Grapevine Response to Pyroligneous Acid: Antifungal, Physiological, and Biochemical Impacts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Pyroligneous Acid (PA)
2.3. Origin and Maintenance of Fungal Strain
2.4. Plant Materials
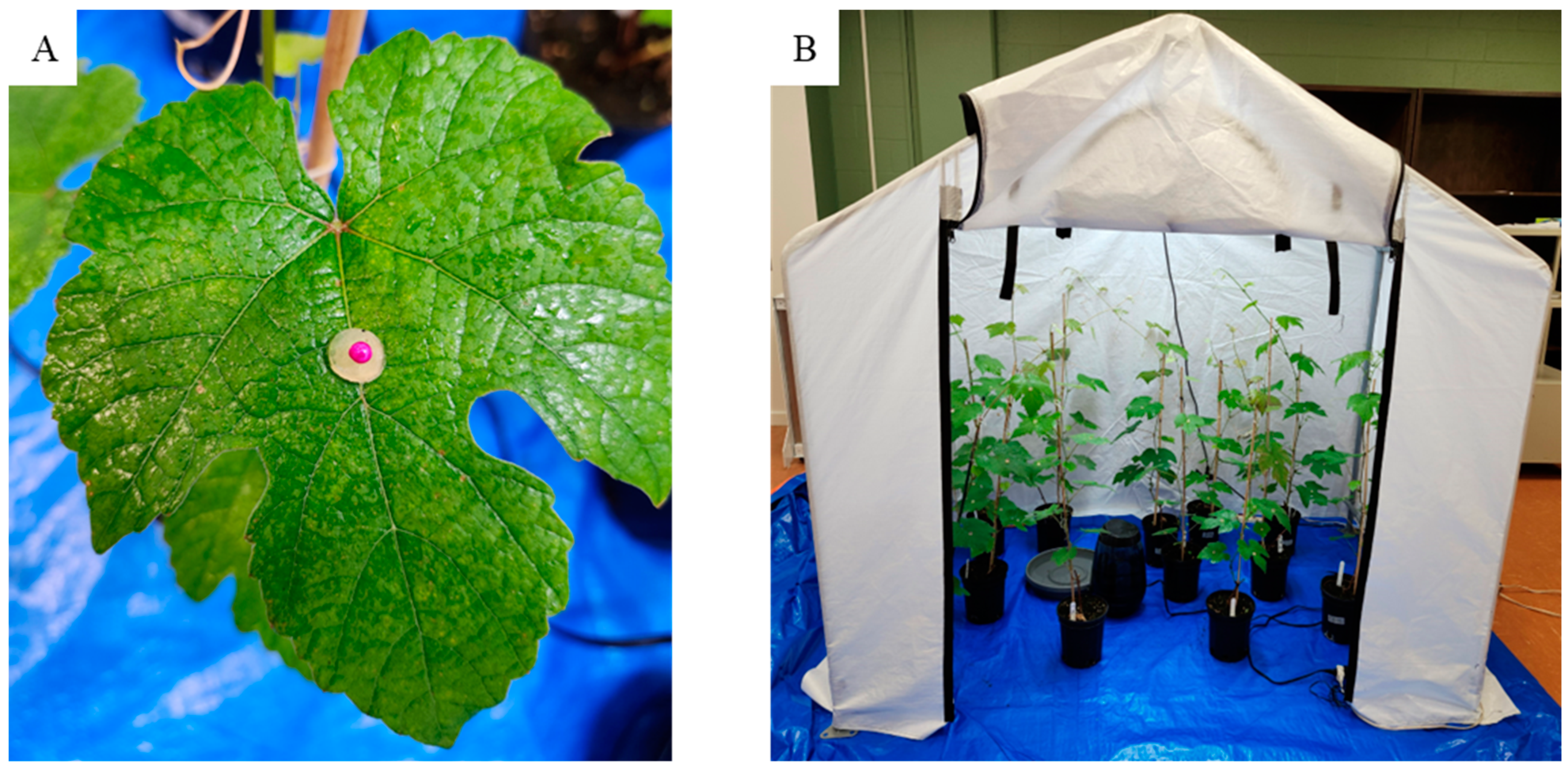
2.5. In Vivo Antifungal Activity Assay
2.6. Plant Physiological Analysis
2.7. Biochemical Analysis
2.7.1. Chlorophylls and Carotenoids
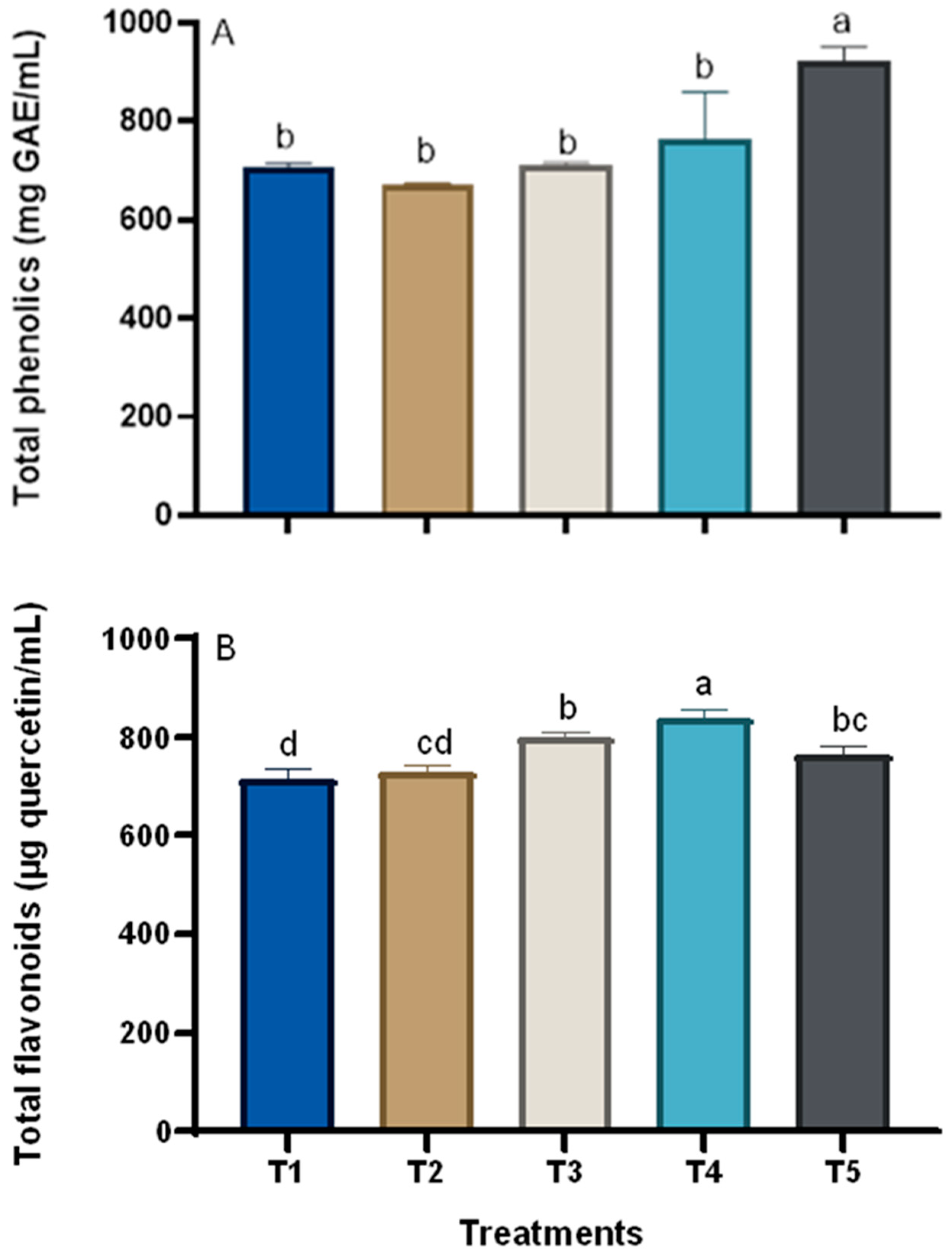
2.7.2. Total Phenolics
2.7.3. Total Flavonoid
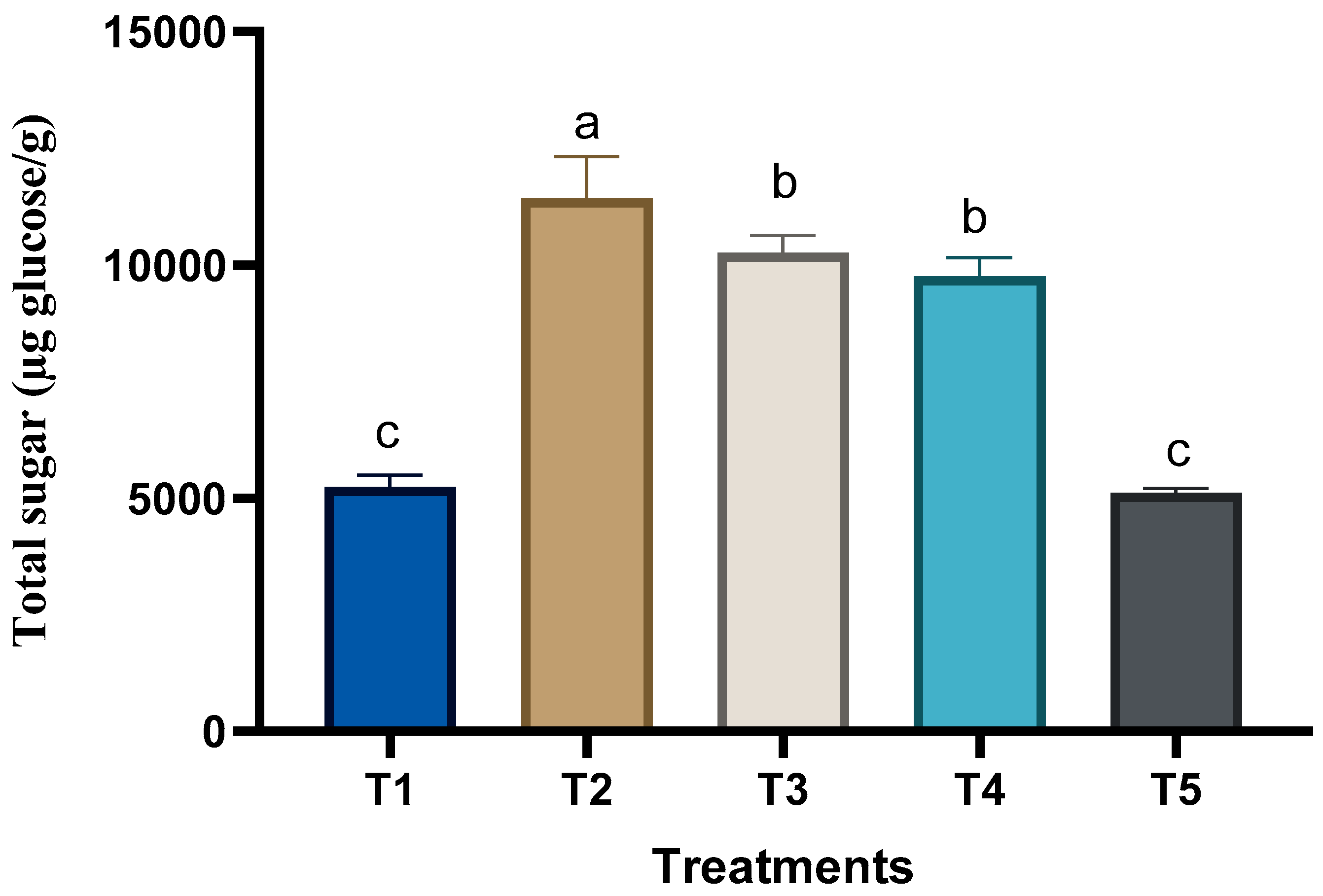
2.7.4. Total Sugar
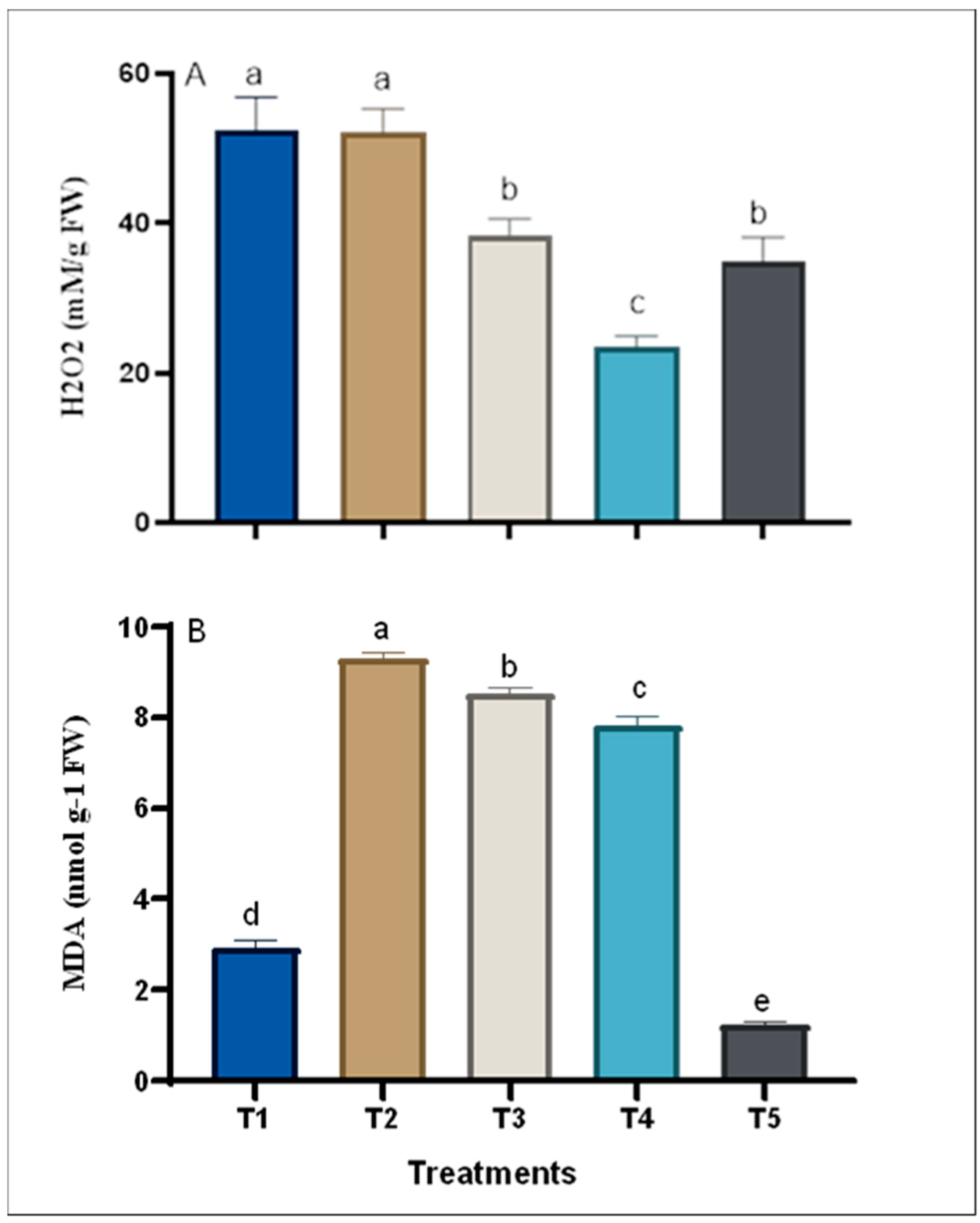
2.7.5. Lipid Peroxidation and Hydrogen Peroxide
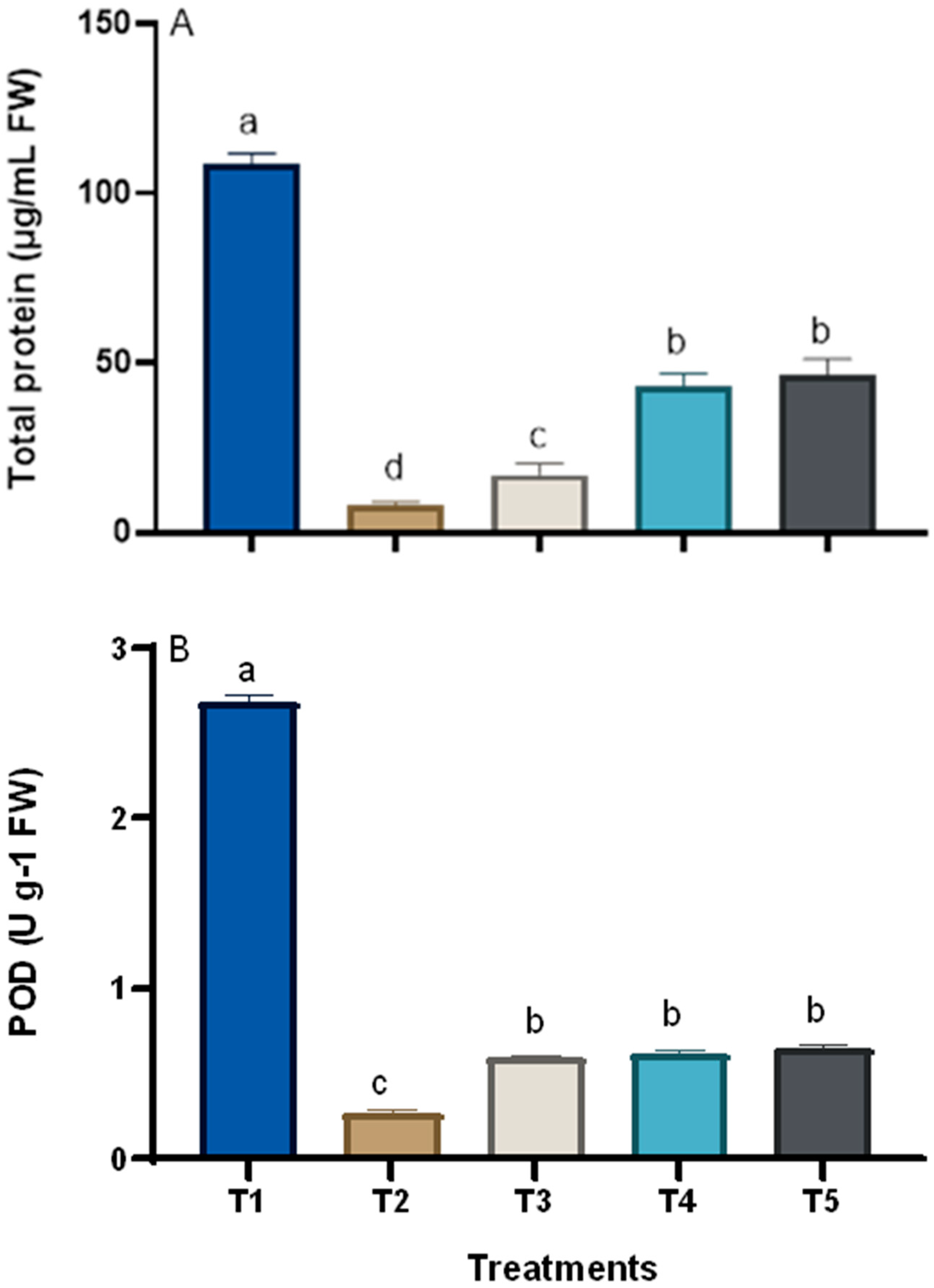
2.7.6. Total Protein and Peroxidase Enzyme Activity
2.8. Data Analysis
3. Results
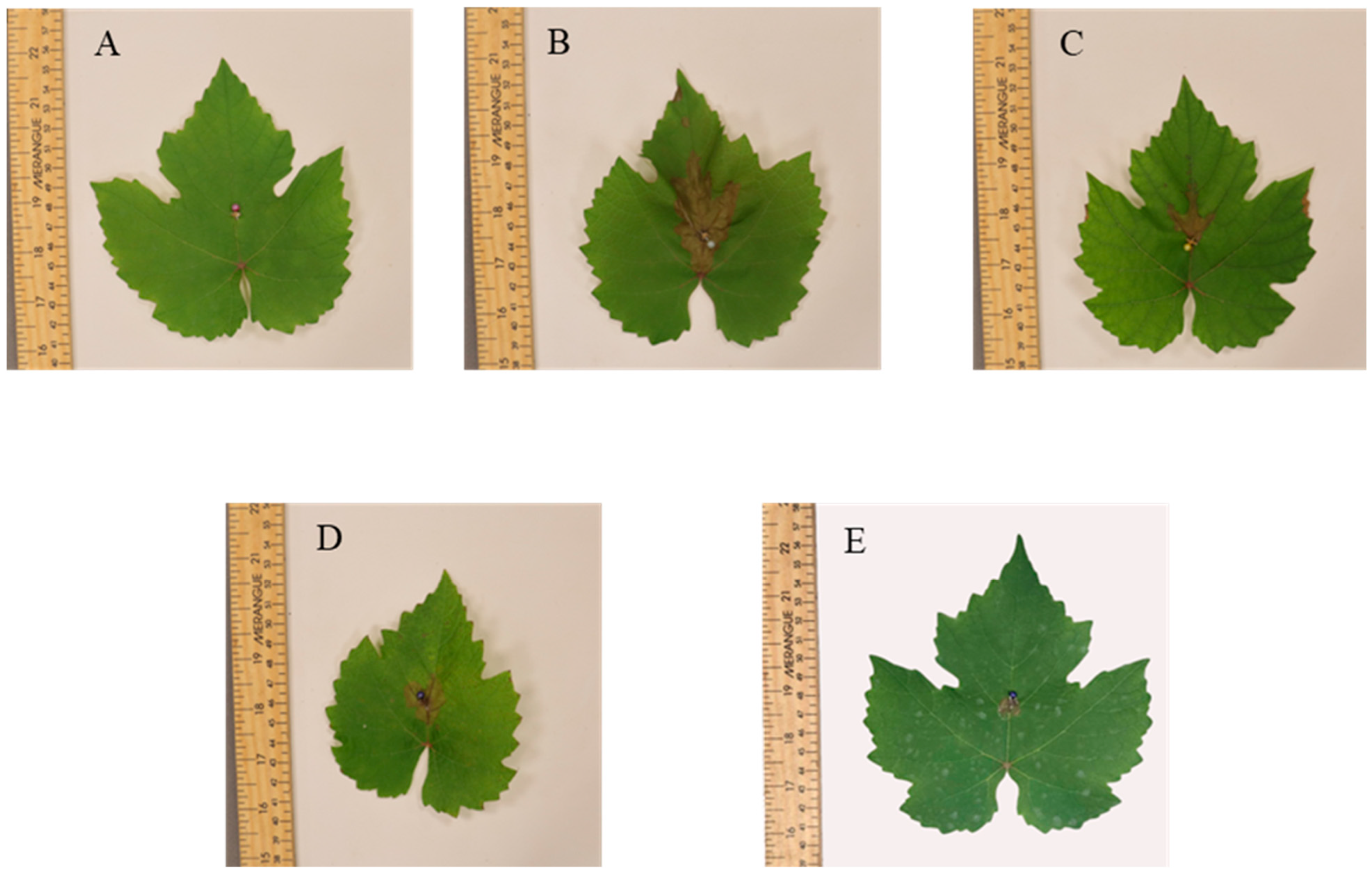
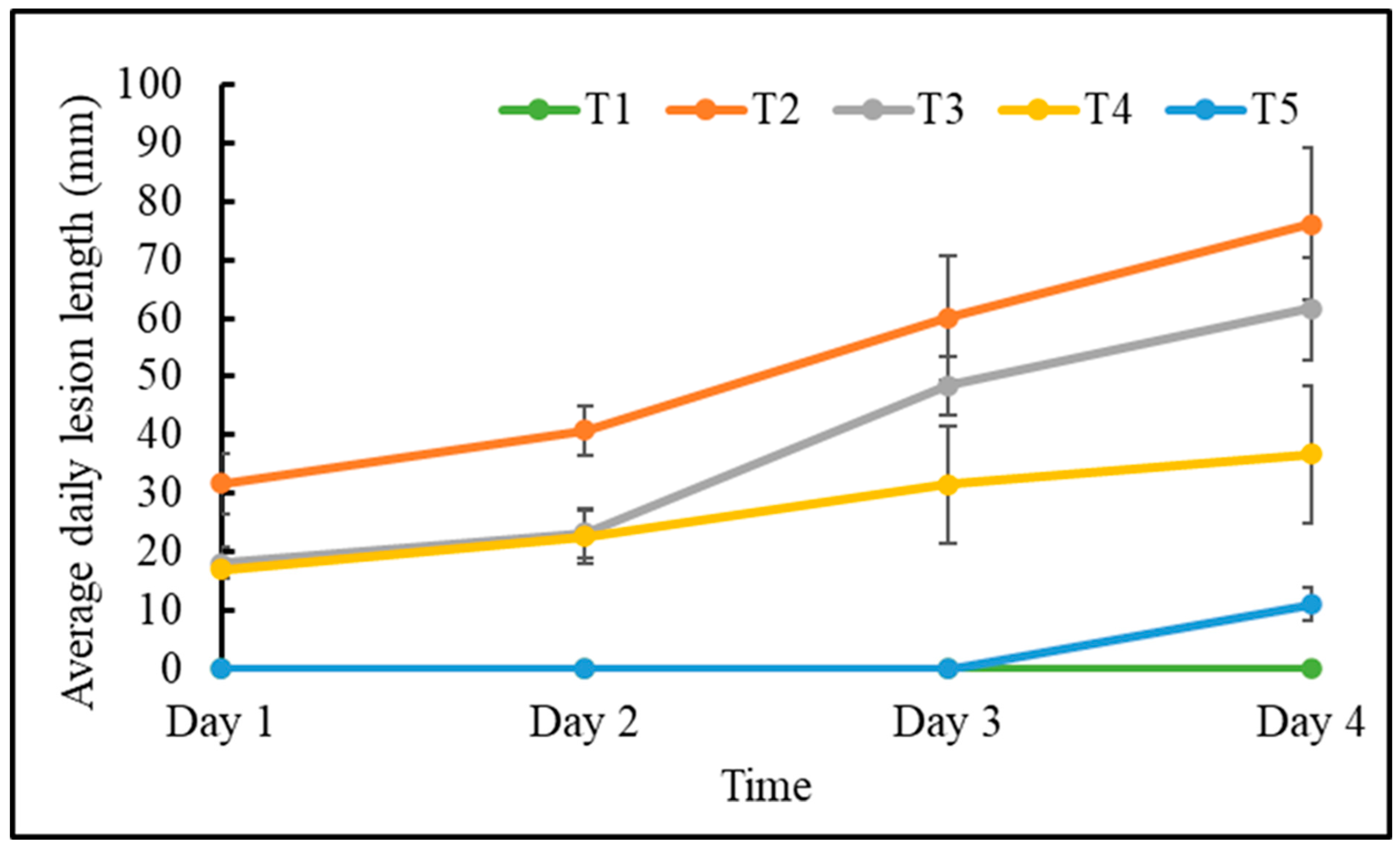
3.1. Botrytis cinerea Necrosis
3.2. Physiological Response of Grape Plants to PA Application and B. cinerea Inoculation
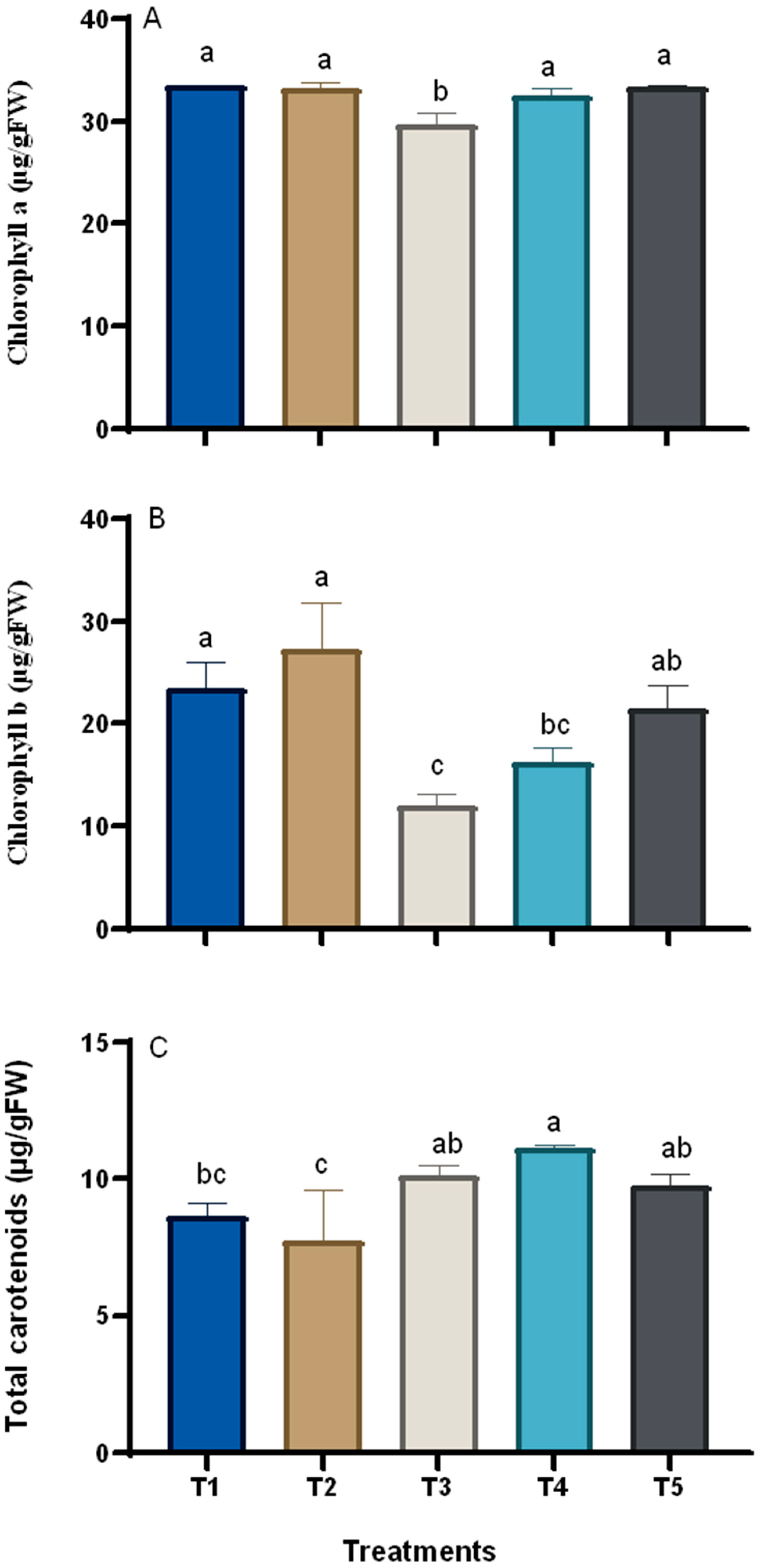
3.3. Changes in the Chemical Composition of Grape Plants in Response to Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bolívar-Anillo, H.J.; Garrido, C.; Collado, I.G. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phytochem. Rev. 2020, 19, 721–740. [Google Scholar] [CrossRef]
- Rodriguez-Moreno, L.; Ebert, M.K.; Bolton, M.D.; Thomma, B.P.H.J. Tools of the crook- infection strategies of fungal plant pathogens. Plant J. 2018, 93, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.W.; Emmanuel, C.J.; Emilda, D.; Terhem, R.B.; Shafia, A.; Tsamaidi, D.; Emblow, M.; van Kan, J.A.L.; Cryptic, A.O. Systemic Botrytis Infections in Symptomless Hosts. Front. Plant Sci. 2016, 7, 625. [Google Scholar] [CrossRef]
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 241–262. [Google Scholar] [CrossRef]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The destructive fungal pathogen Botrytis cinerea—Insights from genes studied with mutant analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef]
- Roca-Couso, R.; Flores-Félix, J.D.; Rivas, R. Mechanisms of action of microbial biocontrol agents against Botrytis cinerea. J. Fungi 2021, 7, 1045. [Google Scholar] [CrossRef]
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- You, Y.; Astudillo-Estévez, I.; Essenstam, B.; Qin, S.; van Kan, J.A.L. Leaf resistance to Botrytis cinerea in wild tomato Solanum habrochaites depends on inoculum composition. Front. Plant Sci. 2023, 14, 1156804. [Google Scholar] [CrossRef]
- Abuqamar, S.F.; Moustafa, K.; Tran, L.S.P. ‘Omics’ and plant responses to Botrytis cinerea. Front. Plant Sci. 2016, 7, 1658. [Google Scholar] [CrossRef]
- Leroch, M.; Kleber, A.; Silva, E.; Coenen, T.; Koppenhöfer, D.; Shmaryahu, A.; Valenzuela, P.D.T.; Hahn, M. Transcriptome profiling of Botrytis cinerea conidial germination reveals upregulation of infection-related genes during the prepenetration stage. Eukaryot. Cell 2013, 12, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.D.C.; Kumar, A.; Fadiji, A.E.; Babalola, O.O.; Puopolo, G.; Santoyo, G. Agroecological Management of the Grey Mould Fungus Botrytis cinerea by Plant Growth-Promoting Bacteria. Plants 2023, 12, 637. [Google Scholar] [CrossRef]
- Pertile, G.; Frąc, M. The Antifungal Effect of Pyroligneous Acid on the Phytopathogenic Fungus Botrytis cinerea. Int. J. Mol. Sci. 2023, 24, 3080. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood vinegar as a complex growth regulator promotes the growth, yield, and quality of rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Aguirre, J.L.; Baena, J.; Martín, M.T.; Nozal, L.; González, S.; Manjón, J.L.; Peinado, M. Composition, Ageing and Herbicidal Properties of Biomass Pyrolysis. Energies 2020, 13, 2418. [Google Scholar] [CrossRef]
- Mathew, S.; Zakaria, Z.A. Pyroligneous acid—The smoky acidic liquid from plant biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef]
- Medeiros, L.C.D.; Gasparotto, L.H.S. Pyroligneous acid and antibacterial activity: Criticism of a paper by Araújo et al. (2018). J. Appl. Microbiol. 2022, 132, 1768–1770. [Google Scholar] [CrossRef]
- Oramahi, H.A.; Yoshimura, T. Antifungal and antitermitic activities of wood vinegar from Vitex pubescens Vahl. J. Wood Sci. 2013, 59, 344–350. [Google Scholar] [CrossRef]
- Pimenta, A.S.; Fasciotti, M.; Monteiro, T.V.C.; Lima, K.M.G. Chemical composition of pyroligneous acid obtained from eucalyptus GG100 clone. Molecules 2018, 23, 426. [Google Scholar] [CrossRef]
- Oğuz, H.İ.; Oğuz, İ. Research on the Use of Wood Vinegar (Pyrolysis Acid) and Biochar in the Horticultural Production. Recent. Headw. Pomol. 2021, 1, 129–150. [Google Scholar]
- Chen, J.; Thilakarathna, W.P.D.W.; Astatkie, T.; Rupasinghe, H.P.V. Optimization of catechin and proanthocyanidin recovery from grape seeds using microwave-assisted extraction. Biomolecules 2020, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Cui, X.; Ji, D.; Xu, Y.; Chen, T.; Tian, S. Exogenous bamboo pyroligneous acid improves antioxidant capacity and primes defense responses of harvested apple fruit. LWT 2020, 134, 110191. [Google Scholar] [CrossRef]
- Ofoe, R.; Gunupuru, L.R.; Abbey, L. Metabolites, elemental profile and chemical activities of Pinus strobus high temperature-derived pyroligneous acid. Chem. Biol. Technol. Agric. 2022, 9, 85. [Google Scholar] [CrossRef]
- Kirkby, K.; Roser, S.; Plett, K. Using Detached Industrial Hemp Leaf Inoculation Assays to Screen for Varietal Susceptibility and Product Efficacy on Botrytis cinerea. Plants 2023, 12, 3278. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987. [Google Scholar] [CrossRef]
- Eb, N.; Ofoe, R.; Ap, K.; Abbey, L. Plant Growth, Yield, and Biochemical Composition of Solanum macrocarpon and Corchorus olitorius under Varying Temperatures. Open Access J. Agric. Res. 2024, 3, 000172. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Ofori, P.A.; Ofoe, R.; Nutsukpo, E.B.; Owusu-Nketia, S.; Kumar, A.P.; Chada, S.; Jiang, Q.; Parks, H.; Basteris, A.; Robinson, J.; et al. Morpho-Physiological and Biochemical Responses of Tomato, Lettuce, Mexican Mint, and Industrial Hemp to Different Temperature Regimes. Open Access J. Agric. Res. 2024, 9, 000378. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Nutsukpo, E.B.; Ofoe, R.; Jiang, Q.; Ofori, P.A.; Asiedu, S.K.; Emenike, C.; Lord, A. Pyroligneous Acid Affects Grapevine Growth, Yield, and Chemical Composition of Leaf, Pomace, and Juice. ACS Agric. Sci. Technol. 2024, 4, 1014–1026. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. 1956. Available online: https://pubs.acs.org/sharingguidelines (accessed on 10 October 2024).
- Negassa, B.; Nebiyu, A.; Garedew, W.; Abbey, L.; Ofoe, R.; Mousavi, N.S.; Nutsukpo, B.; Astatkie, T.; Kuyu, C.G. Evaluation of anchote (Coccinia abyssinica) genotypes and processing methods for mineral and phytochemical composition. J. Agric. Food Res. 2024, 18, 101415. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Env. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrolysis 2010, 87, 24–28. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, Y.F.; Wei, H.; Li, X.X.; Zheng, H.T.; Dong, X.Y.; Xu, T.F.; Meng, J.F. Inhibition efficiency of wood vinegar on grey mould of table grapes. Food Biosci. 2020, 38, 100755. [Google Scholar] [CrossRef]
- Abbey, J.A. Sustainable Management of Botrytis Blossom Blight in Wild Blueberry (Vaccinium angustifolium Aiton). 2017. Available online: https://dalspace.library.dal.ca/items/97b1302c-00bf-433e-a843-e476c1d148a1 (accessed on 30 March 2025).
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Hu, Y.; Zhong, S.; Zhang, M.; Liang, Y.; Gong, G.; Chang, X.; Tan, F.; Yang, H.; Qiu, X.; Luo, L.; et al. Potential role of photosynthesis in the regulation of reactive oxygen species and defence responses to Blumeria graminis f. Sp. tritici in wheat. Int. J. Mol. Sci. 2020, 21, 5767. [Google Scholar] [CrossRef]
- Pedrelli, A.; Ricci, G.P.; Panattoni, A.; Nali, C.; Cotrozzi, L. Physiological and Biochemical Responses Induced by Plum Pox Virus and Plum Bark Necrosis Steam Pitting Associated Virus in Tuscany Autochthonous Plum cv. Coscia di Monaca. Plants 2023, 12, 3264. [Google Scholar] [CrossRef]
- Lamour, J.; Davidson, K.J.; Ely, K.S.; Le Moguédec, G.; Anderson, J.A.; Li, Q.; Calderón, O.; Koven, C.D.; Wright, S.J.; Walker, A.P.; et al. The effect of the vertical gradients of photosynthetic parameters on the CO2 assimilation and transpiration of a Panamanian tropical forest. New Phytol. 2023, 238, 2345–2362. [Google Scholar] [CrossRef]
- Sun, R.; Ma, J.; Sun, X.; Bai, S.; Zheng, L.; Guo, J. Study on a Stomatal Conductance Model of Grape Leaves in Extremely Arid Areas. Sustainability 2023, 15, 8342. [Google Scholar] [CrossRef]
- Suárez, J.C.; Vanegas, J.I.; Contreras, A.T.; Anzola, J.A.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Chlorophyll Fluorescence Imaging as a Tool for Evaluating Disease Resistance of Common Bean Lines in the Western Amazon Region of Colombia. Plants 2022, 11, 1371. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant-Gaveau, N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.D.S.; Moustaka, J.; İşgören, S.; Şaş, B. Harnessing the Role of Foliar Applied Salicylic Acid in Decreasing Chlorophyll Content to Reassess Photosystem II Photoprotection in Crop Plants. Int. J. Mol. Sci. 2022, 23, 7038. [Google Scholar] [CrossRef]
- Gashi, B.; Buqaj, L.; Vataj, R.; Tuna, M. Chlorophyll biosynthesis suppression, oxidative level and cell cycle arrest caused by Ni, Cr and Pb stress in maize exposed to treated soil from the Ferronikel smelter in Drenas, Kosovo. Plant Stress 2024, 11, 100379. [Google Scholar] [CrossRef]
- Llave, C. Dynamic cross-talk between host primary metabolism and viruses during infections in plants. Curr. Opin. Virol. 2016, 19, 50–55. [Google Scholar] [CrossRef]
- Pérez-gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production; metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [PubMed]
- Asaeda, T.; Rahman, M.; Liping, X.; Schoelynck, J. Hydrogen Peroxide Variation Patterns as Abiotic Stress Responses of Egeria densa. Front. Plant Sci. 2022, 13, 855477. [Google Scholar] [CrossRef] [PubMed]
- Taenzer, L.; Toth, G.; Hansel, C.M. Assessment of hydrogen peroxide as a bioindicator of stress in seaweed aquaculture. Sci. Rep. 2024, 14, 1956. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Corpas, F.J.; Ortega-Villasante, C.; Hernandez, L.E.; Tuteja, N.; Sofo, A.; Hasanuzzaman, M.; Fujita, M. Editorial: Recent Insights Into the Double Role of Hydrogen Peroxide in Plants. Front. Plant Sci. 2022, 13, 843274. [Google Scholar] [CrossRef]
- Nurnaeimah, N.; Mat, N.; Mohd, K.S.; Badaluddin, N.A.; Yusoff, N.; Sajili, M.H.; Mahmud, K.; Adnan, A.F.M.; Khandaker, M.M. The Effects of Hydrogen Peroxide on Plant Growth, Mineral Accumulation, as Well as Biological and Chemical Properties of Ficus deltoidea. Agronomy 2020, 10, 599. [Google Scholar] [CrossRef]
- Orange, M.J. Responses of phenological and physiological stages of spring safflower to complementary irrigation. Afr. J. Biotechnol. 2012, 11, 2465–2471. [Google Scholar] [CrossRef]
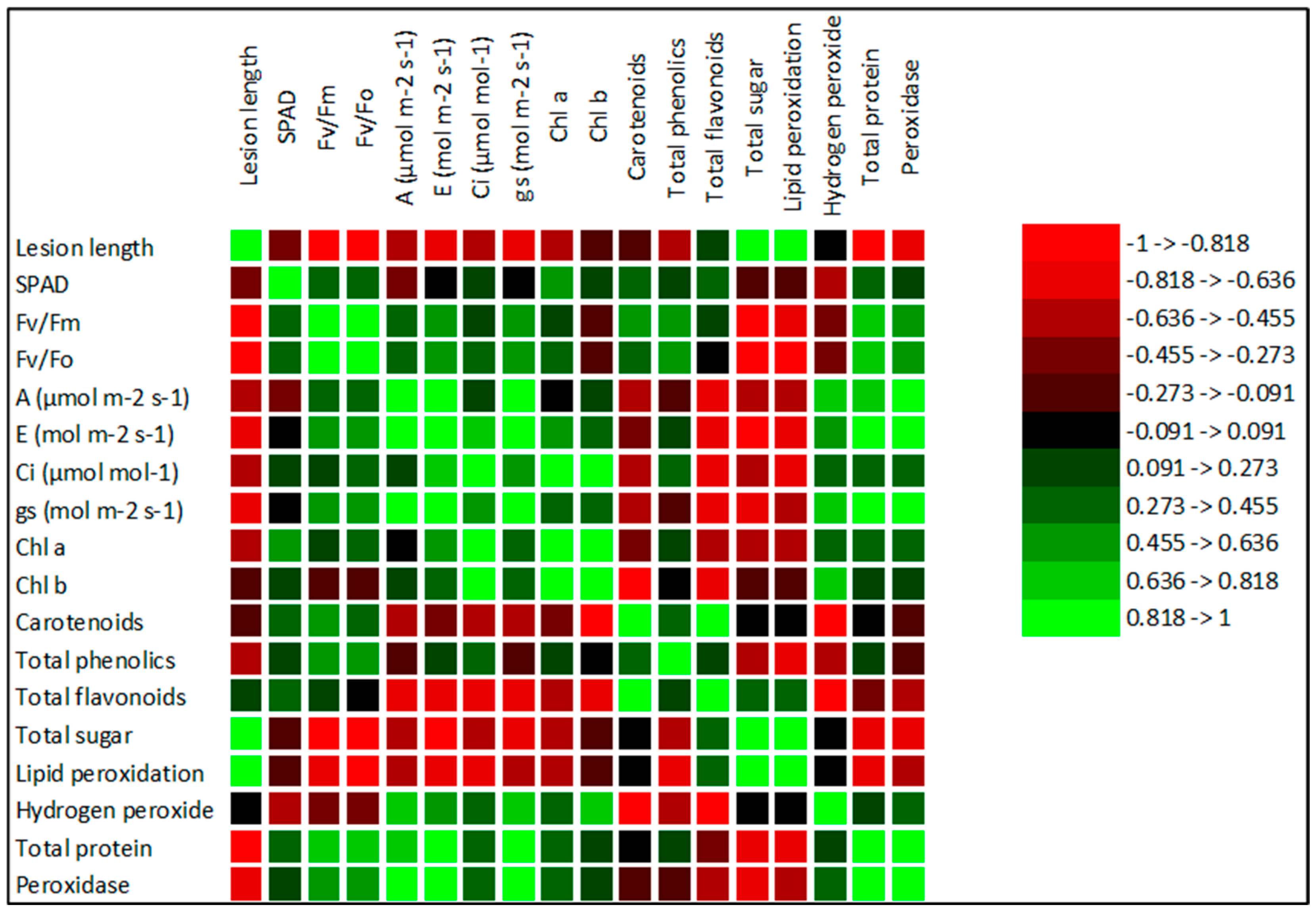
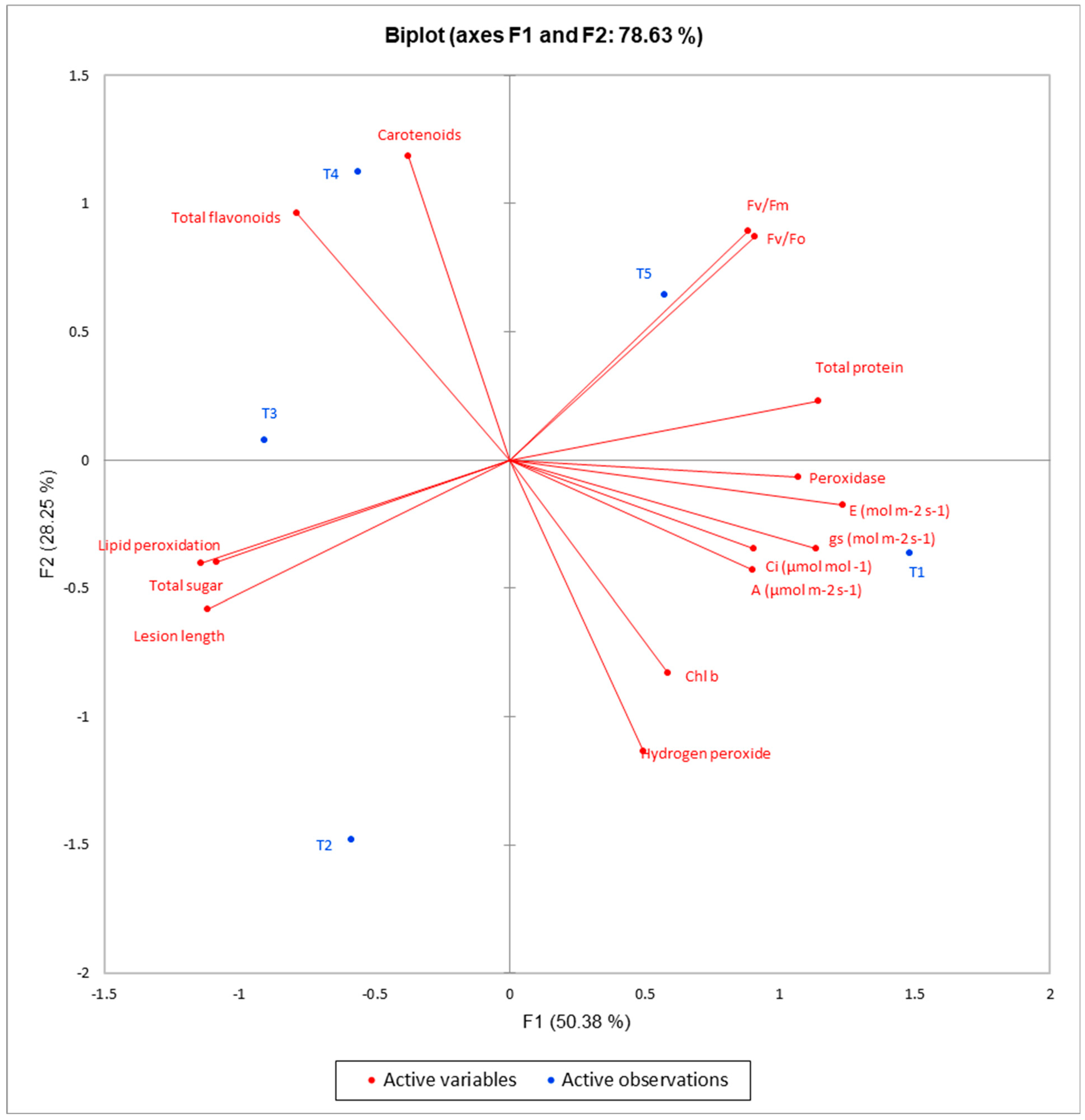
| Treatments | SPAD | Fv/Fm | Fv/Fo | A (μmol m−2 s−1) | E (mol m−2 s−1) | Ci (μmol mol −1) | gs (mol m−2 s−1) |
|---|---|---|---|---|---|---|---|
| T1 | 35.20 ± 3.54a | 0.798 ± 0.00a | 3.97 ± 0.06a | 2.75 ± 1.13a | 1.90 ± 1.33a | 395.20 ± 34.50a | 0.17 ± 0.13a |
| T2 | 34.23 ± 4.25a | 0.778 ± 0.01b | 3.52 ± 0.16b | 1.80 ± 0.87a | 1.08 ± 0.10a | 392.60 ± 20.23a | 0.07 ± 0.01ab |
| T3 | 32.51 ± 2.05a | 0.787 ± 0.00ab | 3.71 ± 0.08ab | 2.12 ± 0.64a | 1.01 ± 0.35a | 363.80 ± 28.60a | 0.06 ± 0.02ab |
| T4 | 37.47 ± 3.16a | 0.794 ± 0.00a | 3.87 ± 0.11a | 1.42 ± 0.27 a | 0.96 ± 0.19a | 378.40 ± 18.80a | 0.05 ± 0.01b |
| T5 | 34.54 ± 0.23a | 0.797 ± 0.00a | 3.95 ± 0.29a | 1.92 ± 0.99a | 1.42 ± 0.34a | 397.80 ± 41.80a | 0.08 ± 0.02ab |
| p-value | 0.168 | 0.001 | 0.001 | 0.187 | 0.153 | 0.370 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nutsukpo, E.B.; Ofori, P.A.; Ofoe, R.; Kumar, A.P.; Asiedu, S.K.; Emenike, C.; Abbey, L. Grapevine Response to Pyroligneous Acid: Antifungal, Physiological, and Biochemical Impacts. Crops 2025, 5, 21. https://doi.org/10.3390/crops5020021
Nutsukpo EB, Ofori PA, Ofoe R, Kumar AP, Asiedu SK, Emenike C, Abbey L. Grapevine Response to Pyroligneous Acid: Antifungal, Physiological, and Biochemical Impacts. Crops. 2025; 5(2):21. https://doi.org/10.3390/crops5020021
Chicago/Turabian StyleNutsukpo, Efoo Bawa, Peter Amoako Ofori, Raphael Ofoe, Anagha Pradeep Kumar, Samuel K. Asiedu, Chijioke Emenike, and Lord Abbey. 2025. "Grapevine Response to Pyroligneous Acid: Antifungal, Physiological, and Biochemical Impacts" Crops 5, no. 2: 21. https://doi.org/10.3390/crops5020021
APA StyleNutsukpo, E. B., Ofori, P. A., Ofoe, R., Kumar, A. P., Asiedu, S. K., Emenike, C., & Abbey, L. (2025). Grapevine Response to Pyroligneous Acid: Antifungal, Physiological, and Biochemical Impacts. Crops, 5(2), 21. https://doi.org/10.3390/crops5020021








