Plasma-Activated Water as a Sustainable Nitrogen Source: Supporting the UN Sustainable Development Goals (SDGs) in Controlled Environment Agriculture
Abstract
1. Introduction
2. Controlled Environment Agriculture
3. Physicochemical Properties and Generation of PAW
4. Techno-Commercial Analysis and Market Potential of PAW
5. PAW and Sustainable Agricultural Potential
6. Expanding PAW’s Utility
7. PAW Alignment with UN SDGs
8. PAW Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAW | Plasma-Activated Water |
| CEA | Controlled Environment Agriculture |
| LEDs | Light-emitting diodes |
| PM2.5 | Particulate matter |
| UV | Ultraviolet |
| UN | United Nations |
| SDG | Sustainable Development Goals |
| EU | European Union |
| U.S. | United States of America |
| DNA | Deoxyribonucleic Acid |
| RNA | Ribonucleic Acid |
References
- Graham, F. Daily Briefing: World Population Estimated to Reach Eight Billion Today. Nature 2022, d41586-022-03739–9. [Google Scholar] [CrossRef] [PubMed]
- Tomšík, K.; Bartoňová, K.; Marušiak, J.; Čermák, M.; Blažek, V.; Malec, K.; Maitah, M. Global Trends in Fertilizer Commerce: A Dual Analysis of General and Nitrogen Fertilizer Markets; Maci, J., Maresova, P., Firlej, K., Soukal, I., Eds.; University of Hradec Králové: Hradec Králové, Czech Republic, 2024; pp. 442–452. [Google Scholar]
- Willer, H.; Trávníček, J.; Schlatter, B. The World of Organic Agriculture: Statistics & Emerging Trends 2024, 25th ed.; Research Institute of Organic Agriculture FiBL and IFOAM—Organics International: Frick/Bonn, Germany, 2024. [Google Scholar]
- U.S. Department of Agriculture, Economic Research Service. Food Availability (Per Capita) Data System: Vegetables (All Uses); Food Availability Data System; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2024; p. 40. [Google Scholar]
- Gülüt, K.Y.; Şentürk, G.G. Impact of Nitrogen Fertilizer Type and Application Rate on Growth, Nitrate Accumulation, and Postharvest Quality of Spinach. PeerJ 2024, 12, e17726. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Peña-Fleitas, M.T.; Gallardo, M.; De Souza, R.; Padilla, F.M.; Thompson, R.B. Sweet Pepper and Nitrogen Supply in Greenhouse Production: Critical Nitrogen Curve, Agronomic Responses and Risk of Nitrogen Loss. Eur. J. Agron. 2020, 117, 126046. [Google Scholar] [CrossRef]
- Yue, W.; Liu, L.; Chen, S.; Bai, Y.; Li, N. Effects of Water and Nitrogen Coupling on Growth, Yield and Quality of Greenhouse Tomato. Water 2022, 14, 3665. [Google Scholar] [CrossRef]
- Him, R.; Romdhane, L.; Nicoletto, C.; Tlili, I.; Ilahy, R. Changes in Morphological and Physiological Parameters Affecting Lettuce Cultivars Due to Nitrogen Fertilizer in Greenhouse Tunnel. J. Postharvest Technol. 2024, 10, 19–34. [Google Scholar]
- Kosson, R.; Felczyński, K.; Szwejda-Grzybowska, J.; Grzegorzewska, M.; Tuccio, L.; Agati, G.; Kaniszewski, S. Nutritive Value of Marketable Heads and Outer Leaves of White Head Cabbage Cultivated at Different Nitrogen Rates. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 67, 524–533. [Google Scholar] [CrossRef]
- Yoder, N.; Davis, J.G. Organic Fertilizer Comparison on Growth and Nutrient Content of Three Kale Cultivars. HortTechnology 2020, 30, 176–184. [Google Scholar] [CrossRef]
- López Martínez, P. Techno-Economic Analysis of a Solar Ammonia and Fertilizer Production. Master’s Thesis, German Aerospace Center, Institute of Future Fuels, Linder Höhe, 51147, Cologne Germany DTU, Process and Systems Engineering Centre, Søltofts Plads, Building 224, 2800 Kgs, Lyngby, Denmark, 2022. [Google Scholar]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; et al. Beyond Fossil Fuel–Driven Nitrogen Transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef]
- Lim, J.; Fernández, C.A.; Lee, S.W.; Hatzell, M.C. Ammonia and Nitric Acid Demands for Fertilizer Use in 2050. ACS Energy Lett. 2021, 6, 3676–3685. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Jardali, F.; Bogaerts, A.; Lefferts, L. From the Birkeland–Eyde Process towards Energy-Efficient Plasma-Based NOX Synthesis: A Techno-Economic Analysis. Energy Environ. Sci. 2021, 14, 2520–2534. [Google Scholar] [CrossRef]
- De Vries, W. Impacts of Nitrogen Emissions on Ecosystems and Human Health: A Mini Review. Curr. Opin. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Achakulwisut, P.; Brauer, M.; Hystad, P.; Anenberg, S.C. Global, National, and Urban Burdens of Paediatric Asthma Incidence Attributable to Ambient NO2 Pollution: Estimates from Global Datasets. Lancet Planet. Health 2019, 3, e166–e178. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Chen, P.; Schiappacasse, C.; Zhou, N.; Anderson, E.; Chen, D.; Liu, J.; Cheng, Y.; Hatzenbeller, R.; Addy, M.; et al. A Review on the Non-Thermal Plasma-Assisted Ammonia Synthesis Technologies. J. Clean. Prod. 2018, 177, 597–609. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of Plasma-Seed Treatments as a Potential Seed Processing Technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhou, L.; Xie, J.; He, C. Plasma-activated Water Production and Its Application in Agriculture. J. Sci. Food Agric. 2021, 101, 4891–4899. [Google Scholar] [CrossRef]
- Song, J.-S.; Kim, S.B.; Ryu, S.; Oh, J.; Kim, D.-S. Emerging Plasma Technology That Alleviates Crop Stress During the Early Growth Stages of Plants: A Review. Front. Plant Sci. 2020, 11, 988. [Google Scholar] [CrossRef]
- Ibendahl, G. The Russia-Ukraine Conflict and the Effect on Fertilizer; Department of Agricultural Economics, Kansas State University: Manhattan, KS, USA, 2022; pp. 1–12. [Google Scholar]
- Vatistas, C.; Avgoustaki, D.D.; Bartzanas, T. A Systematic Literature Review on Controlled-Environment Agriculture: How Vertical Farms and Greenhouses Can Influence the Sustainability and Footprint of Urban Microclimate with Local Food Production. Atmosphere 2022, 13, 1258. [Google Scholar] [CrossRef]
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.-J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled Environment Food Production for Urban Agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Badji, A.; Benseddik, A.; Bensaha, H.; Boukhelifa, A.; Hasrane, I. Design, Technology, and Management of Greenhouse: A Review. J. Clean. Prod. 2022, 373, 133753. [Google Scholar] [CrossRef]
- Oh, S.; Lu, C. Vertical Farming—Smart Urban Agriculture for Enhancing Resilience and Sustainability in Food Security. J. Hortic. Sci. Biotechnol. 2023, 98, 133–140. [Google Scholar] [CrossRef]
- Ojo, M.O.; Zahid, A. Deep Learning in Controlled Environment Agriculture: A Review of Recent Advancements, Challenges and Prospects. Sensors 2022, 22, 7965. [Google Scholar] [CrossRef] [PubMed]
- Ragaveena, S.; Edward, A.S.; Surendran, U. Correction to: Smart Controlled Environment Agriculture Methods: A Holistic Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 915. [Google Scholar] [CrossRef]
- Sharma, A.; Hazarika, M.; Heisnam, P.; Pandey, H.; Devadas, V.S.; Wangsu, M. Controlled Environment Ecosystem: A Plant Growth System to Combat Climate Change through Soilless Culture. Crop Des. 2024, 3, 100044. [Google Scholar] [CrossRef]
- Grand View Research. Indoor Farming Market Size, Share, Growth Report, 2030; Grand View Research: San Francisco, CA, USA, 2024; p. 120. [Google Scholar]
- Dohlman, E.; Maguire, K.; Davis, W.; Husby, M.; Bovay, J.; Weber, C.; Lee, Y. Trends, Insights, and Future Prospects for Production in Controlled Environment Agriculture and Agrivoltaics Systems; U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2024; p. 48. [Google Scholar]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.D.D.; et al. Can Cold Plasma Be Used for Boosting Plant Growth and Plant Protection in Sustainable Plant Production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Cold Plasma Technology for Sustainable Food Production: Meeting the United Nations Sustainable Development Goals. Sustain. Food Technol. 2025, 3, 32–53. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma Agriculture: Review from the Perspective of the Plant and Its Ecosystem. Plasma Process. Polym. 2021, 18, 2000162. [Google Scholar] [CrossRef]
- Rossetti, I. Reactor Design, Modelling and Process Intensification for Ammonia Synthesis. In Sustainable Ammonia Production; Inamuddin, Boddula, R., Asiri, A., Eds.; Green Energy and Technology; Springer: Cham, Switzerland, 2020; pp. 17–48. [Google Scholar] [CrossRef]
- Savi, P.J.; Mantri, A.; Khodaverdi, H.; Zou, Y.; De Moraes, G.J.; Nansen, C. Indirect Effects of Plasma-Activated Water Irrigation on Tetranychus Urticae Populations. J. Pest Sci. 2025, 98, 449–462. [Google Scholar] [CrossRef]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold Plasma in Food Processing: Design, Mechanisms, and Application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. The Role of Different Plasma Forming Gases on Chemical Species Formed in Plasma Activated Water (PAW) and Their Effect on Its Properties. Phys. Scr. 2022, 97, 065003. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.K.; Bazaka, K. Plasma-Activated Water: Generation, Origin of Reactive Species and Biological Applications. J. Phys. Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Jablonowski, H.; Schmidt-Bleker, A.; Weltmann, K.-D.; Von Woedtke, T.; Wende, K. Non-Touching Plasma–Liquid Interaction—Where Is Aqueous Nitric Oxide Generated? Phys. Chem. Chem. Phys. 2018, 20, 25387–25398. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, S.; Ran, C. The Effect of Air-Water-Plasma-Jet-Activated Water on Penicillium: The Reaction of HNO2 and H2O2 Under Acidic Condition. Front. Phys. 2020, 8, 242. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Mordor Intelligence Research & Advisory. Ammonia Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030); Mordor Intelligence: Telangana, India, 2024. [Google Scholar]
- Mohajer, M.H.; Monfaredi, M.; Rahmani, M.; Martami, M.; Razaghiha, E.; Mirjalili, M.H.; Hamidi, A.; Ghomi, H.R. Impact of Dielectric Barrier Discharge Plasma and Plasma-Activated Water on Cotton Seed Germination and Seedling Growth. Heliyon 2024, 10, e38160. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on Formation of Cold Plasma Activated Water (PAW) and the Applications in Food and Agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef]
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive Nitrogen Species in Plasma-Activated Water: Generation, Chemistry and Application in Agriculture. J. Phys. Appl. Phys. 2020, 53, 223001. [Google Scholar] [CrossRef]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal Effects against S. aureus and Physicochemical Properties of Plasma Activated Water Stored at Different Temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef]
- Vlad, I.-E.; Anghel, S.D. Time Stability of Water Activated by Different On-Liquid Atmospheric Pressure Plasmas. J. Electrost. 2017, 87, 284–292. [Google Scholar] [CrossRef]
- Goldstein, S.; Lind, J.; Merényi, G. Chemistry of Peroxynitrites as Compared to Peroxynitrates. Chem. Rev. 2005, 105, 2457–2470. [Google Scholar] [CrossRef]
- Kutasi, K.; Popović, D.; Krstulović, N.; Milošević, S. Tuning the Composition of Plasma-Activated Water by a Surface-Wave Microwave Discharge and a kHz Plasma Jet. Plasma Sources Sci. Technol. 2019, 28, 095010. [Google Scholar] [CrossRef]
- Ghorui, S.; Tiwari, N.; Parab, H. Atmospheric Pressure Portable Catalytic Thermal Plasma System for Fast Synthesis of Aqueous NO3 and NO2 Fertilizer from Air and Water. Plasma Chem. Plasma Process. 2025, 45, 371–402. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.J.; Park, S.; In Yong, H.; Choe, J.H.; Jeon, H.-J.; Choe, W.; Jo, C. The Use of Atmospheric Pressure Plasma-Treated Water as a Source of Nitrite for Emulsion-Type Sausage. Meat Sci. 2015, 108, 132–137. [Google Scholar] [CrossRef]
- Wang, Q.; Salvi, D. Evaluation of Plasma-Activated Water (PAW) as a Novel Disinfectant: Effectiveness on Escherichia Coli and Listeria Innocua, Physicochemical Properties, and Storage Stability. LWT 2021, 149, 111847. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-Phase Chemistry and Bactericidal Effects from an Air Discharge Plasma in Contact with Water: Evidence for the Formation of Peroxynitrite through a Pseudo-Second-Order Post-Discharge Reaction of H2 O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and Future Role of Haber–Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Ekanayake, U.G.M.; Seo, D.H.; Faershteyn, K.; O’Mullane, A.P.; Shon, H.; MacLeod, J.; Golberg, D.; Ostrikov, K.K. Atmospheric-Pressure Plasma Seawater Desalination: Clean Energy, Agriculture, and Resource Recovery Nexus for a Blue Planet. Sustain. Mater. Technol. 2020, 25, e00181. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Khanjani, S. Experimental Study to Improve the Performance of Solar Still Desalination by Hydrophobic Condensation Surface Using Cold Plasma Technology. Sustain. Energy Technol. Assess. 2021, 45, 101129. [Google Scholar] [CrossRef]
- Capdevila-Cortada, M. Electrifying the Haber–Bosch. Nat. Catal. 2019, 2, 1055. [Google Scholar] [CrossRef]
- Winter, L.R.; Chen, J.G. N2 Fixation by Plasma-Activated Processes. Joule 2021, 5, 300–315. [Google Scholar] [CrossRef]
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- United Nations. Progress towards the Sustainable Development Goals; United Nations: New York, NY, USA, 2024; p. 26. [Google Scholar]
- Milhan, N.V.M.; Chiappim, W.; Sampaio, A.D.G.; Vegian, M.R.D.C.; Pessoa, R.S.; Koga-Ito, C.Y. Applications of Plasma-Activated Water in Dentistry: A Review. Int. J. Mol. Sci. 2022, 23, 4131. [Google Scholar] [CrossRef]
- Perez, S.M.; Biondi, E.; Laurita, R.; Proto, M.; Sarti, F.; Gherardi, M.; Bertaccini, A.; Colombo, V. Plasma Activated Water as Resistance Inducer against Bacterial Leaf Spot of Tomato. PLoS ONE 2019, 14, e0217788. [Google Scholar] [CrossRef]
- Pakprom, J.; Santalunai, S.; Charoensiri, W.; Ramjanthuk, S.; Janpangngern, P.; Thongsopa, C.; Thosdeekoraphat, T.; Santalunai, N.; Santalunai, S. Optimizing Nitrate Fertilizer Production Using Plasma-Activated Water (PAW) Technology: An Analysis of Dielectric Properties. Appl. Sci. 2024, 14, 9997. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma Activated Water (PAW): Chemistry, Physico-Chemical Properties, Applications in Food and Agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.-H.; Sun, D.-W. Enhancement of Wheat Seed Germination, Seedling Growth and Nutritional Properties of Wheat Plantlet Juice by Plasma Activated Water. J. Plant Growth Regul. 2023, 42, 2006–2022. [Google Scholar] [CrossRef]
- Wong, K.S.; Chew, N.S.L.; Low, M.; Tan, M.K. Plasma-Activated Water: Physicochemical Properties, Generation Techniques, and Applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Dilip, D.; Modupalli, N.; Rahman, M.M.; Kariyat, R. Atmospheric Cold Plasma Alters Plant Traits and Negatively Affects the Growth and Development of Fall Armyworm in Rice. Sci. Rep. 2025, 15, 3680. [Google Scholar] [CrossRef]
- Doshi, P.; Klas, M.; Kyzek, S.; Zahoranová, A.; Šerá, B. Investigating the Effect of Plasma Activated Water on Entomopathogenic Nematodes under Laboratory Conditions. Heliyon 2025, 11, e42038. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant Hormone Interactions during Seed Dormancy Release and Germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular Mechanisms Underlying Abscisic Acid/Gibberellin Balance in the Control of Seed Dormancy and Germination in Cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pandey, S.; Bhardwaj, R.; Zheng, B.; Tripathi, D.K. The Role of Growth Regulators and Phytohormones in Overcoming Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-323-98332-7. [Google Scholar]
- Barjasteh, A.; Lamichhane, P.; Dehghani, Z.; Kaushik, N.; Gupta, R.; Choi, E.H.; Kaushik, N.K. Recent Progress of Non-Thermal Atmospheric Pressure Plasma for Seed Germination and Plant Development: Current Scenario and Future Landscape. J. Plant Growth Regul. 2023, 42, 5417–5432. [Google Scholar] [CrossRef]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current Advancements in the Molecular Mechanism of Plasma Treatment for Seed Germination and Plant Growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef]
- Lee, G.; Choi, S.-W.; Yoo, M.; Chang, H.-J.; Lee, N. Effects of Plasma-Activated Water Treatment on the Inactivation of Microorganisms Present on Cherry Tomatoes and in Used Wash Solution. Foods 2023, 12, 2461. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Liu, D.; Wang, W.; Niu, J.; Xia, Y.; Qi, Z.; Zhao, Z.; Song, Y. Effect of Nonthermal Plasma-Activated Water on Quality and Antioxidant Activity of Fresh-Cut Kiwifruit. IEEE Trans. Plasma Sci. 2019, 47, 4811–4817. [Google Scholar] [CrossRef]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-Thermal Plasma-Activated Water Inactivation of Food-Borne Pathogen on Fresh Produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Hanbal, S.E.; Takashima, K.; Miyashita, S.; Ando, S.; Ito, K.; Elsharkawy, M.M.; Kaneko, T.; Takahashi, H. Atmospheric-Pressure Plasma Irradiation Can Disrupt Tobacco Mosaic Virus Particles and RNAs to Inactivate Their Infectivity. Arch. Virol. 2018, 163, 2835–2840. [Google Scholar] [CrossRef] [PubMed]
- Ten Bosch, L.; Köhler, R.; Ortmann, R.; Wieneke, S.; Viöl, W. Insecticidal Effects of Plasma Treated Water. Int. J. Environ. Res. Public. Health 2017, 14, 1460. [Google Scholar] [CrossRef]
- Donohue, K.V.; Bures, B.L.; Bourham, M.A.; Roe, R.M. Mode of Action of a Novel Nonchemical Method of Insect Control: Atmospheric Pressure Plasma Discharge. J. Econ. Entomol. 2006, 99, 38–47. [Google Scholar] [CrossRef]
- Lucini, T.; Faria, M.V.; Rohde, C.; Resende, J.T.V.; De Oliveira, J.R.F. Acylsugar and the Role of Trichomes in Tomato Genotypes Resistance to Tetranychus Urticae. Arthropod-Plant Interact. 2015, 9, 45–53. [Google Scholar] [CrossRef]
- Heng, Y.; Wang, M.; Jiang, H.; Gao, S.; Zhang, J.; Wan, J.; Song, T.; Ren, Z.; Zhu, Y. Plasma-Activated Acidic Electrolyzed Water: A New Food Disinfectant for Bacterial Suspension and Biofilm. Foods 2022, 11, 3241. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Hasan, M.S.; Islam, R.; Rana, R.; Sayem, A.; Sad, M.A.A.; Matin, A.; Raposo, A.; Zandonadi, R.P.; Han, H.; et al. Plasma-Activated Water for Food Safety and Quality: A Review of Recent Developments. Int. J. Environ. Res. Public Health 2022, 19, 6630. [Google Scholar] [CrossRef] [PubMed]
- Abuzairi, T.; Ramadhanty, S.; Puspohadiningrum, D.F.; Ratnasari, A.; Poespawati, N.R.; Purnamaningsih, R.W. Investigation on Physicochemical Properties of Plasma-Activated Water for the Application of Medical Device Sterilization; AIP Publishing: Bali, Indonesia, 2018; p. 040017. [Google Scholar]
- Waskow, A.; Avino, F.; Howling, A.; Furno, I. Entering the Plasma Agriculture Field: An Attempt to Standardize Protocols for Plasma Treatment of Seeds. Plasma Process. Polym. 2022, 19, 2100152. [Google Scholar] [CrossRef]
- Locatelli, S.; Triolone, S.; De Bonis, M.; Zanin, G.; Nicoletto, C. Non-Thermal Plasma-Activated Water Enhances Nursery Production of Vegetables: A Species-Specific Study. Agronomy 2025, 15, 209. [Google Scholar] [CrossRef]
- Punith, N.; Harsha, R.; Lakshminarayana, R.; Hemanth, M.; Anand, M.S.; Dasappa, S. Plasma Activated Water Generation and Its Application in Agriculture. Adv. Mater. Lett. 2019, 10, 700–704. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regulation (EU) 2018/848 of the European Parliament and of the Council on Organic Production and Labelling of Organic Products; Official Journal of the European Union: Brussels, Belgium, 2018; pp. L 150/1–L 150/14. [Google Scholar]
- Agricultural Marketing Service; USDA. Synthetic Substances Allowed for Use in Organic Production; Code of Federal Regulations (CFR), Title 7, Part 205; Government Publishing Office: Washington, DC, USA, 2024; pp. 449–451. [Google Scholar]
- USDA; NOP; Agricultural Marketing Service. Decision Tree for Classification of Materials as Synthetic or Nonsynthetic; NOP Guidance Document (NOP 5033-1); USDA: Washington, DC, USA, 2016; pp. 1–3. [Google Scholar]
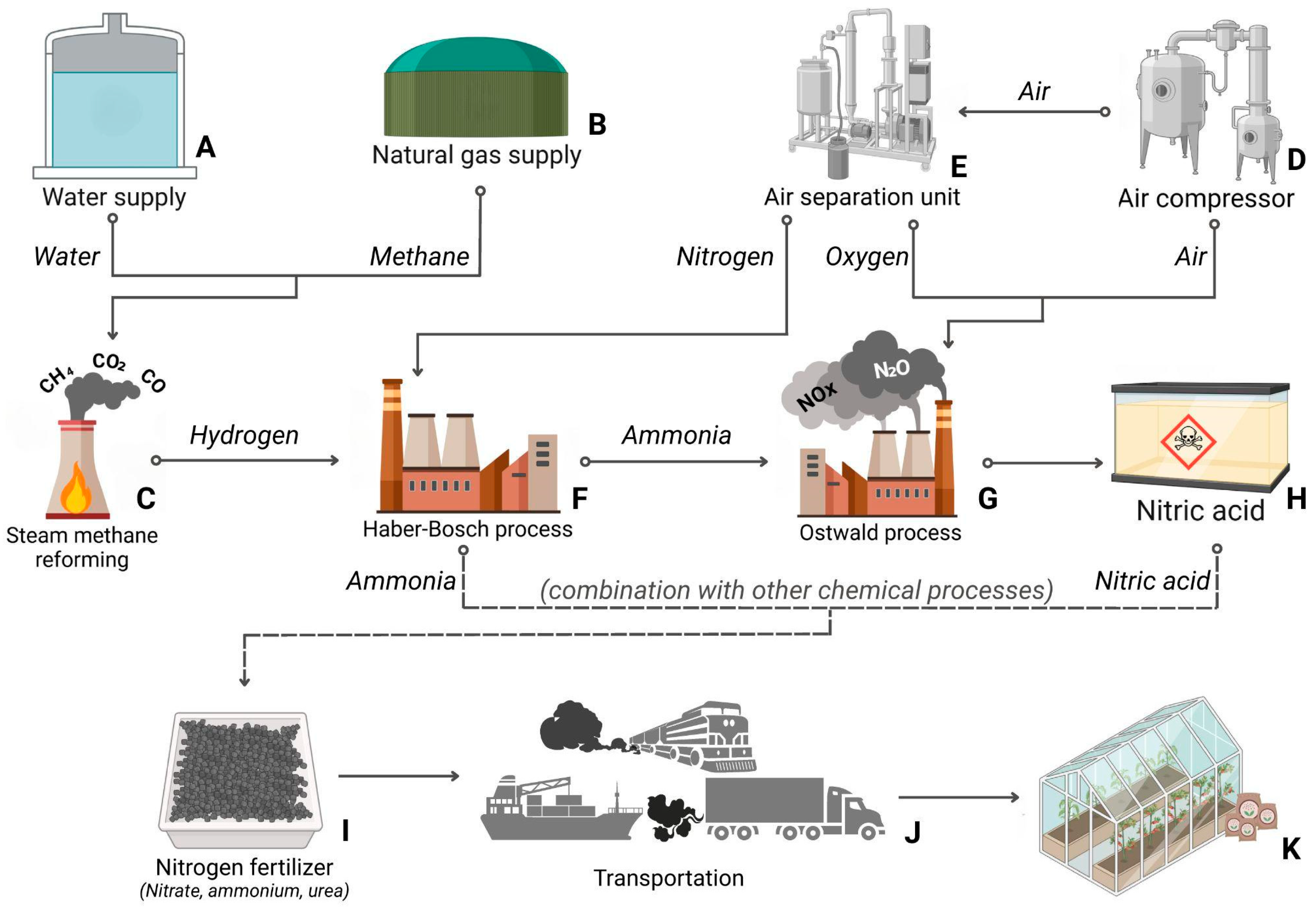

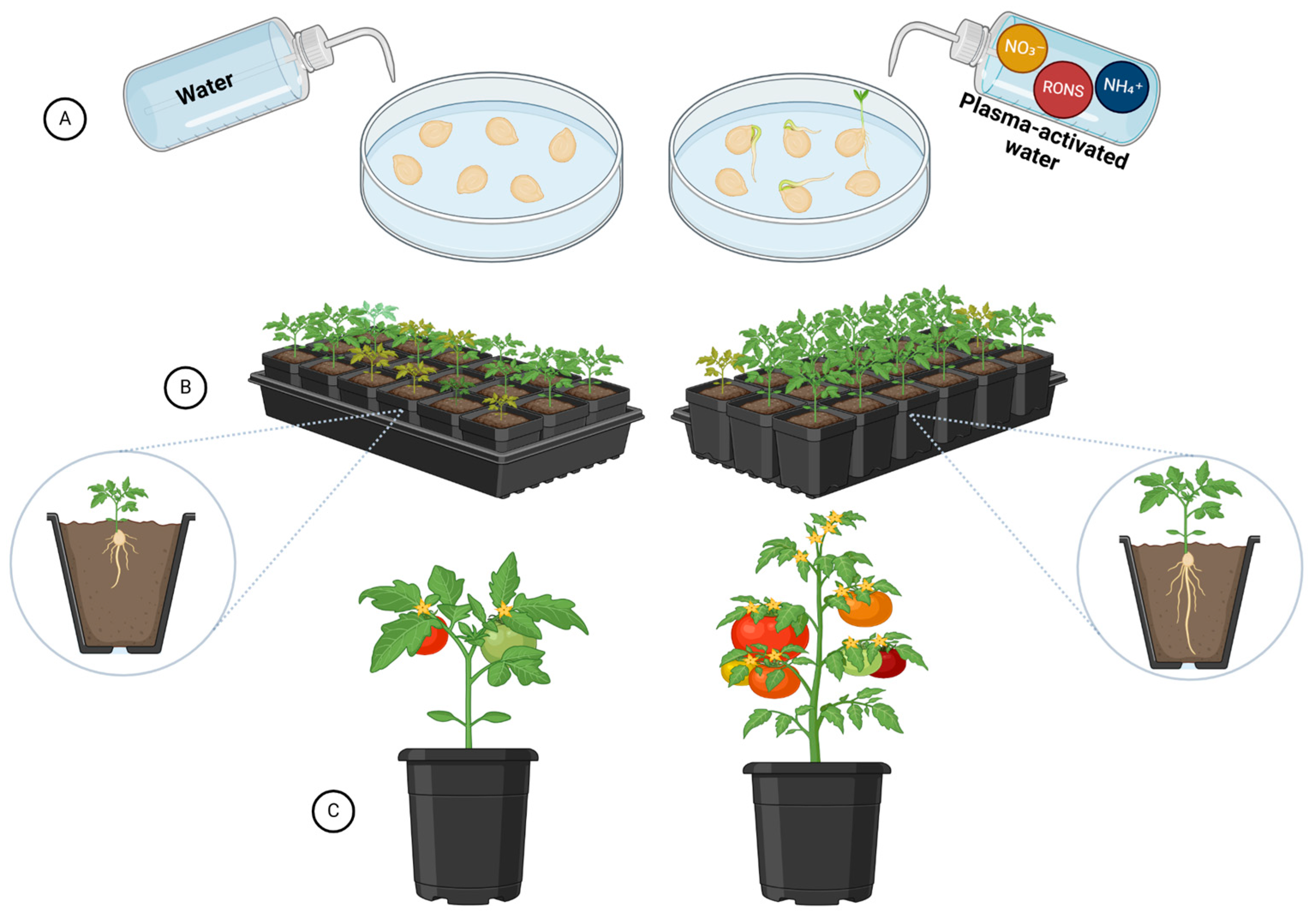
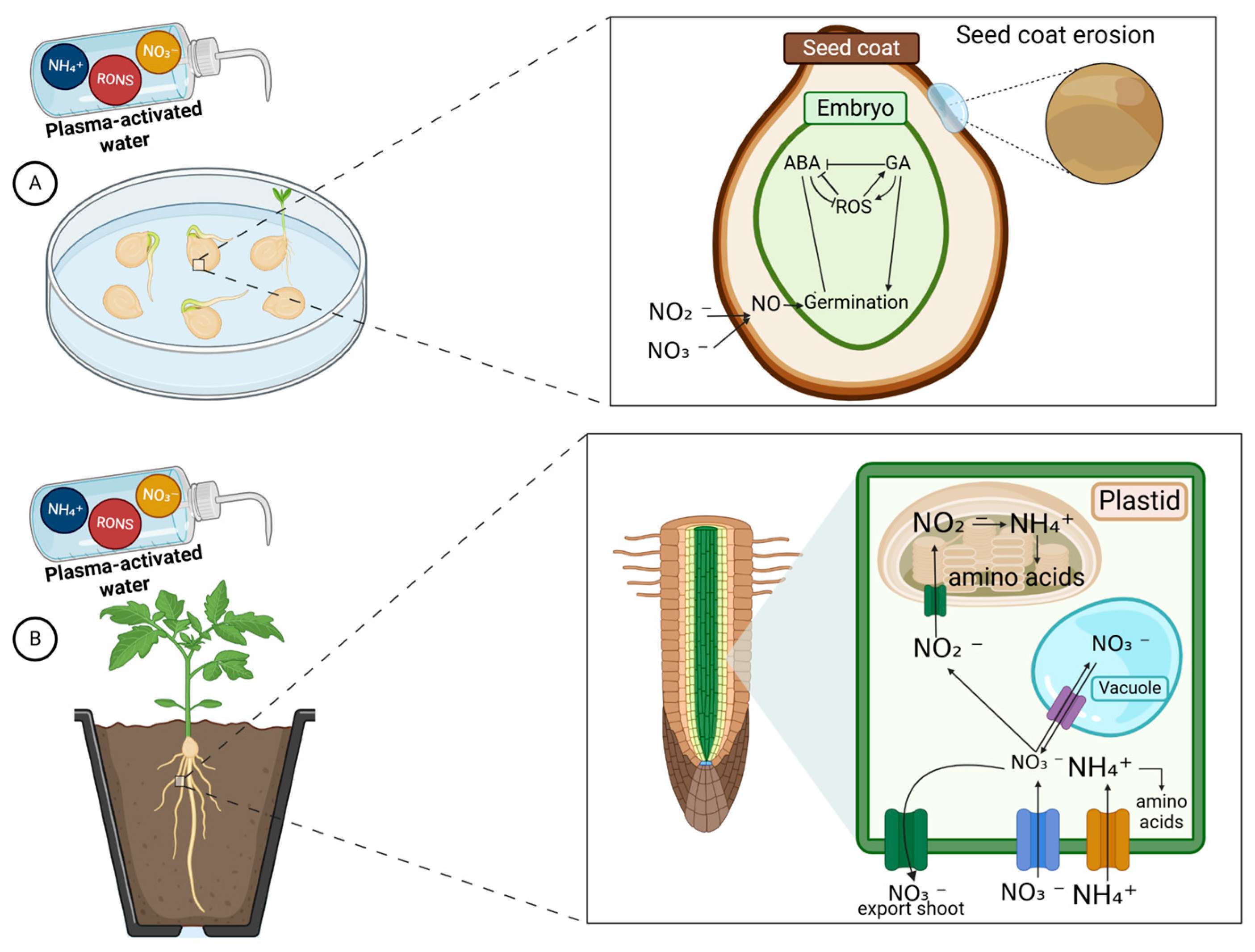
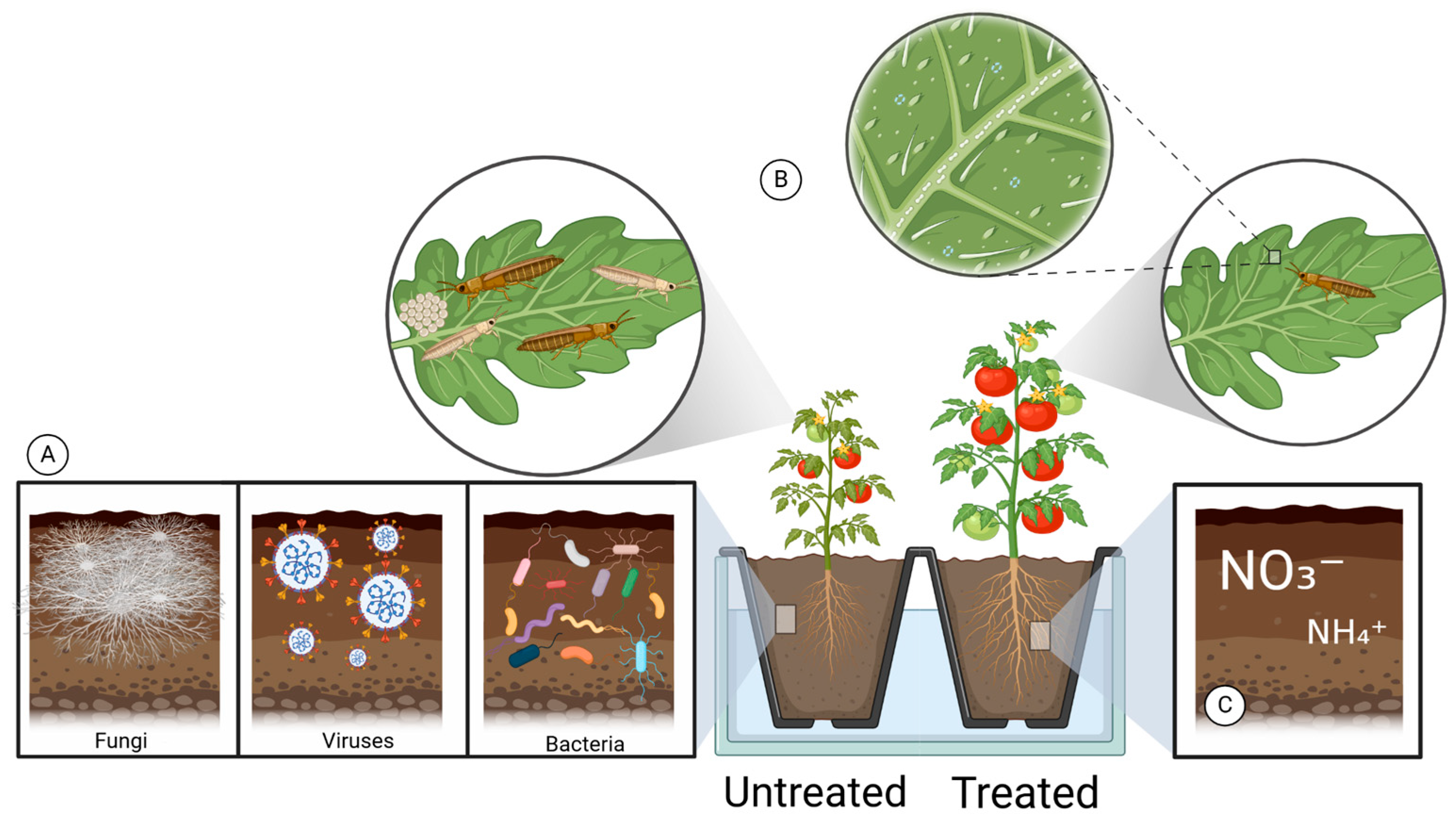
| Crops | Recommended N Fertilization (kg N ha−1) | Average Yield (Mg ha−1) | Annual Consumption per Capita (kg/Person) | References |
|---|---|---|---|---|
| Spinach | 50–150 | 32–50 | 1.30 | [4,5] |
| Bell Pepper | 425 | 93 | 5.02 | [4,6] |
| Tomato | 320 | 53–94 | 34.25 | [4,7] |
| Lettuce (Romain, butterhead, iceberg) | 80 | 27 | 5.76 | [4,8] |
| Cabbage (White head) | 200–500 | 96–197 | 3.24 | [4,9] |
| Kale | 112 | 10–15 | 0.44 | [4,10] |
| Metrics | PAW | Conventional Fertilizers |
|---|---|---|
| Seed germination rate | Enhanced in various species | Indirect effect; not formulated to enhance germination |
| Seedling vigor and uniformity | Improved vigor, uniformity, and root development | No direct impact on uniformity or vigor |
| Root and shoot growth | Increased elongation and biomass in various species | Supports growth via nutrient supply but may not enhance morphology |
| Chlorophyll content | Elevated chlorophyll content | Dependent on nitrate uptake; not always optimized |
| Yield quantity and quality | Improved fruit size, weight, and overall quality | Improves yield quantity, less consistent effect on quality |
| Abiotic stress tolerance | Increased tolerance to drought and salinity | Limited or no stress-mitigating properties |
| Nutrient uptake efficiency | Enhanced uptake of multiple nutrients | Supplies nutrients but may not optimize uptake |
| Hormonal modulation | Activates auxins, cytokinins, and gibberellins for growth and germination | No direct hormonal interaction |
| Pest resistance | Induces trichome development, reducing pest populations | No effect on physical pest defenses |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, P.E.; Savi, P.J.; Almeida, F.S.; Carciofi, B.A.; Pace, A.; Zou, Y.; Eylands, N.; Annor, G.; Mattson, N.; Nansen, C. Plasma-Activated Water as a Sustainable Nitrogen Source: Supporting the UN Sustainable Development Goals (SDGs) in Controlled Environment Agriculture. Crops 2025, 5, 35. https://doi.org/10.3390/crops5030035
Andrade PE, Savi PJ, Almeida FS, Carciofi BA, Pace A, Zou Y, Eylands N, Annor G, Mattson N, Nansen C. Plasma-Activated Water as a Sustainable Nitrogen Source: Supporting the UN Sustainable Development Goals (SDGs) in Controlled Environment Agriculture. Crops. 2025; 5(3):35. https://doi.org/10.3390/crops5030035
Chicago/Turabian StyleAndrade, Pamela Estefania, Patrice Jacob Savi, Flavia Souza Almeida, Bruno Augusto Carciofi, Abby Pace, Yugeng Zou, Nathan Eylands, George Annor, Neil Mattson, and Christian Nansen. 2025. "Plasma-Activated Water as a Sustainable Nitrogen Source: Supporting the UN Sustainable Development Goals (SDGs) in Controlled Environment Agriculture" Crops 5, no. 3: 35. https://doi.org/10.3390/crops5030035
APA StyleAndrade, P. E., Savi, P. J., Almeida, F. S., Carciofi, B. A., Pace, A., Zou, Y., Eylands, N., Annor, G., Mattson, N., & Nansen, C. (2025). Plasma-Activated Water as a Sustainable Nitrogen Source: Supporting the UN Sustainable Development Goals (SDGs) in Controlled Environment Agriculture. Crops, 5(3), 35. https://doi.org/10.3390/crops5030035









