Abstract
The genus Musa L. is one of the most important genera worldwide due to its use in food as a source of carbohydrates. A morphological characterization was performed to evaluate the potential of 100 accessions of Musa spp. from the Amazon region of Ecuador, applying 73 qualitative and quantitative descriptors in addition to the ecogeographic characterization. The multivariate analyses identified four large groups: The first is composed of the Musa AAB Simmonds ecotype “Hartón Plantain” and the “Cuerno Clone”. The second group is composed of the Musa acuminata Colla ecotype “Orito”. The third group is composed of the Musa acuminata ecotype “Malay plantain or red plantain”; and the fourth group is composed of the Musa × paradisiaca L. AAB ecotype “Barraganete” and banana or banana materials and the Musa AAB Simmonds ecotype “Plátano Dominico”. The qualitative descriptors with the highest discriminant value were the shape of the ♂ floret bud, the appearance of the rachis, and the pigmentation of the compound tepal, and the quantitative discriminant characters were the height of the pseudostem, the length of the leaf blade, the width of the leaf blade, and the weight of the raceme. The analysis with CAPFITOGEN of these 100 accessions through the ecogeographic characterization map identified 23 categories, highlighting category 20 with a coverage of 40.35%, which mainly includes the provinces of Orellana, Sucumbíos, part of Napo, Pastaza, and Morona Santiago. This category occurs within an annual temperature range between 21.6 °C and 27 °C, an apparent density of 1.25 to 1.44 g cm−3, and a cation exchange capacity (CEC) of 4 to 29 Cmol kg−1. The morphological characterization of 100 Musa accessions revealed significant phenotypic variability, with four distinct morphological groups identified through cluster analysis. Key differences were observed in traits such as bunch weight, fruit length, and vegetative vigor. This variability highlights the potential of certain accessions for use in genetic improvement programs. The findings contribute valuable information for the efficient conservation, selection, and utilization of the Musa germplasm in Ecuadorian agroecosystems. The results demonstrate the existence of an important genetic variability in the INIAP Musa Germplasm Bank in the Ecuadorian Amazon region.
1. Introduction
The Musaceae family, order Scitamineae, encompasses the genera Musa L., Ensete Bruce ex Horan. and Musella (Franch.) C.Y.Wu ex H.W.Li, but all edible banana and plantain fruits belong to the genus Musa [1,2]. They are monocotyledonous plants from intra- and interspecific crosses between Musa acuminata Colla (genome A) and Musa balbisiana Colla (genome B) from the Southeast Asian center of origin [3,4,5]. In order of economic importance, there are triploid (AAA, AAB, and ABB), diploid (AA and AB), and tetraploid (AAAA, AAAB, and AABB) bananas [6].
The edible banana and plantain were introduced into Africa from the center of origin in Southeast Asia during prehistory as key vegetative crops, valued for their adaptation to the humid tropics and their role in transcontinental agricultural exchange [5,7].
The expansion of the plantains and bananas (Musa spp.) in the Neotropics occurred during the 16th and early 17th centuries, from both the West Indies, Macaronesia, mainly the Canary Islands, and Africa to the Atlantic coast of South America (Brazil) to Cartagena de Indias. These crops have been used by indigenous communities, who promoted their expansion throughout the Meso and South Americas to the ecological limits of their cultivation, as recorded by the chroniclers and settlers [8].
There are no official records of materials introduced into Ecuador; however, through personal communication with Musa experts in the country, they indicate that the materials introduced into Ecuador are the following: Bananas: Formosana (Taiwan–South Africa), Gal (Israel), and Cavendish varieties (Central America) and Plantains: Fhia 21 (Honduras) and Hua moa (Polynesian). Regarding the introduction of banana and plantain materials in Ecuador, the Dominico, Hartón, Barraganete plantain (Central America, Colombia), according to the Ignacio Sotomayor former leader, came from the national Ecuadorian Musa program (Sotomayor, pers. comm., 20 May 2025).
Guerrero [9] studied seven Musa cultigens, among which are Abacá (Australimusa), green Maqueño, curaré (AAB), purple Maqueño (AAA), Cuatrofilos (ABB), Vinces (AAAA), and Manzano (AAA).
Information on specific collections from private plant breeders is scarce and not available to external users.
The acquisition of accessions from the INIAP Germplasm Bank by plant breeders for research purposes is subject to the signing of a document by the requesting entity and the person responsible for the plant material, who represents the INIAP National Department of Plant Genetic Resources (DENAREF). This procedure complies with the Convention on Biological Diversity (CBD), Decision 391 of the Andean Community of Nations, and the International Treaty on Plant Genetic Resources for Food and Agriculture, instruments that recognize genetic resources as national heritage.
Currently, Ecuador has two production systems for the cultivation of Musa spp., one for export and another for local markets. Regarding the export production system, Ecuador ranks fifth worldwide in banana fruit production, with a 5.3% share, and is the second-largest Latin American producer, following Brazil. However, it maintains its leadership as the leading global exporter, with a 28.5% market share [10]. Ecuador’s banana sector GDP contributed 17.4% of the agricultural Gross Value Added (GVA). In 2022, banana cultivation accounted for 12.0% of the area dedicated to permanent crops, distributed across nineteen provinces, with a national production of 6,078,789 tons of fresh fruit [11]. On the other hand, there are also banana and plantain cultivars in the production systems of small and subsistence farmers in the Coast and Amazonian regions for the national market and self-consumption. In 2014, the National Institute of Agricultural Research (INIAP) conducted collecting missions and established an ex situ gene bank of species and varieties of bananas and plantains from the Amazon region of Ecuador at INIAP’s Central Amazon Experimental Station because no national collection of these cultivars was available previously. Plant germplasm is essential for genetic improvement programs, which aim to obtain materials with superior characteristics, high quality, and resistance to major phytosanitary problems [12,13]. Worldwide, the Musa Germplasm Information System (MGIS) contains key information on the genus’ germplasm diversity. Molecular studies and geospatial information (GIS) on 7025 ex situ accessions managed in 32 collections worldwide and 3373 in situ observations in 135 countries make it the most comprehensive source of information on banana genetic resources [14].
INIAP conserves a total of 391 accessions in its germplasm banks, distributed between the Pichilingue and Central Amazonian Experimental Stations. The Pichilingue Experimental Station houses 83 plantain and 108 banana accessions, while the Central Amazonian Experimental Station houses 111 plantain, 77 plantain, and 14 orito (baby banana) accessions.
The Ecuadorian banana boom, which occurred between 1948 and 1965, was driven by the productive expansion and commercialization of bananas in the country, which generated a significant socioeconomic impact and structural transformations throughout its historical development [15].
The cultivation of Musa in Ecuador faces multiple challenges that threaten the sustainability and genetic diversity of the crop, such as climate change, phytosanitary threats, genetic erosion, and limited characterization, among others. Banana and plantain farmers face serious challenges due to climate change, which alters growth and production [16]. Asexual reproduction limits genetic diversity, reducing the capacity to adapt to new threats. Diseases such as black Sigatoka (Mycosphaerella fijiensis (Morelet)) and moko (the Betaproteobacteria Ralstonia solanacearum Race 3), along with pests such as the plantain nematode (Radopholus similis (Cobb) Thorne), threaten the sustainability of the crop [17]. Furthermore, the low genetic variability hinders the development of resistant varieties, limiting advances in genetic improvement.
Morphological characterization refers to the determination of a set of traits using defined descriptors that allow for the taxonomic differentiation of plants [18]. In turn, ecogeographic characterization involves the analysis and description of the environmental, edaphic, and geographic characteristics of a given area to understand the distribution and living conditions of various species or crops [19,20]. This analysis is based on climatic, soil, and geophysical variables and enables the identification of suitable areas for the conservation and production of plant genetic resources.
Both morphological and ecogeographic characterization are essential as they contribute to agrobiodiversity conservation, help identify materials with adaptive potential, and strengthen food security by providing valuable information for breeding programs [21]. In the case of Musa spp. in Ecuador, genetic and technological improvement activities have been concentrated in El Triunfo (Guayas province), a strategic area due to its high banana productivity. Consequently, several initiatives have been developed nationwide to improve harvesting, postharvest practices, and climate change adaptation. However, available sources do not provide detailed information on the specific locations or scope of all implemented projects.
The cultivation and improvement of Musa in Ecuador face several challenges that threaten sustainability and genetic diversity, such as the spread of pests and diseases, vulnerability to climate change, the dominance of a few commercial cultivars, and the insufficient description and use of available genetic resources. In genetic resource management, characterization refers to the systematic description of observable traits (morphological, physiological, or agronomic), while evaluation focuses on assessing the performance and adaptive potential of accessions under specific environmental conditions [22,23,24].
The objective of the Musa collection of the National Institute of Agricultural Research (INIAP) is to establish, conserve, and manage a representative collection of species and varieties present in Ecuador, with the purpose of conserving genetic diversity, supporting scientific research, fostering environmental education, and promoting the sustainable development of the agricultural sector. Currently, the collection consists of 100 accessions conserved in the field at the Central Amazon Experimental Station. These accessions were collected in the six provinces that make up the Ecuadorian Amazon region. Conservation is carried out primarily ex situ through field banks.
INIAP maintains a collection of Musa spp. at the Central Amazon Experimental Station (EECA) in Orellana, with the objective of conserving and characterizing the genetic diversity of bananas and plantains using morphological descriptors established by IPGRI-INIBAP/CIRAD. Globally, between 70% and 85% of the Musa gene pool is concentrated in Asia and the Pacific, while the most important collection is preserved at the International Transit Centre (ITC) in Belgium, with over 1500 accessions under the auspices of Bioversity International. The conservation of these resources is carried out through ex situ methods such as in vitro culture, cryopreservation, and field gene banks, as well as in situ in their natural habitats, in order to address risks such as diseases and genetic erosion [5].
Consequently, this research aimed to study the agro-morphological and ecogeographical diversity of the 100 accessions in the National Musa Collection collected in the six provinces of the Amazon region of Ecuador in 2014, following the protocol of Monteros-Altamirano et al. [25], thus contributing to their conservation and the sustainable management of the priority crop in Ecuador by identifying materials with tolerance to abiotic stress and the climatic, edaphic, and geophysical factors of the sampling areas. Given their strategic value, it was necessary to conduct morphological characterization to identify materials with potential for genetic improvement programs, as well as ecogeographical characterization to allow for planning future collections in areas not yet represented in the preserved collection.
Although bananas and plantains are not native to Ecuador, their economic importance and high level of consumption position them as traditional crops in the country. These products constitute a basic component of the daily diet and represent a symbol of the Ecuadorian agricultural identity. In 2022, annual per capita banana consumption was approximately 2.5 kg, while that of plantains reached 30 kg, according to the Ecuadorian Banana Exporters Association (2021). This marked difference is attributed, in part, to cultural and culinary factors, as plantains are widely consumed in their various forms (ripe or green) and prepared in typical dishes such as ‘patacones’ and ‘bolones’ [26], i.e., daily household consumption. Bananas, meanwhile, being one of the country’s main export products, are mostly destined for the international market, which reduces their availability for domestic consumption.
Ecuador, the world’s largest banana exporter, recorded a 6.7% growth in shipments, with the country’s total exports increasing to approximately 6.2 million tons [27]. Therefore, banana and plantain cultivation are fundamental to the agricultural sector, both economically and nutritionally in terms of gross production value after cocoa, shrimps, and coffee [28]. With more than 370,000 hectares under cultivation, INIAP has led efforts to conserve and improve the Musa germplasm, aiming to address challenges such as disease pressure and environmental stress [28,29,30].
2. Materials and Methods
2.1. Morphological Characterization
The research was conducted at the National Institute of Agricultural Research (INIAP) at the Central Amazon Experimental Station (EECA) located in the province of Orellana, Ecuador (00°21′31.2″ S latitude, 76°52′40.1″ W longitude, and 250 m a.s.l.), with an average annual rainfall of 3500 mm and an average annual temperature of 25 °C. For morphological characterization, the 100 accessions of Musa available were used; these accessions come from collections made in the Ecuadorian Amazon (Figure 1). INIAP has seven experimental stations strategically distributed throughout the country. Among them, the Central Amazon Experimental Station (EECA) is responsible for the territory corresponding to the Ecuadorian Amazon region and is part of the INIAP National Germplasm Bank. The Musa collection is conserved in the field at EECA, which is located in the province of Orellana, Ecuador, at the geographic coordinates of 00°21′31.2″ S latitude and 76°52′40.1″ W longitude at 250 m a.s.l. As mentioned above, germplasm collecting was carried out following the criteria established in the protocol by Monteros-Altamirano et al. [25].
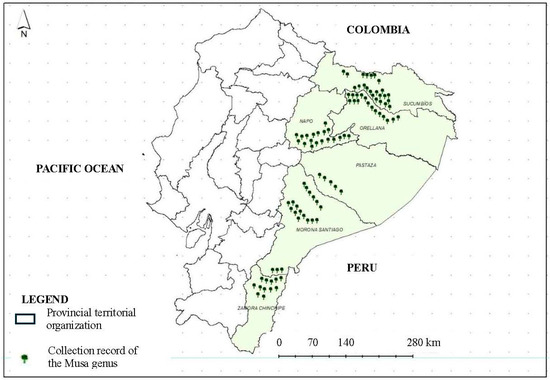
Figure 1.
Origin of the Musa accessions from the Ecuadorian Amazon region.
Two complementary characterization approaches were used for the analysis of the conserved accessions: (i) morphological characterization, understood as the evaluation of a set of phenotypic traits defined by standardized descriptors, which allow for the establishment of taxonomic relationships between the evaluated plant materials [18,19] and (ii) ecogeographic characterization, consisting of the analysis of environmental and geographical conditions, with the purpose of understanding the distribution and adaptation patterns of plant species in relation to their environment in a specific geographical area [19].
Germplasm collecting was carried out through targeted random sampling in the six provinces that make up the Amazon region of Ecuador, with the goal of capturing the greatest possible diversity of plantains and bananas present in traditional production systems. To this end, farms and plots where high phenotypic variability was observed were prioritized.
The mother plants selected for collecting must be vigorous and free of viruses or other diseases; they must also be at least 8 years old and have been cultivated in the locations where they are collected.
Ten individual plants were evaluated, following the standard methodology recommended by IPGRI for Musa characterization. This sample size allows for the adequate representation of inter-accessional variability under field conditions. The ten plants per accession were planted in the field in a single row, with a spacing of 4 × 4 m between plants, and the four central plants were evaluated (to eliminate the edge effect). Note: There is a misunderstanding about the term “intra-accession variability”, since asexual propagation was used during the field collecting process. Specifically, clones of each ecotype present on the farms were collected, and each was individually registered as a distinct accession. These clones were subsequently introduced into the INIAP germplasm collection, located at the Central Amazon Experimental Station. Therefore, each accession represents a single genotype with no internal genetic variability but with inter-accessional variation.
The four plants used for each accession were each evaluated for quantitative and qualitative descriptors. The average of the evaluations of the four plants by quantitative and qualitative descriptor was then calculated, and this average represented the value for each accession. The techniques used were descriptive statistics and multivariate statistics.
For each accession, the morphological data were likewise collected from the four focal plants. For quantitative descriptors, three replicate measurements per trait were taken and averaged per plant. These were then averaged across the four plants in each accession to obtain accession-level means. Standard deviations were also calculated to assess intra-accession variability. For qualitative descriptors, the most frequent category observed among the four plants per accession was recorded, following the IPGRI descriptor scoring guidelines.
The descriptors established by IPGRI/INIBAP/CIRAD [31] and Santo-Pineda et al. [19] were composed of 16 quantitative variables and 57 qualitative variables. To determine the coloration variables of the leaves, flowers, and fruits, the RHS color table [32] was used; the quantitative and qualitative variables are included in Table 1 and Table 2. The data obtained from the morphological characterization were analyzed using the InfoStat/Professional version 2020 program and the R statistical software [33].

Table 1.
Quantitative morphological descriptors of the Musa.

Table 2.
Qualitative morphological descriptors of the Musa.
Qualitative and quantitative variables were hierarchically grouped using Ward’s method and Gower’s distance [34]. Contingency tables were also used for the chi-square test, and multiple correspondence analyses were performed.
2.2. Ecogeographic Characterization
INIAP DENAREF conserves accessions using various strategies, supported by information stored in databases, such as the passport data from the INIAP Germplasm Bank in Ecuador. These standardized data accompany germplasm samples, facilitating their identification and international exchange. They include details on the species, location, and collection date, as well as the geophysical, climatic, and edaphic characteristics of the site of origin. Each sample has a unique identifier, and the information is backed up in the GRIN-Global database, which manages plant genetic resources; therefore, the information used was from the collection site.
The ecogeographic data for each accession were obtained from the geographic coordinates recorded in the passport data at the original collecting sites, i.e., the various locations of the farms where collecting was carried out. Using these coordinates, environmental variables were extracted from global geospatial datasets using the CAPFITOGEN3 tools, ensuring standardization and comparability across accessions. No in situ measurements of climatic or soil variables were performed.
Various tools from the CAPFITOGEN3 program [35] were used. First, Testable was used to verify that the tables with the entered information met all the necessary conditions for correct processing. Subsequently, the quality of the georeferencing of the passport data was evaluated using the GEOQUAL tool. After filtering out records with values above 80% on the GEOQUAL 0–100 evaluation scale, the final database for the analysis of Musa collections consisted of 100%, according to the CAPITOGEN tools. The GEOQUAL tool rates the quality of the georeferenced data according to three criteria: land use, the quality of the data themselves (if the data have complete information: degrees, minutes, and seconds), and the correspondence between the geographic location of the data and their political location. Each criterion is weighted, and the sum of the three defines the general quality of the georeferenced information. In this sense, it was decided to opt for the percentage of 80% (minimum percentage reached by some points after the analysis) so that all collection points were considered in the analysis.
Using the Selecvar tool, fifteen variables were selected: four climatic (annual average temperature, the mean temperature of the warmest quarter, the mean temperature of the coldest quarter, and the precipitation of the wettest quarter), seven geophysical (altitude and the wind speed in the months of February, March, April, August, September, and October), and four edaphic (available water capacity in the soil, water content in the topsoil, bulk density, and cation exchange capacity). To define the environments in which the Musa germplasm is cultivated, an eco-geographical terrain characterization (ELC) map was generated using the ELC-map tool, with a resolution of 1 × 1 km (30 arcseconds). Cultivated germplasm refers to the cultigen that farmers plant and manage within their on-farm production systems.
To define the environmental conditions of the original Musa accession collection sites, ecogeographic characterization was performed using the ELC-map tool. This approach permitted us to identify the ecological contexts in which the germplasm occurs naturally or is traditionally cultivated.
3. Results
3.1. Grouping of Accessions Based on Morphological Variables
Hierarchical clustering using qualitative and quantitative variables identified significant differences among the four groups of Musa accessions. Significance (p < 0.0001) was obtained using multivariate analysis.
Cluster analysis was performed, which allowed variables with similar characteristics to be grouped into homogeneous groups. The four groups identified belong to the Musa AAB Simmonds ecotype “Dominican Banana”, banana or plantain materials, and the Musa paradisiaca AAB ecotype “Barraganete”; the Musa acuminata ecotype “Orito”; the Musa AAB Simmonds ecotype “Hartón Banana” and the “Cuerno Clone”; and the Musa acuminata ecotype “Red Banana”. Although the 100 accessions analyzed represent a significant part of the genetic diversity of the Musa types cultivated in Ecuador, it is estimated that these 100 accessions represent approx. 80% of the diversity present in the Amazon and 40% nationally. Although bananas and plantains are not native to Ecuador, their ‘Andeanization’ and secondary diversification in traditional systems, especially in small areas of the Amazon and the coast, have generated significant local variation.
3.2. Combined Analysis of Qualitative and Quantitative Variables
The taxonomic structure refers to the hierarchical and systematic organization of species based on their morphological characteristics. This structure allows for a better classification and understanding of the diversity within the plantain and banana group; therefore, understanding this taxonomic structure is essential for conservation, germplasm management, and genetic improvement programs. The “systematic organization of species and varieties” to classify and organize plants according to taxonomic and agronomic criteria while considering morphological and ecological characteristics is important.
Hierarchical clustering was performed using Ward’s method and Gower’s distance based on a combined matrix of 73 morphological descriptors (16 quantitative and 57 qualitative variables). This analysis revealed significant phenotypic differentiation among the accessions, resulting in the formation of four well-defined groups (p < 0.0001).
These groupings reflect the underlying genomic and morphological structure of the collection. Group 1 includes AAB types such as the Hartón and Cuerno ecotypes; Group 2 corresponds to the Musa acuminata (AA) ecotype Orito; Group 3 includes other M. acuminata ecotypes such as Malay and the Red Banana; and Group 4 is composed of AAB genotypes including Dominico, Barraganete, and banana-like cultivars. This classification is primarily based on morphological differentiation obtained through multivariate analysis and aligns with known taxonomic and agronomic groupings of the Musa germplasm traditionally described in Ecuador.
The result of grouping the accessions obtained with the Ward method and the Gower distance allowed us to identify the taxonomic structure of the collection between the groupings, forming four genetic groups (Figure 2 and Table 3).
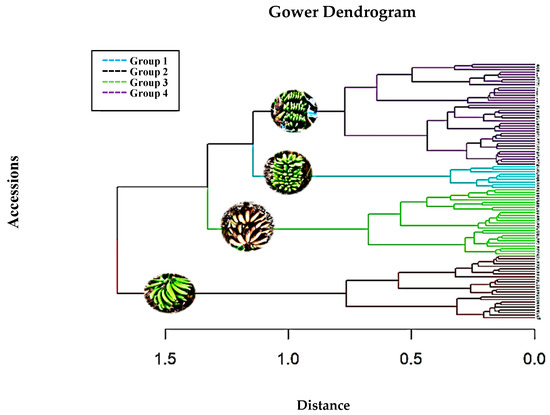
Figure 2.
Dendrogram obtained using hierarchical cluster analysis (Ward’s method and Gower’s distance) based on the qualitative and quantitative morphological descriptors of 100 Musa accessions. Group 1 (Musa AAB “Hartón Plantain” and “Cuerno Clone”), Group 2 (Musa acuminata “Orito”), Group 3 (Musa acuminata “Malay” and “Red Banana”), and Group 4 (Musa paradisiaca “Barraganete” and AAB “Dominican Plantain”).

Table 3.
Accessions from the INIAP Germplasm Bank contained in the 4 groups of accessions in Figure 2.
Within these groups, the accessions of the Musa acuminata ecotype “Malay banana” or “red banana” are present in Group 3. Group 4 contains the most significant number of accessions (40) and includes accessions of the Musa AAB Simmonds ecotype “Dominican banana”, “guineo”, or “banana” materials and the Musa paradisiaca AAB ecotype “Barraganete”. In contrast, Group 2 consists of nine accessions. The groups with the most significant similarity for the qualitative and quantitative variables are Group 3 and Group 4.
To summarize, the plantains in this group are Barraganete, Dominico, Dominico Hartón, Dominico gigante, Maqueño verde, and Limeño. The AAB group has similar characteristics in the color of their peel and flesh (yellow and cream, respectively).
G2: Diploid (AA)—within this group is the orito (Musa acuminata AA), a banana variety grown in Ecuador. It is estimated that there are approx. 8000 ha in cultivation, and its consumption has benefited producers and consumers [36] (Crespo & Guanochanga, 2022). The orito has gone from being a marginal crop to becoming an export product from Ecuador, consumed in the US, Japan, and Europe. This crop grows at altitudes of 200 to 800 m a.s.l., where it produces high-quality fruit [37] (Jiménez-Esparza et al., 2019).
3.3. Grouping of Accessions Based on Quantitative Morphological Characters
Table 4 presents the averages of the most important quantitative characteristics of the four genetic groups identified in the clustering analysis. As a summary, G3, with the variable exceptional vegetative development, presented the greatest vegetative development, standing out with the height of the pseudostem at 4.051 m (the highest value among the groups), the length of the leaf blade at 2.695 m, and the width of the leaf blade at 85.89 cm. In addition, the bunch weight of this group was the largest at 24.93 kg, which suggests a positive correlation between vegetative vigor and bunch weight. In contrast, G2, with the high fruit production variable, stands out for the highest number of fruits per average hand at 20 fruits and the highest total number of fruits per bunch at 143 fruits; however, despite this high production, the fruits of G2 had a shorter external length (13.08 cm); this is because they are from the group of ‘oritos’ or the so-called bananas; regarding G1 with the variable larger size fruits, an external fruit length of 32.13 cm, an internal fruit length of 19.4 cm, and a fruit diameter of 4.42 cm were recorded, as well as a fruit weight of 0.33 kg; this was followed by G4, who presented a number of fruits (107); their fruits were characterized by having an extreme fruit length (23.4 cm external length), a diameter of 3.44 cm, a cluster weight of 16.78 kg, and an individual fruit weight of 0.14 kg fruit−1. The natural grouping observed in the accessions suggests the existence of well-defined patterns of morphological variation within the studied collection. This natural grouping refers to groups of accessions that share similar genetic, morphological, or geographical characteristics and are formed spontaneously by natural and human selection in different regions. Therefore, the clusters observed in the dendrogram represent morphological groupings resulting from hierarchical analysis based on combined qualitative and quantitative traits.

Table 4.
Analysis of the average of the characters evaluated by group (1, 2, 3, and 4).
This type of hierarchical grouping has been previously documented in studies on the genetic divergence of the Musa germplasm, as mentioned in the works by Nadal-Medina et al. [38] and Ukwueze et al. [39], who determined that fruit characteristics are key in the differentiation of genetic groups within the genus Musa.
The observed values (Table 4) show notable morphological variability in several key characteristics. The height of the pseudostem varied between 3.24 m and 4.05 m, reflecting the diversity in the vegetative development of the accessions evaluated; the number of tillers per accession ranged between 2 and 3 per plant; regarding the length of the peduncle, a variation between 46.44 cm and 54.26 cm was recorded; the number of hands per cluster was between 6 and 8, while the number of fruits presented considerable fluctuation, with a range of 36 to 117 fruits, which suggests differences in production capacity between accessions. The weight of the bunch varied between 10.99 kg and 24.93 kg, indicating significant differences in productive capacity. Of the 57 qualitative descriptors analyzed using the Chi-square (X2) test, 20 showed highly significant differences (p < 0.0001). In addition, 10 traits with the greatest discriminatory power were identified, and they are especially useful for distinguishing genetic differences between the groups evaluated. Thus, a marked/significant difference in the number of fruits per plant is apparent based on descriptive statistics.
Note: Although all accessions were grown under homogeneous field conditions, it is important to consider that they come from diverse environmental settings. Therefore, the phenotypic variation observed, particularly in quantitative traits, could reflect underlying genetic differences; however, this interpretation should be made with caution, as genotype × environment interactions could influence the expression of these traits under different conditions. Furthermore, the group with the highest number of fruits per plant was Group 4, composed mainly of AAB genotypes. However, it is important to acknowledge that fruit production is a complex trait that may also be influenced by environmental factors at the original collection sites, such as soil fertility, humidity, and cultivation practices.
Regarding the number of live leaves during the flowering phase, a variation between 9 and 10 leaves was observed, which significantly reduced during harvest, with a range of 3 to 6 leaves, which reflects the natural process of senescence. In relation to the length of the external fruit, values between 13.08 cm (for the group of bananas or oritos) and 32.13 cm (for the banana group) were recorded; The diameter of the fruit varied between 3.24 cm and 4.42 cm, while the weight of the fruit fluctuated between 0.07 kg and 0.32 kg, corresponding to banana materials.
The high variability observed in productivity attributes suggests great potential for selecting and improving genotypes with higher yields. Conservation and sustainable management programs could focus on preserving and promoting accessions with the best values for these characteristics, thus ensuring the long-term availability of this valuable germplasm [40].
3.4. Grouping of Accessions Based on Qualitative Morphological Characters
The genus Musa includes cultivars with varying reproductive biology. Most AAA and AAB genotypes are sterile triploids that exhibit parthenocarpy, producing fruits without seed formation or effective fertilization. Although some cultivars develop both male and female floral structures, fertilization is generally absent or non-functional due to the sterility of gametes. In this study, morphological data were obtained from field-grown plants, and the evaluation of floral traits aimed to characterize phenotypic variation rather than reproductive performance. These traits may contribute to identifying accessions with potential value for future breeding or genetic studies, especially in relation to floral morphology and fruit development mechanisms.
The morphological data were obtained from four plants per accession under field conditions, without experimental replication. Therefore, the variation observed among accessions should be interpreted as descriptive of the phenotypic expression under the evaluation conditions and does not represent confirmatory evidence of genetic differentiation. Therefore, the results presented here should be interpreted as a preliminary phenotypic assessment, which is useful for identifying candidate accessions.
In the case of Musa, the most discriminating qualitative morphological descriptors were those related to the plant, pseudostem, leaves, ♂ bud, bracts, flower, cluster, and fruits.
3.4.1. Plant and Pseudostem
Regarding the leaf habit of the plants, the accessions in G1 and G3 showed 25% and 24% with a normal habit, respectively, while G4 stood out with 38% of plants with this habit. Regarding the color of the pseudostem, G1 presented 24% of the accessions with a yellow-green color, and G4 presented 37%, compared to only 8% for G3, which exhibited a gray-brown color. Regarding the appearance of the pseudostem, G4 showed 40% of accessions with the characteristic of being opaque–waxy. Regarding the development of the offspring, G1 presented 24%, followed by G3 with 26%, while G4 stood out with 40% of the accessions with the character between ¼ and ¾ of the size of the mother plant; Regarding the pigmentation of the internal pods, G3 presented 15% with a purple color, G1 8% with a grayish violet color, and G4 26% with a red-purple color; Finally, in the wax character in the pods, G4 presented 22% with little wax, while G3 showed 18% with the waxy characteristic.
The predominance of waxy and pigmented traits in Group 4, which is composed mainly of AAB genotypes, suggests a possible link between these phenotypic characteristics and the genomic background of this group, particularly the inherited Musa balbisiana traits of wax content [41].
Note: The frequency percentages presented for qualitative descriptors are based on the dominant trait observed among the four plants per accession. In cases where intra-accession variability was observed, the most frequent category was recorded as representative, following IPGRI-INIBAP scoring guidelines. While the total number of evaluated accessions (n = 100) provides a broad overview of diversity, the limited within-accession sample size may reduce the precision of frequency estimates, especially for traits with visually similar states such as pulp color or fruit shape. Therefore, these results should be interpreted as indicative rather than definitive.
3.4.2. Leaves
For the variable spots at the base of the petiole, the character “small spots” was presented in G1 (16%) and G4 (18%), while the character “large spots” was observed in G3 (8%); Regarding the color of the spots, the “brown” color appeared in G3 (12%), while the “grayish violet” color appeared in G1 (21%) and G4 (18%). Regarding the canal of the leaf petiole, the “open with erect margin” character was recorded in G3 (15%), while the “narrow with erect margin” character was presented in G1 (23%) and G4 (27%); regarding the color of the vein in the beam, the “yellow-green” character was observed in G1 (23%) and G4 (37%), while the “green” color was presented in G3 (10%); In relation to the color of the dorsal face of the candle, the “yellow-green” color was presented in G1 and G3 (24%), with G4 standing out with 39% of the accessions; in the variable spots on the epidermis of the tillers, the character “without spot” was presented in G3 (24%), while the character “small or narrow spots” was observed in G1 (18%), with G4 standing out with 35% of the accessions evaluated and concerning the number of living leaves at flowering; G2, G3, and G4 presented 10%, while G1 registered 9%. Finally, regarding the number of living leaves at harvest, G3 and G4 presented 4%, while G3 presented 6% of living leaves at harvest. The variability observed in the number of live leaves at harvest (with a coefficient of variation of 35.94%) suggests significant differences in the rate of leaf senescence. This phenomenon could be closely related to tolerance to biotic and abiotic stresses [42].
3.4.3. ♂ Flower
Regarding the variable type of male bud, the “normal” character (presence) was manifested in G3 with 26%, highlighting G4 with 40%. On the other hand, the character “degenerated before maturity” (false horn banana) was presented in G1 with 14%; regarding the shape of the male bud, the “lanceolate” character was observed in G1 with 16%, while the “intermediate” character was presented in G2 with 15%. The “ovoid” character was recorded in G4 with 22%.
3.4.4. Bracts
The shape of the apex of the bracts presented various characteristics among the groups evaluated: the intermediate character was observed in G3 (14%) and G4 (30%), while the slightly pointed character was presented in G1 (7%); regarding the imbrication of young bracts, the character “slightly covers it” was observed in G4 (27%), followed by G1 (16%), and the character “they cover it completely” was recorded in G3 (13%); regarding the color of the inner face of the bracts, the grayish violet color appeared in G4 (24%) and G1 (13%), while the grayish red color appeared in G3 (17%).
Regarding the attenuated coloration of the base of the bracts, the character of discontinuous coloration towards the insertion with the rachis (pigmented area at the base of the bracts) was observed in G2 (9%); on the other hand, the character of homogeneous coloration (continuous and uniform pigmentation to the base) was present in G3 (26%) and G4 (25%). Regarding the behavior of the bract before falling, the “revolute” character (it rolls up) was presented in G3 (24%), while the “non-revolute” character (it does not roll up) was observed in G1 (16%) and G4 (22%). Finally, regarding the presence of wax on the bracts, the “very little waxy” character was present in G1 (6%), while the “waxy” character was more frequent in G3 (22%) and G4 (28%).
3.4.5. ♀ Flower
The variable color of the compound tepal, the “white” character, was presented in G3 (21%) and G4 (19%); for the variable pigmentation of the compound tepal, the character “very little or no visible sign of pigmentation” was presented in G4 (34%), while the character “presence of pink color” was recorded in G3 (20%). Regarding the color of the compound tepal locule, the “yellow” character was presented in G3 (16%), and the “orange-yellow” character was observed in G4 (20%); regarding the appearance of the free tepal, the character “folded under the apex” was presented in G1 (16%) and G3 (26%), while the character “very folded under the apex (corrugated)” was recorded in G4 (18%); regarding the shape of the style, the “erect” character was present in all groups, standing out especially in G3 (26%) and G4 (40%). Regarding the color of the stigma, the “yellow orange” character was presented in G4 (14%), the “grayish yellow” character was observed in G1 (9%), and “white yellow” was presented in G3 (10%); regarding the basic color of the ovary, the “yellow-green” character was presented in G4 (30%), while the “white-green” character was observed in G3 (14%); finally, for the pigmentation of the ovary, the character “very little or no visible sign of pigmentation” was presented in G1 (16%) and G4 (39%), while the character “purple-red” was presented in G3 (20%).
3.4.6. Raceme
Regarding the position of the raceme, the “vertically pendulous” character was present in the four groups, standing out in G1 (19%), G3 (20%), and G4 (24%). Regarding the type of rachis, the character “present in the male bud, which may be degenerated or persistent” was observed in all four groups, being more prominent in G4 (40%). In the position of the spine, the “vertically pendulous” character was mainly presented in G3 (26%) and G4 (36%), while the “curved” character was recorded in G2 (8%). Regarding the appearance of the rachis, the character “male flower or bracts persistent above the male bud” (the rachis is bare on top) was presented in G3 (14%); the character “sterile or male flower throughout the rachis, without persistent bracts” was presented in G1 (16%), Figure 3. Finally, the character “small cluster of hermaphrodite flowers (hermaphrodite just above the male bud)” was recorded in G4 (21%).


Figure 3.
Morphological characteristics of the different genetic groups included in the Musa collection. Fruiting plants of each of the four groups collected. Photos. Nelly Paredes, INIAP, DENAREF. 2025.
3.4.7. Fruit
Regarding the shape of the fruit, the “straight” character (not very marked curve) was presented in G3 with 14%, and the “straight in the distal part” character was recorded in G1 (24%) and G4 (22%). Regarding the cross-section of the fruit, the character “pronounced edges” was presented in G1 (25%) and G4 (22%), while the character “weakly pronounced edges” was observed in G3 (21%); regarding the apex of the fruit, the “long pointed” character was presented in G1 (25%) and G4 (21%), while the “truncated” character was recorded in G3 (18%); for the variable floral vestige at the apex of the fruit, the character “without floral vestige” was presented in G3 (22%), while the character “prominent style base” was observed in G1 (25%) and G4 (22%).
Regarding the variables related to the peel, pulp, and flavor in the genetic groups, for the color of the immature peel, the yellow-green color occurred in the four groups, being more predominant in G1 (24%) and G4 (38%); for the color of the mature peel, the yellow character also predominated in G1 (24%) and G4 (38%); for the peel adhesion character easily comes off, it was presented in G3 (23%), and does not come off easily was presented in G1 (25%) and G4 (23%); regarding the color of the pulp before maturity, the white characters was presented in G2 (4%), while the white orange color was more predominant in G3 (15%) and G4 (17%); regarding the color of the pulp at maturity, the yellow-orange color was mainly presented in G1 (17%) and G4 (20%). Finally, regarding the predominant flavor, the mild flavor was presented in G3 (22%), while the sweet and sour flavor (like apple) was more common in G1 (25%) and G4 (23%).
3.5. Discriminating Capacity of Descriptors
The identification of the most discriminating qualitative descriptors among the four genetic groups was based on Chi-square significance values (p < 0.0001) and the strength of association measured by Cramer’s V. As previously indicated, the four genetic groups were defined using quantitative morphological variables through hierarchical cluster analysis. The qualitative traits were subsequently used to interpret and describe the phenotypic distinctiveness of each group.
Table 5 presents the distribution of the 20 most significant qualitative descriptors across the four groups. Group 4 exhibited high relative frequencies for traits such as purple pigmentation in the compound tepal, purple bract color, horizontal fruit position, and overlapping hands. In contrast, Group 2 showed consistently low frequencies across most traits, highlighting its distinct morphological profile rather than a sampling imbalance—an observation supported by its clear separation in the dendrogram. Group 1 was characterized by a high frequency of the yellow pulp color, and Group 3 was characterized by the predominance of an ellipsoidal fruit shape.

Table 5.
Analysis in percentage of the characters evaluated by group (1, 2, 3, and 4).
Among the most discriminant traits, male bud shape, rachis appearance, and compound tepal pigmentation presented the highest χ2 values (149.34, 138.31, and 113.16, respectively) and had Cramer’s V values of 0.71, 0.68, and 0.61. Additional traits such as fruit cross-section (χ2 = 137.46; Cramer’s V = 0.83), fruit shape (χ2 = 28.72; V = 0.38), and ovarian pigmentation (χ2 = 93.16; V = 0.68) also contributed substantially to the discrimination among groups (Table 6). Overall, G3 and G4 were most strongly associated with male bud shape, rachis appearance, and compound tepal pigmentation, confirming their relevance as key morphological markers.

Table 6.
Eigenvalues determined by the canonical discriminant function that discriminates the grouping of Musa accessions.
3.6. Evaluation of the Ecogeographic Diversity of Musa Using the ELC Map
The ELC-map tool was used to characterize the eco-geographical distribution and model the distribution of Musa species. This tool generates frequency maps for specific categories, facilitating the identification of priority areas for germplasm conservation and collection. Clustering procedures were applied to classify occurrence data, and the results were integrated with the Modela tool to generate species distribution models (SDMs) and evaluate their potential use in biodiversity conservation.
The data to prepare this map were obtained from the coordinates of the 100 accessions collected, and the bioclimatic, soil, and geophysical data were obtained from WorldClim and the global harmonized databases of HWSD, SRTM v 3 DEM, and SoilGrids, which are part of the databases held by CAPFITOGEN.
The ELC map identified a total of 23 ecogeographic categories (Figure 4), of which some stand out in terms of their frequency and distribution, so the most frequent category (Category 20) presented a frequency of 40.35% and a total coverage of 188,763 cells, with ranges of characteristics such as the annual temperature oscillating between 21.6 °C to 27 °C, the apparent density from 1.25 to 1.44 g cm−3, and a cation exchange capacity (CEC) of 4 to 29 cmol kg−1. Regarding the geographical distribution, category 20 covers most of the Amazon region, specifically in the provinces of Orellana, Sucumbíos, part of Napo, a large part of Pastaza, and approximately half of Morona Santiago (Table 7). On the other hand, the least frequent categories were 4, 9, 10, 22, and 23, which represent less than 0.01% of the coverage; regarding the ranges of characteristics, they presented the following: average annual temperature varying from 18.2 °C to 22.4 °C, apparent density from 10.12 to 14.19 g/cm3, and cation exchange capacity (CEC) from 13.82 to 45.67 Cmol kg−1. Regarding the geographical distribution, these categories are found in the Amazon and Litoral regions; on the other hand, category 12 stands out, which is located in the central area of the Litoral region, specifically in the provinces of Esmeraldas, Manabí, Guayas, and Los Ríos. In the same way, in relation to category 11, which is found in the foothills of the western mountain range in the provinces of Esmeraldas, Manabí, Santo Domingo de los Tsáchilas, Guayas, and Los Ríos, in addition to the foothills of the eastern mountain range in the provinces of Napo and Pastaza, category 6 also stands out, which is covering areas towards the Pacific Ocean in the provinces of Manabí, Guayas, and El Oro, as well as in the southwestern part of the province of Loja.
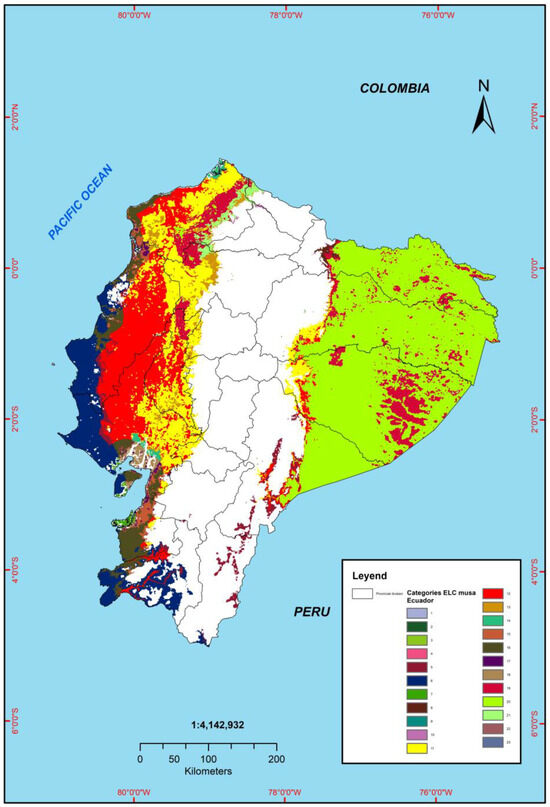
Figure 4.
Ecogeographic diversity in reference to the ELC map based on the morphological characterization of 100 Musa accessions.

Table 7.
The range of characteristics for each ecogeographic category.
In terms of geographic distribution, these categories are found in the Amazon and Litoral regions. Category 12 stands out, located in the central zone of the Litoral region, specifically in the provinces of Esmeraldas, Manabí, Guayas, and Los Ríos. Category 11 is found in the foothills of the western mountain range in the provinces of Esmeraldas, Manabí, Santo Domingo de los Tsáchilas, Guayas, and Los Ríos, in addition to the foothills of the eastern mountain range in the provinces of Napo and Pastaza. Finally, category 6 covers areas towards the Pacific Ocean in the provinces of Manabí, Guayas, and El Oro, as well as in the southwestern part of the province of Loja.
The morphological and ecogeographic analyses of Musa germplasm collections allow for the assessment of genetic diversity, the identification of variations between accessions, and the selection of adapted or resistant cultivars. Furthermore, ecogeographic studies help us understand species distribution and identify gaps in the collection, facilitating the conservation and prioritization of key areas to preserve genetic diversity.
4. Discussion
The variables with the greatest discriminating power identified in the present study include the shape of the male bud, the appearance of the rachis, the pigmentation of the compound tepal, the cross-section of the fruit, the shape and apex of the fruit, the color of the immature shell, the pigmentation of the ovary, the type of male bud, the height of the pseudostem, the length of the leaf blade, the weight of the cluster, the number of fruits, and the length of the fruit on its axis, both internally and externally. These results contrast with the findings by Caicedo Arana [43], who determined that the most relevant variables for the characterization of edible Musa materials were the inflorescence an the male bud, made up of variables such as the length, diameter, color, and pubescence of the peduncle; the position, shape, appearance, female flowers, and fruits of the cluster; the type, position, and appearance of the spine; and the type, shape, and size of the male bud (the male part of the raceme). This difference may be due to the morphological composition of the accessions from the Amazon region, where floral and pigmentation traits tend to be more stable and informative for group differentiation. This difference may be attributed to the morphological composition of the Amazonian accessions, in which floral and pigmentation traits exhibited lower phenotypic variability and higher discriminatory power for group differentiation under the evaluated conditions.
Multivariate statistics is a powerful tool to explain the genetic diversity observed through morphological descriptors, allowing us to group information of greater similarity, as well as the high indices of morphological variability of the bank; in this work and in addition to the descriptors of the male flower, bracts and inflorescence are those that contribute to more than 90% of the variability. The greatest plant height was recorded in G3 with 4.05 m, while G1, G2, and G4 presented average heights ranging between 3.25 m and 3.62 m. These values coincided with values reported by Deras et al. [44], who recorded heights of 2.80 and 3.50 m; like Aldana Leyva et al. [45], they mention that in their research, they found banana materials with plant heights between 3.00 and 3.50 m; also, Dela Cruz et al. [46] indicated that cultivars from FHIA [47] reached an average height of 3.06 m for Philippine conditions; on the other hand, research carried out by de la Torres-Cabrera et al. [48] reported an average height of 2.22 m for the “Grande Naine” cultivar, which facilitates crop management (application of products, phytosanitary controls, leaf removal, and harvest) due to its smaller stature.
The height differences observed between groups may also reflect the combined effect of plant traits and the specific environmental conditions under which accessions were grown, as no controlled environmental replication was applied.
The greatest number of functional leaves at the time of flowering was observed in G1, G2 and G3 with 10 functional leaves, while G4 presented 9 functional leaves. It is important to highlight that the number of functional leaves at this stage is a crucial variable since it has been determined that it is essential for the growth and development of fruits in plantains and bananas. According to Hernández et al. [49], a minimum of 7 to 10 functional sheets are required. Furthermore, the results of this study coincide with those by Torres-Cabrera et al. [47] and Brenes-Gamboa [50], who reported averages of 9.6 and 10 functional leaves in the cultivars “Reacido al Rey” and “Williams”, respectively. Rodríguez et al. [51] suggested that banana or plantain plants can maintain between 6 and 12 leaves from flowering to harvest without affecting the weight of the bunch, the quality of the fruit, or post-harvest ripening. Likewise, the capacity for development and fruit filling depends directly on the number of healthy leaves present during flowering [52].
The study by Chundawat et al. [53] shows that phenotypic variability in the traits evaluated (such as plant height, hands per bunch, fingers per bunch, bunch weight, and finger weight) is predominantly influenced by genetic variance, which is reflected in high broad heritability values and genetic coefficients of variation. This suggests strong genetic control over these traits, allowing phenotypic selection to be an effective strategy for their improvement. In contrast, the leaves-per-plant trait showed a greater contribution of environmental variance compared to genetic variance, indicating that its expression is more subject to external conditions (such as agricultural management or the environment). Therefore, for this trait, selection based solely on phenotype would be less efficient, requiring approaches such as selection or environmental control for its genetic improvement.
The study by Nayar et al. [54] reveals that traits such as hand weight and fruit length exhibit high genotypic variation and significant genetic coefficients of variation, indicating significant potential for selection and genetic improvement. Likewise, traits such as plant height, leaves per plant, hands and fingers per bunch, and fruits per hand showed high heritability values, confirming that their phenotypic variability is largely determined by genetic factors that favor their advancement through direct selection. However, fruit length exhibited low heritability, suggesting a greater influence of environmental factors or genotype–environment interactions on its expression. This implies that, for this trait, strategies such as marker-assisted selection or specific agronomic management could be more effective than conventional phenotypic selection.
The results indicate that the traits seedling height, leaf blade width, and leaf blade length exhibit high broad-sense heritability, suggesting strong genetic control over their expression. This suggests that early seedling selection based on these traits would be highly effective, since the observed phenotypic variability primarily reflects genetic differences. Therefore, these traits can be considered reliable criteria for breeding programs aimed at their improvement. On the other hand, variables such as bunch weight showed low heritability, indicating a greater influence of environmental factors on their expression [55].
The results indicate phenotypic variability among the genotypic groups (G1–G4), as reflected by their differing yields (36–143 fruits per bunch) under the same environmental conditions. Although the trial was conducted in a single environment, the observed differences may be attributed to genetic divergence among the groups and their distinct responses to local conditions.
These findings suggest that, under the evaluated conditions, traits such as plant height, the number of hands and fingers per bunch, bunch weight, and finger weight exhibited consistent phenotypic expression across accessions. While these traits are known to be influenced by environmental factors, their observed variation may still be useful for preliminary selection purposes in breeding programs.
The Musa materials or ecotypes were collected through asexual propagation, which guarantees the preservation of the mother plant’s genetic characteristics. This ensures genetic uniformity and high heritability in the evaluated traits.
The predominance of waxy and pigmented traits in Group 4, composed mainly of AAB genotypes, suggests a possible linkage between these phenotypic features and the genomic background of this group, particularly traits inherited from Musa balbisiana.
G1 is composed of the Musa AAB Simmonds, “Plátano Hartón” and “Clon Cuerno” ecotypes, with an average weight of 11.57 kg; G2 is composed of the Musa acuminata ecotype “Orito”, which recorded an average weight of 10.99 kg; G3 includes the Musa acuminata ecotype “Malay Banana” or “Red Banana”, with an average weight of 24.94 kg; G4 is made up of the Musa AAB Simmonds ecotype “Plátano Dominico” materials, banana or guineo materials, and the Musa paradisiaca AAB ecotype “Barraganete”, which recorded an average of 16.78 kg. These results do not agree with those recorded by Hoyos-Leyva et al. [56], who reported that the Cachaco Espermo variety produced bunches with an average weight of 8 kg, as well as studies by Smith et al. [57], who recorded a bunch weight of 28.07 kg, while the Bocadillo Chileno variety reached an average weight of 43 kg. These contrasts show that the characteristics of the bunch in Musa depend on various factors, such as genetic, agroclimatic factors, and the age of the crop. In fact, these plants’ morphological characteristics result from the interaction between the genotype and the environment [58].
Note: The analysis of quantitative traits is complex in nature, as the variables attributable to Musa sp. production and quality (fruit length and bunch weight) are highly sensitive to the environment, while heritability varies according to the Musaceae ecotypes and the environment. Agroclimatic factors (precipitation and temperature) have a nonlinear effect. Evaluations related to yield and bunch weight (kg) show that G1 and G2 had average weights of 11.57 kg and 10.99 kg, respectively. G3 recorded the highest weight per bunch at 24.93 kg, while G4 recorded a weight of 16.78 kg. These results differ from those obtained by Torres-Cabrera et al. [47], who reported lower average weights, between 16.72 kg and 17.52 kg, and they also differ from what was reported by Aldana Leyva et al. [44], who recorded bunch weights (kg) of 21.37 kg for “FHIA-17” agamic propagation, 24.05 kg for “FHIA-17” micropropagation, and 18.41 kg for “Grande naine” micropropagation. Regarding the number of hands, the four groups presented an average of between 6 and 8 hands per bunch, which coincides with the findings by Brenes-Gamboa [49], who found an average of between 6.8 and 13 hands per bunch.
Despite these limitations, the quantitative traits were evaluated under uniform agronomic management within a common environment, which minimizes random variation and allows for exploratory discrimination among accessions. While not conclusive for genetic differentiation, this phenotypic classification provides a useful framework for identifying promising materials for further study under controlled or replicated conditions.
Finally, regarding the number of fruits per cluster, G1 showed the lowest number of fruits per cluster with a total of 36; while G4 recorded an average of 107 fruits, G3 reached 117 fruits, and G2 presented 143 fruits per cluster. However, the number of fruits was lower than that reported by Aldana Leyva et al. [19], who found a number of total fruits/cluster of 156.15 for ‘FHIA-17’ agamic propagation, 164.67 for ‘FHIA-17’ micropropagation, and 136.38 total fruits/cluster for “Grande naine” micropropagation, agreeing with what was stated by Buitrago-Bitar et al. [59], who state that the number of fruits with their length has a direct correlation, considering that the greater the number of fruits, the shorter their length, and this is an essential characteristic to take into account in plant improvement programs.
The results demonstrate a clear genotype–environment interaction because the variability between the genotypes of the evaluated materials (G1–G4) showed different yields (36–143 fruits bunch−1) under the same conditions; this genetic variation is confirmed by the high heritability values previously reported, which also indicates that the cultivation environment (soil, climate, and management) differentially affected each genetic material, which implies that the selection must consider both the genetic component and the cultivation conditions; it is evident that the genetic potential is only fully expressed in optimal environments.
In G2, G3, and G4, the average fruit length in all accessions was 13.08 cm for the “Orito” ecotype, 22.07 cm for the “Plátano Malayo” or “Plátano Rojo” ecotype, and 23.40 cm for the “Plátano Dominico” ecotype, banana or banana materials, and the “Barraganete” ecotype. These results agree with the findings by Hoyos-Leyva et al. [56], who reported that in their twenty varieties of Musa with different genetic compositions (AB, BB, AAA, AAB, ABB, AAAA, and AAAB) from the germplasm bank, all except the plantain subgroup (Mbindi and Africa-1) presented an average fruit length of less than 25 cm. However, for commercial purposes, especially in industry, bananas must be large in size, with greater length and diameter.
The fruits that presented the maximum values of these variable fruit length and related size attributes corresponded to G1, with an average length of 32.13 cm, which coincides with the findings by Dufour et al. [60] and Gibert et al. [61], who indicated that bananas from the AAB group have lengths greater than 23 cm. However, in this study, it was observed that the G1 accessions, corresponding to the “Plátano Hartón” and “Clon Cuerno” ecotypes, recorded a fruit length greater than 32 cm, which constitutes an outstanding characteristic in terms of genetic improvement, so they should be studied in more detail as promising materials for agroindustry; in addition, banana and plantain production is constantly threatened by the effects of climate change and the incidence of diseases. Therefore, it is essential to have better knowledge about the morphological characteristics of materials and production parameters to develop a varietal strategy that allows for increasing the yields.
Banana improvement in Ecuador is a joint effort between the private sector, public institutions, and research centers. Although the private sector has historically had a greater influence in terms of direct investment, the public sector’s role is crucial for the evaluation and local adaptation of new varieties.
In Ecuador, such strategies are aligned with the objectives of national plant breeding programs, which are mainly conducted by public institutions like INIAP, with limited involvement from private sector initiatives.
Evaluations on yield and bunch weight (kg) showed that G1 and G2 had average weights of 11.57 kg and 10.99 kg, respectively. G3 recorded the highest weight per bunch at 24.93 kg, while G4 presented a weight of 16.78 kg. These results differ from those reported by Torres-Cabrera et al. [47], who recorded lower average weights, between 16.72 kg and 17.52 kg. Regarding the number of hands, the four groups presented an average of between 6 and 8 hands per bunch, which coincides with the findings by Brenes-Gamboa [49], who reported an average of between 6.8 and 13 hands per bunch. Finally, regarding the number of fruits per cluster, G1 presented the lowest number of hands, with a total of 36, while G4 registered 107, G3 reached 117, and G2 presented 143 hands per cluster.
Regarding the ecogeographic characterization, category 16, located in the Amazon and the coast, is distinguished by its high rainfall and humidity, with high values throughout the year. In contrast, category 2, located in the coastal zone, has the lowest levels of precipitation, greater aridity, and higher wind speeds. On the other hand, category 1, located in the Andean foothills, is characterized by a higher apparent density, better cation exchange capacity, and a greater amount of organic carbon in the soil compared to the other categories. These findings are consistent with previous studies by Ferguson et al. [62], Wang et al. [63], Williams et al. [64], and Parra-Quijano et al. [65], who point out that environmental, edaphic, and geophysical variables are fundamental for the preparation of ecogeographic maps.
For example, in Iowa, USA, these types of maps were used to determine crop capacity. In contrast, Tapia et al. [28] reported that the ELC map identified non-homogeneous adaptive scenarios, which were classified into 24 ecogeographic categories for the biodiversity of the genus Musa, finding low-frequency categories that share only specific characteristics in their climatic, geophysical, or edaphic components with the high-frequency categories. The ELC map allowed us to correctly discriminate adaptive scenarios using key ecogeographic variables, which directly influence the abiotic adaptation of the Musa genus and, therefore, determine its distribution. This approach is crucial for the efficient collection, conservation, and utilization of plant genetic resources.
Although no formal correlation analysis was performed, preliminary observations suggest that G1 and G4 were more frequently associated with lowland and humid ecotypes, while G2 and G3 appeared in higher-altitude or transitional zones. This ecogeographic variation complements the morphological differentiation observed among the accessions and highlights the relevance of environmental data for understanding the distribution and potential adaptation of Musa genotypes in Ecuador.
Morphological characterization is not necessarily related to ecogeographic characterization [34]. Regarding the ecogeographic characterization, for G1, composed of the Musa AAB Simmonds ecotypes, “Plátano Hartón” and “Clon Cuerno”, which develops at an altitude of 438.76 m a.s.l., with an average annual temperature of 23.93 °C, ±9.85, the apparent density is 1.34 g cm−3, ±0.03 and has a cation exchange capacity (CEC) of 13.44 cmol kg−1, ±3.47; G2, composed of the Musa acuminata ecotype “Orito”, develops at 527.44 m a.s.l., with an average annual temperature of 23.55 °C, ±10.03; its apparent density is 1.33 g cm−3, ±0.04, and has a cation exchange capacity (CIC) of 14.73 cmol kg−1, ±3.30; G3 includes the Musa acuminata ecotype “Red Banana”, and it develops at 591.85 m a.s.l., with average annual temperature of 23.39 °C, ±12.46; its apparent density is 1.31 g cm−3, ±0.05, and has a cation exchange capacity (CEC) of 15.42 cmol kg−1, ±5.94, while group G4 is made up of the Musa AAB Simmonds ecotype “Dominican Banana”, banana materials, and the Musa paradisiaca AAB ecotype “Barraganete”; it develops at 563.25 m a.s.l., with an average annual temperature of 23.57 °C, ±11.48; its apparent density is 1.32 g cm−3, ±0.04, and has a cation exchange capacity (CEC) of 14.67 cmol kg−1, ±3.94. Ambient temperature is the main determinant of vegetative development, productive yield, and flowering processes in the banana (Musa spp.), with an optimal temperature range identified between 21 and 33 °C to ensure its physiological and metabolic efficiency [66]. In banana plantations in Brazil, average cation exchange capacity (CEC) values of 7.26 cmol kg−1 were observed for the cultivar ‘Prata’ and 6.00 cmol kg−1 for ‘Cavendish’ [67]. These values are lower than those of all four groups regarding the variable cation exchange capacity (CEC), implying that the higher the organic matter content, the greater the soil’s capacity to retain essential cations such as Ca2+, Mg2+, K+, and NH4+ [68].
5. Conclusions
Quantitative morphological analysis of the 100 Musa accessions has revealed significant variation in key characteristics, such as vegetative development, fruit number, and cluster weight. The evaluated groups show well-defined patterns of variation that reflect notable differences in their productive potential. G3 stood out for its vegetative vigor, while G2 stood out for its high fruit production, and G1 presented the largest fruits. This observed variability provides a solid basis for the selection and genetic improvement of accessions with high yields.
The variability observed in morphological and physiological characteristics between accessions G1, G3, and G4 suggests important genetic and adaptive differences. G4 excelled in key variables, such as pseudostem development, pigmentation, and the number of distinctive features on the leaves. These results could be relevant for genetic selection and improvement, indicating that G4 could be more favorable for certain breeding or research programs.
Among the floral traits, male bud shape and rachis position were consistent across accessions within groups, which, given the clonal propagation, suggests a strong genetic basis. In contrast, traits such as pseudostem height and bunch weight showed wider variation, likely reflecting genotype–environment interactions.
While the results provide promising insights into the morphological and productive variability of Musa accessions, it is necessary to interpret their potential utility for breeding in light of the environmental influence on measured traits. Further evaluation under controlled or replicated conditions would help to confirm the genetic basis of the observed variation.
The analysis of the ELC map reveals a clear distribution of ecogeographic categories, with category 20 predominating in terms of frequency and coverage, especially in the Amazon region, suggesting an ecological environment characterized by moderate annual temperatures and relatively high soil apparent density. In contrast, the less frequent categories, namely 4, 9, 10, 22, and 23, have limited geographic coverage, and they are found mainly in the coastal zone and in the foothills of the eastern and western mountain ranges, which could indicate more specific or less widespread ecosystems. These variations in the frequency and distribution of categories reflect the complex ecological heterogeneity of the region.
Author Contributions
N.A.P.: research, field data collection, writing, and editing; R.A.V.: statistical morphological analysis; L.L.T.: germplasm collection, field data collection; Á.M.-A.: conceptualization, writing, and editing; C.T.B.: methodology; S.M.F.: ecogeographic analysis; M.S.: writing (review and editing); N.P.A.: writing (original draft preparation). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Special thanks to the research fund for Agrobiodiversity, Seeds, and Sustainable Agriculture—FIASA, which financed the project FIASA—EESC-2024-022, “Conservation and management of the INIAP Germplasm Bank”, within which this research work was carried out in its germplasm bank in the Ecuadorian Amazon.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Langhe, E.; Vrydaghs, L.; de Maret, P.; Perrier, X.; Denham, T. Why Bananas Matter: An introduction to the history of banana domestication. Ethnobot. Res. Appl. 2009, 7, 165–177. [Google Scholar] [CrossRef]
- Campos, H.; Caligari, P.D.; Brown, A.; Tumuhimbise, R.; Amah, D.; Uwimana, B.; Swennen, R. Bananas and plantains (Musa spp.). In Genetic Improvement of Tropical Crops; Campos, H., Caligari, P.D.S., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; pp. 219–240. [Google Scholar]
- Vásquez, R.; Romero, A.; Figueroa, J. Paquete Tecnológico para el Cultivo de Plátano; Gobierno del Estado de Colima: Colima, Mexico, 2005; N° 001; 72p. [Google Scholar]
- Cayón, S.D.G. (Ed.) Post Cosecha y Agroindustria del Plátano en el eje Cafetero de Colombia; CORPOICA: Cali, Colombia, 2000; 145p, ISBN 9589688500. [Google Scholar]
- Debnath, S.; Khan, A.A.; Das, A.; Murmu, I.; Khan, A.; Mandal, K.K. Genetic Diversity in Banana. In Genetic Diversity in Horticultural Plants. Sustainable Development and Biodiversity; Nandwani, D., Ed.; Springer: Cham, Switzerland, 2019; Volume 22, pp. 217–241. [Google Scholar] [CrossRef]
- Robinson, J.; Galán Saúco, V. Banana and Plantains, 2nd ed.; CAB International: Cambridge, UK, 2010; 297p, ISBN 978-1-84593-658-7. [Google Scholar]
- Power, R.C.; Güldemann, T.; Crowther, A.; Boivin, N. Asian Crop Dispersal in Africa and Late Holocene Human Adaptation to Tropical Environments. J. World Prehist. 2019, 32, 353–392. [Google Scholar] [CrossRef]
- Salas-Pascual, M.; Cáceres-Lorenzo, T. The Dispersal of Bananas (Musa spp.) to the Americas in the Sixteenth Century. Econ. Bot. 2022, 76, 354–367. [Google Scholar] [CrossRef]
- Guerrero, S. Características Morfométricas de Cultivares de Musáceas Establecidos en la Finca Experimental “La Maria”. Pregrade Project Thesis. Quevedo State Technical University: Quevedo, Ecuador, 2016. 93p. Available online: https://repositorio.uteq.edu.ec/handle/43000/3259 (accessed on 22 February 2025).
- MAG [Ministerio de Agricultura y Ganadería]. Boletín Situacional Cultivo de Banano. 2022. Available online: http://sipa.agricultura.gob.ec/index.php/platano/boletines-situacionales-platano-ecuador (accessed on 22 February 2025).
- INEC [Instituto Nacional de Estadística y Censos]. Boletín Técnico Encuesta de Superficie y Producción Agropecuaria Continua (ESPAC). 2023. Available online: https://www.ecuadorencifras.gob.ec/estadisticas-agropecuarias-2/ (accessed on 22 February 2025).
- Engels, J.M.M.; Visser, L. (Eds.) Guía para el manejo eficaz de un banco de germoplasma. In Manuales para Bancos de Germoplasma; Bioversity International: Rome, Italy, 2007; Volume 6, 192p, Available online: https://cropgenebank.sgrp.cgiar.org/images/file/learning_space/genebankmanual6_spa.pdf (accessed on 22 February 2025).
- Travez Guanotuña, X.D. Creación de Bancos Locales de Semillas Ancestrales como Estrategia para Enfrentar el Cambio Climático en la Parroquia de Chugchilán e Isinliví. Thesis Ing. Ambiental [BSc Environment]. Universidad Técnica de Cotopaxi: Latacunga, Ecuador, 2024. 94p. Available online: https://repoadmin.utc.edu.ec/bitstreams/054a25a5-8f31-431c-9adb-56801ac4dbad/download (accessed on 24 February 2025).
- MGIS [Musa Germplasm Information System]. 2023. Available online: https://www.crop-diversity.org/mgis/ (accessed on 28 May 2025).
- Cedeño Cedeño, L.M. El Boom Bananero y la Movilidad Humana en la Sierra Centro Ecuatoriana en el Período 1948-1965. Bachelor’s Thesis, Universidad Central del Ecuador, Quito, Ecuador, 2017; 103p. Repositorio UCE. Available online: http://www.dspace.uce.edu.ec/handle/25000/13992 (accessed on 24 February 2025).
- Jiffy Growing Solutions. ¿Cuáles Son los Desafios del Cultivo de Plátano? Available online: https://jiffygroup.com/es/noticias/cuales-son-los-desafios-del-cultivo-de-platano/Torres (accessed on 6 May 2025).
- Torres Jaramillo, L.A.; Centanaro Quiroz, P.H.; Raffo Folleco, L.A.; Nava Luzardo, J.C. Factores Limitantes del Desarrollo Agroecológico en el Cultivo de Banano (Musa AAA), Provincia del Guayas, Ecuador. Rev. Técnica Fac. Ing. Univ. Zulia 2023, 46, e234601. [Google Scholar] [CrossRef]
- Hernandez Villarreal, A.E. Caracterización morfológica de recursos fitogenéticos. Rev. Bio Cienc. 2013, 2, 113–118. Available online: https://revistabiociencias.uan.edu.mx/index.php/BIOCIENCIAS/article/view/41/133 (accessed on 24 February 2025).
- Santo-Pineda, U.; Torres-Vargas, L.; Santamaria-Guerra, J.; Thomas, G.; Montezuma, V. Caracterización morfológica de 21 Accesiones de musáceas colectadas en la Comarca Ngäbe-Buglé, Panamá. Cienc. Agropecu. 2024, 38, 7–26. Available online: http://revistacienciaagropecuaria.ac.pa/index.php/ciencia-agropecuaria/article/view/624 (accessed on 24 February 2025).
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Review. Applications of ecogeography and geographic information systems in conservation and utilization of plant genetic resources. Span J. Agric. Res. 2012, 10, 419–429. [Google Scholar] [CrossRef]
- Elbehri, A.; Calberto, G.; Staver, C.; Hospido, A.; Roibas, L.; Skully, D.; Siles, P.; Arguello, J.; Sotomayor, I.; Bustamante, A. Ecuador’s Banana Sector Under Climate Change—An Economic and Biophysical Assessment to Promote a Sustainable and Climate-Compatible Strategy; Food and Agriculture Organization of the United Nations [FAO]: Rome, Italy, 2016; 164p, ISBN 978-92-5-109249-1. [Google Scholar]
- Cedeño-Zambrano, J.R.; García-Párraga, J.V.; Solórzano-Cobeña, C.M.; Jiménez-Flores, L.A.; Ulloa-Cortazar, S.M.; López-Mejía, F.X.; Avellán-Vásquez, L.E.; Bracho-Bravo, B.; Sánchez-Urdaneta, A.B. Fertilización con magnesio en plátano Barraganete (Musa AAB) Ecuador. Granja Rev. Cienc. Vida 2022, 35, 8–19. Available online: https://lagranja.ups.edu.ec/pdf/granja/fertilizante_platano_esp.pdf (accessed on 26 February 2025). [CrossRef]
- Torres, S.J.P.; Cedeño, F.J.A.; Segura, R. Estado actual del mejoramiento genético en el cultivo del banano (Musa AAA, subgrupo Cavendish), y plátano (Musa AAB). Acorbat Rev. Tecnol. Cienc. 2024, 1, 2. [Google Scholar] [CrossRef]
- Beaton, K.; Mazadza, A.; Chikwambi, Z. Identificación de cultivares locales de banano (Musa spp.) en Zimbabue mediante morfología y secuenciación genómica. J. Genet. Eng. Biotechnol. 2023, 21, 118. [Google Scholar] [CrossRef]
- Monteros-Altamirano, A.; Tacán, M.; Peña Monserrate, G.R.; Tapia, B.C.; Paredes, N.; Lima, L. Guía para el Manejo y Conservación de Recursos Fitogenéticos en Ecuador: Protocolos; INIAP, Estación Experimental Santa Catalina, Departamento Nacional de Recursos Fitogenéticos: Mejía, Ecuador, 2018; Volume 432, pp. 1–104, Publicación Miscelánea; Available online: https://repositorio.iniap.gob.ec/bitstream/41000/4889/1/iniapscpm432.pdf (accessed on 26 February 2025).
- Asociación de Exportadores de Banano [AEBE]. Guía Culinaria del Banano—Banana Culinary Guide; Imprenta Mariscal: Guayaquil, Ecuador, 2021; 111p, ISBN 978-9942-962-09-6. Available online: https://www.aebe.com.ec/_files/ugd/f4cd67_21aef8d33457473f997eea1dc297f638.pdf?index=true (accessed on 26 February 2025).
- FAO. Banano. In Análisis del Mercado 2023; FAO: Rome, Italy, 2023; 10p, Available online: https://openknowledge.fao.org/server/api/core/bitstreams/318cda0c-dae9-4c24-a8cb-44afd40b90c3/content (accessed on 26 February 2025).
- León Ajila, J.P.; Espinosa Aguilar, M.A.; Carvajal Romero, H.R.; Quezada Campoverde, J. Análisis de la producción y comercialización de banano en la provincia de El Oro en el periodo 2018–2022. Cienc. Lat. 2023, 7, 7494–7507. [Google Scholar] [CrossRef]
- Tapia, C.; Paredes, N.; Lima, L. Representatividad de la diversidad del género Musa en el Ecuador. Repos. Digit. INIAP 2019, 6, 53–58. [Google Scholar]
- FAO [Food and Agriculture Organization of the United Nations]. Medium-Term Outlook: Prospects for World Production and Trade in Bananas and Tropical Fruits 2019–2028; FAO: Rome, Italy, 2020; Available online: https://www.fao.org/home/en/ (accessed on 26 February 2025).
- IPGRI/INIBAP/CIRAD [International Plant Genetic Resources Institute (IPGRI); International Network for the Improvement of Banana and Plantain (INIBAP); Centre de Cooperation Internationale en Recherche Agronomique pour le Developpement (CIRAD)]. Descriptores para el Banano (Musa spp.); International Plant Genetic Resources Institute: Rome Italy, 1996; 55p, Available online: https://alliancebioversityciat.org/es/node/3726 (accessed on 26 February 2025).
- Royal Horticultural Society (RHS). The RSH Colour Chart, 6th ed.; Royal Horticultural Society (RHS): London, UK, 2007; Available online: https://www.rhs.org.uk/ (accessed on 26 February 2025).
- Infostat. Infostat—Software Estadístico. 2020. Available online: https://www.infostat.com.ar/ (accessed on 28 February 2025).
- Flores, C.; Salomé, D. Estudio de la Diversidad Morfológica y Eco-Geográfica en tres Cultivos Andinos del Ecuador: El caso del Tarwi (Lupinus mutabilis Sweet.), Jícama (Smallanthus sonchifolius (Poepp. & Endl.) H.Robinson), y Miso (Mirabilis expansa Ruiz & Pav. Standley.); Universidad de Santiago de Compostela: España, Spain, 2021; Available online: http://hdl.handle.net/10347/27229 (accessed on 28 February 2025).
- Parra-Quijano, M.; Iriondo, J.M.; Torres, M.E.; López, F.; Maxted, N.; Kell, S.P. Capftogen3: A Toolbox for the Conservation and Promotion of the Use of Agricultural Biodiversity; Universidad Nacional de Colombia: Bogotá, Colombia, 2021; p. 45194. Available online: https://repositorio.unal.edu.co/handle/unal/85787 (accessed on 28 February 2025).
- Crespo, C.F.; Guanochanga, J. Diseño de un Proceso para la Obtención de Pulpa Congelada a Partir de Orito (Musa acuminata AA). Rev. Cienc. Tecnol. 2022, 22. [Google Scholar] [CrossRef]
- Jiménez-Esparza, L.O.; Decker-Campuzano, F.E.; González-Parra, M.M.; Mera-Andrade, R. Abonos orgánicos una alternativa en el desarrollo de cormos de orito (Musa acuminata AA). J. Selva Andina Biosph. 2019, 7, 54–62. [Google Scholar] [CrossRef]
- Nadal-Medina, R.; Manzo-Sánchez, G.; Orozco-Romero, J.; Orozco-Santos, M.; Guzmán-González, S. Diversidad genética de bananos y plátanos (Musa spp.) determinada mediante marcadores rapd/Genetic diversity of bananas and plantains (Musa spp.) determined by rapd markers. Rev. Fitotec. Mex. 2009, 32, 1–7. [Google Scholar]
- Ukwueze, C.K.; Oselebe, H.O.; Igwe, D.O.; Mekonnen, T.B. Morphological characterization and genetic diversity assessment of accessions of Musa spp. Res. Sq. 2024, preprint. [Google Scholar] [CrossRef]
- Christelová, P.; De Langhe, E.; Hřibová, E.; Čížková, J.; Sardos, J.; Hušáková, M.; Van den Houwe, I.; Sutanto, A.; Kepler, A.K.; Swennen, R.; et al. Molecular and cytological characterization of the global Musa germplasm collection provides insights into the treasure of banana diversity. Biodivers. Conserv. 2017, 26, 801–824. [Google Scholar] [CrossRef]
- Sampangi-Ramaiah, M.H.; Ravishankar, K.V.; Shivashankar, K.S.; Roy, T.K.; Rekha, A.; Hunashikatti, L.R. Developmental changes in the composition of leaf cuticular wax of banana influenced by wax biosynthesis gene expression: A case study in Musa acuminata and Musa balbisiana. Acta Physiol. Plant 2019, 41, 141. [Google Scholar] [CrossRef]
- Chaves-Barrantes, N.F.; Gutiérrez-Soto, M.V. Respuestas al estrés por calor en los cultivos. II. Tolerancia y tratamiento agronómico. Agron. Mesoam. 2016, 28, 255–271. [Google Scholar] [CrossRef]
- Caicedo Arana, Á. Caracterización y Evaluación Morfológica, Física y Química de Introducciones del Banco de Germoplasma de Musáceas en el Centro de Investigación Corpoica Palmira. Master’s Thesis, Universidad Nacional de Colombia, Palmira, Colombia, 2015. Available online: https://repositorio.unal.edu.co/bitstream/handle/unal/58986/Alvaro_Caicedo_Arana.pdf?sequence=1&isAllowed=y (accessed on 12 March 2025).
- Deras, M.; Durán, L.F.; Mercadal, L.; Rivera, M. Avances del proyecto evaluación y diseminación de híbridos de Musa con resistencia a Sigatoka Negra. In Informe Técnico Programa de Banano y Plátano 2003; FHIA, Ed.; Federación Hondureña de Investigaciones Agrícolas: La Lima, Honduras, 2004; pp. 45–58. [Google Scholar]
- Aldana Leyva, F.; Fernández Martínez, O.; García-Águila, L.; Sarría, Z.; Hurtado Ribalta, O. Respuesta agronómica de plantas de banano cultivar ‘FHIA-17’ (Musa AAAA) obtenidas por cultivo de tejidos y por propagación agámica. Biotecnol. Veg. 2020, 20, 83–91. [Google Scholar]
- Dela Cruz, F.S.; Gueco, L.S.; Damasco, O.P.; Huelgas, V.C.; dela Cueva, F.M.; Dizon, T.O.; Sison, M.L.J.; Banasihan, I.G.; Sinohin, V.O.; Molina, A.B., Jr. Farmers’ Handbook on Introduced and Local Banana Cultivars in the Philippines; Bioversity International: Rome, Italy, 2008; 68p, Available online: https://www.researchgate.net/publication/317932686_Farmer%27s_handbook_on_introduced_and_local_banana_cultivars_in_the_Philippines (accessed on 28 February 2025).
- FHIA [La Fundación Hondureña de Investigación Agricola]. ‘FHIA-17’ Programa de Bananos y Plátanos; Instituto Patria: La Lima, Honduras, 2016; Available online: http://www.fhia.org.hn (accessed on 20 April 2019).
- Torres-Cabrera, D.; García-Águila, L.; Bermúdez-Caraballoso, I.; Sarría, Z.; Hurtado Ribalta, O.; Delgado, E.; Pérez, A.; Fernández Martínez, O. Respuesta morfo-agronómica y organoléptica de cinco cultivares de banano (Musa spp.) en condiciones de campo. Biotecnol. Veg. 2020, 20, 43–50. [Google Scholar]
- Hernández, Y.; Marín, M.; García, J. Respuesta en el rendimiento del plátano (Musa AAB cv. Hartón) en función de la nutrición mineral y su ciclo fenológico Parte, I. Crecimiento y producción. Rev. Fac. Agron. 2007, 24, 607–626. [Google Scholar]
- Brenes-Gamboa, S. Production and quality parameters of three banana cultivars FHIA-17, FHIA-25 and Yangambi. Agron. Mesoam. 2017, 28, 719–733. [Google Scholar] [CrossRef]
- Rodríguez, C.; Cayón, G.D.; Mira, J.J. Effect of number of functional leaves at flowering on yield of banana Grand Naine (Musa AAA Simmonds). Rev. Fac. Nac. Agron. Medellín 2012, 65, 6591–6597. [Google Scholar]
- Martínez, A.M.; Cayón, D.G. Dinámica del crecimiento del banano (Musa AAA, Simmons cvs. ‘Grande naine’ y Valery). Rev. Fac. Nal. Agr. Medellín 2011, 64, 6055–6064. [Google Scholar]
- Chundawat, B.S.; Patel, N.L.; Dave, S.K.; Tikka, S.B.S. Quantitative variability for yield and other characters in banana. Indian J. Hortic. 1988, 45, 8–12. [Google Scholar]
- Nayar, N.K.; Lyla, K.R.; Mathew, V. Genetic variability in dessert type of banana. Indian J. Agric. Sci. 1979, 49, 414–416. [Google Scholar]
- Rodríguez Cabriales, J.C. Estimación de la Variación Somaclonal y la Inducida por Rayos Gamma en Banano (Musa spp.): Determinación de Índices para el Proceso de Selección. Ph.D. Thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Mexico, 2000. Available online: https://cdigital.dgb.uanl.mx/te/1080110328.PDF (accessed on 28 February 2025).
- Hoyos-Leyva, J.D.; Jaramillo-Jiménez, P.A.; Giraldo-Toro, A.; Dufour, D.; Sánchez, T.; Lucas-Aguirre, J.C. Caracterización física, morfológica y evaluación de las curvas de empastamiento de musáceas (Musa spp.). Acta Agronómica 2012, 61, 214–229. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-28122012000300003&lng=en&tlng=es (accessed on 11 March 2025).
- Smith, M.K.; Langdon, P.W.; Pegg, K.G.; Daniells, J.W. Growth, yield and Fusarium wilt resistance of six FHIA tetraploid bananas (Musa spp.) grown in the Australian subtropics. Sci. Hortic. 2014, 170, 176–181. [Google Scholar] [CrossRef]
- Vuylsteke, D.R.; Ortiz, R.; Shaun, R.; Ferris, B.; Crouch, J.H. Plantain improvement. Plant Breed. Rev. 1997, 14, 267–320. [Google Scholar]
- Buitrago-Bitar, M.A.; Enríquez-Valencia, A.L.; Londoño-Caicedo, J.M.; Muñoz-Flórez, J.E.; Villegas-Estrada, B.; Santana-Fonseca, G.E. Molecular and morphological characterization of Musa spp. (Zingiberales: Musaceae) cultivars. Boletín Científico Cent. Mus. Mus. Historia Natural 2020, 24, 33–47. [Google Scholar] [CrossRef]
- Dufour, D.; Giraldo, A.; Gibert, O.; Sánchez, T.; Reynes, M.; González, A.; Fernández, A.; Díaz, A. Propiedades físico-químicas y funcionales de los bananos de postre, plátanos de cocción y FHIA híbridos: Preferencia varietal de los consumidores en Colombia. In Proceedings of the CD-Proceedings, Acorbat 2008, XVIII International Meeting, Guayaquil, Ecuador, 11–14 November 2008; Borja, J.S., Nogales, C., Orrantia, C., Paladines, R., Quimi, V., Tazan, L., Eds.; p. 33. Available online: https://agritrop.cirad.fr/547229/ (accessed on 28 February 2025).
- Gibert, O.; Dufour, D.; Giraldo, A.; Sánchez, T.; Reynes, M.; Pain, J.-P.; González, A.; Fernández, A.; Díaz, A. Differentiation between cooking bananas and dessert bananas. 1. Morphological and compositional characterization of cultivated Colombian Musaceae (Musa sp.) in relation to consumer preferences. J. Agric. Food Chem. 2009, 57, 7857–7869. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.E.; Jarvis, A.; Stalker, H.T.; Williams, D.E.; Guarino, L.; Valls, J.F.M.; Pittman, R.N.; Simpson, C.E.; Bramel, P.J. Biogeography of wild Arachis (Leguminosae): Distribution and environmental characterization. Biodivers. Conserv. 2005, 14, 1777–1798. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, G.; Yang, L.; Li, Z. Distribution, species diversity and life-form spectra of plant communities along an altitudinal gradient in the northern slopes of Qilianshan Mountains, Gansu, China. Plant Ecol. 2003, 165, 169–181. [Google Scholar] [CrossRef]
- Williams, C.L.; Hargrove, W.W.; Liebman, M.; James, D.E. Agro-ecoregionalization of Iowa using multivariate geographical clustering. Agric. Ecosyst. Environ. 2008, 123, 161–174. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Ecogeographical land characterization maps as tool for assessing plant adaptation and their implications in agrobiodiversity studies. Genet. Resour. Crop. Evol. 2011, 59, 205–217. [Google Scholar] [CrossRef]
- Lobo, M.G.; Rojas, F.J.F. Biology and postharvest physiology of banana. In Handbook of Banana Production, Postharvest Science, Processing Technology, and Nutrition; Siddiq, M., Ahmed, J., Lobo, M.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 19–44. [Google Scholar] [CrossRef]
- Lima Neto, A.J.; Deus, J.A.L.; Rodrigues Filho, V.A.; Natale, W.; Parent, L.E. Nutrient diagnosis of fertigated “Prata” and “Cavendish” banana (Musa spp.) at plot-scale. Plants 2020, 9, 1467. [Google Scholar] [CrossRef]
- Solly, E.F.; Weber, V.; Zimmermann, S.; Walthert, L.; Hagedorn, F.; Schmidt, M.W.I. A Critical Evaluation of the Relationship Between the Effective Cation Exchange Capacity and Soil Organic Carbon Content in Swiss Forest Soils. Front. For. Glob. Change 2020, 3, 98. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).