Abstract
Bluehead gilia or bluefield gilia (Gilia capitata Sims, Polemoniaceae) is an annual herbaceous plant widely distributed in the western regions of North America but cultivated as an ornamental flower in various regions to support pollinators. The comprehensive chemical composition of this plant has not been previously reported. Essential oils (EOs) were obtained by hydrodistillation from different parts of the gilia plants. The yield of EOs ranged from 0.42 to 1.97 mL/kg, with the largest yields being obtained from the seeds; smaller yields obtained from the flowers, fruits, and leaves; and the lowest quantity obtained from the stems, roots, and shells. Using the GC-MS method, we identified 116 compounds. Hexahydrofarnesyl acetone was dominant in most parts of the G. capitata. The EO of flowers was dominated by hexahydrofarnesyl acetone (19.1%), fruits by hexahydrofarnesyl acetone (18.2%), seeds by hexahydrofarnesyl acetone (15.2%), fruit by (+)-epi-bicyclosesquiphellandrene (15.4%), leaves by phytol (23.3%), stems by isomanool (8.3%), and roots by (-)-myrtenol (25.7%). Triterpenoid saponins were identified, and 21 compounds were quantified (by HPLC). Saponin levels were high in aerial parts (excluding stems) and the lowest in plant roots. Based on the contents of EO and saponins, the aerial parts of G. capitata may have pharmaceutical properties, but saponins might be the main value of G. capitata.
1. Introduction
Plant parts used for medicinal applications vary greatly regarding the qualitative and quantitative contents of biologically active substances [1]. The chemical composition of some plant species is still poorly studied. One of them is the genus Gilia and the family Polemoniaeae in general [2,3,4]. Gilia capitata Sims, with the common name bluehead gilia or blue field gilia or globe gilia, is a close relative to phloxes and polemonium [5,6,7], which suggests that the level and composition of essential oils could be analogous to Phloxes. However, there are differences in the genus Gilia as it has a high level of saponins [2,8].
Gilia capitata (Figure 1) is native to all western states of the USA [9,10]. The native habitats of G. capitata are sunny rocky hillslopes and open fields with well-drained soils at an elevation range of up to 2000 m [11,12]. It is widely introduced throughout Western North America, Alaska, Northern Mexico, and Europe as an ornamental flower [13]. Bluehead gilia is declared one of the pollinator magnets by gardeners, e.g., the Blog of Oregon Native Plants for Pollinators [14]. It is included in many wildflower seed mixes as a pollinator attractor. G. capitata flowers are visited by a wide range of small-sized pollinator insects, including wild bees, bumblebees, flies, and butterflies [15,16].

Figure 1.
Gilia capitata. Photo: J. Liira.
In Estonia, G. capitata is cultivated as an ornamental plant, but recently, it was included in the list of bee-friendly intercrop species, and a farmer can obtain extra agri-environmental support for growing it in fields near beehives [17]. This makes the bluehead gilia a potential production species for additional agricultural goods, such as essential oils or saponins.
G. capitata is a polytypic species and phenotypically plastic in response to environmental conditions [11,18,19]. Eight defined subspecies form a morphological gradation throughout the Pacific coast of North America [11]. The flagship sub-species, G. capitata subsp. capitata, is a herbaceous annual plant with a slender 20–100 cm erect stem. The stem can be simple or branched in the upper third, glabrous, glandular, or slightly floccose. The root system consists of a relatively shallow main root. On the base and stem, cauline leaves are bipinnately dissected, usually glabrous, 4–10 cm long, and with 5–20 mm lobes; the upper leaves are more straightforward and linear. Blue (or blue-violet) campanulate flowers are clustered into globose to conoidal inflorescences with (25) 50–100 flowers at the terminal position of each stem or branch. The inflorescence diameter varies between 14 and 40 mm. Individual flowers therein are 0.6–2.5 mm [18] with acute lobes 0.6–1 mm wide and 2 mm long, straight-tipped, with a 6–8 mm corolla. The pollen has a light blue colour. The flowers of the gilia are protandrous, i.e., the anthers mature first, and stigmas rise a few days later [6,20].
Bluehead gilia is mostly autogamous and self-compatible; only some of its native populations or races are facultative outcrossers [6,20] or even self-incompatible [18]. The fruit is a three-celled globose or obovoid capsule, which splits at maturity only partly as three valves (i.e., it has an indehiscent capsule). The capsule contains 1–10 (25) seeds, with 1 or 2 in each cell. The capsule remains indehiscent in dry weather and splits only partly open with the rain. When dry, the capsules break off easily by touch. The seeds are hard, brown, ovoid, or ovoid-angled and 1–2.3 mm long. When wet, the verrucose seed coat becomes a sticky, mucilaginous coat; this feature probably serves to stick seeds to animals for dispersal and later to substrate or avoid its forage by birds or animals [2,18].
G. capitata is easily grown from seed. No stratification or scarification is required (though two weeks of cold stratification has been suggested to speed up germination). Seeds can be sown directly into the garden in spring. Still, we observed that they self-seeded reasonably well in autumn, overwintered in the Estonian winter, and underwent intensive germination in early spring. The soil should be moist when seeds are planted. In nature, bluehead gilia profits from wildfires, removing mature vegetation and opening the ground for light [21]. Its germination probably reacts to the signals from fire residues [22]. During growth, minimal maintenance is required except for weeding. The flowering process can be long-term and continuous depending on soil moisture and temperature conditions. Cutting deadhead flowers will encourage reflowering. It grows in gardens alone or with other low-competition annuals in sunny open locations. It grows well in well-drained medium to coarse soil with a sub-neutral pH (6.0–7.0). Adult plants are drought tolerant.
The attractiveness of bluehead gilia to pollinators has been partly explained by its tight capitate inflorescence structure, composed of up to 80 flowers, which forms a convenient landing pad for all insects [15]. Some of its essential oils could also be one of the reasons why the flower’s scent attracts pollinators [23,24] or the target of foraging [25]. Therefore, the authors of this study were interested in the content of essential oil in different parts of G. capitata, as well as the composition of the oil. The presence and a sufficient amount of essential oil in the plant may provide an opportunity to use the species to benefit human health, such as through medicinal plants, the food industry, perfumery, etc. Alternatively, earlier studies in the literature hint that saponins can be the main value of the species [2,18].
The detailed chemical composition of G. capitata has not been studied before. The foam produced during the distillation of the drug and the irritating effect of the dust produced during the grinding of the drug on mucous membranes indicate the possible presence of saponins in the plant. Therefore, this work aimed to study the contents and compositions of EOs and saponins of different morphological groups of raw materials of G. capitata.
2. Materials and Methods
2.1. Plant Material
This study aimed to obtain the first generalised estimates about the content and composition of essential oils (EOs) in G. capitata and to pinpoint the main source of EOs. This means that the variability and environmental effects were suppressed by mixing plant material grown in field conditions at three sites around Tartu, Estonia. The sites differed in soil conditions: one was heavily clay-rich, one was light clay, and one was sand-rich clay. More detailed studies can be conducted when EO sources within the plants are successfully explored.
Seeds of G. capitata were obtained from different commercial flower suppliers in Europe. Seeds were mixed to obtain more general estimates about the species’ chemical properties and to avoid strong bottleneck effects based on the genetic type grown by a single seed producer. Mixed seeds were sown in spring in mid-May (15–22 May) 2023. Species’ taxonomic identity was re-assessed at the flowering stage using keys available on the Internet. During the growth stage, plant growth was supported with commercial complex fertilisers aimed at flowers.
Plant material was collected twice during the summer. The bluehead gilia is an annual plant species, meaning it has the best EO content status at its flowering stage, except for fruits. Therefore, plant material was mainly collected during mass flowering. We observed two waves of mass flowering—the first wave of inflorescences formed at the top of the main stem, and the second wave of mass flowering occurred several weeks later, when inflorescences on lateral stems bloomed. The material was collected in both rounds and later mixed. Depending on the specific site phenology, the first forage was performed at the end of June and early July (30 June–15 July), and the second in the early weeks of July, with some continued collection being carried out until early August.
Plant parts (flowers, stems with leaves, roots, and fruits) were collected separately in the field. Later, after drying, the leaves and stems were separated. All collected material from different fields was mixed in relatively equal proportions before the analysis to ensure a more general estimate of EOs for the species. The fruits (including seeds and surrounding valves) matured continuously and were therefore collected over several weeks in late summer in August and September 2023. Fruits were defined as ripe for collection when they were yellow and started to dry or were already dry. The heads and clusters of fruit capsules were collected and dried. Later, fruit capsules were crushed, and seeds were separated. Capsule residues containing mainly fruit valves and walls were kept as a separate fraction, as shells of the fruit wall (hereafter called shells). The plant material was dried for 14 days at room temperature in a well-ventilated room. It was stored at room temperature in paper bags before the distillation of essential oil and the extraction of saponins. The voucher sample specimens (No Polemon/Gil1-7) are available at the Institute of Pharmacy, University of Tartu, Estonia.
This study used plant material from only one year of growth, as the aim was to provide a preliminary assessment of the pharmaceutical potential of this plant, which had not been fully analysed phytochemically.
2.2. Hydrodistillation of Essential Oil
Before analysis, the plant material to be examined was ground in a coffee grinder and sieved (diameter 3 mm). The essential oils (EOs) were hydrodistilled from the different dried parts of G. capitata using the method described in the European Pharmacopoeia [1]. The plant materials (20 g) were hydrodistilled in a 1000 mL round-bottom flask for 2 h with 400 mL of distilled water (3–4 mL/min). Hexane (0.5 mL) was added to a graduated tube to remove the distilled oil [26,27]. Due to the content of saponins, intense foam was produced during the distillation of most plant parts, and 30 g of potassium chloride was added to the flask to reduce the foam (except for the roots and stems).
The yield of EOs was measured using the European Pharmacopoeia method with 0.5 mL of xylol [1]. The essential oil content (mL/kg) was calculated based on the volume of the distillate (mL), which was multiplied by 1000 g and divided by the mass of the plant material taken for distillation (20 g).
2.3. Gas Chromatography/Mass Spectrometry
The samples of EO were analysed by gas chromatography–mass spectrometry (GC-MS) using an Agilent 6890/5973 G-CMS system controlled by MSD Chemstation (Agilent Technologies, Inc., Santa Clara, CA, USA). An amount of 1 µL of the sample was automatically injected at an injector temperature of 280 °C in split mode (10:1), using He as the carrier gas onto Agilent HP-5MSI column (Agilent Technologies, Inc., Santa Clara, CA, USA) (30 m length, 0.25 mm inner diameter, and 0.25 µm film thickness). The carrier gas was held at a constant flow rate of 1 mL/min. The oven was held at 50 °C for 2 min, followed by a ramp of 4 °C/min to a final temperature of 280 °C and held at 280 °C for 5 min. The MSD was operated in EI mode at 70 eV. After a delay time of 4 min, mass spectra were recorded in the range of 29–400 m/z at a rate of 3.8 scans per second. The data were analysed using Agilent Masshunter Data Analysis Software G1617DA using a deconvolution algorithm with different window size factors. Obtained compounds were identified by using NIST23 library with a Match Factor of ≥90 and using retention indexes (relative to n-alkanes C8–C30). The area percentages of each peak were calculated from the total areas in the chromatograms without using correction factors [26,27].
2.4. Extraction of Saponins
The saponins were extracted as follows: 0.5 g of crushed raw materials was placed into a 100.0 mL conical flask equipped with a reflux condenser, and then 25 mL of methyl alcohol was added and boiled for 30 min in a water bath. After cooling the sample to room temperature (20 °C), the extract was filtered into a 25 mL volumetric flask. The obtained extract was then topped up to 25 mL with methyl alcohol.
2.5. Identification of Saponins
The class identification of components was carried out by matching UV spectra to standard substances. The spectra of triterpene saponins have a characteristic absorption maximum at (200–210) nm, corresponding to the absorption spectra of ursolic and oleanolic acids.
The study using HPLC [28,29,30] was conducted on a Shimadzu LC20 Prominence liquid chromatograph in a modular system equipped with a four-channel LC20AD pump, a CTO20A column thermostat, an SIL20A automatic sampler, an SPDM20A diode-array detector, and ChemStation LC20 under the following conditions: column: X-Bridge C18, 150 mm × 4.6 mm, 5 µm particle size (Waters); column temperature: 30 °C; detection wavelength: 205 nm; mobile phase flow rate: 1.0 mL/min; injected sample volume: 20 µL; mobile phase composition: methanol for HPLC, 0.2% ammonium acetate solution (pH 6.75) in a ratio of 80:20; elution mode: isocratic.
Component classification was performed by matching the UV spectra of substances with those of standards. The spectra of triterpenoid saponins exhibit maximum absorption at 200–210 nm, so the detection of this group of compounds was conducted at 205 nm.
2.6. Quantification of Saponins
The quantitative determination of the saponin content in the methanol extracts of the studied raw materials was carried out using ursolic acid as a reference standard. Retention time for ursolic acid was 17.45 min; λmax (nm) 200.
The content of the substance in raw materials during extraction with the specified extractant under the given conditions for the liquid extract or the content of the substance in the dry extract (X) as a percentage is determined as follows:
where
Apr—the peak area of the substance on the chromatogram of the test solution;
Ast—the peak area of the substance on the chromatogram of the standard solution;
mst—the mass of the standard substance sample in the standard solution, mg;
mpr—the mass of the preparation (raw material), mg;
Vpr—the dilution of the test solution (volume of extractant used), mL;
Vst—the dilution of the standard solution, mL;
P—the purity of the standard, %.
2.7. Foam Index of Saponins
The foam index is determined by measuring the height of the foam produced by the equivalent of 1 g of herbal drug under the stated test conditions according to the European Pharmacopoeia method [1]. The foam index test indirectly indicates the amount of saponins in the material to be determined. During the analysis, an aqueous extract of the plant material is allowed to fall from a pipette, which is 45 cm in height, into a measuring cylinder, where the height of the foam column formed is measured. The higher (cm) the foam column, the higher the foam index value, and the more saponins the drug contains. The aqueous extract was prepared from 1 g of drug powder, adding 50 mL of distilled water and allowing it to stand for 30 min, and mixing 3–5 times with a spatula to disperse the powder without producing any foam. The spatula and the internal walls of the beaker were rinsed with 50 mL of distilled water to ensure that as much of the herbal drug or herbal drug preparation as possible was contained within the liquid. The mixture was allowed to stand undisturbed for 30 min and filtered through a filter paper 125 mm in diameter. Further analysis of the filtrate as the test solution was performed following the instructions of the European Pharmacopoeia using a filled pipette and measuring cylinder. The average of 3 determinations was recorded as a result.
2.8. Data Analysis
The multitude of EO compounds does not allow for an easy assessment of the uniformity of compounds from the table or lists. Therefore, the similarity of plant parts in their EO composition was analysed and illustrated using cluster analysis, which is implemented in PC-ord v7 [31]. The Sorensen (Bray–Curtis) distance measure of similarity was used to build the cluster tree by applying the flexible beta linkage method with a parameter of −0.25 (a default value). The log-transformed proportion of oils was used, and only components with at least 1% were included in the data set to reduce noise. The tree branch length illustrates the EO compositional dissimilarity between plant parts.
The foam index of saponins was compared between plant parts using a single-factor general linear model in Statistica ver 9 (StatSoft, Inc. Tulsa, OK, USA), and model-predicted least-square means were compared using Tukey’s multiple comparison test.
3. Results and Discussion
3.1. Analysis of Essential Oil
Different parts of G. capitata yielded concentrations of EO ranging from 0.42 mL/kg (shells) to 1.97 mL/kg (seeds) (Figure 2). The seeds contained the greatest proportion of essential oil instead of the flowers, which is the traditional expectation. The EO content was practically the same in the flowers, fruits, and leaves, but there was minimal EO in the stems, roots, and shells. Since the European Pharmacopoeia method (2.8.12. essential oils in herbal drugs [1]) does not require multiple parallel hydrodistillations, this work was limited to a single distillation. In our previous experience, the experimental error of this method is in the order of 10–15%, which we can also take into account when interpreting the results.
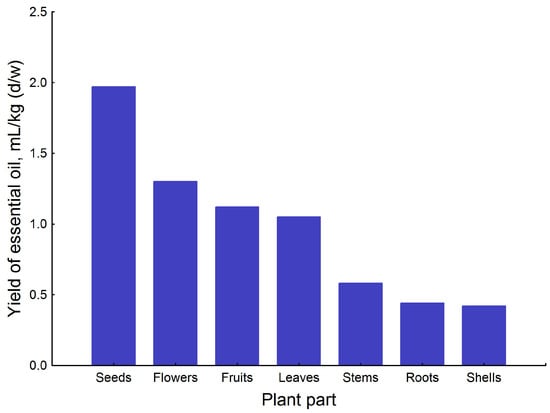
Figure 2.
Yield of essential oils (mL/kg (d/w)) in different parts of G. capitata.
Gilia parts contain relatively modest levels of oils in comparison to some other well-known medicinal plants [32]. For instance, the aerial parts of Origanum vulgare from different countries contained 2–11 mL/kg of EO [33]. In the Thymus vulgaris herb, the yield of EO was 3–28 mL/kg [34]; in commercial samples of Valeriana officinalis roots from various countries of different origins, the yield was 2–10 mL/kg [35]; and in the herb of Artemisia absinthium from different origins, the yield was 2–4 mL/kg [36]. The branches of Juniperus communis shrubs were collected from 27 different habitats in Estonia and contained 0.3–6 mL/kg of EO [37].
We aimed to first estimate the possible pharmaceutical potential of the phytochemically completely unstudied species G. capitata. A more thorough study of the plant should include three parallel distillations over 2–3 years of growth. Based on the results obtained in this work, we see that the quantitative content of essential oil in the plant G. capitata does not deserve a more thorough study in the future before its other important groups of active ingredients, which may affect the plant’s pharmaceutical potential, have been investigated.
The composition of EOs was studied using GC-MS (Table 1). In total, 116 compounds were identified, representing 80.50–92.16% of the extracted essential oils (Figure 3, Table 1). All parts of the plant contain phenylacetaldehyde, nonanal, (E)-2-nonenal, (-)-myrtenol, (E,E)-2,4-decadienal, tetradecanal, pentadecanal, and hexahydrofarnesyl acetone.

Table 1.
The content (%) of volatile compounds (>0.1%) in different parts of G. capitata.
Figure 3.
GC-MS chromatogram of essential oil hydrodistilled from G. capitata fruits.
The composition of essential oils in different parts of G. capitata is illustrated with a cluster tree, with the branch lengths showing the similarity–dissimilarity between plant parts (Figure 4). The fruits and seeds were most similar in composition, being almost identical. The flowers were next, similar to the fruits and seeds. They all contain high levels of hexahydrofarnesyl acetone and palmitic acid, but there are many other common EOs with lower levels (Table 1, Figure 3). Leaves and stems formed another narrow cluster with flagship compounds phytol and isomanool, as well as (E)-beta-ionone, manoyl oxide, and sclareol. The most distant from them were the shells of the fruit, showing that seeds contain the most EO in a fruit. Shells can be characterised by β-elemene and (+)-epi-bicyclosesquiphellandrene. The composition of roots was the most distinct from all other plant parts (Figure 4), mostly determined by the prevalence of (-)-myrtenol and (E)-myrtanol (Figure 5).
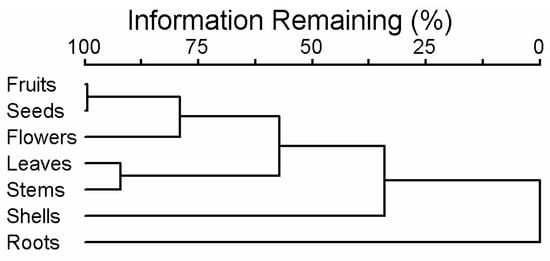
Figure 4.
A cluster tree characterising the essential oil composition of different parts of G. capitata.
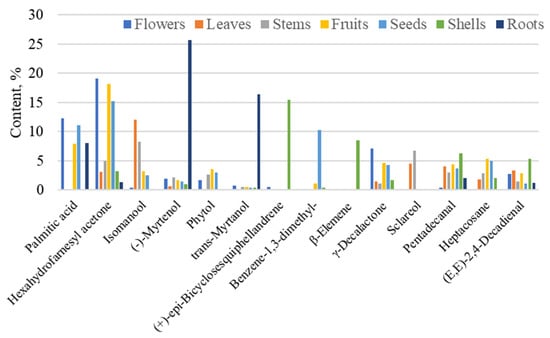
Figure 5.
Dominant substances (%) in different parts of G. capitata. Compounds with at least 5% are presented.
We have not found detailed scientific articles on the content and composition of the essential oil (EO) of G. capitata, though there are a few available studies for other plants of the Gilia genus (Polemoniaceae) [2,3], and the essential oils in Polemonium caeruleum and/or other species of the Polemonium genus have not been studied [8,24]. The most studied in the family is the Phlox genus [24]. Thus, we cannot compare our results with previous studies conducted by other authors or with the EO compositions of other related plant species. Interestingly, we detected many different EOs, which have been reported as potential pollinator attractors or flower oils for forage [24,25,38], but all were found at very low concentrations. However, the diversity of EOs studied was rather high—116 compounds were identified in different parts of G. capitata. We detected lower EO diversity using the same method in these plant species, except in the roots of V. officinalis, in which we found 150 compounds [35].
All parts of the G. capitata plant contained eight common components: phenylacetaldehyde, nonanal, (E)-2-nonenal, (-)-myrtenol, (E,E)-2,4-decadienal, tetradecanal, pentadecanal, and hexahydrofarnesyl aceton. These are rather nontypical components in other plant species, at least in classical EO-bearing plants.
We can only state that in the case of G. capitata, the same components are not always detected in all plant parts. This shows the importance of addressing the plant in detail and avoiding analysing a single part of the plant or the overall herb (Table 1). The composition of more prominent EOs (at least 5%) was similar in flowers and fruits (specifically in seeds). The vegetative parts of the plant, namely the stems and leaves, had a slightly different composition. The least valuable parts in EO quantity and composition were the shells of the fruits and roots. The analysis results with the assessment ‘nd’ (not detected) do not necessarily mean the complete absence of these substances in EO but may indicate very low contents of the components (probably less than 0.03%, the reliability level of the GC-MS method).
The essential oil of flowers was dominated by hexahydrofarnesyl acetone (19.1%), followed by palmitic acid (12.2%), γ-decalactone (7.1%), and (Z)-2-p-menthen-1-ol (3.6%) (Figure 5). In addition to the flowers, hexahydrofarnesyl acetone was also the dominant component in fruits (18.2%) and seeds (15.2%), and it was found in significant quantities in the stems (4.9%) of the G. capitata (Figure 5). Hexahydrofarnesyl acetone has been proven to exhibit potent antimicrobial, anti-inflammatory, and cytotoxic activity and is used in pain relief research [39,40,41,42]. Palmitic acid, also present in seeds (11.1%) and fruits (8.0%), has antioxidant and antibacterial activities [43,44].
Lactones, including γ-decalactone, constitute an essential group of fatty acid-derived volatile organic compounds conferring a peach-like aroma to a few essential oils and fruits, including peach, plum, pineapple, and strawberry [45]. γ-Decalactone inhibits strawberry pathogen growth and achene germination [46].
Menthane monoterpenoids (Z)-2-p-menthen-1-ol and (E)-p-2-menthen-1-ol are found in all organs of the aboveground part of the plant. They make up 5.7% of flowers, 2.2% of leaves, 6.0% of stems, 5.6% of fruits, 3.3% of shells, and 4.6% of seeds. Menthenol derivatives have potentially different biological properties. 1-Methyl-4-(1-methylethenyl)-2-cyclohexen-1-ol is an acetal reagent synthesising desoxy cannabidiols and THC-related psychoactive compounds. It is formed from (+)-limonene using a photosynthesised O2 transfer [47].
In addition to the dominant compounds in the flowers, (Z)-γ-bisabolene was also found (though it is similarly abundant in stems and fruits). The anti-tumor activity of γ-bisabolene in human neuroblastoma cells has been proven through the induction of p53-mediated mitochondrial apoptosis [48]. γ-Bisabolene has demonstrated antiproliferative activities against several human cancer cell lines. Another study [29] disclosed the antiproliferative and apoptosis-inducing activities of γ-bisabolene toward human neuroblastoma TE671 cells, and the CC50 value of γ-bisabolene was 8.2 μM toward TE671 cells [49].
Caryophyllene oxide was found in flowers (0.9%), while caryophyllene was distributed more widely in flowers, leaves, fruits, and shells (1.3, 0.5, and 2.8%, respectively). Its anti-cancer, antioxidant, and antimicrobial properties have been proven [50].
Linalool was found in flowers (0.3%), leaves (0.5%), stems (0.3%), shells (0.6%), and seeds (0.3%). Linalool and (E)-caryophyllene exhibited high cytotoxic activity against amelanotic melanoma and renal adenocarcinoma cells [51].
The predominant compounds in the EOs of the leaves were phytol (23.3%) and diterpene alcohols isomanool (12.1%), sclareol (4.5%), and (E)-β-ionone (4.5%). Phytol has antibacterial and antioxidant activities, inhibiting the growth of Staphylococcus aureus [52,53]. Phytol is used in fragrance and cosmetics to produce shampoos, toilet cleaners, household cleaners, and detergents [54].
Labdane diterpenoids are the most common types of diterpenoids isolated in minute amounts from higher plants [55]. Labdane diterpenoids are interesting for their cytotoxic, antifungal, anti-inflammatory, antiparasitic, and analgesic properties [56,57,58]. Labdane diterpenoid sclareol has a wide range of bioactivities, including anti-tumour, anti-inflammation, and anti-pathogenic microbes, and even anti-diabetes and anti-hypertension properties [59]. Sclareol can also kill human leukaemic cells and colon cancer cells in vitro by apoptosis [60,61]. Labdanum-type diterpenoids are usually found in the plant as a mixture of components. Therefore, we found it necessary to provide all the structural formulas of labdanum diterpenoids contained in G. capitata raw materials (Figure 6). Labdan-type diterpenes, such as manoyl oxide, 13-epi-manoyl oxide, epi-13-manool, kolavelool, isomanool, and sclareol, make up a significant part of oils in leaves and stems—22.6% and 20.2%, respectively. Essential oils from fruits and seeds contain less—3.9% and 3.0%, respectively. The main component among labdan diterpenes is isomanool (labda-8,14-diene-13β-ol); it is found in flowers (0.4%), leaves (12.1%), stems (8.3%), fruits (3.2%), and seeds (2.5%). It is followed by manoyl oxide, which is found in leaves (2.3%), stems (3.0%), fruits (0.6%), and seeds (0.5%). Both are absent in shells and roots. Nor-labdane diterpenoids ambrial is found in trace amounts only in stems.
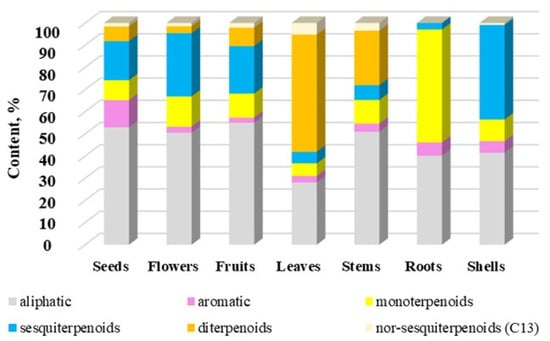
Figure 6.
The contents (%) of group compounds in volatile components of different parts of G. capitata. The total% from Table 1 has been rescaled to 100%.
C-13-isoprenoid, apocarotenoid β-ionone had fungicidal activities against Aspergillus fumigatus. It also ameliorated fungal keratitis in mice by reducing inflammation, which LOX-1, p-p38MAPK, and p-JNK regulated [62]. Data in the literature indicate that β-ionone and its derivatives have a lot of important pharmacological activities, including antibacterial, antifungal, antioxidant, anti-inflammatory, antiproliferative, and anti-cancer effects [63].
The most dominant compounds in EO in stems are isomanool (8.3%), sclareol (6.7%), and (E,E)-2,4-decadienal (2.9%). It is known that 2,4-decadienal has nematicidal [64] and insecticidal activities against the barn bug [65].
In addition to hexahydrofarnesyl acetone, the dominant compounds in the fruits were palmitic acid (8.0%), heptacosane (5.3%), and γ-decalactone (4.6%). The dominant compounds in the shell were (+)-epi-bicyclosesquiphellandrene (15.4%), β-elemene (8.5%), and pentadecanal (6.3%). β-Elemene exhibits anti-tumour properties, as well as anti-inflammatory and antioxidant effects [66,67]. β-Elemene and β-elemene piperazine derivatives have been shown to inhibit tumour cell growth in vitro and in vivo [68]. The antibacterial authorities of pentadecanal were established [69,70].
In addition to hexahydrofarnesyl acetone, the dominant compounds in the seeds were palmitic acid (11.1%), 1,3-dimethylbenzene (10.3%), and heptacosane (5.0%). Heptacosan can improve P-glycoprotein-mediated drug transport, demonstrating the ability to retain the substrate doxorubicin inside the cell and enhancing its cytotoxic effects [71].
The dominant compounds in the roots were (-)-myrtenol (25.7%), (E)-myrtanol (16.4%), palmitic acid (8.1%), and 2-pentylfuran (2.9%). Several reports have demonstrated the pharmacological properties of myrtenol, including its antioxidant, antibacterial, antifungal, antidiabetic, anxiolytic, and gastroprotective activities [72,73]. (-)-(E)-Myrtanol is an antimicrobial and acaricide agent [74,75]. 2-Pentylfuran has been suggested as a repellent for spotted-wing drosophila as it can significantly reduce fruit infestations under field conditions [76].
Many volatile compounds are aliphatic (24.4–48.6%). They are the dominant compounds in flowers, stems, fruits, and seeds (Figure 6). At the same time, diterpenoids are the dominant group in the composition of volatile compounds in leaves (45.9%), sesquiterpenoids in shells (34.7%), and monoterpenoids in roots (46.4%). It should also be noted that the contents of mono- and sesquiterpenoids are quite high in flowers, stems, fruits, and seeds. Figure 6 clearly shows that the EO content of roots is much different from that of aerial parts.
Sesquiterpenoids are represented by derivatives of naphthalene, such as copaene, humulene, (+)-epi-bicyclosesquiphellandrene, β-selinene, τ-muurolol, α-cadinol, and azulene, such as 7-epi-α-cedrene series. Antiviral activity against dengue virus (VDEN) was established for a few sesquiterpenoids, including isomers of copaene, β-caryophillene, caryophillene oxide, and (+)-epi-bicyclosquiphellandrene [65]. Norsesquiterpenoids or apocarotenoids are represented by two compounds, namely (E)-β-ionone and (E)-β-damascenone. They are absent in the roots. β-Damascenone inhibits the expression of pro-inflammatory cytokines and leukocyte adhesion molecules [66]. β-Damascenol effectively prevents skin sunburn [67].
Aromatic compounds represent an insignificant part of the composition of volatile compounds, and they are found in all parts of the plant and accumulate in fruits to the maximum extent (11.0%).
3.2. Analysis of Saponins
3.2.1. Identification of Saponins
The class identification of components was carried out by matching UV spectra to standard substances. The spectra of triterpene saponins have a characteristic absorption maximum of 200–210 nm, corresponding to the absorption spectra of ursolic and oleanolic acids. The analysis report of saponins in different G. capitata parts is shown in the Supplementary File.
Triterpene saponins are quite common in medicinal plants [32]. In previous studies, the presence of a variety of triterpene saponins, as well as oleanane derivatives such as polemoniumsaponines [77], polemonioside C, polemoniumgenin A, and polemoniogenin, were found in underground parts of Polemonium caeruleum [8]. It contains pentacyclic saponins of the β-amyrin type, the aglycones of which are mostly represented by esters of strongly hydroxylatedtriterpene alcohols [78]. Hypothetically, the structures of saponins in G. capitata may be similar, but further analysis is needed.
3.2.2. Quantification of Saponins
The results of the quantification of saponins are shown in Table 2. The analysis detected 21 different saponins. The highest levels of different saponins were found in the flowers and seeds, and minimal concentrations were found in the stems and roots. A similar tendency in the quantitative content of saponins in different parts of G. capitata is also confirmed by the results of foam index determination (Figure 7). The maximum number of individual saponins was typical of aerial parts (excluding stems) of G. capitata (Table 2). The foam index differed between plant parts (ANOVA F6,10-test = 29.9, p ≤ 0.0001). But it was not statistically different among flowers, leaves, fruits, and shells (index mean values of 2.9–3.4); it was somewhat lower in the seeds (2.6) and stems (1.9) (Figure 7). Surprisingly, the foam index was significantly lower in the plant’s roots (1.0). However, it is known that the roots of many plants rich in saponins are used as herbal drugs in medicinal practice [31]. These results are similar to the content of individual saponins found in different parts of G. capitata.

Table 2.
Saponin contents in different parts of Gilia capitata.
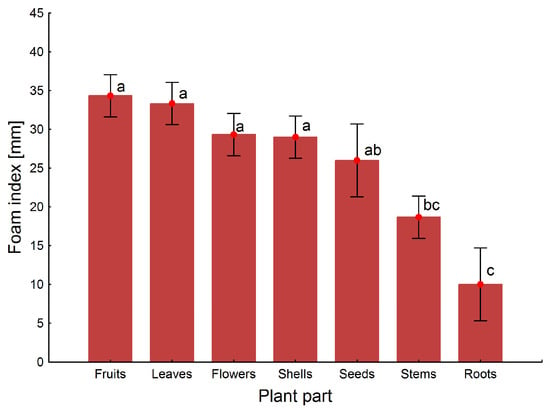
Figure 7.
Foam index of different parts of G. capitata. Note: Shared letter labels indicate homogeneity of groups according to Tukey’s test.
Since HPLC and GC are analytical methods with very low standard deviation (SD) values, Table 1 does not include SD parameters. The data obtained are sufficient to provide a preliminary assessment of the pharmaceutical potential of G. capitata.
No studies are available on the qualitative and quantitative contents of saponins in G. capitata and the entire plant genus Gilia. We can draw parallels with the relatively well-studied medicinal plant Polemonium caeruleum, which has been named Polemoniaceae. Because the hemolytic index of P. caeruleum is 1100 for roots and rhizomes, 1000 for herbs, and 3000 for seeds [78], we can expect a high content of saponins in the aboveground parts of the plant, especially the seeds.
Saponin distribution has been found to vary in individual plant parts [79]. The richest parts of Medicago truncatula [80] and Allium nigrum are the roots [81]. The total saponin concentration has been reported to be the highest in the leaves of Maesa lanceolata [82] and Panicum virgatum [83]. Thus, the aboveground parts of plants may also be a rich source of saponins, as appears to be the case in G. capitata. The localisation of EO also supports their preference for underground parts. According to the European Pharmacopoeia [1], the foam index of Senega root (Polygala radix) must be no less than 3.5. Moreover, senega root is a classic saponin drug containing 6–10% triterpene saponins [84]. Thus, the aerial parts of G. capitata contain a remarkable amount of saponins. These results suggest that if G. capitata has potential as a medicinal plant, the herb from which the thin stems have been isolated should be used as a source of the herbal drug.
3.2.3. The Limitations of the Study
This is the first explorative study about the chemical composition of G. capitata, and we were not able to cover the variability in the composition and quantities of substances, driven by yearly variation in weather conditions, soil types, or seasonality. G. capitata is known to have genetic and morphological variability within its native geographic range [11,12], and therefore, there might be differences between native populations and ecological conditions. It is also somewhat problematic to perform EO distillations only once from each plant part. It would be more correct to perform four distillations and find the SD value based on them. But on the other hand, even in its current form, it is convincingly clear in which part of the plant the EO yield is minimal and where it is maximal.
4. Conclusions
The composition of volatile compounds and saponins of various parts of G. capitata was studied for the first time. The analysis of bluehead gilia essential oil made it possible to detect and identify important potentially active compounds with various biological properties, including antioxidant, anti-cancer, and antidepressant properties, among others, which indicates the prospects for further phytochemical and pharmacological studies of the genus Gilia. The main sources of essential oils and saponins are the flowers, seeds, and potentially leaves; these parts should be given more attention in future studies. However, saponins in bluehead gilia are the most abundant compound for future interest.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/crops5030033/s1. Spectra of saponins from G. capitata.
Author Contributions
Conceptualisation, A.R., J.L. and O.K.; methodology, A.R., M.L., T.I., A.K., A.G. and O.K.; software, T.I., A.K. and A.R.; validation, A.R., M.L., T.I. and A.K.; formal analysis, A.R., M.L., P.S., A.G. and O.K.; investigation, A.R., M.L., P.S., A.G. and O.K.; resources, A.R. and J.L.; data curation, A.R., M.L., P.S. and O.K.; writing—original draft preparation, A.R., J.L., T.I., A.K. and O.K.; writing—review and editing, A.R., J.L., M.L., T.I., A.K. and O.K.; visualisation, J.L., T.I., A.G. and A.K.; supervision, A.R.; project administration, A.R.; funding acquisition, A.R. and O.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Union in the MSCA4Ukraine project titled “Design and development of 3D-printed medicines for bioactive materials of Ukrainian and Estonian medicinal plants origin” (ID number 1232466) and the Estonian Research Council grant PRG123. The views and opinions expressed in this study are only those of the author(s) and do not necessarily reflect those of the European Union. Neither the European Union nor the MSCA4Ukraine Consortium as a whole nor any individual member institutions of the MSCA4Ukraine Consortium can be held responsible for the views presented in this study.
Data Availability Statement
The data supporting the results of this study can be obtained from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- Smith, D.M.; Glennie, C.W.; Harborne, J.B.; Williams, C.A. Flavonoid Diversification in the Polemoniaceae. Biochem. Syst. Ecol. 1977, 5, 107–115. [Google Scholar] [CrossRef]
- Grant, V. Primary Classification and Phylogeny of the Polemoniaceae, with Comments on Molecular Cladistics. Am. J. Bot. 1998, 85, 741–752. [Google Scholar] [CrossRef] [PubMed]
- California Native Plant Society. Gilia Capitata. In Calscape; California Native Plant Society: Sacramento, CA, USA, 2024. [Google Scholar]
- Grant, V. Taxonomy of the Polemoniaceae: Gilia and Lathrocasis. SIDA Contrib. Bot. 2004, 21, 531–546. [Google Scholar]
- Grant, V. Genetic and Taxonomic Studies in Gilia: VI. Interspecific Relationships in the Leafy-Stemmed Gilias. Aliso 1954, 3, 35–49. [Google Scholar] [CrossRef]
- Grant, V.; Grant, A. Genetic and Taxonomic Studies in Gilia: X. Conspectus of the Subgenus Gilia. Aliso J. Syst. Evol. Bot. 1956, 3, 297–300. [Google Scholar] [CrossRef]
- Łaska, G.; Sieniawska, E.; Świątek, Ł.; Zjawiony, J.; Khan, S.; Boguszewska, A.; Stocki, M.; Angielczyk, M.; Polz-Dacewicz, M. Phytochemistry and Biological Activities of Polemonium caeruleum L. Phytochem. Lett. 2019, 30, 314–323. [Google Scholar] [CrossRef]
- Jaramillo, Z.; Leigh, J. Revision of the Genus Gilia of Utah. J. Undergrad. Res. 2019, 2019, 105. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. Propagation Protocol for Production of Container (Plug) Gilia capitata Sims Plants. In Native Plant Network; University of Kentucky: Lexington, KY, USA, 2002. [Google Scholar]
- Grant, V. Genetic and Taxonomic Studies in Gilia: I. Gilia capitata. Aliso 1950, 2, 239–316. [Google Scholar] [CrossRef]
- Porter, J.M. Gilia capitata subsp. Capitata. In Jepson eFlora 2023; Jepson Flora Project, Ed.; University of California: Berkeley, CA, USA, 2023. [Google Scholar]
- Gilia capitata Sims. Kew Plants of the World. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:110013-2 (accessed on 10 May 2025).
- Hayes, J. Top 10 Oregon Native Plants for Pollinators: Week 8. Available online: https://blogs.oregonstate.edu/gardenecologylab/2022/01/10/top-10-oregon-native-plants-for-pollinators-week-8/ (accessed on 27 March 2024).
- Grant, V.; Grant, A. Flower Pollination in the Phlox Family; Columbia University Press: New York, NY, USA, 1965. [Google Scholar]
- Nagy, E.S. Selection for Native Characters in Hybrids Between Two Locally Adapted Plant Subspecies. Evolution 1997, 51, 1469. [Google Scholar] [CrossRef]
- Runge, T.; Latacz-Lohmann, U.; Schaller, L.; Todorova, K.; Daugbjerg, C.; Termansen, M.; Liira, J.; Le Gloux, F.; Dupraz, P.; Leppanen, J.; et al. Implementation of Eco-schemes in Fifteen European Union Member States. EuroChoices 2022, 21, 19–27. [Google Scholar] [CrossRef]
- Nagy, E.S.; Rice, K.J. Local Adaptation in Two Subspecies of an Annual Plant: Implications for Migration and Gene Flow. Evolution 1997, 51, 1079. [Google Scholar] [CrossRef] [PubMed]
- Kruckeberg, A.R. Intraspecific variability in the response of certain native plant species to serpentine soil. Am. J. Bot. 1951, 38, 408–419. [Google Scholar] [CrossRef]
- Brown, H.S. Differential Chiasma Frequencies in Self-Pollinating and Cross-Pollinating Species of the Genus Gilia. Aliso J. Syst. Florist. Bot. 1961, 5, 67–81. [Google Scholar] [CrossRef][Green Version]
- Grant, V. Seed Germination in Gilia capitata and Its Relatives. Madroño 1949, 10, 87–93. [Google Scholar]
- Keeley, J.E.; Keeley, S.C. Role of Fire in the Germination of Chaparral Herbs and Suffrutescents. Madroño 1987, 34, 240–249. [Google Scholar]
- Cseke, L.J.; Kaufman, P.B.; Kirakosyan, A. The Biology of Essential Oils in the Pollination of Flowers. Nat. Prod. Commun. 2007, 2, 1934578X0700201225. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Dötterl, S.; Schäffler, I. Flower Scent of Floral Oil-Producing Lysimachia punctata as Attractant for the Oil-Bee Macropis fulvipes. J. Chem. Ecol. 2007, 33, 441–445. [Google Scholar] [CrossRef]
- Raal, A.; Ilina, T.; Kovalyova, A.; Koshovyi, O. Volatile Compounds in Distillates and Hexane Extracts from the Flowers of Philadelphus coronarius and Jasminum officinale. Sci. Pharm. Sci. 2024, 6, 37–46. [Google Scholar] [CrossRef]
- Hrytsyk, Y.; Koshovyi, O.; Lepiku, M.; Jakštas, V.; Žvikas, V.; Matus, T.; Melnyk, M.; Grytsyk, L.; Raal, A. Phytochemical and Pharmacological Research in Galenic Remedies of Solidago canadensis L. Herb. Phyton 2024, 93, 2303–2315. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, K.; Zhao, E.; Li, Y.; Yao, L.; Yang, X.; Xie, X. Determination of Oleanolic Acid and Ursolic Acid in Chinese Medicinal Plants Using HPLC with PAH Polymeric C18. Pharmacogn. Mag. 2013, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Xia, Z.H.; Tan, R.X. High-Performance Liquid Chromatographic Analysis of Bioactive Triterpenes in Perilla frutescens. J. Pharm. Biomed. Anal. 2003, 32, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data; MjM Software Design: Gleneden Beach, OR, USA, 2018. [Google Scholar]
- Raal, A. Pharmacognosy, 2025; Tartu Ülikooli Kirjastus: Tartu, Estonia, 2025. [Google Scholar]
- Raal, A.; Gontova, T.; Ivask, A.; Orav, A.; Koshovyi, O. Yield, Composition, and Chemotypes of Essential Oils from Origanum vulgare L. Aerial Parts Cultivated in Different European Countries. Agronomy 2024, 14, 3046. [Google Scholar] [CrossRef]
- Raal, A.; Gontova, T.; Palmeos, M.; Orav, A.; Sayakova, G.; Koshovyi, O. Comparative Analysis of Content and Composition of Essential Oils of Thymus vulgaris L. from Different Regions of Europe. Proc. Estonian Acad. Sci. 2024, 73, 332–344. [Google Scholar] [CrossRef]
- Raal, A.; Kokitko, V.; Odyntsova, V.; Orav, A.; Koshovyi, O. Comparative Analysis of the Essential Oil of the Underground Organs of Valeriana spp. from Different Countries. Phyton 2024, 93, 1365–1382. [Google Scholar] [CrossRef]
- Ain, R.; Ilina, T.; Kovaleva, A.; Orav, A.; Karileet, M.; Džaniašvili, M.; Koliadzhyn, T.; Grytsyk, A.; Koshovyi, O. Variation in the Composition of the Essential Oil of Commercial Artemisia absinthium L. Herb Samples from Different Countries. Sci. Pharm. Sci. 2024, 2, 19–28. [Google Scholar] [CrossRef]
- Raal, A.; Komarov, R.; Orav, A.; Kapp, K.; Grytsyk, A.; Koshovyi, O. Chemical Composition of Essential Oil of Common Juniper (Juniperus communis L.) Branches from Estonia. Sci. Pharm. Sci. 2022, 6, 66–73. [Google Scholar] [CrossRef]
- Andersson, S.; Nilsson, L.A.; Groth, I.; Bergström, G. Floral Scents in Butterfly-Pollinated Plants: Possible Convergence in Chemical Composition. Bot. J. Linn. Soc. 2002, 140, 129–153. [Google Scholar] [CrossRef]
- Filipowicz, N.; Kamiński, M.; Kurlenda, J.; Asztemborska, M.; Ochocka, J.R. Antibacterial and Antifungal Activity of Juniper Berry Oil and Its Selected Components. Phytother. Res. 2003, 17, 227–231. [Google Scholar] [CrossRef]
- Razavi, S.M.; Nejad-Ebrahimi, S. Phytochemical Analysis and Allelopathic Activity of Essential Oils of Ecballium elaterium A. Richard Growing in Iran. Nat. Prod. Res. 2010, 24, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, S.; Shi, J.; Sun, Z.; Lei, Z.; Yin, Z.; Qian, Z.; Tang, H.; Xie, H. Genotypic and Environmental Effects on the Volatile Chemotype of Valeriana jatamansi Jones. Front. Plant Sci. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Avoseh, O.N.; Mtunzi, F.M.; Ogunwande, I.A.; Ascrizzi, R.; Guido, F. Albizia lebbeck and Albizia zygia Volatile Oils Exhibit Anti-Nociceptive and Anti-Inflammatory Properties in Pain Models. J. Ethnopharmacol. 2021, 268, 113676. [Google Scholar] [CrossRef]
- Ganesan, T.; Subban, M.; Christopher Leslee, D.B.; Kuppannan, S.B.; Seedevi, P. Structural Characterization of N-Hexadecanoic Acid from the Leaves of Ipomoea eriocarpa and Its Antioxidant and Antibacterial Activities. Biomass Convers. Biorefinery 2024, 14, 14547–14558. [Google Scholar] [CrossRef]
- Johannes, E.; Litaay, M.; Syahribulan, S. The Bioactivity of Hexadecanoic Acid Compound Isolated from Hydroid Aglaophenia cupressina Lamoureoux as Antibacterial Agent against Salmonella typhi. Int. J. Biol. Med. Res. 2016, 7, 5469–5472. [Google Scholar]
- Sánchez-Sevilla, J.F.; Cruz-Rus, E.; Valpuesta, V.; Botella, M.A.; Amaya, I. Deciphering Gamma-Decalactone Biosynthesis in Strawberry Fruit Using a Combination of Genetic Mapping, RNA-Seq and eQTL Analyses. BMC Genom. 2014, 15, 218. [Google Scholar] [CrossRef]
- Chambers, A.H.; Evans, S.A.; Folta, K.M. Methyl Anthranilate and γ-Decalactone Inhibit Strawberry Pathogen Growth and Achene Germination. J. Agric. Food Chem. 2013, 61, 12625–12633. [Google Scholar] [CrossRef]
- Gong, X.; Sun, C.; Abame, M.A.; Shi, W.; Xie, Y.; Xu, W.; Zhu, F.; Zhang, Y.; Shen, J.; Aisa, H.A. Synthesis of CBD and Its Derivatives Bearing Various C4′-Side Chains with a Late-Stage Diversification Method. J. Org. Chem. 2020, 85, 2704–2715. [Google Scholar] [CrossRef]
- Jou, Y.-J.; Hua, C.-H.; Lin, C.-S.; Wang, C.-Y.; Wan, L.; Lin, Y.-J.; Huang, S.-H.; Lin, C.-W. Anticancer Activity of γ-Bisabolene in Human Neuroblastoma Cells via Induction of P53-Mediated Mitochondrial Apoptosis. Molecules 2016, 21, 601. [Google Scholar] [CrossRef]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a Sesquiterpene from the Essential Oil Extract of Opoponax (Commiphora guidottii), Exhibits Cytotoxicity in Breast Cancer Cell Lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic Activity of Essential Oils from Labiatae and Lauraceae Families against in Vitro Human Tumor Models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar] [PubMed]
- Yoshihiro, I.; Toshiko, H.; Akiko, S.; Kazuma, H.; Hajime, H.; Shigeki, K. Biphasic Effects of Geranylgeraniol, Terpenone and Phytolon Thegrowth of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 1770–1774. [Google Scholar]
- Sabudak, T.; Ozturk, M.; Goren, A.C.; Kolak, U.; Topcu, G. Fatty Acids and Other Lipid Composition of Five Trifolium Species with Antioxidant Activity. Pharm. Biol. 2009, 47, 137–141. [Google Scholar] [CrossRef]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Phytol. Food Chem. Toxicol. 2010, 48, S59–S63. [Google Scholar] [CrossRef]
- Demetzos, C.; Dimas, K.S. Labdane-Type Diterpenes: Chemistry and Biological Activity. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2001; Volume 25, pp. 235–292. ISBN 978-0-08-044001-9. [Google Scholar]
- Tran, Q.T.N.; Wong, W.S.F.; Chai, C.L.L. Labdane Diterpenoids as Potential Anti-Inflammatory Agents. Pharmacol. Res. 2017, 124, 43–63. [Google Scholar] [CrossRef]
- Villamizar, J.E.; Juncosa, J.; Pittelaud, J.; Hernández, M.; Canudas, N.; Tropper, E.; Salazar, F.; Fuentes, J. Facile Access to Labdane-Type Diterpenes: Synthesis of Coronarin C, Zerumin B, Labda-8(17), 13(14)-Dien-15,16-Olide and Derivatives from (+)-Manool. J. Chem. Res. 2007, 6, 342–346. [Google Scholar] [CrossRef]
- Cyr, A.; Wilderman, P.R.; Determan, M.; Peters, R.J. A Modular Approach for Facile Biosynthesis of Labdane-Related Diterpenes. J. Am. Chem. Soc. 2007, 129, 6684–6685. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, X.; Tang, H.; Peng, C.; Peng, F. The Bioactivities of Sclareol: A Mini Review. Front. Pharmacol. 2022, 13, 1014105. [Google Scholar] [CrossRef]
- Jameel, S.; Bhat, K.A. Sclareol: Isolation, Structural Modification, Biosynthesis, and Pharmacological Evaluation—A Review. Pharm. Chem. J. 2024, 57, 1568–1579. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Jung, E.; Kang, S.; Kim, Y.J. Sclareol Isolated from Salvia officinalis Improves Facial Wrinkles via an Antiphotoaging Mechanism. J. Cosmet. Dermatol. 2016, 15, 475–483. [Google Scholar] [CrossRef]
- Yin, M.; Li, C.; Zhang, L.; Lin, J.; Jiang, N.; Wang, Q.; Xu, Q.; Zheng, H.; Gu, L.; Jia, Y.; et al. Mechanism of Antifungal Activity and Therapeutic Action of β-Ionone on Aspergillus fumigatus Keratitis via Suppressing LOX1 and JNK/P38 MAPK Activation. Int. Immunopharmacol. 2022, 110, 108992. [Google Scholar] [CrossRef]
- Kang, C.-H.; Jayasooriya, R.G.P.T.; Choi, Y.H.; Moon, S.-K.; Kim, W.-J.; Kim, G.-Y. β-Ionone Attenuates LPS-Induced pro-Inflammatory Mediators Such as NO, PGE2 and TNF-α in BV2 Microglial Cells via Suppression of the NF-κB and MAPK Pathway. Toxicol. In Vitro 2013, 27, 782–787. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal Activity of (E,E)-2,4-Decadienal and (E)-2-Decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef]
- Mirek, J.; Walkowiak-Nowicka, K.; Słocińska, M. The Effect of (E,E)-2,4-Decadienal, (E)-2-Decenal, 2-Undecanone and Furfural on Reproduction of Tenebrio molitor. In Proceedings of the 1st International Electronic Conference on Entomology, Online, 1–15 July 2021; p. 10540. [Google Scholar]
- Feng, Y.; An, Q.; Zhao, Z.; Wu, M.; Yang, C.; Liang, W.Y.; Xu, X.; Jiang, T.; Zhang, G. Beta-Elemene: A Phytochemical with Promise as a Drug Candidate for Tumor Therapy and Adjuvant Tumor Therapy. Biomed. Pharmacother. 2024, 172, 116266. [Google Scholar] [CrossRef]
- Chen, X.; Huang, C.; Li, K.; Liu, J.; Zheng, Y.; Feng, Y.; Kai, G. Recent Advances in Biosynthesis and Pharmacology of β-Elemene. Phytochem. Rev. 2023, 22, 169–186. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, R.; Xu, L.; Xie, S.; Dong, J.; Jing, Y. β-Elemene Piperazine Derivatives Induce Apoptosis in Human Leukemia Cells through Downregulation of c-FLIP and Generation of ROS. PLoS ONE 2011, 6, e15843. [Google Scholar] [CrossRef]
- Ricciardelli, A.; Casillo, A.; Papa, R.; Monti, D.M.; Imbimbo, P.; Vrenna, G.; Artini, M.; Selan, L.; Corsaro, M.M.; Tutino, M.L.; et al. Pentadecanal Inspired Molecules as New Anti-Biofilm Agents against Staphylococcus epidermidis. Biofouling 2018, 34, 1110–1120. [Google Scholar] [CrossRef]
- Casillo, A.; Papa, R.; Ricciardelli, A.; Sannino, F.; Ziaco, M.; Tilotta, M.; Selan, L.; Marino, G.; Corsaro, M.M.; Tutino, M.L.; et al. Anti-Biofilm Activity of a Long-Chain Fatty Aldehyde from Antarctic Pseudoalteromonas haloplanktis TAC125 against Staphylococcus epidermidis Biofilm. Front. Cell. Infect. Microbiol. 2017, 7, 46. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Poma, P.; Tutone, M.; McCubrey, J.A.; Sajeva, M.; Notarbartolo, M. Phytol and Heptacosane Are Possible Tools to Overcome Multidrug Resistance in an In Vitro Model of Acute Myeloid Leukemia. Pharmaceuticals 2022, 15, 356. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Jaouadi, I.; Zeouk, I.; Ghchime, R.; El Menyiy, N.; Omari, N.E.; Balahbib, A.; Al-Mijalli, S.H.; Abdallah, E.M.; El-Shazly, M.; et al. Biological and Pharmacological Properties of Myrtenol: A Review. Curr. Pharm. Des. 2023, 29, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Barbhuiya, P.A.; Pathak, M.P. Myrtenol: A Promising Terpene with Potent Pharmacological Properties. Pharmacol. Res. Nat. Prod. 2024, 4, 100067. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lee, H.-S. Verbenone Structural Analogues Isolated from Artemesia aucheri as Natural Acaricides against Dermatophagoides spp. and Tyrophagus putrescentiae. J. Agric. Food Chem. 2013, 61, 12292–12296. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lee, H.-W.; Lee, H.-S. Growth Inhibitory Activities of Myrtanol and Structural Analogues from Thymus tosevii against Intestinal Bacteria. Food Sci. Biotechnol. 2015, 24, 169–174. [Google Scholar] [CrossRef]
- Cha, D.H.; Roh, G.H.; Hesler, S.P.; Wallingford, A.; Stockton, D.G.; Park, S.K.; Loeb, G.M. 2-Pentylfuran: A Novel Repellent of Drosophila suzukii. Pest Manag. Sci. 2021, 77, 1757–1764. [Google Scholar] [CrossRef]
- Reznicek, G.; Schroder, H.; Schubertzsilavecz, M.; Schopke, T.; Lehrkinder, S.; Haslinger, E.; Hiller, K.; Jurenitsch, J.; Kubelka, W. Polemonium caeruleum L.—The Main Saponins of Lower Polarity. Pharmazie 1994, 49, 58–61. [Google Scholar]
- Frolova, L.N.; Kovaleva, E.L.; Shelestova, V.V.; Kuteynikov, V.Y.; Flisyuk, E.V.; Pozharitskaya, O.N.; Shikov, A.N. Comparison of Analytical Methods Used for Standardization of Triterpenoid Saponins in Herbal Monographs Included in the Russian and Other Pharmacopeias. Phytochem. Anal. 2025, pca.3516. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/pca.3516 (accessed on 10 May 2025). [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and Quantification of Saponins: A Review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Huhman, D.V.; Berhow, M.A.; Sumner, L.W. Quantification of Saponins in Aerial and Subterranean Tissues of Medicago truncatula. J. Agric. Food Chem. 2005, 53, 1914–1920. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Theunis, M.H.B.L.; Foubert, K.; Pollier, J.; Gonzalez-Guzman, M.; Goossens, A.; Vlietinck, A.J.; Pieters, L.A.C.; Apers, S. Determination of Saponins in Maesa lanceolata by LC-UV: Development and Validation. Phytochemistry 2007, 68, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Mitchell, R.B.; Wang, Z.; Heiss, C.; Gardner, D.R.; Azadi, P. Isolation, Characterization, and Quantification of Steroidal Saponins in Switchgrass (Panicum virgatum L.). J. Agric. Food Chem. 2009, 57, 2599–2604. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines: A Guide for Healthcare Professionals; Pharmaceutical Press: London, UK, 2002. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).