Sesame Diseases and Pests: Assessment of Threats to the Establishment of an Australian Industry
Abstract
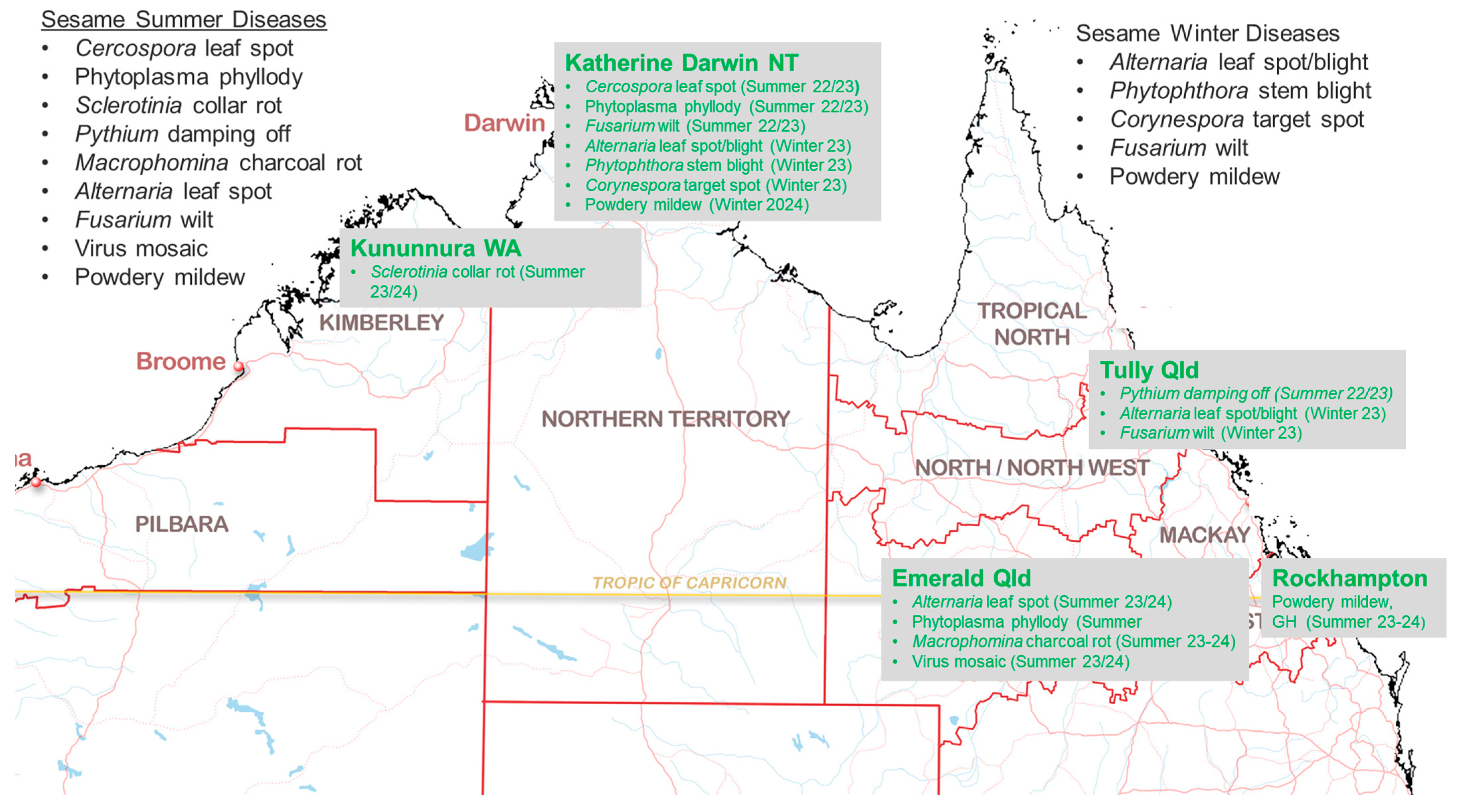
1. Introduction
2. Disease Threats
3. Fungal and Oomycete Diseases
3.1. Fusarium Wilt
3.2. Charcoal Rot/Dry Root Rot
3.3. Root/Stem Rot, Seedling Blight, and Damping-Off
3.4. Collar Rot, Southern Blight, and Stem/Root Rot
3.5. Alternaria Leaf Spot, Alternaria Leaf Blight, and Stem Necrosis
3.6. Cercospora Leaf Spot
3.7. Angular Leaf Spot/Brown Angular Spot
3.8. Powdery Mildews
3.9. Corynespora Blight/Target Spot
3.10. Anthracnose
3.11. Bipolaris Leaf Spot
3.12. Helminthosporium Leaf Spot, Helminthosporium Blight or Brown Leaf Spot, Aerial Stem Rot
3.13. Ascochyta Blight/Leaf Spot
3.14. Cladosporium Leaf Spot
3.15. Wet Rot of Seedlings
3.16. Phytophthora Blight
3.17. Damping Off
4. Bacterial and Phytoplasma Diseases
4.1. Bacterial Blight
4.2. Bacterial Leaf Spot
4.3. Phyllody
4.4. Virus Diseases
4.5. Pest Threats
4.5.1. International Situation
4.5.2. Sesame Pest Threats in Australia
4.5.3. Management Options for Sesame Pests in Australia
4.6. Pest Species Profiles
4.6.1. Sesame Leaf Webber/Capsule Borer Antigastra catalaunalis Duponchel (Lepidoptera: Crambidae)
4.6.2. Green Peach Aphid (Myzus persicae) (Hemiptera: Aphididae)
4.6.3. Green Vegetable Bug Nezara viridula Linnaeus (Hemiptera: Pentatomidae)
4.6.4. Tomato Bug, Nesidiocoris tenuis Reuter (Hemiptera: Miridae)
4.6.5. Silverleaf Whitefly Bemisia tabaci/B. argentifolii (Hemiptera: Aleyrodidae)
4.6.6. Green Mirid Creontiades dilutus Stål (Hemiptera: Miridae)
4.6.7. Rutherglen Bug Nysius vinitor Bergroth (Hemiptera: Lygaeidae)
5. Discussion
6. Limitations and Future Research Directions
- Rapid Disease Diagnostics and Assessment Tools: Develop tools that provide immediate results by leveraging modern molecular biology approaches, satellite imagery, and remote sensing technologies.
- Disease Incidence and Distribution: Identify the incidence and distribution of each sesame disease and assess their impact on production to ensure effective management in the field.
- Understanding Pathosystems: Gain insights into the interactions between host, pathogen, and environment to make informed decisions in disease management, without compromising agronomic or economic goals.
- Diversity of Plant Pathogens: Research the diversity of different plant pathogens to aid breeding programmes aimed at enhancing resistance and tolerance.
- Site-Specific Screening Tests: Conduct screening tests on local and introduced varieties and strains to select for resistance to local diseases.
- Genetic and Breeding Approaches: Develop varieties with resistance or tolerance to priority diseases through genetic and breeding methods, including generating genetic variability by transferring alien genes from closely related or unrelated sources using wide hybridization and biotechnological tools. Genetic resistance or tolerance is the most effective solution from both economic and environmental perspectives.
- Reliable Disease Screening Protocols: Establish well-planned and executed protocols for reliable disease screening focused on priority diseases.
- Utilizing Online Databases: Exploit available online sesame databases that provide valuable information on molecular functions, genome components, gene expression, SSR, SNP, QTL (quantitative trait locus), functional genes, transposons, and genetic maps to support the sesame improvement breeding program.
- Biochemical and Physiological Studies: Investigate the biochemical and physiological basis of resistance against major sesame diseases.
- Epidemiological Studies: Conduct epidemiological studies on various priority diseases to inform disease management strategies.
- Chemical Registration: Screen available chemicals for registration by the Australian Pesticides and Veterinary Medicines Authority (APVMA) for use on priority diseases in Queensland.
- Biological Agents Evaluation: Survey, identify, and evaluate various biological agents for their efficacy against priority sesame diseases to minimize chemical use in IDM.
- Development of IDM Modules: Create IDM modules for priority sesame diseases based on current and new knowledge of best management practices.
- Integration of Resources: Integrate genomic resources, crop production and protection techniques, postharvest practices, crop improvement programs, and capacity building to ensure the successful production of sesame in Queensland.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SNMV | Sesame Necrotic Mosaic Virus |
| CABMV | Cowpea Aphid-Borne Mosaic Virus |
| TAV | Tomato Aspermy Virus |
| SeCTV | Sesame Curly Top Virus |
| CSIRO | Commonwealth Scientific and Industrial Research Organisation |
| NTDPIF | Northern Territory Department of Primary Industry and Fisheries |
| EC | Emulsifiable Concentrate |
| ITS | Internal Transcribed Spacer |
| APVMA | Australian Pesticides and Veterinary Medicines Authority |
| SSR | Simple Sequence Repeat |
| SNP | Single Nucleotide Polymorphisms |
| QTL | Quantitative Trait Locus |
| IDM | Integrated Disease Management |
| IPM | Integrated Pest Management |
References
- Bennett, M.R. Handbook for Farmers and Investors; Nothern Territory Department of Primary Industry and Fisheries: Darwin, NT, Australia, 1998; pp. 361–367. [Google Scholar]
- Decker, C.; Kurnik, B. Scan of New and Emerging Agricultural Industry Opportunities and Market Scoping: A Custom Report Compiled by Euromonitor International; AgriFutures: Wagga Wagga, NSW, Australia, 2018. [Google Scholar]
- Mordor Intelligence, Sesame Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030). 2021. Available online: https://www.mordorintelligence.com/industry-reports/sesame-seeds-market (accessed on 15 January 2025).
- McCulloch, M. 15 Health and Nutrition Benefits of Sesame Seeds. Healthline 2023. 14 February 2023. Available online: https://www.healthline.com/nutrition/sesame-seeds#:~:text=Medically%20reviewed%20by%20Kim%20Rose,known%20for%20supporting%20digestive%20health (accessed on 7 April 2025).
- Meng, Z.; Liu, D.; Li, S.; Xu, Z.; Deng, Q.; Liu, Y. A fast multi-residue analysis of twenty-four classes of pesticide in sesame (Sesamum indicum L.) and their migration into processed products. Food Res. Int. 2023, 173, 113322. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Bhattrai, S.; Akbar, D.; Thomson; Trotter, T.; Timilsina, S. The prospect of developing sesame industry in northern Australia through analysing market opportunity. Aust. J. Reg. Stud. 2020, 26, 347378. [Google Scholar]
- Gupta, K.; Naik, K.; Bisen, R. Status of sesame diseases and their integrated management using indigenous practices. Int. J. Chem. Stud. 2018, 6, 1945–1952. [Google Scholar]
- Min, Y.Y.; Toyota, K. Occurrence of different kinds of diseases in sesame cultivation in Myanmar and their impact to sesame yield. J. Exp. Agric. Int. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Weiss, E.A. Castor, Sesame and Safflower; Leonard Hill Books: London, UK, 1971; 901p. [Google Scholar]
- Kindeya, Y.B.; Negash, W.; Kebede, A.; Baraki, F. Survey and identification of major sesame diseases in low land areas of western zone of Tigray, Ethiopia. J. Biomater. 2018, 2, 58–64. [Google Scholar]
- Meena, B.; Indiragandhi, P.; Ushakumari, R. Screening of sesame (Sesamum indicum L.) germplasm against major diseases. J. Pharmacogn. Phytochem. 2018, 7, 1466–1468. [Google Scholar]
- Saharan, G. Sesame diseases: An annotated bibliography from the 1900–1988 literature. In Manuscript Report/IDRC 227e; Haryana Agricultural University: Hisar, India, 1989. [Google Scholar]
- Verma, M.; Mehta, N.; Sangwan, M. Fungal and Bacterial Diseases of Sesame. In Diseases of Oilseed Crops; Saharan, G.S., Mehta, N., Sangwan, M.S., Eds.; Indus Publishing Company: New Delhi, India, 2005; pp. 269–303. [Google Scholar]
- Vikaspedia. Sesame: Diseases and Symptoms. 2021. Available online: https://vikaspedia.in/agriculture/crop-production/integrated-pest-managment/ipm-for-oilseeds/ipm-strategies-for-sesame/sesame-diseases-and-symptoms (accessed on 15 March 2021).
- Damania, A.B.; Valkoun, J.; Willcox, G.; Qualset, C.O. The Origins of Agriculture and Crop Domestication. 352 p. ISBN: 978-92-9127-084-2, ISBN: 92-9127-084-9. 1998. Available online: https://cgspace.cgiar.org/items/eaf3fe4c-7c51-4e65-ac39-954ebad747f6 (accessed on 20 January 2025).
- Langham, D.R.; Cochran, K.A. Fungi, Oomycetes, Bacteria, and Viruses Associated with Sesame (Sesamum indicum L.); Sesame Research: San Angelo, TX, USA, 2021; 747p. [Google Scholar]
- Gaikwad, S.; Kapgate, D. Biological control of sclerotium root rot of sesamum. PKV Res. J. 1990, 14, 87–89. [Google Scholar]
- Shivas, R.G.; Brockway, C.A.; Beilharz, V.C. First record in Australia of Cercospora sesami and Pseudocercospora sesami, two important foliar pathogens of sesame. Australas. Plant Pathol. 1996, 25, 212. [Google Scholar] [CrossRef]
- Rathaiah, Y.; Pavgi, M. Development of Sclerotia and Spermogonia in Cercospora sesamicola and Ramularia carthami. Sydowia, 1978. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19781351276 (accessed on 5 October 2024).
- Ayiecho, P.O.; Nyabundi, J.O.; Nyanapah, J.O. Nyanapah Evaluation of Sesame Cultivars for Resistance to Cercospora Leaf Spot; College of Agriculture and Veterinary Sciences, University of Nairobi Research Archive: Nairobi, Kenya, 2011. [Google Scholar]
- Zhao, H.; Liu, H.Y.; Yang, X.S.; Liu, Y.X.; Ni, Y.X.; Wang, F.; Tang, L. First report of Nigrospora leaf blight on sesame caused by Nigrospora sphaerica in China. Plant Dis. 2014, 98, 842. [Google Scholar] [CrossRef]
- Agrifutures Australia. AgriFutures Australia’s Post. LinkedIn. Available online: https://www.linkedin.com/posts/agrifutures-australia_growing-sesame-in-australia-pest-management-activity-7178250821539704832-RC3k#:~:text=Pest%20Alert:%20Meet%20the%20major%20culprits%20threatening,pose%20risks%2C%20especially%20during%20early%20establishment%20phases (accessed on 20 November 2024).
- González-Segnana, L.R.; Farina, A.E.; Gonzalez, D.D.; Mello, A.P.O.A.; Rezende, J.A.M.; Kitajima, E.W. Alternative hosts of Cowpea aphid-borne mosaic virus (CABMV) in sesame (Sesamum indicum) crops grown in Paraguay. Trop. Plant Pathol. 2013, 38, 539–542. [Google Scholar] [CrossRef]
- Wang, H.; Gong, H.H.; Yan, Z.Y.; Tang, W.; Zhu, T.S.; Zhao, M.; Li, X.D. First report of tobacco vein banding mosaic virus Infecting Sesame in China. Plant Dis. 2017, 101, 850. [Google Scholar] [CrossRef]
- Jones, R.A.; Sharman, M.; Trebicki, P.; Maina, S.; Congdon, B.S. Virus diseases of cereal and oilseed crops in Australia: Current position and future challenges. Viruses 2021, 13, 2051. [Google Scholar] [CrossRef] [PubMed]
- Zi-Lin, T.; Ze-Yong, H. Studies on Sesame Virus Diseases II. Identification of the Pathogen of Sesame Yellow Mosaic Disease. Virol. Sin. 1993, 8, 277–283. [Google Scholar]
- Gunning, R.V.; Byrne, F.J.; Condé, B.D.; Connelly, M.I.; Hergstrom, K.; Devonshire, A.L. First report of B-biotype Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) in Australia. J. Aust. Entomol. Soc. 1995, 34, 116–120. [Google Scholar] [CrossRef]
- Bennett, M.R.; Wood, I.M. (Eds.) Proceedings of the First Australian Sesame Workshop, Darwin-Katherine, NT, Austarlia, 21–23 March 1995; Betta Concepts: Northbridge, NSW, Australia, 1995. Available online: https://trove.nla.gov.au/work/21455186 (accessed on 15 June 2024).
- Bennett, M.; Lang, M. Sesame Research Report 1988–1989 Wet Season Katehrine. Technical Bulletin 1991(166). Available online: https://daf.nt.gov.au/__data/assets/pdf_file/0009/233289/tb166.pdf (accessed on 15 June 2024).
- Matchett, T. The Effect of Soil-borne Fungi on the Growth of Sesame (Sesamum indicum) Cultivars. In Proceedings of the First Australian Sesame Workshop, Darwin-Katherine, NT, Australia, 21–23 March 1995; Betta Concepts: Artarmon, NSW, Australia, 1995. [Google Scholar]
- Beech, D.F.; Imrie, B.C. Breeding for Mechanised Sesame Production in Australia; International Atomic Energy Agency (IAEA): Vienna, Austria, 2001; pp. 63–70. [Google Scholar]
- Bennett, M.; Conde, K.; Conde, B. Sesame Recommendations for the Northern Territory in AgNote; Department of Primary Industry, Fisheries and Mines © Northern Territory Government: Darwin, NT, Australia, 2003; p. 4. [Google Scholar]
- Harris, G.; Pendergast, L.; Owens, L.; Nastassi, C. Developing a Broadacre Cropping Sector in North Queensland; CRCNA: Holland, MI, USA; CRCC for Developing Northern Australia: London, UK, 2024; p. 100. [Google Scholar]
- Alemaw, G.; Terefe, G.; Zewdie, K.; Weyesa, B. Lowland Oilcrops A Three-Decade Research Experience in Ethiopia; Research Report No. 31; Ethiopian Institute of Agricultural Research: Addis Ababa, Ethiopia, 1997; 31p. [Google Scholar]
- Ara, A. Histopathological studies of sesame (Sesamum indicum) seedlings infected with Fusarium oxysporum. Plant Pathol. Quar. 2017, 7, 82–90. [Google Scholar] [CrossRef]
- Anon. Diseases of Sesamum. Available online: http://eagri.org/eagri50/PATH272/lecture12/index.html (accessed on 1 May 2025).
- Joshi, M.; Srivastava, R.; Sharama, A.K.; Prakash, A. Screening of Resistant Varieties and Antagonistic Fusarium oxysporum for Biocontrol of Fusarium Wilt of Chilli. J. Plant Pathol. Microbiol. 2012, 3, 1–6. [Google Scholar] [CrossRef]
- Kavak, H.; Boydak, E. Screening of the Resistance Levels of 26 Sesame Breeding Lines to Fusarium Wilt Disease. Plant Pathol. J. 2006, 5, 157–160. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium Wilt of Banana: Current Knowledge on Epidemiology and Research Needs Toward Sustainable Disease Management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2008; 369p. [Google Scholar]
- Stover, R.H. Studies on Fusarium wilt of bananas: VIII. Differentiation of clones by cultural interaction and volatile substances. Can. J. Bot. 1962, 40, 1467–1471. [Google Scholar] [CrossRef]
- Mahdy, R.; Gaber, D.A.; Hashem, M.; Alamri, S.; Mahdy, E.E. Improving Sesame (Sesamum indicum L.) Seed Yield through Selection under Infection of Fusarium oxysporum f. sp. sesami. Plants 2022, 11, 1538. [Google Scholar] [CrossRef]
- Mihail, J.; Taylor, S. Interpreting variability among isolates of Macrophomina phaseolina in pathogenicity, pycnidium production, and chlorate utilization. Can. J. Bot. 1995, 73, 1596–1603. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General Characteristics of Pathogenicity and Methods of Control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef]
- Bashir, M.R. Impact of Global Climate Change on Charcoal Rot of Sesame Caused by Macrophomina phaseolina. J. Hortic. 2017, 4, 100–106. [Google Scholar]
- Prasad, R.; Padmaja, D.; Balram, N.; Omprakash, S. Management of stem and root rot of sesame caused by Macrophomina phaseolina. Pharma Innov. J. 2022, 11, 6121–6123. [Google Scholar]
- Chamorro, M.; Miranda, L.; Dominguez, P.; Medina, J.J.; Sona, C.; Romero, F.; Aranda, J.M.L.; De los Santos, B. Evaluation of biosolarization for the control of charcoal rot disease (Macrophomina phaseolina) in strawberry. Crop Prot. 2015, 67, 279–286. [Google Scholar] [CrossRef]
- Khamari, B.; Satapathy, S.; Patra, C. Effect of inoculum load and duration of exposure to Macrophomina phaseolina on disease incidence of sesame. Int. J. Chem. Stud. 2019, 7, 1728–1730. [Google Scholar]
- Choudhary, K.; Meena, A.K.; Dhakar, H.; Kumar, M. Management of charcoal rot of sesame [Macrophomina phaseolina (Tassi.) Goid.] by different bio-agent and fungicide. Int. J. Chem. Stud. 2018, 6, 1556–1559. [Google Scholar]
- Bashir, M.R. Evaluation of New Chemistry Fungicides against Charcoal Rot of Sesame Caused by Macrophomina phaseolina in Pakistan. J. Hortic. 2018, 5, 1000e109. [Google Scholar] [CrossRef]
- Choudhary, R.; Rai, S.; Singh, K. Economic injury level of the sesame leaf webber, Antigastra catalaunalis (Dup.) in Delhi. Indian J. Plant Prot. 1987, 15, 136–141. [Google Scholar]
- Gupta, K.N.; Ranganatha, A.R.G. Biological control for charcoal rot (Macrophomina phaseolina) of sesame. In International Conference on Agriculture & Horticulture, 3rd ed.; Agrotechnology; Hyderabad International Convention Centre: Kothaguda India, 2014. [Google Scholar]
- Radhakrishnanp, R.; Sathasivam, R.; Rengarajan, R.L.; Hashem, A.; Allah, E.F.A. Isolation and Identification of Charcoal Rot Disease Causing Agent in Sesame (Sesamum indicum L.) and Their Growth Inhibition by Bacillus methylotrophicus Ke2. Pak. J. Bot. 2017, 49, 2495–2497. [Google Scholar]
- Siddiq, J.A.; Kalpana, K.; Ebenezar, E.G.; Chinniah, C. In vitro efficacy of soluble silicon against sesame (Sesamum indicum L.) charcoal rot disease caused by Macrophomina phaseolina (Tassi) Goid. J. Pharmacogn. Phytochem. 2019, 8, 3532–3536. [Google Scholar]
- Amen, S.G.; El-Sharawy, A.A.; Hassan, T.H.A.; Abdalla, M.Y. Use of Biofumigation for Controlling Sesame Root Rot in North Sinai. Asian J. Plant Pathol. 2020, 14, 21–26. [Google Scholar] [CrossRef]
- Khamari, B.; Beura, S.; Ranasingh, N. Status of sesame diseases grown in different agroclimatic zones of Odisha. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 945–948. [Google Scholar] [CrossRef]
- Dutta, D.; Awon, V.K.; Gangopadhyay, G. Transcriptomic dataset of cultivated (Sesamum indicum), wild (S. mulayanum), and interspecific hybrid sesame in response to induced Macrophomina phaseolina infection. Data Brief 2020, 33, 106448. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Awon, V.K.; Gangopadhyay, G. Comparative Transcriptome Analysis of Susceptible and Resistant Sesame Against Charcoal Rot. Mendeley Data, V1. 2020. Available online: https://data.mendeley.com/datasets/nk27dkn5d7/1 (accessed on 30 June 2024).
- Yan, W.; Ni, Y.; Liu, X.; Zhao, H.; Chen, Y.; Jia, M.; Liu, M.; Liu, H.; Tian, B. The mechanism of sesame resistance against Macrophomina phaseolina was revealed via a comparison of transcriptomes of resistant and susceptible sesame genotypes. BMC Plant Biol. 2021, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Shivas, R.G.; Adorada, D.L.; Barbetti, M.J.; Bithell, S.L.; Kelly, L.A.; Moore, N.; Sparks, A.H.; Tan, Y.P.; Thomas, G.; et al. Hidden diversity of Macrophomina associated with broadacre and horticultural crops in Australia. Eur. J. Plant Pathol. 2021, 161, 1–23. [Google Scholar] [CrossRef]
- Cochran, K.A.; Tolbert, A.C.; Spurlock, T.N. First Report of Rhizoctonia solani AG4 Causing Stem Necrosis in Sesame in Southwest Texas. Plant Dis. 2018, 102, 2039. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Durr, C.; Schawanck, A.A.; Robin, M.-H.; Sarthou, J.-P.; Cellier, V.; Messean, A.; Aubertot, J.-N. Integrated management of damping-off diseases. A review. Agron. Sustain. Dev. 2017, 37, 10. [Google Scholar] [CrossRef]
- Kator, L.; Hosea, Z.Y.; Oche, O.D. Sclerotium rolfsii: Causative organism of southern blight, stem rot, white mold and sclerotia rot disease. Ann. Biol. Res. 2015, 6, 78–79. [Google Scholar]
- Shamsi, S.; Naher, N. In vitro management of Sclerotium rolfsii Sacc.—The causal agent of stem rot of sesame (Sesamum indicum L.). Int. J. Bioinform. Biol. Sci. 2020, 8, 1–6. [Google Scholar] [CrossRef]
- Praveenkumar, N. Studies on Biological Management of Collar Rot of Sesame Caused by Sclerotium rolfsii Sacc. Master’s Thesis, Department of Plant Pathology, University of Agricultural Sciences, Dharwad, India, 2009; p. 67. [Google Scholar]
- Hernández-Morales, J.; Ochoa-Martínez, D.L.; Ayala-Escobar, V. First report of southern blight caused by Sclerotium rolfsii on sesame in Mexico. J. Plant Pathol. 2018, 100, 323. [Google Scholar] [CrossRef]
- Paparu, P.; Acur, A.; Kato, F.; Acam, C.; Nakibuule, J.; Nkuboye, A.; Musoke, S.; Mukankusi, C. Morphological and Pathogenic Characterization of Sclerotium rolfsii, the Causal Agent of Southern Blight Disease on Common Bean in Uganda. Plant Dis. 2020, 104, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Eslami, A.; Khodaparast, S.A.; Mousanejad, S.; Dehkaei, F.P. Evaluation of the virulence of Sclerotium rolfsii isolates on Arachis hypogaea and screening for resistant genotypes in greenhouse conditions. Hell. Plant Prot. J. 2015, 8, 1–11. [Google Scholar] [CrossRef]
- Naik, M.; Chennappa, G.; Amaresh, Y.S.; Sudha, S.; Chowdappa, P.; Patil, S. Characterization of phytotoxin producing Alternaria species isolated from sesame leaves and their toxicity. Indian J. Exp. Biol. 2017, 55, 36–43. [Google Scholar][Green Version]
- Ojiambo, P.S.; Ayiecho, P.O.; Nyabundi, J.O. Severity of Alternaria Leaf Spot and Seed Infection by Alternaria sesami (Kawamura) Mohanty and Behera, as Affected by Plant Age of Sesame (Sesamum indicum L.). J. Phytopathol. 1999, 147, 403–407. [Google Scholar] [CrossRef]
- Ojiambo, P.S.; Narla, R.D.; Ayiecho, P.O.; Nyabundi, J.O. Effect of infection level of sesame (Sesamum indicum L.) seed by Alternaria sesami on severity of Alternaria leaf spot. Trop. Agric. Res. Ext. 1998, 1, 125–130. [Google Scholar]
- Prakash, V.R.N. Alternaria Leaf Spot of Sesame (Sesamum indicum L.) Caused by Alternaria sesami (Kawamura) Mohanty and Behera. APS Publication No. IW000081, 2007. Available online: https://www.researchgate.net/publication/305656337_Alternaria_leaf_spot_of_sesame_Sesamum_indicum_L_caused_by_Alternaria_sesami_Kawamura_Mohanty_and_Behera (accessed on 13 March 2024).
- Ramamohana Rao, N.; Vijayalakshmi, M. Studies on Alternaria sesami pathogenic to sesame. Microbiol. Res. 2000, 155, 129–131. [Google Scholar] [CrossRef]
- Sahu, U.; Verma, K. Isolation, identification and pathogenicity of A. sesami causing Alternaria leaf spot of sesame. Ann. Plant Soil Res. 2016, 18, 86–87. [Google Scholar]
- Choi, Y.P.; Paul, N.C.; Lee, H.B.; Yu, S.H. First Record of Alternaria simsimi Causing Leaf Spot on Sesame (Sesamum indicum L.) in Korea. Mycobiology 2014, 42, 405–408. [Google Scholar] [CrossRef]
- Nayyar, B.G.; Woodward, S.; Mur, L.A.J.; Akram, A.; Arshad, M.; Naqvi, S.M.S.; Akhund, S. The incidence of Alternaria species associated with infected Sesamum indicum L. seeds from fields of the Punjab, Pakistan. Plant Pathol. J. 2017, 33, 543. [Google Scholar] [CrossRef]
- Ojiambo, P.S.; Mibey, R.K.; Narla, R.D.; Ayiecho, P.O. Field transmission efficiency of Alternaria sesami in sesame from infected seed. Crop Prot. 2003, 22, 1107–1115. [Google Scholar] [CrossRef]
- Rotem, J. The Genus Alternaria: Biology, Epidemiology, and Pathogenicity; American Phytopathological Society: St. Paul, MN, USA, 1994; ISBN 978-0-89054-152-4. [Google Scholar]
- Yu, S.-H.; Mathur, S.; Neergaard, P. Taxonomy and pathogenicity of four seed-borne species of Alternaria from sesame. Trans. Br. Mycol. Soc. 1982, 78, 447–458. [Google Scholar]
- Sahani, P.; Mahapatra, S.S. Chemical management of Alternaria leaf spot disease of sesame in Odisha. J. Plant Prot. Environ. 2014, 11, 64–66. [Google Scholar]
- Marri, N.; Lodhi, A.M.; Hajano, J.; Shah, G.S.; Maitlo, S.A. Response of different sesame (Sesamum indicum L.) cultivars to alternaria leaf spot disease (Alternaria sesami) (kawamura) mohanty & behera. Pak. J. Phytopathol. 2012, 24, 129–132. [Google Scholar]
- Tozlu, E.; Tekiner, N.; Örtücü, S. Investigation on the biological control of Alternaria alternata. Indian J. Agric. Sci. 2018, 88, 1241–1248. [Google Scholar] [CrossRef]
- Roco, A.; Pérez, L.M. In vitro biocontrol activity of Trichoderma harzianum on Alternaria alternata in the presence of growth regulators. Electron. J. Biotechnol. 2001, 4, 1–2. [Google Scholar]
- Zaker, M. Screening of some medicinal plant extracts against Alternaria sesami, the causal agent of Alternaria leaf spot of sesame. J. Ornam. Plants Hortic. Plants 2013, 3, 1–8. [Google Scholar]
- Lubaina, A.S.; Murugan, K. Physiological and biochemical characterization of Senna alata (L.) Roxb. leaf extract—A plant based fungicide against Alternaria leaf spot in sesame. World J. Pharm. Pharm. Sci. 2013, 2, 5790–5801. [Google Scholar]
- Guleria, S.; Kumar, A. Azadirachta indica leaf extract induces resistance in sesame against Alternaria leaf spot disease. J. Cell Mol. Biol. 2006, 5, 81–86. [Google Scholar]
- TNAU Agritech. Cercospora Leaf Spot/White Spot: Cercospora sesami, C. sesamicola. Crop Protection. 2015. Available online: https://agritech.tnau.ac.in/crop_protection/sesame_diseases/sesame_2.html (accessed on 1 July 2021).
- Londase, V. Cercospora Leaf Spot in Sesame: Cercospora sesami; PlantwisePlus Knowledge Bank, 2013. Available online: https://plantwiseplusknowledgebank.org/doi/full/10.1079/pwkb.20147801434 (accessed on 17 September 2024).
- Tamil Nadu Agricultural University. Sesamum Major Disease Sesamum Index: Diseases of Sesamum [Lecture]. 2021. Available online: http://eagri.org/eagri50/PATH272/lecture12/006.html (accessed on 19 July 2021).
- Teshome, E.; Kor, D.; Hasen, T.; Merga, H. A case report on Cercospora leaf spot (Cercospora sesami zimm.) disease occurrence in the mid and lowland sesame growing districts of Bale zone, South-east Oromia, Ethiopia. Acad. Lett. 2022; 4564. [Google Scholar] [CrossRef]
- Teshome, E.; Kora, D. Field evaluation of different fungicides for their effectiveness against cercospora leaf spot (Cercospora sesame Zimm.) of Sesame (Sesamum indicum L.). Acta Entomol. Zool. 2021, 2, 102–107. [Google Scholar] [CrossRef]
- Enikuomehin, O.; Peters, O. Evaluation of crude extracts from some Nigerian plants for the control of field diseases of sesame (Sesamum indicum L.). Trop. Oilseeds J. 2002, 7, 84–93. [Google Scholar]
- Palakshappa, M.; Banu, H.; Patil, L.; Vanishree, C.J.K. Biopriming and integrated management of Cercospora leaf spot of Sesame caused by Cercospora sesamicola. J. Pharmacogn. Phytochem. 2020, 9, 3130–3133. [Google Scholar]
- EPPO. PseudoCercospora sesami. EPPO Global Database 2002. 15 May 2021. Available online: https://gd.eppo.int/taxon/CERSSE (accessed on 31 March 2021).
- Reddy, C.D.R.; Haripriya, S. Heterosis for tolerance of powdery mildew in sesame. Indian J. Mycol. Plant Pathol. 1990, 20, 160–161. [Google Scholar]
- Rajpurohit, T. Occurrence, varietal reaction and chemical control of new powdery mildew (Erysiphe orontii Cast) of sesame. Indian J. Mycol. Plant Pathol. 1993, 23, 207–209. [Google Scholar]
- Patel, M.K.; Kamat, M.N.; Bhide, V.P. Fungi Bombay Supplemeny I. Indian Phytopathol. 1949, 2, 142–155. [Google Scholar]
- Venkatakrishnaiya, N.S. Powdery Mildew Some New Hostsin Mysore. Mysore Agric. J. 1958, 33, 5–6. [Google Scholar]
- Puzari, K.C.; Sarbhoy, A.K.; Ahmad, N.; Agarwal, D.K. New species of powdery mildews from North Eastern Region of India. Indian Phytopathol. 2006, 59, 72–79. [Google Scholar]
- Srinivasulu, U.; Bagyanarayana, G.; Raju, M. Two new Oidium species from India. Indian Phytopathol. 2003, 56, 96–97. [Google Scholar]
- Gemawat, P.D.; Verma, O.P. A new powdery mildew of Sesamum indicum incited by Sphaerotheca fuliginea. Indian J. Mycol. Plant Pathol. 1972, 2, 94. [Google Scholar]
- Chen, R.-S.; Chu, C.; Cheng, W.; Chen, W.-Y.; Tsay, J.-G. Differentiation of two powdery mildews of sunflower (Helianthus annuus) by a PCR-mediated method based on ITS sequences. Eur. J. Plant Pathol. 2008, 121, 1–8. [Google Scholar] [CrossRef]
- Sujatha, M.; Soni, P.; Jatothu, J. Identification of Podosphaera xanthii causing powdery mildew on sesame (Sesamum indicum L.). J. Oilseeds. Res. 2015, 32, 183–185. [Google Scholar] [CrossRef]
- Egonyu, J.; Kyamanywa, S.; Anyanga, W.; Seekamebmbe, C.K. Review of pests and diseases of sesame in Uganda. In African Crop Science Conference Proceedings; African Crop Science Society: Kampala, Uganda, 2005. [Google Scholar]
- Venkata, R.R.P.; Anuradha, G.; Prasuna, K.; Gouri, S.V.; Siddiq, E.A. Inheritance of Powdery Mildew Resistance in Sesame (Sesamum indicum L.)—A Review. Int. J. Bio-Resour. Stress Manag. 2013, 4, 614–619. [Google Scholar]
- PlantwisePlus Knowledge Bank, C.I. Antigastra catalaunalis (sesame webworm). 2021. Available online: https://plantwiseplusknowledgebank.org/doi/10.1079/PWKB.Species.5750 (accessed on 31 March 2023).
- Jia, M.; Liu, X.; Zhao, H.; Ni, Y.; Liu, H.; Tian, B. Cell-wall-degrading enzymes produced by sesame leaf spot pathogen Corynespora cassiicola. J. Phytopathol. 2021, 169, 186–192. [Google Scholar] [CrossRef]
- Qi, Y.X.; Zhang, X.; Pu, J.J.; Liu, X.M.; Lu, Y.; Zhang, H.; Zhang, H.Q.; Lu, Y.C.; Xie, Y.X. Morphological and molecular analysis of genetic variability within isolates of Corynespora cassiicola from different hosts. Eur. J. Plant Pathol. 2011, 130, 83–95. [Google Scholar] [CrossRef]
- Lopez, D.; Ribeiro, S.; Label, P.; Fumanal, B.; Venisse, J.-S.; Kohler, A.; de Oliveira, R.R.; Labutti, K.; Lipzen, A.; Lail, K.; et al. Genome-Wide Analysis of Corynespora cassiicola Leaf Fall Disease Putative Effectors. Front. Microbiol. 2018, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.R.; Saraiva, O.F.; Farias, J.R.B.; Gaudencion, C.A.; Torres, E. Survival of pathogens on soybean debris under no-tillage and conventional tillage systems. Pesqui. Agropecu. Bras. 2001, 36, 1231–1238. [Google Scholar] [CrossRef]
- Oliveira, R.R.; Aguilar, B.D.; Tessmann, D.J.; Pujade-Renaud, V.; Vida, J.B. Chlamydospore formation by Corynespora cassiicola. Trop. Plant Pathol. 2012, 37, 415–418. [Google Scholar] [CrossRef]
- Hartman, G.L.; Rupe, J.C.; Sikora, E.J.; Domier, L.L.; Davis, J.A.; Steffey, K.L. Compendium of Soya Bean Diseases and Pests; American Phytopathological Society: St. Paul, MI, USA, 2015. [Google Scholar]
- Galbieri, R.; Araujo, D.C.E.B.; Kobayasti, L.; Girotto, L.; Matos, J.N.; Marangoni, M.S.; Mehta, Y.R. Corynespora leaf blight of cotton in Brazil and its management. Am. J. Plant Sci. 2014, 5, 3805. [Google Scholar] [CrossRef]
- Gao, D.-X.; Sun, H.-J.; Na, Y.-B.; Cheng, H.-S.; Yang, G.; Xu, J.; Xu, X.-D. First Report of Root Rot Caused by Corynespora cassiicola on Sesame in China. Plant Dis. 2018, 102, 1664. [Google Scholar] [CrossRef]
- Vawdrey, L.L.; Grice, K.R.E.; Westerhuis, D. Field and laboratory evaluations of fungicides for the control of brown spot (Corynespora cassiicola) and black spot (Asperisporium caricae) of papaya in far north Queensland, Australia. Australas. Plant Pathol. 2008, 37, 552–558. [Google Scholar] [CrossRef]
- Teppa, P. Anthracnose. Plant the Future. 2021. Available online: https://www.plantthefuture.com/anthracnose/ (accessed on 9 August 2021).
- Abo-Ghazala, M.; El-Shazly, A.; Tolba, I.H. Characterization of Bipolaris sorokiniana and Alternaria sesami isolates obtained from sesame (Sesamum indicum L.) in Egypt. Al-Azhar J. Agric. Res. 2019, 44, 74–87. [Google Scholar] [CrossRef]
- El-Fawy, M.M.; El-Said, M.A.A. Effect of Foliar Application of some Zinc and Phosphorus Sources on Controlling Helminthosporium Leaf Spot Disease and Production of Sesame. J. Plant Prot. Pathol. 2018, 9, 201–207. [Google Scholar] [CrossRef]
- Wasnikar, A.R.; Sharma, S.M.; Prasad, K.V.V. Seed-borne microflora of sesame and their significance. J. Oil Seeds Res. 1987, 4, 141–144. [Google Scholar]
- Bayer Australia. Ascochyta Blight. Available online: https://www.crop.bayer.com.au/find-crop-solutions/by-pest/diseases/ascochyta-blight (accessed on 30 June 2021).
- Harveson, R.M.; Markell, S.G. Ascochyta Blight. Ben IPM: Legume IPM PIPE Diagnostic Series. Available online: https://beanipm.pbgworks.org/ascochyta-blight (accessed on 9 August 2021).
- Davidson, J.; Kimber, R. Integrated disease management of Ascochyta blight in pulse crops. Eur. J. Plant Pathol. 2007, 119, 99–110. [Google Scholar] [CrossRef]
- Kolke, S.T.; LeStrange, M. Cladosporium Leaf Spot (Cladosporium variabile). Agriculture: Spinach Pest Management. Available online: https://www2.ipm.ucanr.edu/agriculture/spinach/Cladosporium-Leaf-Spot/ (accessed on 9 August 2021).
- Srikantappa, N.O.; Somashekar, A.G.; Malammanavar, G.; Krishnappa, K. Seed-borne fungi of sesame (Sesamum indicum L.) seeds in Davanagere district and their effect on germination. Res. Rev. Biosci. 2009, 3, 157–163. [Google Scholar]
- CABI. Choanephora Fruit Rot. PlantWise Knowledge Bank. Available online: https://www.plantwise.org/KnowledgeBank/datasheet/13038#HostPlantsSection (accessed on 9 August 2021).
- Alchetron, Choanephora cucurbitarum. 2018. Available online: https://alchetron.com/Choanephora-cucurbitarum (accessed on 25 September 2024).
- Jackson, G.; Mua, M. Cucurbit Wet Rot. Pacific Pests, Pathogens & Weeds—Fact Sheets 2020. 2025. Available online: https://apps.lucidcentral.org/ppp_v9/text/web_full/entities/cucurbit_wet_rot_144.htm (accessed on 7 May 2025).
- Sharma, R.L.; Mishra, T.; Bhagat, R.; Swarinkar, V.K. Management of Phytopthora Blight of Sesame on Farmers’ Field by Chemical Fungicides. Trends Biosci. 2017, 10, 2068–2072. [Google Scholar]
- Monpara, B. A Black Sesame Variety Gujarat Til 10 (GT 10) Field Resistance to Phytophthora Blight Disease. Int. J. Curr. Res. Biosci. Plant Biol. 2015, 2, 53–63. [Google Scholar]
- Folnovic, T. Pythium Disease Management. Pests and Diseases. Available online: https://blog.agrivi.com/post/pythium-disease-management (accessed on 9 August 2021).
- Felix-Gastelum, R.; Maldonado-Mendoza, I.E.; Olivas-Peraza, N.G.; Penuelas-Rubio, O.; Leyva-Madrigal, K.Y.; Cervantes-Gamez, R.; Lizarraga-Sanchez, G.L.; Longoria-Espinoza, R.M. First report of sesame spot caused by Xanthomonas campestris pv. sesami in Sinaloa, Mexico. Can. J. Plant Pathol. 2019, 41, 296–300. [Google Scholar] [CrossRef]
- Kottle, S. Diseases of edible oil seed crops. In Rapeseed-Mustard and Sesame Diseases; CRC Press Inc.: Boca Raton, FL, USA, 1985; Volume II. [Google Scholar]
- Firdous, S.; Asghar, R.; Ul-Haque, M.I.; Waheed, A.; Afzal, S.; Mirza, M. Pathogenesis of Pseudomonas syringae pv. sesami associated with sesame (Sesamum indicum L.) bacterial leaf spot. Pak. J. Bot. 2009, 41, 927–934. [Google Scholar]
- Prathuangwong, S.; Yowabutra, P. Approach to Control Measure of Sesame Bacterial Leaf Spot in Thailand. In Pseudomonas Syringae Pathovars and Related Pathogens; Rudolph, K., Ed.; Springer: Dordrecht, The Netherlands, 1997; pp. 617–622. [Google Scholar]
- Rao, G.P.; Nabi, S.U. Overview on a century progress in research on sesame phyllody disease. Phytopathog. Mollicutes 2015, 5, 74–83. [Google Scholar] [CrossRef]
- Kumari, S.; Nagendran, K.; Rai, A.W.; Bijendra, S.; Rao, G.P.; Bertaccini, A. Global status of phytoplasma diseases in vegetable crops. Front. Microbiol. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Jagadeswar, R.; Babu, G. Evaluation of sesame (Sesamum indicum) genotypes for their reactions to powdery mildew and phyllody diseases. Plant Dis. Res. 2005, 20, 126–130. [Google Scholar]
- Banerjee, S.; Gangopadhyay, G. Untargeted metabolomics reveals altered pathways in phytoplasma-infected sesame plants. Plant Mol. Biol. Rep. 2024, 43, 392–410. [Google Scholar] [CrossRef]
- Mohammed, Z.H.; Khobe, E.P. Assessment of seedling damping-off and wilts diseases of irrigated sesame (Sesamum indicum L.) in Maidugur, Nigeria. Fudma J. Agric. Agric. Technol. 2018, 4, 248–253. [Google Scholar]
- Dilipsundar, N.; Chitra, N.; Gowtham, V. Checklist of insect pests of sesame. Indian J. Entomol. 2019, 81, 928–944. [Google Scholar] [CrossRef]
- Ahuja, D.; Bakhetia, D. Bio-ecology and management of insect pets of sesame, a Review. J. Insect Sci. 1995, 8, 1–19. [Google Scholar]
- Myint, D.; Gilani, S.A.; Kawase, M.; Watanabe, K.N. Sustainable sesame (Sesamum indicum L.) production through improved technology: An overview of production, challenges, and opportunities in Myanmar. Sustainability 2020, 12, 3515. [Google Scholar] [CrossRef]
- Kinati, K. The survey on field insect pests of sesame (Sesamum indicum L.) in east wollega and horo guduru wollega zones, west Oromia, Ethiopia. Int. J. Entomol. Res. 2017, 2, 22–26. [Google Scholar]
- Akinyemi, A.O.; Pitan, O.O.R.; Osipitan, A.A.; Adebisi, M.A. Susceptibility of sesame (Sesamum indicum L.) to major field insect pests as influenced by insecticide application in a sub-humid environment. Afr. Entomol. 2015, 23, 48–58. [Google Scholar] [CrossRef]
- Simoglou, K.B.; Anastasiades, A.I.; Baizeras, J.; Roditakis, E. First report of Antigastra catalaunalis on sesame in Greece. Entomol. Hell. 2017, 26, 6–12. [Google Scholar] [CrossRef]
- Thangjam, R.; Vastrad, A. Studies on pest complex of sesame and their natural enemies in North Karnataka, India. J. Entomol. Zool. Stud. 2018, 6, 57–60. [Google Scholar]
- Roy, N. Population dynamics and economic thresholds based time series for smart pest management of sesame. Int. J. Trop. Insect Sci. 2021, 41, 2573–2584. [Google Scholar] [CrossRef]
- Kebede, A.; Taferee, Z.; Negash, W. Assessment of Prevalence and Incidence of Sesame Gall Midge (Asphondylia sesami Felt) in Kafta-Humera District Tigray, Ethiopia. J. Agric. Ecol. Res. Int. 2020, 21, 29–36. [Google Scholar] [CrossRef]
- Biswas, G.; Kabir, S.; Das, G. Insect pests of sesame, Sesamum indicum Linn. in Bangladesh, their succession and natural enemies. Indian J. Entomol. 2001, 63, 117–124. [Google Scholar]
- Zemedkun, A.W. Taye, Pest management of sesame in Ethiopia: A review. Peruv. J. Agron. 2022, 6, 210–221. [Google Scholar]
- Berhe, M.; Subramanyam, B.; Chichaybelu, M.; Abera, F.A.; Mahroof, R.; Harvey, J. Insect species dynamics and associated losses in on-farm stored sesame (Sesamum indicum L.) seeds in major sesame growing areas in Ethiopia. Int. J. Trop. Insect Sci. 2024, 44, 855–871. [Google Scholar] [CrossRef]
- Panday, A.K.; Dwarka, R.B.; Jain, S. Major insect pests of sesame and their management. In Insect Pest Management: Concept and Approaches; Mishra, Y.K., Ed.; AkiNik Publications: New Delhi, India, 2021. [Google Scholar]
- Gupta, M.; Rai, H.; Chaurasia, S. Incidence and avoidable loss due to leaf roller/capsule borer, Antigastra catalaunalis Dup. in sesame. Ann. Plant Prot. Sci. 2002, 10, 202–206. [Google Scholar]
- Nayak, G.S.; Samal, T.; Dohling, P.N.K.; Reshma, M. The Impact of Various IPM Modules on the Management of Major Insect Pests of Sesame in Madhya Pradesh’s Bundhelkhand Zone. Int. J. Plant Soil Sci. 2023, 35, 77–85. [Google Scholar]
- Langham, D.R. Sesame Pests—A Review Part 1 (Sesamum indicum L.). 2019. Available online: https://www.researchgate.net/publication/333732200 (accessed on 10 February 2023).
- Habeck, D.H.; Mead, F.W.; Fasula, T.R. Lantana Lace Bug, Teleonemia scrupulosa Stål (Insecta: Hemiptera: Tingidae); Rhodes, E., Ed.; IFAS Extension University of Florida: Gainesville, FL, USA, 2024. [Google Scholar]
- Premdas, M.C.; Hariprasad, K.V.; Manjula, K.; Reddy, D.M.; Sumathi, P. Management of lepidopteran pests of sesamum with certain insecticides. Andhra Pradesh J. Agric. Sci. 2018, 4, 7–13. [Google Scholar]
- Gebregergis, Z.; Assefa, D.; Fitwy, I. Sesame sowing date and insecticide application frequency to control sesame webworm Antigastra catalaunalis (Duponchel) in Humera, Northern Ethiopia. Agric. Food Secur. 2018, 7, 39. [Google Scholar] [CrossRef]
- Khidher, K.Q.; Mohamed, A.M.A.; Kadir, N.B. Seasonal incidence of sesame webworm, Antigastra catalaunalis (Dup.) and evaluation of selected cultural control practices, on sesame crop in Erbil city. Agric. Sci. Dig. 2023, 43, 713–717. [Google Scholar] [CrossRef]
- Egonyu, J.; Kyamanywa, S.; Ssekabembe, C. Integration of time of planting and insecticide application schedule to control sesame webworm and gall midge in Uganda. J. Appl. Biosci. 2009, 18, 967–975. [Google Scholar]
- Karuppaiah, V.; Nadarajan, L. Host plant resistance against sesame leaf webber and capsule borer, Antigastra catalaunalis Duponchel (Pyraustidae: Lepidoptera). Afr. J. Agric. Res. 2013, 8, 4674–4680. [Google Scholar]
- Thakur, K.; Panday, A.K. Morphological Parameters of Sesame in Relation to Susceptibility to Major Sucking Insect Pests. Indian J. Entomol. 2024, 87, 1–4. [Google Scholar] [CrossRef]
- Manisegaran, S.; Manimegalai, M.; Venkatesan, S.; Mohammed, S.E.N. Effect of intercropping on the incidence of shoot webber Antigastra catalaunalis in sesame. Ann. Plant Prot. Sci. 2001, 9, 131–133. [Google Scholar]
- Azimi, S.; Amini, R.; Hosseingolizadeh, M. Suppression of weed and insect populations by living and straw mulches in sesame (Sesamum indicum L.). Sci. Rep. 2023, 13, 21586. [Google Scholar] [CrossRef]
- Baskaran, R.; Mahadevan, N.; Thangavelu, S. Influence of intercropping on infestation of shoot-webber (Antigastra catalaunalis) in sesame (Sesamum indicum). Indian J. Agric. Sci. 1991, 61, 440–442. [Google Scholar]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2016, 2, 16014. [Google Scholar] [CrossRef]
- Ahirwar, R.; Gupta, M.; Banerjee, S. Evaluation of natural products and endosulfan against incidence of Antigastra catalaunalis (Dup.) in sesame. Ann. Plant Prot. Sci. 2008, 16, 25–28. [Google Scholar]
- Ahirwar, R.; Gupta, M.; Banerjee, S. Field efficacy of natural and indigenous products on sucking pests of sesame. Indian J. Nat. Prod. Resour. 2010, 1, 221–226. [Google Scholar]
- Ahmed, K.N.; Pramanik, S.H.A.; Khatun, M.; Hasan, M.R.; Mohanta, L.C.; Hoq, T.; Ghose, S.K. Suppression of dominant insect pests and yield of sesame with plant materials in different climatic conditions. Bangladesh J. Sci. Ind. Res. 2014, 49, 31–34. [Google Scholar] [CrossRef]
- Jones, L.C.; Rafter, M.A.; Walter, G.H. Host plant acceptance in a generalist insect: Threshold, feedback or choice? Behaviour 2020, 157, 1059–1089. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Ibrahim, M.; Koraim, A. Survey and population density of sesame pests and associated natural enemies. J. Product. Dev. 2024, 29, 9–21. [Google Scholar]
- Sarazú-Pillado, R.A.; Gonzalez-Hernandez, H.; Lomeli-Flores, J.R.; Valdez-Carrasco, J.M.; Cortez-Mondaca, E.; Guzman-franco, A. Parasitoid wasps associated with Antigastra catalaunalis (Lepidoptera, Crambidae) in Northern Sinaloa, Mexico. J. Hymenopt. Res. 2024, 97, 741–754. [Google Scholar] [CrossRef]
- Hallman, G.; Sanchez, G. Possibilities for biological control of Antigastra catalaunalis [Lep.: Pyralidae], a new pest of sesame in the western hemisphere. Entomophaga 1982, 27, 425–429. [Google Scholar] [CrossRef]
- Mukhtar, Y.; Shankar, U.; Singh, R.; Singh, A.K.; Kumar, S.; Peshin, R. Can effective pest management practices for Antigastra catalaunalis Duponchel (Crambidae: Lepidoptera) aid non-Apis bee conservation in sesame field? Phytoparasitica 2025, 53, 47. [Google Scholar] [CrossRef]
- Schaffers, J. Reconstruction of the origin of Antigastra catalaunalis, a new moth for the Dutch fauna (Lepidoptera: Crambidae). Entomol. Ber. 2009, 69, 36–45. [Google Scholar]
- Ahirwar, R.; Gupta, M.P.; Smita Banerjee, S.B. Bio-ecology of leaf roller/capsule borer Antigastra catalaunalis Duponchel. Adv. Bio Res. 2010, 1, 90–104. [Google Scholar]
- Ramdas Menon, M.G.; Rattan Lal, R.L.; Bhattacherjee, N.S. Studies on Antigastra catalaunalis (Duponchel), The TIL Leaf-Roller. Indian J. Entomol. 1960, 22, 1–7. [Google Scholar]
- Chaitra, H.; Deb, S.; Borad, P. Biology of Sesame Leaf Webber Antigastra catalaunalis Duponchel. Indian J. Entomol. 2022, 84, 837–839. [Google Scholar]
- Singh, G.; Sinha, R.P.; Singh, S.P.; Hameed, S.F. Population dynamics and biology of sesamum shoot and leaf webber, Antigastra catalaunalis Dup.(Lepidoptera: Pyralidae). J. Entomol. Res. 1992, 16, 305–310. [Google Scholar]
- Kumar, R.; Ali, S.; Dhoray, U.C.R. Incidence of Antigastra catalaunalis, Dup. in different varieties of sesame. Mol. Entomol. 2012, 3. Available online: https://www.proquest.com/docview/1875187408?pq-origsite=gscholar&fromopenview=true&sourcetype=Scholarly%20Journals (accessed on 15 May 2025).
- Saravanaraman, M.; Selvanarayanan, V.; Saravanan, K. Sesame webworm, Antigastra catalaunalis duponchel (Crambidae: Lepidoptera) survives on a new alternate host in Southern India. Int. J. Entomol. Res. 2016, 1, 46–48. [Google Scholar]
- Narayanan, U.S. Reproductive Biology and Sex Communication in Sesame Leaf Webber Antigastra catalaunalis (Duponchel) (Lepidoptera: Pyraustidae). Master’s Thesis, Department of Agricultural Entomology and Plant Nematology, Tamil Nadu Agricultural University, Coimbatore, India, 2002. [Google Scholar]
- Narayanan, U.S.; Nadarajan, L. Evidence for a male-produced sex pheromone in sesame leaf webber, Antigastra catalaunalis Duponchel (Pyraustidae, Lepidoptera). Curr. Sci. 2005, 88, 631–634. [Google Scholar]
- Phelan, P.L.; Baker, T.C. Evolution of male pheromones in moths: Reproductive isolation through sexual selection? Science 1987, 235, 205–207. [Google Scholar] [CrossRef]
- Athya, D.P.; Panday, A.K. Economic injury level of sesame leaf webber and capsule borer Antigastra catalaunalis (Duponchel). Indian J. Entom. 2020, 82, 735–738. [Google Scholar] [CrossRef]
- Akbari, D.; Patel, R.; Barad, A.; Mohaptara, A. Evaluation of different ready-mix insecticides against the insect pest of sesame. Plant Arch. 2024, 24, 2674–2678. [Google Scholar]
- Naveen, B.; Sushila, N.; Ashoka, J.; Sreenivasa, A.G. Bio efficacy of novel insecticides against capsule borer Antigastra catalunalis (Duponchel) in sesame. Int. J. Curr. Microbiol. Appl. Sci. Spec. 2019, 9, 279–284. [Google Scholar]
- Divya, P.; Dhurua, S.; Chalam, M.S.V.; Rao, S.G. Evaluation of insecticides against leaf webber and capsule borer (Antigastra catalaunalis Dup.) in sesamum. J. Exp. Zool. India 2022, 25, 1161–1164. [Google Scholar]
- Omprakash, S.; Reddy, C.N.; Sree, M.S.; Babu, T.K.; Sreedhar, M. Evaluation of certain insecticides against sesame leaf webber and capsule borer (Antigastra catalaunalis duponchel). Int. J. Bio-Resour. Stress Manag. 2022, 13, 1162. [Google Scholar] [CrossRef]
- GBIF Secretariat, GBIF Backbone Taxonomy. 2023. Available online: https://www.gbif.org/dataset/d7dddbf4-2cf0-4f39-9b2a-bb099caae36c (accessed on 15 May 2025).
- Emden, H.V.; Harrington, R. Aphids as Crop Pests; CABI Digital Library: Oakland, CA, USA, 2017. [Google Scholar] [CrossRef]
- Blackman, R.; Eastop, V. Aphids on the World’s Crops: An Identification and Information Guide; John Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Narváez, Z.; Notz, A. Population parameters of green sesame aphid Myzus persicae (Sulzer), on potato, Solanum tuberosum L. and sesame, Sesamum indicum L. in Venezuela. Bol. Entomolgia Venez. 1996, 11, 39–47. [Google Scholar]
- Blackman, R. Life-cycle variation of Myzus persicae (Sulz.) (Hom. Aphididae) in different parts of the world, in relation to genotype and environment. Bull. Entomol. Res. 1974, 63, 595–607. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gubrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]
- Ward, S.; Jalali, T.; van Rooyen, A.; Reidy-Crofts, J.; Moore, K.; Edwards, O.; Umina, P.A. The evolving story of sulfoxaflor resistance in the green peach aphid, Myzus persicae (Sulzer). Pest Manag. Sci. 2024, 80, 866–873. [Google Scholar] [CrossRef]
- Scopes, N. The potential of Chrysopa carnea as a biological control agent of Myzus persicae on glasshouse chrysanthemums. Ann. Appl. Biol. 1969, 64, 433–439. [Google Scholar] [CrossRef]
- Shan, L.T.; Feng, M.G. Evaluation of the biocontrol potential of various Metarhizium isolates against green peach aphid Myzus persicae (Homoptera: Aphididae). Pest Manag. Sci. Former. Pestic. Sci. 2010, 66, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Hatcher, P.E. Combining entomopathogenic fungi and parasitoids to control the green peach aphid Myzus persicae. Biol. Control 2017, 110, 44–55. [Google Scholar] [CrossRef]
- Todd, J. Ecology and behavior of Nezara viridula. Annu. Rev. Entomol. 1989, 34, 273–292. [Google Scholar] [CrossRef]
- Wilson, F. A Review of the Biological Control of Insects and Weeds in Australia and Australian New Guinea; CABI Digital Library: Oakland, CA, USA, 1960; p. 102. [Google Scholar]
- Kiritani, K.; Hokyo, N. Studies on the life table of the southern green stink bug, Nezara viridula. Jpn. J. Appl. Entomol. Zool. 1962, 6, 124–140. [Google Scholar] [CrossRef]
- Velasco, L.; Walter, G. Availability of different host plant species and changing abundance of the polyphagous bug Nezara viridula (Hemiptera: Pentatomidae). Environ. Entomol. 1992, 21, 751–759. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Hirose, E. Survival, reproduction, and starvation resistance of adult southern green stink bug (Heteroptera: Pentatomidae) reared on sesame or soybean. Ann. Entomol. Soc. Am. 1995, 88, 661–665. [Google Scholar] [CrossRef]
- de Lima, A.K.; Soares, J.J.; Soares, M.A.; Zanuncia, J.C.; Bicho, C.; da Silva, C.A.D. Development, Survival and Reproduction of Nezara viridula (Hemiptera: Pentatomidae) in Sesame Cultivars and Implications for the Management. Plants 2024, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, A.; Lucini, T. What happened to Nezara viridula (L.) in the Americas? Possible reasons to explain populations decline. Neotrop. Entomol. 2016, 45, 619–628. [Google Scholar] [CrossRef]
- Turner, J. Influence of plant species on the movement of Trissolcus basaus Woolaston (Hymenoptera: Scelionidae)—A parasite of Nezara viridula L. Aust. J. Entomol. 1983, 22, 271–272. [Google Scholar] [CrossRef]
- Knight, K.M.; Gurr, G.M. Review of Nezara viridula (L.) management strategies and potential for IPM in field crops with emphasis on Australia. Crop Prot. 2007, 26, 1–10. [Google Scholar] [CrossRef]
- Brookes, D.R.; Hereward, J.P.; Wilson, L.J.; Walter, G.H. Multiple invasions of a generalist herbivore—Secondary contact between two divergent lineages of Nezara viridula Linnaeus in Australia. Evol. Appl. 2020, 13, 2113–2129. [Google Scholar] [CrossRef]
- Tillman, P.G. Susceptibility of pest Nezara viridula (Heteroptera: Pentatomidae) and parasitoid Trichopoda pennipes (Diptera: Tachinidae) to selected insecticides. J. Econ. Entomol. 2006, 99, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.; Denecke, S.; Livadaras, I.; Geibel, S.; Nauen, R.; Vontas, J. Development of efficient RNAi in Nezara viridula for use in insecticide target discovery. Arch. Insect Biochem. Physiol. 2020, 103, e21650. [Google Scholar] [CrossRef]
- CAB International. Nesidiocoris tenuis (Tomato Bug). 2021. Available online: https://plantwiseplusknowledgebank.org/doi/10.1079/pwkb.species.16251 (accessed on 10 May 2025).
- Calvo, J.; Bolckmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Lacasa, A.; Arno, I.; Castañe, C.; Alomar, O. Life history parameters for Nesidiocoris tenuis (Reuter)(Het. Miridae) under different temperature regimes. J. Appl. Entomol. 2009, 133, 125–132. [Google Scholar] [CrossRef]
- Sanchez, J.A. Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric. For. Entomol. 2008, 10, 75–80. [Google Scholar] [CrossRef]
- Castillo, J.; Roda, A.; Qureshi, J.; Perez-Hedo, M.; Urbaneja, A.; Stansly, P. Sesame as an alternative host plant to establish and retain predatory mirids in open-field tomatoes. Plants 2022, 11, 2779. [Google Scholar] [CrossRef]
- Konan, K.A.J.; Monticelli, L.S.; Ouali-N’Goran, S.-W.M.; Ramirez-Romero, R.; Martin, T.; Desneux, N. Combination of generalist predators, Nesidiocoris tenuis and Macrolophus pygmaeus, with a companion plant, Sesamum indicum: What benefit for biological control of Tuta absoluta? PLoS ONE 2021, 16, e0257925. [Google Scholar] [CrossRef] [PubMed]
- Nakaishi, K.; Fukui, Y.; Arakawa, R. Reproduction of Nesidiocoris tenuis (Reuter) on sesame. Jpn. J. Appl. Entomol. Zool. 2011, 55, 199–205. [Google Scholar] [CrossRef]
- Chailleux, A.; Ndjiliw, S.; Diakhate, M.; Akodjetin, G.F.; Correa, P.; Deletre, E.; Brevault, T. Approaches to conservation of Nesidiocoris tenuis for biological control of pests in field-grown tomato in Senegal. Biol. Control 2022, 172, 104984. [Google Scholar] [CrossRef]
- Rim, H.; Uefune, M.; Ozawa, R.; Yoneya, K.; Takabayashi, J. Experience of plant infestation by the omnivorous arthropod Nesidiocoris tenuis affects its subsequent responses to prey-infested plant volatiles. BioControl 2017, 62, 233–242. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, M. Seasonal incidence of major insect pests of sesame. Indian J. Entomol. 2023, 85, 201–204. [Google Scholar] [CrossRef]
- Yano, E.; Nakauchi, M.; Watanabe, T.; Watanabe, H.; Hosaka, S.; Nishimori, S.; Miura, S.; Kandori, I.; Hinomoto, N. Life history traits of Nesidiocoris tenuis on Bemisia tabaci and Thrips palmi. BioControl 2020, 65, 155–164. [Google Scholar] [CrossRef]
- Passos, L.C.; Ricupero, M.; Gugliuzzo, A.; Soares, M.A.; Desneux, N.; Campolo, O.; Carvalho, G.A.; Biondi, A.; Zappala, L. Sublethal effects of plant essential oils toward the zoophytophagous mirid Nesidiocoris tenuis. J. Pest Sci. 2022, 95, 1609–1619. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Disndale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Fang, C.; Hopkinson, J.E.; Balzer, J.; Frese, M.; Tay, W.T.; Walsh, T. Screening for insecticide resistance in Australian field populations of Bemisia tabaci (Hemiptera: Aleyrodidae) using bioassays and DNA sequencing. Pest Manag. Sci. 2022, 78, 3248–3259. [Google Scholar] [CrossRef] [PubMed]
- Wongnikong, W.; Hereward, J.P.; van Brunschot, S.L.; Cappadonna, J.K.; Walter, G.H. Assessment of relative host plant quality for three cryptic species of the Bemisia tabaci species complex in Australia. Arthropod-Plant Interact. 2021, 15, 845–859. [Google Scholar] [CrossRef]
- Wongnikong, W.; van Brunschot, S.L.; Hereward, J.P.; De Barro, P.J.; Walter, G.H. Testing mate recognition through reciprocal crosses of two native populations of the whitefly Bemisia tabaci (Gennadius) in Australia. Bull. Entomol. Res. 2020, 110, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Wongnikong, W.; Hereward, J.P.; van Brunschot, S.L.; Walter, G.H. Multiple invasions of Bemisia argentifolii into Australia and its current genetic connectivity across space. J. Pest Sci. 2021, 94, 1331–1343. [Google Scholar] [CrossRef]
- Hopkinson, J.; Pumpa, S.; van Brunschot, S.; Fang, C.; Frese, M.; Tay, W.T.; Walsh, T. Insecticide resistance status of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae) in Australian cotton production valleys. Austral. Entomol. 2020, 59, 202–214. [Google Scholar] [CrossRef]
- Iram, A.; Khan, J.K.J.; Aslam, N.A.N.; Ehasan-Ul-Haq, E.-U.-H.; Javed, H.I.; Irlan, M.I.M.; Rasool, A.R.A.; Aslam, S.A.S. Efficacy of plant derived oils and extracts against whitefly, Bemisia tabaci (Gennadius) on sesame crop. Pak. J. Agric. Res. 2014, 27, 250–254. [Google Scholar]
- Laurentin, H.; Pereira, C.; Sanabria, M. Phytochemical characterization of six sesame (Sesamum indicum L.) genotypes and their relationships with resistance against the sweetpotato whitefly Bemisia tabaci Gennadius. Agron. J. 2003, 95, 1577–1582. [Google Scholar] [CrossRef]
- de Almeida Marques, M.; Quintela, E.D.; Mascarin, G.M.; Fernandes, P.M.; Arthurs, S.P. Management of Bemisia tabaci biotype B with botanical and mineral oils. Crop Prot. 2014, 66, 127–132. [Google Scholar] [CrossRef]
- Hori, K.; Miles, P. The etiology of damage to lucerne by the green mirid, Creontiades dilutus (Stal). Aust. J. Exp. Agric. 1993, 33, 327–331. [Google Scholar] [CrossRef]
- Whitehouse, M. IPM of mirids in Australian cotton: Why and when pest managers spray for mirids. Agric. Syst. 2011, 104, 30–41. [Google Scholar] [CrossRef]
- McColl, S.A.; Khan, M.; Umina, P.A. Review of the biology and control of Creontiades dilutus (Stål) (Hemiptera: Miridae). Aust. J. Entomol. 2011, 50, 107–117. [Google Scholar] [CrossRef]
- Mensah, R.; Khan, M. Use of Medicago sativa (L.) interplantings/trap crops in the management of the green mirid, Creontiades dilutus (Stal) in commercial cotton in Australia. Int. J. Pest Manag. 1997, 43, 197–202. [Google Scholar] [CrossRef]
- Khan, M.; Gregg, P.; Mensah, R. Effect of temperature on the biology of Creontiades dilutus (Stål) (Heteroptera: Miridae). Aust. J. Entomol. 2009, 48, 210–216. [Google Scholar] [CrossRef]
- Hereward, J.P. Molecular Ecology of the Green Mirid Creontiades Dilutus Stål (Hemiptera: Miridae)-Movement and Host Plant Interactions Across Agricultural and Arid Environments. Ph.D. Thesis, School of Biological Science, The University of Queensland, Brisbane, QLD, Australia, 2012. [Google Scholar]
- Hereward, J.; Walter, G.H.; De Barro, P.J.; Lowe, A.J.; Riginos, C. Gene flow in the green mirid, Creontiades dilutus (Hemiptera: Miridae), across arid and agricultural environments with different host plant species. Ecol. Evol. 2013, 3, 807–821. [Google Scholar] [CrossRef]
- Atlas of Living Australia. Creontiades dulutus (Stal, 1859). Available online: https://bie.ala.org.au/species/https://biodiversity.org.au/afd/taxa/36fcd47e-c0cb-47a4-96e9-24336b966809 (accessed on 16 December 2024).
- Parry, H.R.; Marcora, A.; Macfadyen, S.; Hopkinson, J.; Hulthen, A.D.; Neave, M.; Bianchi, F.J.J.A.; Franzamann, B.A.; Lloyd, R.J.; Miles, M. A native with a taste for the exotic: Weeds and pasture provide year-round habitat for Nysius vinitor (Hemiptera: Orsillidae) across Australia, with implications for area-wide management. Austral. Entomol. 2019, 58, 237–247. [Google Scholar] [CrossRef]
- Kehat, M.; Wyndham, M. Flight activity and displacement in the Rutherglen bug Nysius vinitor (Hemiptera: Lygaeidae). Aust. J. Zool. 1973, 21, 413–426. [Google Scholar] [CrossRef]
- Smith, J.H. Life History Notes on the Rutherglen Bug. Qld. Agric. J. 1927, 27, 285–302. [Google Scholar]
- Baker, G. Developing Strawberry IPM: Testing OP-Resistant Predatory Mites; South Australia Research & Development Institute (SARDI): Adelaide, SA, Australia, 2012. [Google Scholar]
- Moradi-Vajargah, M.; Parry, H.R. Environmental and biological drivers of flight initiation in a sporadic pest, Rutherglen bug, Nysius vinitor Bergroth (Hemiptera: Orsillidae). Austral. Entomol. 2017, 56, 225–234. [Google Scholar] [CrossRef]
- Murray, D. Facilitating IPM adoption in northern region broadacre farming systems. Final Report 2009, Queensland Department of Food and Fisheries. Available online: https://era.dpi.qld.gov.au/id/eprint/2941/1/GRDC_final_report_DAQ00074.pdf (accessed on 10 January 2025).
- McDonald, G.; Broadley, R.H.; Smith, A.M.; Blackburn, M.D. Evaluation of insecticides for‘Nysius vinitor’Bergroth (Hemiptera: Lygaeidae). Gen. Appl. Entomol. J. Entomol. Soc. N.S.W. 1986, 18, 11–16. [Google Scholar]
- Cotton Research and Development Corporation (CRDC). CottonInfo. Cotton Pest Management Guide 2024–2025; CRDC: Shanghai, China, 2024. [Google Scholar]
- Mishra, S.K.; Kumar, R.; Pandey, S.; Rai, A.; Mishra, R.K. Sesame leaf roller and capsule borer, Antigastra catalaunalis (Dup.) (Lepidoptera: Crambidae): A review. Pharma Innov. Int. J. 2023, 12, 2220–2224. [Google Scholar]
- Panday, A.; Rajani, B.; Surabhi, J.; Ranganatha, A. Efficacy and economics of different insecticidal treatments for the management of major sucking insect pests of sesame. J. Entomol. Zool. Stud. 2018, 6, 1247–1252. [Google Scholar]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [PubMed]








| Disease | Causal Pathogen | Countries Where Reported |
|---|---|---|
| A. Fungi | ||
| Charcoal rot/Dry root rot | Macrophomina phaseolina | Australia, Bangladesh, Brazil, China, Colombia, Cuba, Cyprus, Ecuador, Egypt, Ethiopia, Greece, Honduras, India, Iran, Iraq, Israel, Japan, Kenya, Mexico, Myanmar, Nicaragua, Nigeria, Pakistan, Paraguay, Republic of Korea, Sri Lanka, Sudan, Syria, Tanzania, Thailand, Turkey, Uganda, USA, and Venezuela [16] |
| Wilts | Fusarium oxysporum f. sp. sesame/Fusarium spp. | Australia, Bangladesh, Brazil, Bulgaria, China, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Egypt, Ethiopia, Greece, Guatemala, Honduras, India, Iran, Iraq, Israel, Italy, Japan, Kenya, Malawi, Mexico, Nicaragua, Nigeria, Pakistan, Paraguay, Philippines, Republic of Korea, Saudi Arabia, Sierra Leone, Sudan, Tanzania, Thailand, Turkey, Uganda, Ukraine, USA, Uzbekistan, and Venezuela [16] |
| Verticillium spp. | Australia, Bulgaria, China, Egypt, Ethiopia, India, Pakistan, Turkey, Uganda, USA, and Uzbekistan [16] | |
| Neocosmospora spp. | Uzbekistan [16] | |
| Root rot/Stem rot | Rhizoctonia solani | Australia, Bolivia, Brazil, China, Colombia, Costa Rica, Dominican Republic, Egypt, India, Iraq, Japan, Myanmar, Nicaragua, Pakistan, Panama, Republic of Korea, Uganda, USA, and Venezuela [16] |
| Sclerotinia spp. | India, and Mexico [16] | |
| Phymatotrichopsis spp. | USA [16] | |
| Thielaviopsis spp. | Egypt and USA [16] | |
| Helicobasidium spp. | China [16] | |
| Gibberella spp. | India, Iran, Iraq, Nigeria, Pakistan, Republic of Korea, Sudan, and Uganda [16] | |
| Root/stem/collar rot, southern blight | Sclerotium rolfsii | China, Costa Rica, Greece, Honduras, India, Italy, Japan, Mexico, Nicaragua, Nigeria, Philippines, Sudan, USA, and Venezuela [16] |
| Alternaria leaf spot/leaf blight | Alternaria sesame/A. alternata/A. simsimi/Alternaria spp. | Australia, Bolivia, Brazil, Burkina Faso, China, Costa Rica, Cuba, Egypt, Ethiopia, Greece, Guatemala, Honduras, India, Iran, Iraq, Israel, Japan, Kenya, Mexico, Myanmar, Nicaragua, Nigeria, Pakistan, Paraguay, Republic of Korea, Russia, Saudi Arabia, Sudan, Tanzania, Turkey, Uganda, Ukraine, USA, and Venezuela [16,17] |
| Cercospora leaf spot | Cercospora sesame | Australia, Brazil, Burkina Faso, China, Colombia, Dominican Republic, Egypt, Ethiopia, Guatemala, Honduras, India, Israel, Italy, Japan, Kenya, Mexico, Myanmar, Nicaragua, Nigeria, Pakistan, Panama, Paraguay, Philippines, Somalia, Sri Lanka, Sudan, Surinam, Tanzania, Thailand, Turkey, Uganda, USA, and Venezuela [16] |
| Angular leaf spot | Cercospora sesamicola | Australia [18], India [19], Kenya [20] |
| Powdery mildew | Oidium spp. | Australia, China, Ethiopia, Greece, India, Israel, Japan, Mexico, Myanmar, Nigeria, Sri Lanka, Sudan, Tanzania, Uganda, USA, and Venezuela [16] |
| Podosphaera xanthii (=Sphaeroteca fuliginea) | Australia, China, Ethiopia, India, Iraq, Japan, Malawi, Mexico, Somalia, Sudan, Tanzania, and Turkey [16] | |
| Leveillula taurica/Leveillula spp. | India, Italy, Japan, Mexico, Pakistan, USA, and Venezuela [16] | |
| Erysiphe cichoracearum (syn. Golovinomyces cichoracearum)/Erysiphe orontii/Erysiphe spp. | China, Ethiopia, India, Japan, Mexico, Sudan, Thailand, Republic of Korea, and Uganda [16] | |
| Podosphaera spp. | Australia, China, Ethiopia, India, Iraq, Japan, Malawi, Mexico, Somalia, Sudan, Tanzania, and Turkey [16] | |
| Corynespora blight and target spot | Corynespora cassiicola | Australia, Brazil, China, Colombia, Costa Rica, Cuba, Ecuador, India, Japan, Mexico, Republic of Korea, USA, and Venezuela [16] |
| Brown angular spot | Cylindrosporium sesame/Pseudocercospora sesami | Australia, Ecuador, Mexico, Venezuela [16] |
| Aerial stem rot/Leaf spot | Helminthosporium sesame/Helminthosporium spp. | China, Costa Rica, Egypt, India, Italy, Japan, Kenya, Nigeria, Philippines, Saudi Arabia, Tanzania, and USA [16] |
| Leaf spots | Ascochyta spp. | China, Japan, and Sudan [16] |
| Cladosporium spp. | Cuba, Egypt, India, Iran, Iraq, Nigeria, Pakistan, Saudi Arabia, and Venezuela | |
| Curvularia spp. | Bangladesh, Cuba, India, Nigeria, Pakistan, Paraguay, Saudi Arabia, and Sudan | |
| Colletotrichum spp. | China, India, Italy, Japan, Mexico, Myanmar, Nigeria, Paraguay, Republic of Korea, Thailand, Uganda, and USA [16] | |
| Drechslera spp. | Brazil, Egypt, India, Mexico, Pakistan, Saudi Arabia, and Sudan [16] | |
| Cercoseptoria spp. | India, USA, and Venezuela [16] | |
| Cochliobolus spp. | Cuba, India. and Nigeria [16] | |
| Gloeosporium spp. | Italy [16] | |
| Exserohilum spp. | Egypt and Saudi Arabia [16] | |
| Phoma spp. | Brazil, China, Cuba, Egypt, India, Italy, Japan, Mexico, Nigeria, Philippines, Republic of Korea, Sudan, and Venezuela [16] | |
| Pseudocercosporella spp. | India and Turkey [16] | |
| Myrothecium spp. | Egypt and India [16] | |
| Paramyrothecium spp. | Cuba, India, and Pakistan [16] | |
| Pestalotiopsis spp. | Nigeria [16] | |
| Stem blight | Didymella spp. | Cambodia, India, and Mexico [16] |
| Wet rot of seedlings | Choanephora cucurbitarum | India [16] |
| Leaf blight | Nigrospora sphaerica | China [21]; Egypt, and Pakistan [16] |
| Anthracnose | Colletotrichum spp. | China, India, Italy, Japan, Mexico, Myanmar, Nigeria, Paraguay, Republic of Korea, Thailand, Uganda, and USA [16] |
| Sphaeronema spp. | India [16] | |
| Rust/Warts | Synchitrium spp. | India and Mexico [16] |
| B. Oomycetes | ||
| Phytophthora blight | Phytophthora spp. | Argentina, China, Dominican Republic, Egypt, Guatemala, Honduras, India, Iran, Japan, Kenya, Malawi, Mexico, Nicaragua, Nigeria, Paraguay, Peru, Republic of Korea, Sri Lanka, Tanzania, Thailand, Turkey, USA, and Venezuela [16]; Australia [22] |
| Damping-off | Pythium spp. | Australia, Costa Rica, Egypt, India, Iraq, Kenya, Mexico, Pakistan, Republic of Korea, Thailand, USA, and Venezuela [16] |
| C. Bacteria | ||
| Bacterial leaf spot | Pseudomonas spp. | Australia, Brazil, Bulgaria, Burkina Faso, China, Cuba, Ethiopia, Greece, Guatemala, India, Japan, Kenya, Macedonia, Malawi, Mexico, Myanmar, Nigeria, Pakistan, Paraguay, Republic of Korea, Somalia, Sudan, Tanzania, Thailand, Turkey, USA, and Venezuela [16] |
| Bacterial blight | Xanthomonas campestris pv. sesami | Myanmar [8]; Brazil, Burkina Faso, China, Ecuador, Ethiopia, Honduras, India, Japan, Malawi, Mexico, Myanmar, Nicaragua, Nigeria, Pakistan, Paraguay, Republic of Korea, Sudan, Turkey, USA, and Venezuela [16] |
| Pseudomonas solanacearum = Ralstonia solanacearum | China, India, Iraq, Japan, Mexico, Republic of Korea, Thailand, and USA [16] | |
| Bacterial wilt | Erwinia spp. | Ethiopia [16] |
| Phyllody, Witches’ broom | Phytoplasma | Myanmar [8]; Australia, Brazil, Burkina Faso, China, Egypt, Ethiopia, India, Iran, Iraq, Israel, Italy, Japan, Kenya, Malawi, Mexico, Myanmar, Niger, Nigeria, Oman, Pakistan, Paraguay, Philippines, Republic of Korea, Senegal, Sierra Leone, Sri Lanka, Sudan, Syria, Taiwan, Tanzania, Thailand, Turkey, Uganda, USA, Venezuela, and Vietnam [16] |
| Yellows | Spiroplasma spp. | Iran and Turkey [16] |
| Seed-borne disease | Memnoniella spp. | India [16] |
| D. Viruses | ||
| Mosaic | Cowpea Aphid-Borne Mosaic Virus (CABMV) | Ivory Coast, Mexico, Paraguay, and USA [16,23] |
| Tobacco vein banding mosaic virus (TVBMV) | China [16,24] | |
| Watermelon Mosaic Virus (WMV) | China, Japan, and Republic of Korea [16]; Australia [25] | |
| Bean Common Mosaic Virus (BCMV) | China [16] | |
| Zucchini yellow mosaic virus (ZYMV) | China [16] | |
| Sesame yellow mosaic virus (YMo-I) | China [26] | |
| Turnip mosaic virus (TuMV) | China and Japan [16] | |
| Pepper mild mosaic virus (PMMoV) | China [16] | |
| Tobacco mosaic virus (TMV) | Nigeria [16] | |
| Stripe | Peanut stripe virus (PSV) | China [16] |
| Leaf curl | Sesame curly top virus (SeCTV) | Iran [16] |
| Tomato yellow leaf curl virus (TYLCV) | Nigeria [16] | |
| Tobacco leaf curl virus (TLCV) | China, India, Mexico, Myanmar, Pakistan, Nigeria, Sierra Leone, Sudan, Tanzania, and Venezuela [16] | |
| Spotted wilt | Tomato spotted wilt virus (TSWV) | Mexico [16] |
| Yellow spot | Melon yellow spot virus (MYSV) | Mexico [16] |
| Bud necrosis | Groundnut bud necrosis virus (GBNV) | India [16] |
| Family | Species | Global Significance to Sesame | Status in Australian Sesame |
|---|---|---|---|
| Lepidoptera | |||
| Crambidae | Antigastra catalaunalis | Major pest of foliage and pods in Africa, India, and SE Asia | Actual and Possible Major |
| Maruca vitrata | Minor pest of pods in Uganda | ||
| Noctuidae | Agrotis ipsilon | Destructive but sporadic foliage and stem pest | Possible Major |
| Argyrogramma signata | Foliage pest India | ||
| Chrysodeixis acuta | Foliage pest Nigeria | ||
| Helicoverpa armigera | Global generalist pest, foliage and pods | Actual (scarce) | |
| Helicoverpa punctigera | Australian endemic, foliage and pods | Actual (scarce) | |
| Spodoptera exigua | Global generalist pests, minor foliage pests on sesame in India, Turkey, US, and Japan | Actual (scarce) | |
| Spodoptera littoralis | |||
| Spodoptera litura | |||
| Spodoptera frugiperda | |||
| Thysanoplusia orichalcea | Foliage pest, India, Bangladesh | ||
| Hemiptera | |||
| Aleyrodidae | Bemisia tabaci (or B. argentifolii) | Major foliage pest in India, Africa, and South America | Actual and Possible Major |
| Alydidae | Leptocorisa acuta | Pod pest, Bangladesh | |
| Aphididae | Aphis craccivora | Foliage pest, South America | |
| Aphis gossypii | Foliage pest, reported major pest in India, minor pest elsewhere | ||
| Myzus persicae | Major foliage pest India, China, Africa, South America | Actual and Possible Major | |
| Cicadellidae | Amrasca biguttula | Minor foliage pest, India | |
| Balclutha incisa | Minor foliage pest, India | ||
| Batracomorphus angustatus | Foliage pest India | ||
| Cicadulina bipunctata | Foliage pest Turkey | ||
| Cofana spectra | Foliage pest Bangladesh | ||
| Deltocephalus sp. | Foliage pest and phyllody vector, Africa, and Asia | Possible Major # | |
| Exitianus sp. | Foliage pest, India | ||
| Orosius orientalis | Foliage pest and phyllody vector, India, Japan, Middle East | Possible Major # | |
| Cixiidae | Oliarus sp. | Foliage pest, India | |
| Delphacidae | Cemus sp. | Foliage and pod pest, India | |
| Lyaeidae | Elasmolomus pallens (sordidus) | Pod pest, Africa, India, and Bangladesh | |
| Nysius vinitor | Australian endemic | Actual (pods) | |
| Miridae | Campylomma spp. | Foliage pest, India | |
| Creontiades dilutus | Australian endemic | Actual (foliage, pods) | |
| Nesidiocoris tenuis | Australian endemic | Actual (foliage, pods) | |
| Poppiocapsidea biseratense | Foliage pest (minor), India | ||
| Taylorilygus sp. | Foliage | ||
| Pentatomidae | Chinavia hilaris | Pods | |
| Nezara viridula | Pods | Actual and Possible Major | |
| Plautia affinis | Australian endemic | Actual | |
| Pseucoccidae | Phenacoccus solenopsis | Global generalist pest, pest on sesame in Pakistan, Ethiopia | |
| Pyrrhocoridae | Dysdercus cingulatus | Minor pod pest Bangladesh | |
| Tingidae | Telenonemia scrupulosa | Incidental foliage pest, East Africa * | |
| Thripidae | Frankliniella occidentalis | Flower pest, Turkey, USA | |
| Frankliniella schultzei | Flower pest, Bangladesh | ||
| Scirtothrips dorsalis | Foliage pest, India | ||
| Thrips hawaiiensis | Foliage pest, India | ||
| Thrips palmi | Flower pest, India, Cuba | ||
| Thrips tabaci | Foliage pest, India, Nigeria | ||
| Orthoptera | |||
| Acrididae | Gastrimargus musicus | Australian endemics, observed 1970–1980s, foliage feeders | Actual |
| Austracris guttulosa | Actual | ||
| Hymenoptera | |||
| Formicidae | Pheidole ampla | Australian endemic, observed 1980–1990s, seed harvester ant | Actual |
| Coleoptera | |||
| Cerambycidae | Oberea sp. | Stem pest, India | |
| Chrysomelidae | Aulocophora sp. | Foliage pest, India | |
| Monolepta signata | Foliage pest, Bangladesh | ||
| Diptera | |||
| Psilidae | Chyliza sp. | Stem pest, India |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adorada, D.L.; Jones, L.C.; Liu, J.; Gurr, G.M. Sesame Diseases and Pests: Assessment of Threats to the Establishment of an Australian Industry. Crops 2025, 5, 44. https://doi.org/10.3390/crops5040044
Adorada DL, Jones LC, Liu J, Gurr GM. Sesame Diseases and Pests: Assessment of Threats to the Establishment of an Australian Industry. Crops. 2025; 5(4):44. https://doi.org/10.3390/crops5040044
Chicago/Turabian StyleAdorada, Dante L., Lachlan C. Jones, Jian Liu, and Geoff M. Gurr. 2025. "Sesame Diseases and Pests: Assessment of Threats to the Establishment of an Australian Industry" Crops 5, no. 4: 44. https://doi.org/10.3390/crops5040044
APA StyleAdorada, D. L., Jones, L. C., Liu, J., & Gurr, G. M. (2025). Sesame Diseases and Pests: Assessment of Threats to the Establishment of an Australian Industry. Crops, 5(4), 44. https://doi.org/10.3390/crops5040044







