Allelopathic Effect of Salvia pratensis L. on Germination and Growth of Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Collection and Preparation of Water Extracts
2.2. Petri Dish Bioassay
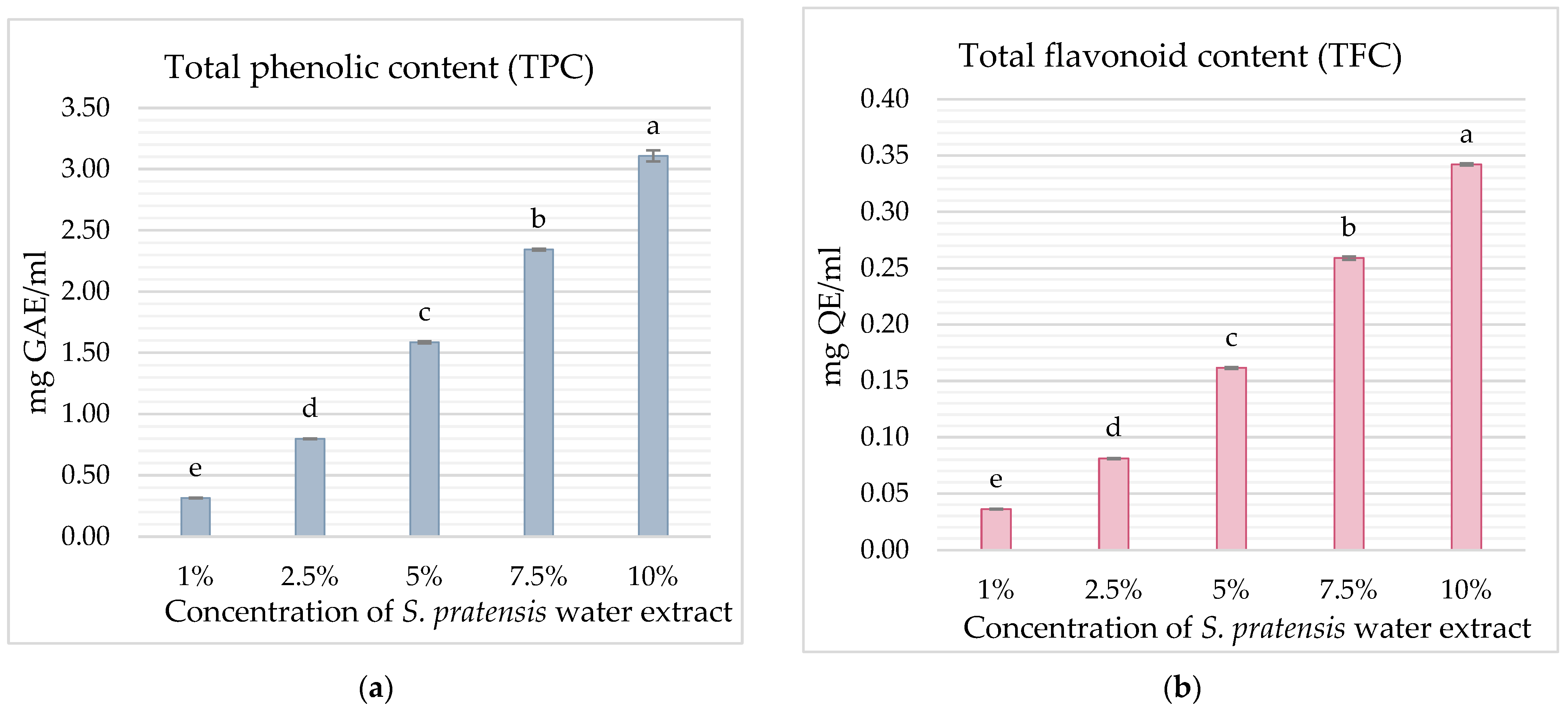
2.3. Determination of Total Phenolic and Flavonoid Content
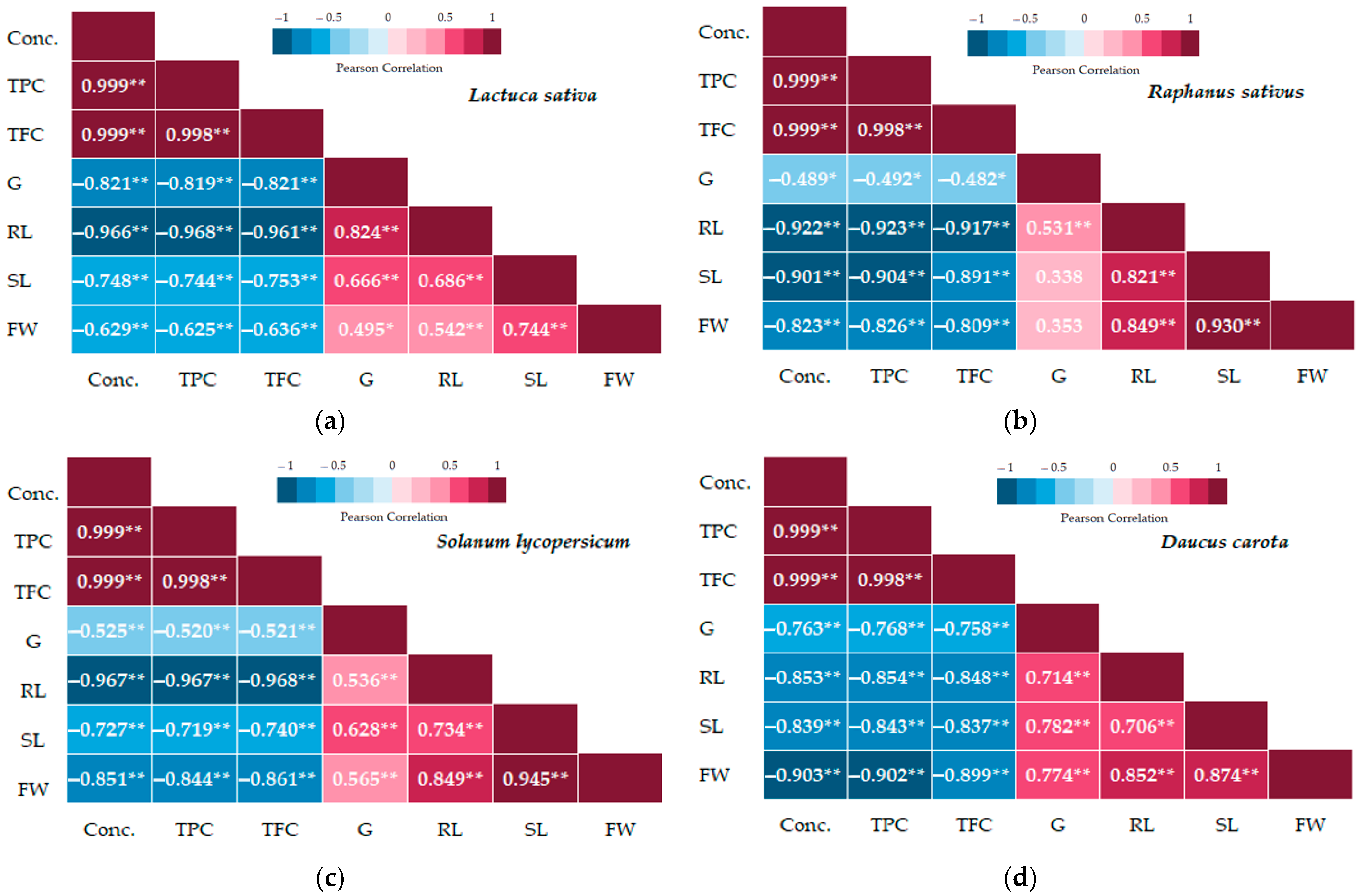
2.4. Statistical Analysis
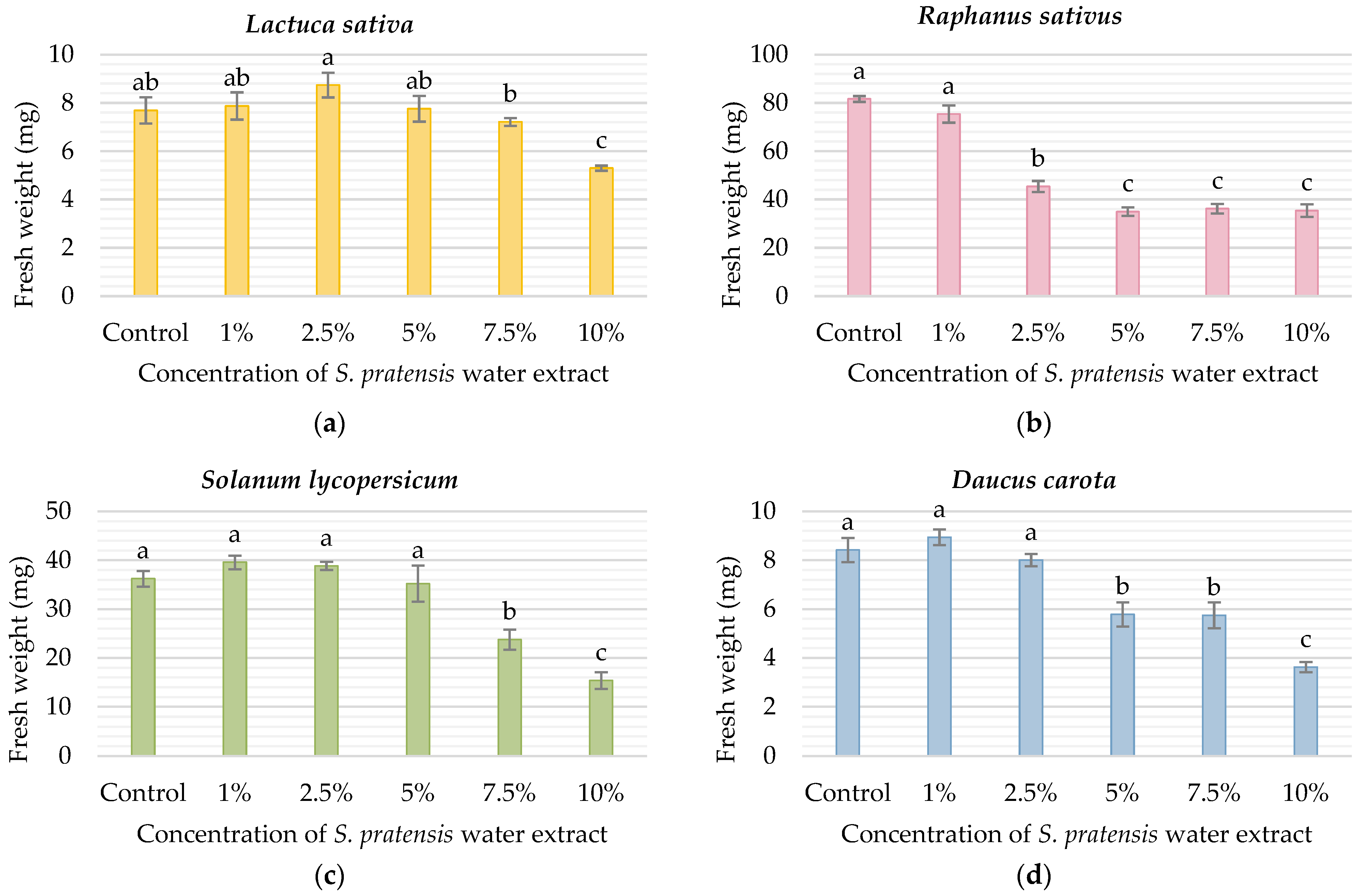
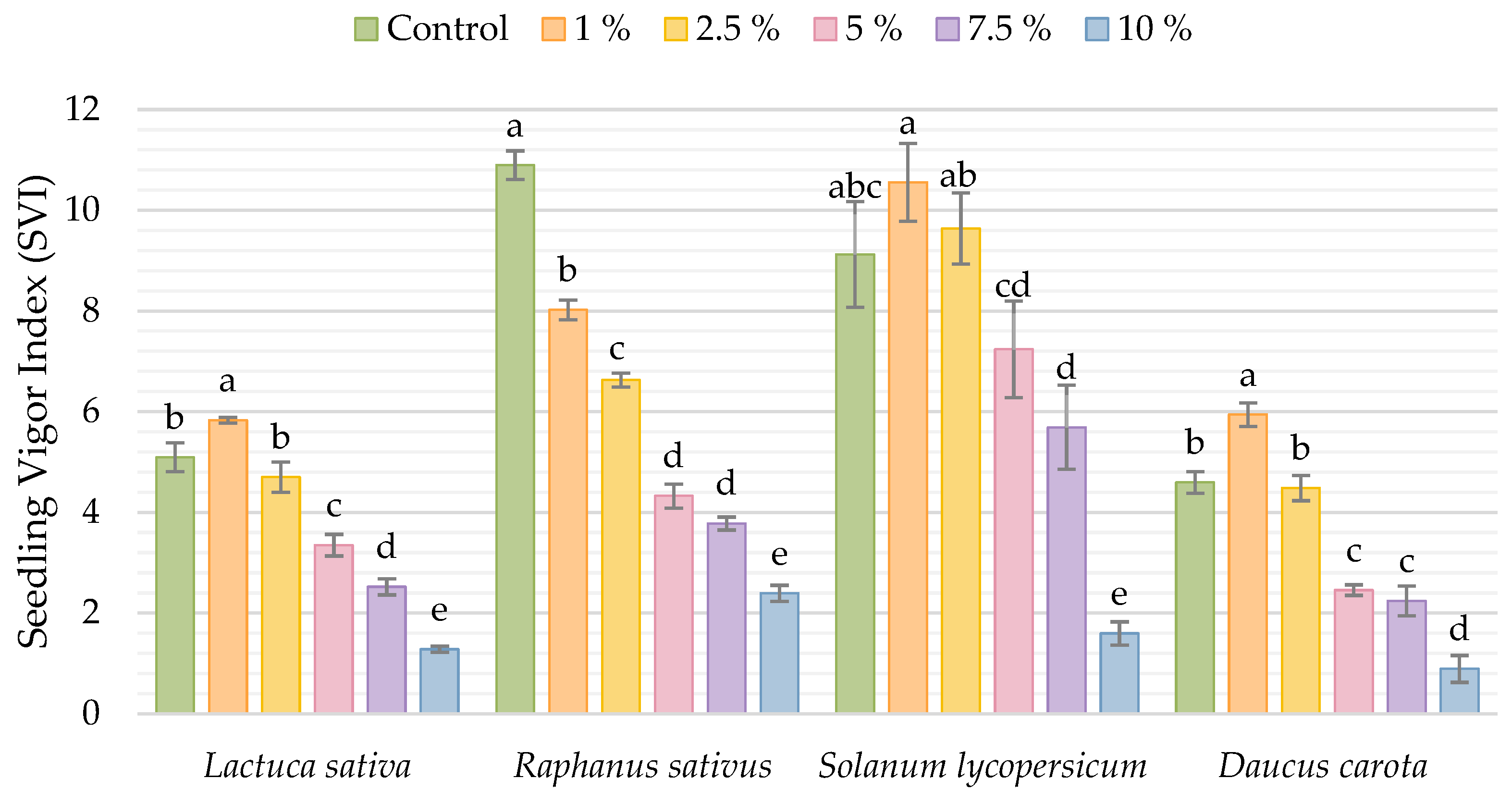
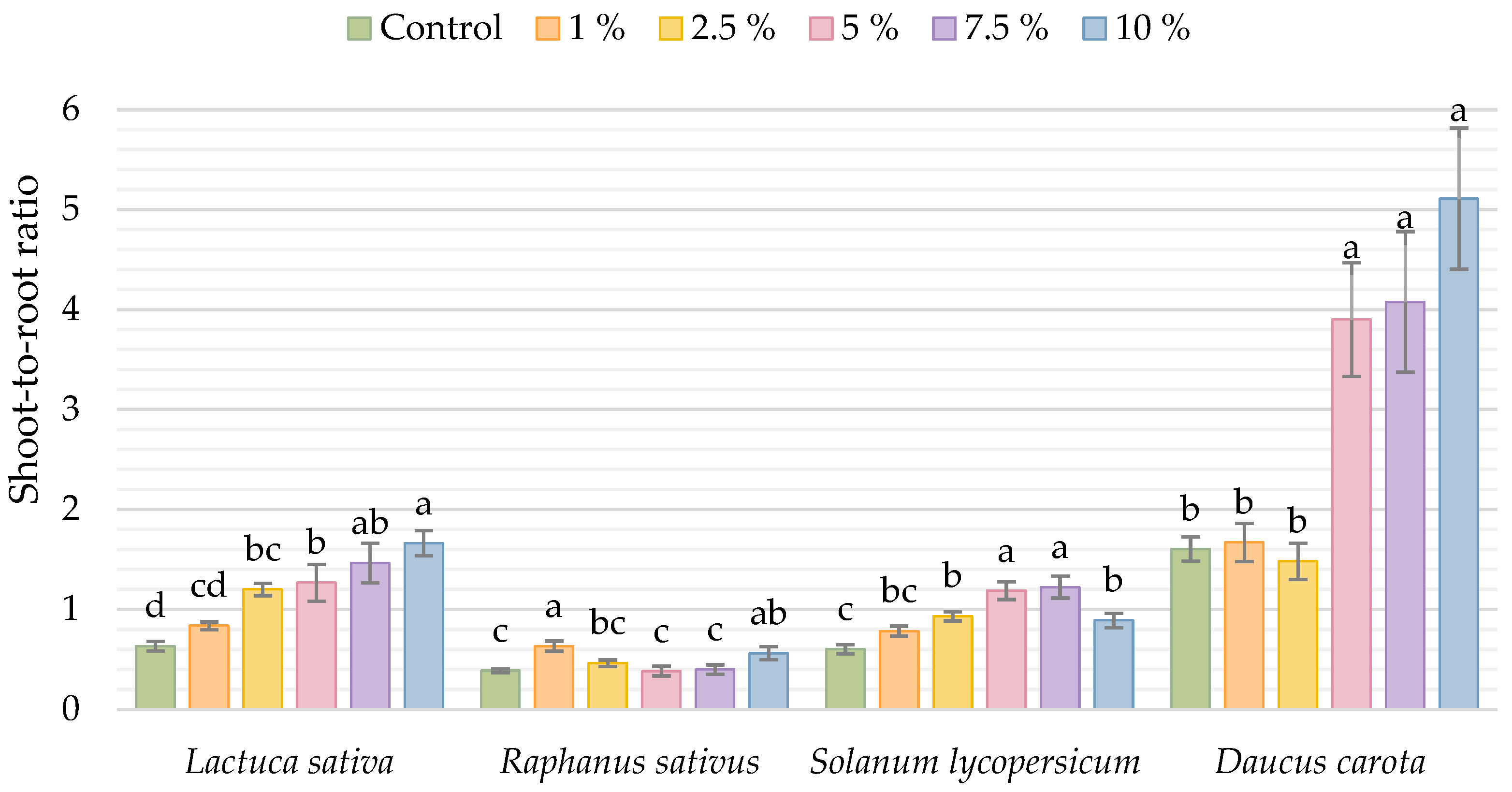
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; p. 400. [Google Scholar]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and its Application as a Weed Management Tool: A Review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef] [PubMed]
- Uzelac Božac, M.; Poljuha, D.; Dudaš, S.; Bilić, J.; Šola, I.; Mikulič-Petkovšek, M.; Sladonja, B. Phenolic Profile and Antioxidant Capacity of Invasive Solidago canadensis L.: Potential Applications in Phytopharmacy. Plants 2025, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant Allelochemicals and Their Various Applications. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2020; pp. 441–465. [Google Scholar] [CrossRef]
- Šćepanović, M.; Košćak, L.; Šoštarčić, V.; Pismarović, L.; Milanović-Litre, A.; Kljak, K. Selected Phenolic Acids Inhibit the Initial Growth of Ambrosia artemisiifolia L. Biology 2022, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; He, F.; Liang, Q.; Bo, Y.; Lin, X.; Javed, Q.; Ullah, M.S.; Sun, J. Allelopathic Effects of Caffeic Acid and Its Derivatives on Seed Germination and Growth Competitiveness of Native Plants (Lantana indica) and Invasive Plants (Solidago canadensis). Agriculture 2023, 13, 1719. [Google Scholar] [CrossRef]
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The Ecological Importance of Allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Nath, C.P.; Singh, R.G.; Choudhary, V.K.; Datta, D.; Nandan, R.; Singh, S.S. Challenges and Alternatives of Herbicide-Based Weed Management. Agronomy 2024, 14, 126. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Moradinezhad, F. Evaluation of Allelopathic Activity of 68 Medicinal and Wild Plant Species of Iran by Sandwich Method. Int. J. Hortic. Sci. Technol. 2016, 3, 243–253. [Google Scholar] [CrossRef]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef]
- Mervić, M.; Bival Štefan, M.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative Antioxidant, Anti-Acetylcholinesterase and Anti-α-Glucosidase Activities of Mediterranean Salvia Species. Plants 2022, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Sadeqifard, S.; Mirmostafaee, S.; Joharchi, M.R.; Zandavifard, J.; Azizi, M.; Fujii, Y. Evaluation of Allelopathic Activity Interactions of Some Medicinal Plants Using Fractional Inhibitory Concentration and Isobologram. Agronomy 2022, 12, 3001. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Vinković, Ž.; Brozović, B.; Sarajić, A.; Kranjac, D. The Allelopathic Potential of Ruderal Plant Species on Tomato and Lettuce. Poljoprivreda 2024, 30, 3–9. [Google Scholar] [CrossRef]
- Li, B.; Cantino, P.D.; Olmstead, R.G.; Bramley, G.L.C.; Xiang, C.-L.; Ma, Z.-H.; Tan, Y.-H.; Zhang, D.-X. A Large-Scale Chloroplast Phylogeny of the Lamiaceae Sheds New Light on Its Subfamilial Classification. Sci. Rep. 2016, 6, 34343. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.K.M.M.; Suttiyut, T.; Anwar, M.P.; Juraimi, A.S.; Kato-Noguchi, H. Allelopathic Properties of Lamiaceae Species: Prospects and Challenges to Use in Agriculture. Plants 2022, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Bouajaj, S.; Benyamna, A.; Bouamama, H.; Romane, A.; Falconieri, D.; Piras, A.; Marongiu, B. Antibacterial, Allelopathic, and Antioxidant Activities of Essential Oil of Salvia officinalis L. Growing Wild in the Atlas Mountains of Morocco. Nat. Prod. Res. 2013, 27, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Silva, C.T.A.; Nóbrega, L.H.P.; Dellagostin, S.M.; Silva, C.F.G. Salvia officinalis L. Coverage on Plant Development. Rev. Bras. Plantas Med. 2016, 18, 488–493. [Google Scholar] [CrossRef]
- Bonea, D. Allelopathic Effect of Sage on Germination and Initial Growth of Maize. An. Univ. Craiova Agric. Montanol. Cad. 2018, 48, 54–60. [Google Scholar]
- Amani, M.; Younessi-Hamzekhanlu, M.; Nojadeh, M.S.; Karkaj, E.S. Evaluation of antioxidant properties of phenol, flavonoid, and allelopathy of Achillea wilhelmsii and Salvia officinalis L. extracts. J. Environ. Sci. Stud. 2021, 6, 3793–3802. [Google Scholar]
- Politi, M.; Ferrante, C.; Menghini, L.; Angelini, P.; Flores, G.A.; Muscatello, B.; Braca, A.; De Leo, M. Hydrosols from Rosmarinus officinalis, Salvia officinalis, and Cupressus sempervirens: Phytochemical analysis and bioactivity evaluation. Plants 2022, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Knežević, M. Atlas korovne, Ruderalne i Travnjačke Flore, 3rd ed.; University of Josip Juraj Strossmayer of Osijek, Faculty of Agriculture in Osijek: Osijek, Croatia, 2006; p. 249. [Google Scholar]
- Moughan, J.; McGinn, K.J.; Jones, L.; Rich, T.C.G.; Waters, E.; de Vere, N. Biological Flora of the British Isles: Salvia pratensis. J. Ecol. 2021, 109, 4171–4190. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Puricelli, C.; Ciuchi, D.; Segale, A.; Fico, G. Traditional knowledge on medicinal and food plants used in Val San Giacomo (Sondrio, Italy)—An alpine ethnobotanical study. J. Ethnopharmacol. 2013, 145, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Veličković, A.S.; Ristić, M.S.; Veličković, D.T.; Ilić, S.N.; Mitić, N.D. The possibilities of the application of some species of sage (Salvia L.) as auxiliaries in the treatment of some diseases. J. Serb. Chem. Soc. 2003, 68, 435–445. [Google Scholar] [CrossRef]
- Smékalová, K.; Dušek, K.; Dušková, E. Salvia verticillata L. and Salvia pratensis L.—The variability of essential oil content in the Czech Republic. Acta Hortic. 2010, 860, 51–60. [Google Scholar] [CrossRef]
- Srećković, N.; Mišić, D.; Gašić, U.; Matić, S.L.; Katanić Stanković, J.S.; Mihailović, N.R.; Monti, D.M.; D’Elia, L.; Mihailović, V. Meadow sage (Salvia pratensis L.): A neglected sage species with valuable phenolic compounds and biological potential. Ind. Crops Prod. 2022, 189, 115841. [Google Scholar] [CrossRef]
- Gruľová, D.; Baranová, B.; Eliašová, A.; Brun, C.; De Martino, L.; Caputo, L.; Poračová, J.; Nastišin, Ľ.; Fejér, J.; Elshafie, H.S.; et al. Salvia pratensis L. Extracts as Potential Eco-Friendly Herbicides for Sustainable Agricultural Applications. Sci. Rep. 2025, 15, 659. [Google Scholar] [CrossRef] [PubMed]
- Itani, T.; Nakahata, Y.; Kato-Noguchi, H. Allelopathic Activity of Some Herb Plant Species. Int. J. Agric. Biol. 2013, 15, 1359–1362. [Google Scholar]
- Ravlić, M.; Baličević, R.; Svalina, T.; Posavac, D.; Ravlić, J. Herbicidal potential of meadow sage (Salvia pratensis L.) against velvetleaf (Abutilon theophrasti Med.) and common corn-cockle (Agrostemma githago L.). Glasnik Zaštite Bilja 2023, 46, 116–121. [Google Scholar] [CrossRef]
- Hess, M.; Barralis, G.; Bleiholder, H.; Buhr, H.; Eggers, T.; Hack, H. Use of the extended BBCH scale—General for the description of the growth, stages of mono- and dicotyledonous species. Weed Res. 1997, 37, 433–441. [Google Scholar] [CrossRef]
- Norsworthy, J.K. Allelopathic potential of wild radish (Raphanus raphanistrum). Weed Technol. 2003, 17, 307–313. [Google Scholar] [CrossRef]
- Islam, A.; Anuar, N.; Yaakob, Z. Effect of Genotypes and Pre-Sowing Treatments on Seed Germination Behavior of Jatropha. Asian J. Plant Sci. 2009, 8, 433–439. [Google Scholar] [CrossRef]
- Smith, O.P. Allelopathic potential of the invasive alien Himalayan Balsam (Impatiens glandulifera Royle). Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2013. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Isla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Bisio, A.; Fraternale, D.; Giacomini, M.; Giacomelli, E.; Pivetti, S.; Russo, E.; Caviglioli, G.; Romussi, G.; Ricci, D.; De Tommasi, N. Phytotoxicity of Salvia spp. Exudates. Crop Prot. 2010, 29, 1434–1446. [Google Scholar] [CrossRef]
- Erez, M.E.; Fidan, M. Allelopathic effects of sage (Salvia macrochlamys) extract on germination of Portulaca oleracea seeds. Allelopathy J. 2015, 35, 285–296. [Google Scholar]
- Šućur, J.; Popovıć, A.; Petrovıć, M.; Anačkov, G.; Malenčıć, D.; Prvulovıć, D. Allelopathic Effects and Insecticidal Activity of Salvia sclarea L. Studia UBB Chem. 2015, 60, 253–264. [Google Scholar]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a Source of Bioherbicides: Challenges and Prospects for Sustainable Agriculture. Rev. Environ. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Staszek, P.; Krasuska, U.; Ciacka, K.; Gniazdowska, A. ROS Metabolism Perturbation as an Element of Mode of Action of Allelochemicals. Antioxidants 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Šoln, K.; Klemenčič, M.; Koce, J.D. Plant cell responses to allelopathy: From oxidative stress to programmed cell death. Protoplasma 2022, 259, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Erhatić, R.; Horvat, D.; Zorić, Z.; Repajić, M.; Jović, T.; Herceg, M.; Habuš, M.; Srečec, S. Aqueous Extracts of Four Medicinal Plants and Their Allelopathic Effects on Germination and Seedlings: Their Morphometric Characteristics of Three Horticultural Plant Species. Appl. Sci. 2023, 13, 2258. [Google Scholar] [CrossRef]
- Findura, P.; Kocira, S.; Hara, P.; Pawłowska, A.; Szparaga, A.; Kangalov, P. Extracts from Artemisia vulgaris L. in Potato Cultivation—Preliminary Research on Biostimulating Effect. Agriculture 2020, 10, 356. [Google Scholar] [CrossRef]
- Pannacci, E.; Baratta, S.; Falcinelli, B.; Farneselli, M.; Tei, F. Mugwort (Artemisia vulgaris L.) Aqueous Extract: Hormesis and Biostimulant Activity for Seed Germination and Seedling Growth in Vegetable Crops. Agriculture 2022, 12, 1329. [Google Scholar] [CrossRef]
- Ali, Q.; Perveen, R.; El-Esawi, M.A.; Ali, S.; Hussain, S.M.; Amber, M.; Iqbal, N.; Rizwan, M.; Alyemeni, M.N.; El-Serehy, H.A.; et al. Low Doses of Cuscuta reflexa Extract Act as Natural Biostimulants to Improve the Germination Vigor, Growth, and Grain Yield of Wheat Grown under Water Stress: Photosynthetic Pigments, Antioxidative Defense Mechanisms, and Nutrient Acquisition. Biomolecules 2020, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Kasim, S.; Yang, Z.; Deng, X.; Saidi, N.B.; Uddin, M.K.; Shuib, E.M. Plant Extracts as Biostimulant Agents: A Promising Strategy for Managing Environmental Stress in Sustainable Agriculture. Phyton—Int. J. Exp. Bot. 2024, 93, 2149–2166. [Google Scholar] [CrossRef]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Generalić, I.; Skroza, D.; Šurjak, J.; Smole Možina, S.; Ljubenkov, I.; Katalinić, A.; Šimat, V.; Katalinić, V. Seasonal variations of phenolic compounds and biological properties in sage (Salvia officinalis L.). Chem. Biodivers. 2012, 9, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Horvat, D.; Tucak, M.; Viljevac Vuletić, M.; Čupić, T.; Krizmanić, G.; Kovačević Babić, M. Phenolic Content and Antioxidant Activity of the Croatian Red Clover Germplasm Collection. Poljoprivreda 2020, 26, 3–10. [Google Scholar] [CrossRef]
- Appiah, K.S.; Mardani, H.K.; Omari, R.A.; Eziah, V.Y.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Amoatey, C.A.; Kawada, K.; Katsura, K.; Oikawa, Y.; et al. Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus officinalis Leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Tsytsiura, Y. The assessment of allelopathic sensitivity of oilseed radish (Raphanus sativus L. var. oleiformis Pers.) to the main weeds of its agrocenoses at the stage of initial growth. Ştiinţa Agric. 2021, 2, 40–48. [Google Scholar]
- Ravlić, M.; Markulj Kulundžić, A.; Baličević, R.; Marković, M.; Viljevac Vuletić, M.; Kranjac, D.; Sarajlić, A. Allelopathic Potential of Sunflower Genotypes at Different Growth Stages on Lettuce. Appl. Sci. 2022, 12, 12568. [Google Scholar] [CrossRef]
- Medina-Villar, S.; Uscola, M.; Pérez-Corona, M.E.; Jacobs, D.F. Environmental stress under climate change reduces plant performance, yet increases allelopathic potential of an invasive shrub. Biol. Invasions 2020, 22, 2859–2881. [Google Scholar] [CrossRef]
- Appiah, K.S.; Omari, R.A.; Onwona-Agyeman, S.; Amoatey, C.A.; Ofosu-Anim, J.; Smaoui, A.; Arfa, A.B.; Suzuki, Y.; Oikawa, Y.; Okazaki, S.; et al. Seasonal Changes in the Plant Growth-Inhibitory Effects of Rosemary Leaves on Lettuce Seedlings. Plants 2022, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Tojić, T.; Đorđević, T.; Đurović-Pejčev, R.; Aćimović, M.; Božić, D.; Radivojević, L.; Sarić-Krsmanović, M.; Vrbničanin, S. Allelopathic Potential of Artemisia absinthium and Artemisia vulgaris from Serbia: Chemical Composition and Bioactivity on Weeds. Plants 2025, 14, 1663. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Schwambach, J.; Soares, G.L.G. Seed Size Correlation with Phytotoxic Effects of Baccharis psiadioides Essential Oil during Seeds Germination. Allelopathy J. 2018, 44, 107–118. [Google Scholar] [CrossRef]
- Belz, R.G.; Hurle, K.; Duke, S.O. Dose-response-a challenge for allelopathy? Nonlinearity Biol. Toxicol. Med. 2005, 3, 173–211. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Matloob, A.; Cheema, Z.A.; Farooq, M. Allelopathic Activity of Crop Residue Incorporation Alone or Mixed Against Rice and Its Associated Grass Weed Jungle Rice (Echinochloa colona [L.] Link). Chilean J. Agric. Res. 2011, 71, 418–423. [Google Scholar] [CrossRef]



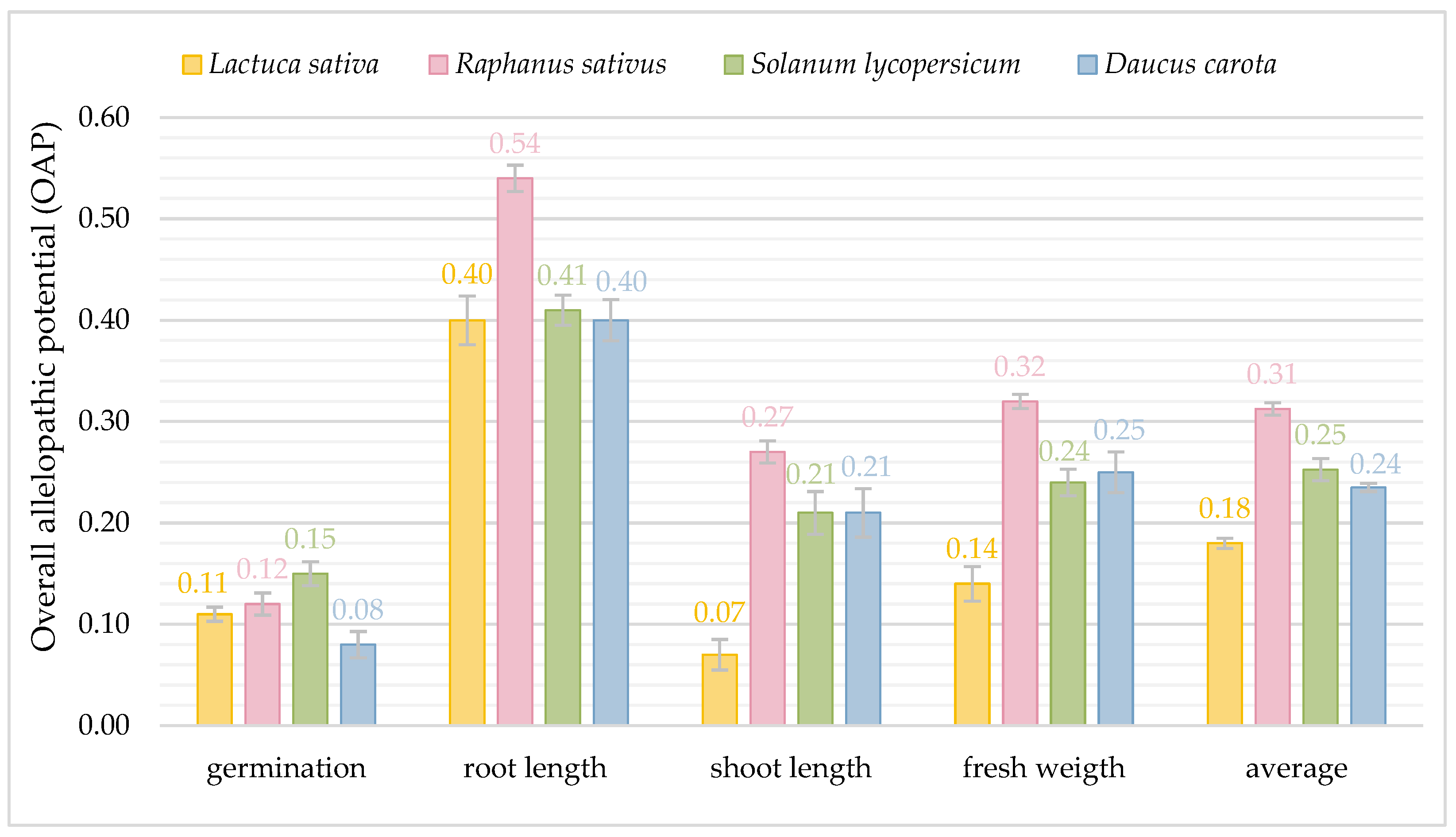
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravlić, M.; Baličević, R.; Lisjak, M.; Vinković, Ž.; Ravlić, J.; Županić, A.; Svitlica, B. Allelopathic Effect of Salvia pratensis L. on Germination and Growth of Crops. Crops 2025, 5, 45. https://doi.org/10.3390/crops5040045
Ravlić M, Baličević R, Lisjak M, Vinković Ž, Ravlić J, Županić A, Svitlica B. Allelopathic Effect of Salvia pratensis L. on Germination and Growth of Crops. Crops. 2025; 5(4):45. https://doi.org/10.3390/crops5040045
Chicago/Turabian StyleRavlić, Marija, Renata Baličević, Miroslav Lisjak, Željka Vinković, Jelena Ravlić, Ana Županić, and Brankica Svitlica. 2025. "Allelopathic Effect of Salvia pratensis L. on Germination and Growth of Crops" Crops 5, no. 4: 45. https://doi.org/10.3390/crops5040045
APA StyleRavlić, M., Baličević, R., Lisjak, M., Vinković, Ž., Ravlić, J., Županić, A., & Svitlica, B. (2025). Allelopathic Effect of Salvia pratensis L. on Germination and Growth of Crops. Crops, 5(4), 45. https://doi.org/10.3390/crops5040045




