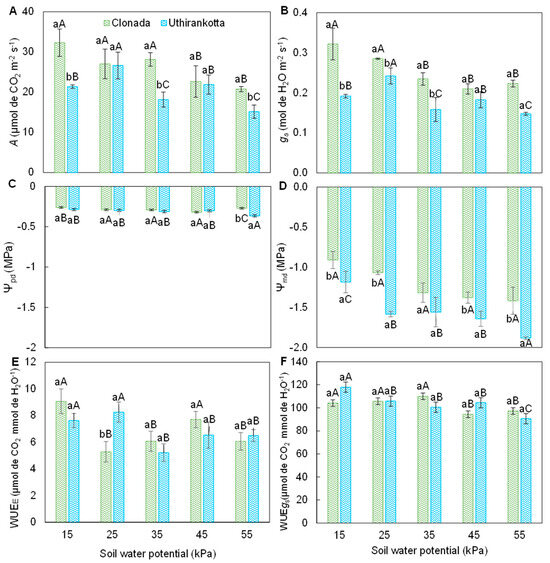
Figure 3.
Net CO2 assimilation rate (A), stomatal conductance (B), predawn water potential (C), midday water potential (D), instantaneous water-use efficiency (E), and intrinsic water-use efficiency (F) of two black pepper cultivars (Clonada and Uthirankotta) cultivated under five soil water potentials. Different capital letters indicate statistical differences among soil water potentials within the same cultivar. Different lowercase letters denote statistical differences between cultivars at the same soil water potential. Means comparison was accomplished by the Tukey test (p > 0.05).
The stomatal conductance (gs) (Figure 3B) was significantly affected by soil water potential and varied between cultivars. In Clonada, the gs was observed at 15 and 25 kPa (approximately 0.3 mol H2O m−2 s−1). The gs declined significantly with increasing soil water potential, reaching its lowest value at 55 kPa (0.1 mol H2O m−2 s−1). In Uthirankotta, A similar trend was observed, with maximum stomatal conductance at 15 kPa (0.25 mol H2O m−2 s−1) and minimum at 55 kPa (0.08 mol H2O m−2 s−1). Uthirankotta consistently showed lower stomatal conductance than Clonada at all soil water potentials.
The predawn leaf water potential (Ψpd) (Figure 3C) became more negative with increasing soil water potential for both cultivars. In Clonada, Ψpd ranged from approximately −0.5 MPa at 15 kPa to −1.5 MPa at 55 kPa. There were no significant differences in Ψpd between 25, 35, and 45 kPa, but a significant drop occurred at 55 kPa. In Uthirankotta, Ψpd followed a similar trend, ranging from −0.6 MPa at 15 kPa to −1.8 MPa at 55 kPa. At all soil water potentials, Uthirankotta showed more negative Ψpd as compared to Clonada.
Midday leaf water potential (Ψmd) (Figure 3D) exhibited similar trends to Ψpd but was more negative overall. In Clonada, Ψmd ranged from −1.0 MPa at 15 kPa to −2.5 MPa at 55 kPa. Significant declines were observed with increasing soil water potential. In Uthirankotta, Ψmd ranged from −1.2 MPa at 15 kPa to −3.0 MPa at 55 kPa. Uthirankotta consistently showed more negative values than Clonada, with significant differences at all soil water potentials.
Instantaneous water use efficiency (WUEE) (Figure 3E) increased initially but declined at the highest soil water potential. In Clonada, WUEE was highest at 25 kPa (approximately 5 µmol CO2 mmol−1 H2O) and decreased significantly at 55 kPa (3 µmol CO2 mmol−1 H2O). In Uthirankotta, WUEE followed a similar trend, with the highest value at 25 kPa (4.5 µmol CO2 mmol−1 H2O) and a decline at 55 kPa (2.5 µmol CO2 mmol−1 H2O). Uthirankotta consistently had lower WUEE than Clonada at all soil water potentials.
Intrinsic water-use efficiency (WUEi) (Figure 3F) displayed a similar trend across soil water potentials. In Clonada, WUEi was highest at 25 and 35 kPa (120–130 µmol CO2 mmol−1 H2O) and declined slightly at 55 kPa (110 µmol CO2 mmol−1 H2O). In Uthirankotta, the highest WUEi was at 25 and 35 kPa (110–115 µmol CO2 mmol−1 H2O) and declined significantly at 55 kPa (90 µmol CO2 mmol−1 H2O). Uthirankotta showed lower WUEi compared to Clonada at all soil water potentials.
3.4. Physiological Responses of Black Pepper Cultivars to Soil Water Potential
The Principal Component Analysis (PCA) biplot illustrates the relationships among physiological traits and their contributions to the variance in the data for the two cultivars of Piper nigrum. The first two principal components (PC1 and PC2) explain 68.7% of the total variability, with PC1 accounting for 43% and PC2 for 25.7% (Figure 4).
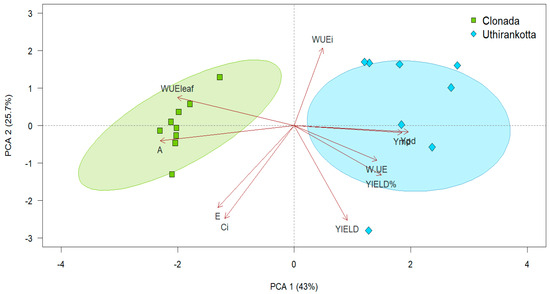
Figure 4.
Principal Component Analysis biplot presenting major axes of variation for parameters related to physiological traits. The arrows are the vectors of correlation among variables, and ellipses represent delineated groups based on normally distributed data in different watering regimes.
The clustering indicates that Clonada is primarily associated with physiological traits such as net CO2 assimilation rate (A), transpiration rate (E), and stomatal conductance (gₛ). The arrow directions (A, E, WUEleaf, and Ci) show that these variables significantly drive the separation of Clonada along PC1. On the other hand, Uthirankotta is closely associated with traits related to yield (YIELD), water-use efficiency (WUE), and percent yield (YIELD%). These variables contribute to the separation of Uthirankotta from Clonada along PC1, as indicated by the arrows pointing in the positive PC1 direction.
Yield-related traits, including total yield (YIELD), yield percentage (YIELD%), and whole-plant water-use efficiency (WUE), were strongly associated with Uthirankotta and contributed predominantly to the positive direction of PC1. In contrast, photosynthetic parameters such as net CO2 assimilation rate (A), leaf-level WUE (WUEₗₑₐf), stomatal conductance (gₛ), and intercellular CO2 concentration (Cᵢ) were closely aligned with Clonada, driving variation along the negative axis of PC1. PC2 accounted for additional variability by separating secondary traits within each cultivar group. Although yield-related traits contributed less to PC2, they still helped distinguish individual responses within Uthirankotta.
4. Discussion
Water-use efficiency (WUE) is a critical parameter in sustainable agricultural systems and has been the focus of numerous studies due to its implications for resource conservation and crop productivity [1,2,3]. While substantial evidence supports improvements in WUE across various plant species, most studies predominantly rely on instantaneous WUE measurements, often neglecting yield-related aspects [29,30]. Recent findings indicate that the cultivar Uthirankotta exhibits significantly higher WUE, demonstrating an efficiency approximately 80% higher than that observed in Clonada [10]. However, no direct correlation was observed between short-term and long-term WUE in either cultivar.
In this study, we further demonstrate that cultivar responses to temporal variations in water availability differ significantly. Both cultivars exhibited enhanced yield and WUE as soil water potential declined from 15 to 35 kPa, with peak performance observed at 35 kPa. However, beyond this threshold, a decline in performance was noted. While Clonada generally achieved higher net CO2 assimilation rates (A) at lower soil water potentials (e.g., 15 and 25 kPa) as compared to Uthirankotta, this did not lead to higher yields (GPY and BPY). To confirm that, the Principal Component Analysis (PCA) further revealed distinct physiological and agronomic profiles between the cultivars. Clonada showed a tendency to favor traits associated with enhanced gas exchange, whereas Uthirankotta displayed characteristics more strongly linked to yield optimization and water-use efficiency. These findings highlight the complex interplay between structural and functional adaptations in shaping cultivar-specific responses to water availability.
4.1. Soil Water Potential Modulates Yield and WUE Through Cultivar-Specific Physiological Strategies in Black Pepper
Water availability is a critical determinant of yield and WUE in pepper cultivars, with optimal performance typically observed within a moderate range of soil water potential [29,30,31]. The observed peak in yield and WUE at 35 kPa (Figure 1) aligns with previous findings that moderate water deficits enhance physiological efficiency and resource partitioning in many crop species [29,30,31]. However, as soil water potential declines beyond this threshold (45–55 kPa), both cultivars exhibited a significant reduction in yield and WUE, likely due to increased hydraulic limitations and consequently photosynthesis impairments [32,33,34]. In this vein, differences in performance between Clonada and Uthirankotta suggest cultivar-specific adaptations to soil moisture levels [34,35]. Clonada showed lower yields at lower soil water potentials (15 and 25 kPa), despite its superior stomatal regulation and higher A under mild stress conditions. In contrast, Uthirankotta consistently demonstrated superior WUE across all soil water potentials, suggesting a better strategy for water conservation, likely linked to its anatomical and hydraulic traits [34].
4.2. Temporal Physiological Adjustments and Cultivar-Specific Stress Responses in Black Pepper
The temporal decline in A, gs, E, and Ψpd observed in both cultivars (Figure 2) aligns with well-documented trends in plant water relations, where prolonged exposure to water deficits induces physiological adjustments to minimize water loss [36,37]. Interestingly, despite such declines, both WUEE and WUEi increased over time, suggesting an adaptive trade-off where plants prioritize water conservation over maximizing carbon assimilation [38,39]. The inter-cultivar comparison further highlights Clonada’s superior A, gs, and E across most time points, which may contribute to its higher yields under moderate stress [40,41]. Conversely, Uthirankotta’s consistently lower water potential values indicate a higher susceptibility to water stress, likely due to differences in root hydraulic conductivity or osmotic adjustment abilities [10,34,42].
4.3. Gas Exchange Dynamics and Water-Use Trade-Offs Between Black Pepper Cultivars
The decline in A, gs, and E and Ψpd with increasing soil water potential (Figure 3) reflects the physiological constraints imposed by limited water availability [43,44]. Stomatal closure under high soil water tension (55 kPa) reduces transpiration but also limits CO2 diffusion, thereby decreasing photosynthesis and overall plant productivity [43,44,45,46]. The observed peak in WUEE and WUEi at intermediate soil water potentials (25–35 kPa) aligns with findings that mild water stress can improve intrinsic water-use efficiency by promoting an optimal balance between carbon assimilation and water loss [47,48]. Clonada’s consistently higher photosynthetic performance (A, E, gₛ) suggests a superior ability to maintain gas exchange under varying moisture conditions, likely due to a combination of efficient stomatal control and photosynthetic machinery [49,50]. Uthirankotta’s more negative leaf water potential values reinforce its greater susceptibility to water stress, yet its enhanced WUE suggests a strategy focused on productivity rather than maximizing physiological resilience [47,51].
4.4. Principal Component Analysis Reveals Contrasting Physiological and Agronomic Strategies Between Black Pepper Cultivars
The clear separation between Clonada and Uthirankotta along PC1 in the PCA (Figure 4) confirms that these cultivars employ distinct physiological and agronomic strategies under soil tension conditions [10]. This clustering pattern underscores how different traits dominate the response mechanisms of each variety, reflecting fundamental trade-offs between yield and water loss regulation. Clonada’s stronger association with gas exchange parameters (A, E, gₛ) highlights its ability to sustain photosynthetic activity under varying water conditions, likely supporting its yields at lower soil water potentials [10,34]. In contrast, Uthirankotta’s alignment with yield and water-use efficiency traits suggests a prioritization of biomass accumulation and water conservation over sustained physiological activity [39,50]. These findings evidence the idea that cultivar selection should consider the balance between physiological robustness and water-use strategies to optimize performance under different environmental constraints.
5. Conclusions
This study demonstrates that moderate soil water potentials (25–35 kPa) optimize yield and water-use efficiency (WUE) in Piper nigrum, but cultivar-specific strategies drive differential performance under a gradient of water availability. Clonada maintains higher gas exchange capacity, yet this did not result in higher yields. In contrast, Uthirankotta prioritizes water conservation and targeted biomass allocation, sustaining higher yields and WUE across all conditions despite lower leaf water potentials. Together, these findings reveal a physiological trade-off between yield and water-use efficiency in Piper nigrum, highlighting the importance of selecting genotypes with complementary traits to enhance performance under drought. A deeper understanding of the genetic and functional basis of these responses will be instrumental for guiding breeding strategies aimed at improving resilience and yield under adverse climatic conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/crops5040054/s1, Figure S1: Soil water balance [A], and excess, deficit, loss, and water reposition [B] obtained during 2022–2023. The meteorological variables were obtained from the platform of the National Institute of Meteorology (INMET) automatic station A202, located in the municipality of Castanhal, PA, Brazil; Figure S2: Soil water retention curve for irrigation management [A] and water amount per irrigation during 2022–2023 [B]; Table S1: Soil chemical characterization; Table S2: Summary of irrigation management for two black pepper cultivars (Clonada and Uthirankotta) cultivated under five soil water potentials. Irrigation depths (Irrig.), precipitation (Prec.), total amount of water applied (total), number of irrigations (NI), irrigation frequency (IF) and daily water demand (DWD)e; Table S3: Summary of analysis of variance for net CO2 assimilation rate (AN), stomatal conductance (gs), transpiration rate (E), substomatal CO2 concentration (Ci), substomatal to ambient CO2 concentration ratio (Ci/Ca), water-use efficiency (A/E) and predawn water potential (Ψpd) in two black pepper cultivars (Clonada and Uthirankotta) cultivated under five soil water potential over the time of evaluation; Table S4: Summary of analysis of variance for midday water potential (Ψmd) in two black pepper cultivars (Clonada and Uthirankotta) cultivated under five soil water potentials.
Author Contributions
Conceptualization: H.C.A.S. and J.A.L.J.; methodology: H.C.A.S. and J.A.L.J.; investigation: H.C.A.S., O.P.S., R.S.G., M.C.A., D.B.d.S., W.L.C.d.A., M.d.B.C.L.M., J.P.C.L.B. and O.F.L.; formal analysis: H.C.A.S. and L.M.L.; writing—original draft preparation: H.C.A.S.; writing—review and editing: H.C.A.S., J.A.L.J. and L.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES)-Brazil-Finance Code 001.
Data Availability Statement
Dataset available upon request from the authors.
Acknowledgments
The authors gratefully acknowledge the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) for providing a scholarship to HCAS. We also thank the Universidade Federal Rural da Amazônia (UFRA) for access to core research facilities, and Empresa de Produtos Tropicais de Castanhal LTDA (TROPOC) for financial support and access to field facilities.
Conflicts of Interest
Author Deiviane B. da Silva was employed by the Agropalma S/A, William L. C. de Aviz was employed by the Acelen Renováveis, Oriel F. Lemos and João P. C. L. Both were employed by the Embrapa Amazônia Oriental. The remaining authors declare no conflicts of interest. The authors have no competing interests to disclose, either financial or non-financial.
Abbreviations
The following abbreviations are used in this manuscript:
| A | Net CO2 assimilation rate |
| ANOVA | Analysis of variance |
| BPY | Black pepper yield |
| Ci | Intercellular CO2 concentration |
| CV | Coefficient of variation |
| E | Transpiration rate |
| GPY | Green pepper yield |
| gs | Stomatal conductance |
| kPa | Kilopascal |
| PAR | Photosynthetically active radiation |
| PCA | Principal component analysis |
| TLA | Total leaf area |
| WUE | Water-use efficiency |
| WUEE | Instantaneous water-use efficiency (A/E) |
| WUEᵢ | Intrinsic water-use efficiency (A/gs) |
| Ψpd | Predawn leaf water potential |
| Ψmd | Midday leaf water potential |
| Ψm | Matric potential |
References
- Liang, J.; Krauss, K.W.; Finnigan, J.; Stuart-Williams, H.; Farquhar, G.D.; Ball, M.C. Linking Water Use Efficiency With Water Use Strategy From Leaves to Communities. New Phytol. 2023, 240, 1735–1742. [Google Scholar] [CrossRef]
- Alharbi, S.; Felemban, A.; Abdelrahim, A.; Al-Dakhil, M. Agricultural and Technology-Based Strategies to Improve Water-Use Efficiency in Arid and Semiarid Areas. Water 2024, 16, 1842. [Google Scholar] [CrossRef]
- Li, F.; Xiao, J.; Chen, J.; Ballantyne, A.; Jin, K.; Li, B.; Abraha, M.; John, R. Global water use efficiency saturation due to increased vapor pressure deficit. Science 2023, 381, 672–677. [Google Scholar] [CrossRef]
- Cernusak, L.A. Gas exchange and water use efficiency in plant canopies. Plant Biol. 2020, 22, 52–67. [Google Scholar] [CrossRef]
- Lobo, F.d.A.; Previl, R.; Gonzalez-Meler, M.A.; Pereira, B.L.C.; Moura, L.C.d.; Ortíz, C.E.R.; Genuncio, G.d.C.; Vourlitis, G.L. Is Intrinsic Water Use Efficiency Independent of Leaf-to-Air Vapor Pressure Deficit. Theor. Exp. Plant Physiol. 2023, 35, 65–80. [Google Scholar] [CrossRef]
- Głowacka, K.; Kromdijk, J.; Kucera, K.; Xie, J.; Cavanagh, A.P.; Leonelli, L.; Leakey, A.D.B.; Ort, D.R.; Niyogi, K.K.; Long, S.P. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 2018, 9, 868. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Jubery, T.Z.; Ganapathysubramanian, B.; Gilbert, M.E.; Attinger, D. Integrating optimization with thermodynamics and plant physiology for crop ideotype design. arXiv 2017, arXiv:1704.05885. [Google Scholar]
- Kang, J.; Hao, X.; Zhou, H.; Ding, R. An integrated strategy for improving water use efficiency by understanding physiological mechanisms of crops responding to water deficit: Present and prospect. Agric. Water Manag. 2021, 255, 107008. [Google Scholar] [CrossRef]
- Santos, H.C.A.; Lima, J.A.L., Jr.; Silva, O.P.; Guerino, R.S.; Alves, M.C.; Sousa, D.P.; Romariz, R.N.V.; Martins, J.S.; Gonçalves, M.A.S.; Lemos, O.F.; et al. Morpho-physiological traits associated with contrasting water-use efficiency in Piper nigrum. Res. Sq. 2024, rs-4412806. [Google Scholar]
- Rasanjali, K.G.A.I.; Silva, A.C.S.; Priyadarshani, K.D.N. Influence of super absorbent polymers (Saps) on irrigation interval and growth of black pepper (Piper nigrum L.) in nursery management. OUSL J. 2019, 14, 7–25. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Biological role of Piper nigrum L. (Black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 2, 1945–1953. [Google Scholar] [CrossRef]
- Petrík, P.; Petek-Petrik, A.; Mukarram, M.; Schuldt, B.; Lamarque, L.J. Leaf physiological and morphological constraints of water-use efficiency in C3 plants. AoB Plants 2023, 15, plad047. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Flexas, J.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Nakayama, L.H.I. Pimenteira-do-reino. In Recomendações de Calagem e Adubação Para o Estado do Pará, 1st ed.; Brazil, E.C., Cravo, M.d.S., Viegas, I.d.J.M., Eds.; Embrapa: Brasília, Brazil, 2007; pp. 175–177. [Google Scholar]
- Souza, E.B.d.; Ferreira, D.B.d.S.; Guimarães, J.T.F.; Azevedo, F.T.M.d.; Souza, P.G.d.O.P.d. Padrões climatológicos e tendências da precipitação nos regimes chuvoso e seco da Amazônia oriental. Rev. Bras. Climatol. 2017, 21, 81–93. [Google Scholar]
- Cardoso Júnior, E.Q.; Kato, O.R.; Kato, M.d.S.A.; Lopes, S.d.C.; Sá, T.D.d.A. Métodos de Preparo de Área Sobre Algumas Características Físicas do Solo e da Produção do Maracujazeiro (Passiflora edulis) no Nordeste do Pará, 1st ed.; Embrapa Amazônia Oriental-Boletim de Pesquisa e Desenvolvimento: Belém, Brazil, 2007; 23p. [Google Scholar]
- Thornthwaite, C.W.; Mather, J.R. The Water Balance; Laboratory of Climatology: Centerton, NJ, USA, 1955; 104p. [Google Scholar]
- Vieira, G.H.S.; Nascimento, D.P.; Mônaco, P.A.V.L.; Haddade, I.R.; Rosado, T.L.; Chambela Neto, A. Eficiência de irrigação em cafeeiros conilon na região Centro Serrana do Espírito Santo. Rev. Ifes Ciência 2020, 6, 22–34. [Google Scholar] [CrossRef]
- Franco, H.H.S. Abordagem Metodológica Envolvendo Tensiometria e Determinação da Curva de Retenção de Água num Solo de Textura Média. Master’s Thesis, Universidade de São Paulo, Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba-SP, Brazil, 2015. [Google Scholar]
- Richards, L.A. A pressure membrane extraction apparatus for soil solution. Soil Sci. 1941, 51, 377–386. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 3 March 2023).
- Marouelli, W.A. Tensiômetros Para o Controle de Irrigação em Hortaliças; Circular técnica; Embrapa Hortaliças: Brasília, Brazil, 2008; 15p. [Google Scholar]
- Doorenbos, J.; Kassam, A.H. Efeito da Água no Rendimento das Culturas; Gheyi, H.R.; de Souza, A.A.; Damasco, F.A.V.; de Medeiro, J.F., Translators; UFPB: Campina Grande, Brazil, 1994; 306p. [Google Scholar]
- Ee, K.P.; Shang, C.Y. Novel farming innovation for high production of black pepper (Piper nigrum L.). Planting Materials. J. Agric. Sci. Technol. B 2017, 7, 301–308. [Google Scholar]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Chen, L.; Jia, G. Whole-plant instantaneous and short-term water-use efficiency in response to soil water content and CO2 concentration. Plant Soil 2019, 444, 281–298. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Jones, M.P.; Turner, N.C. Instantaneous versus long-term WUE: A review of methodologies and implications. J. Exp. Bot. 2019, 70, 1123–1135. [Google Scholar]
- Lee, H.; Santos, J.P.; Martinez, R. Structural and functional adaptations influencing WUE in contrasting pepper cultivars. Plant Physiol. Biochem. 2023, 195, 203–217. [Google Scholar]
- Smith, J.D.; Brown, R.T.; Wang, X. Advances in water-use efficiency in crop production. Agric. Water Manag. 2018, 202, 45–57. [Google Scholar]
- Patel, R.; Kumar, S.; Devi, L. Comparative assessment of water-use efficiency in pepper cultivars under different irrigation regimes. HortScience 2021, 56, 989–997. [Google Scholar]
- Yildirim, e.; Ekinci, M.; Turan, M.; Agar, G.; Ors, S.; Dursun, A.; Kul, R.; Akgul, G. Physiological and biochemical changes of pepper cultivars under combined salt and drought stress. J. Appl. Bot. Food Qual. 2022, 95, 123–130. [Google Scholar] [CrossRef]
- Boughalleb, F.; Abdellaoui, R.; Brahim, N.B.; Neffati, M. Growth, Photosynthesis, Water Use Efficiency, and Osmoregulation of the Halophyte Atriplex gombiformis Under Water Deficit Conditions. Braz. J. Bot. 2016, 39, 147–156. [Google Scholar] [CrossRef]
- Duah, S.A.; Souza, C.S.e.; Nagy, Z.; Pék, Z.; Neményi, A.; Daood, H.G.; Vinogradov, S.; Helyes, L. Effect of Water Supply on Physiological Response and Fruit Quality of Four Pepper (Capsicum annuum L.) Cultivars. Water 2021, 13, 1284. [Google Scholar] [CrossRef]
- Leal, M.P.d.S.; Dias, T.J.; Sousa, V.F.d.O.; Silva, T.I.d.; Ribeiro, J.E.d.S.; Pereira, W.E.; Souza, A.d.G.; Smiderle, O.J.; Alves, E.U. Physiology and Production of Colored Bell Pepper Cultivars in a Semi-Hydroponic System. Rev. Bras. Eng. Agrícola Ambient. 2024, 28, 90–97. [Google Scholar]
- Erwin, J.; Hussein, T.; Baumler, D.J. Pepper Photosynthesis, Stomatal Conductance, Transpiration, and Water Use Efficiency Vary with Variety and Growth Stage. HortScience 2019, 54, 1662–1670. [Google Scholar] [CrossRef]
- Araz, O.; Ekinci, M.; Yildirim, E. Physiological, Biochemical and Molecular Response of Pepper Genotypes to Water Deficit. J. Crop Health 2025, 77, 45–56. [Google Scholar] [CrossRef]
- Bhattacharya, A. Soil Water Deficit and Physiological Issues in Plants; Springer: Berlin/Heidelberg, Germany, 2021; 702p. [Google Scholar]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain filling of cereals under soil drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential-are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Lawlor, D.W. Genetic engineering to improve plant performance under drought: Physiological evaluation of achievements, limitations, and possibilities. J. Exp. Bot. 2013, 64, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Senthamil, E.; Halli, H.M.; Basavaraj, P.S.; Angadi, S.S.; Gangana Gowdra, V.M.; Harisha, C.B.; Boraiah, K.M.; Sandeep, A.B.; Salainkoppa, S.R.; Mohite, G.; et al. Waterlogging Effects on Root Morphology, Yield, and Stress Tolerance in Cowpea (Vigna unguiculata L. Walp) Grown on Semi-Arid Vertisols. J. Agron. Crop Sci. 2025, 211, e70014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).