Wood Distillate as a Solution for Growing Crops Under Water Deficiency
Abstract
1. Introduction
2. Materials and Methods
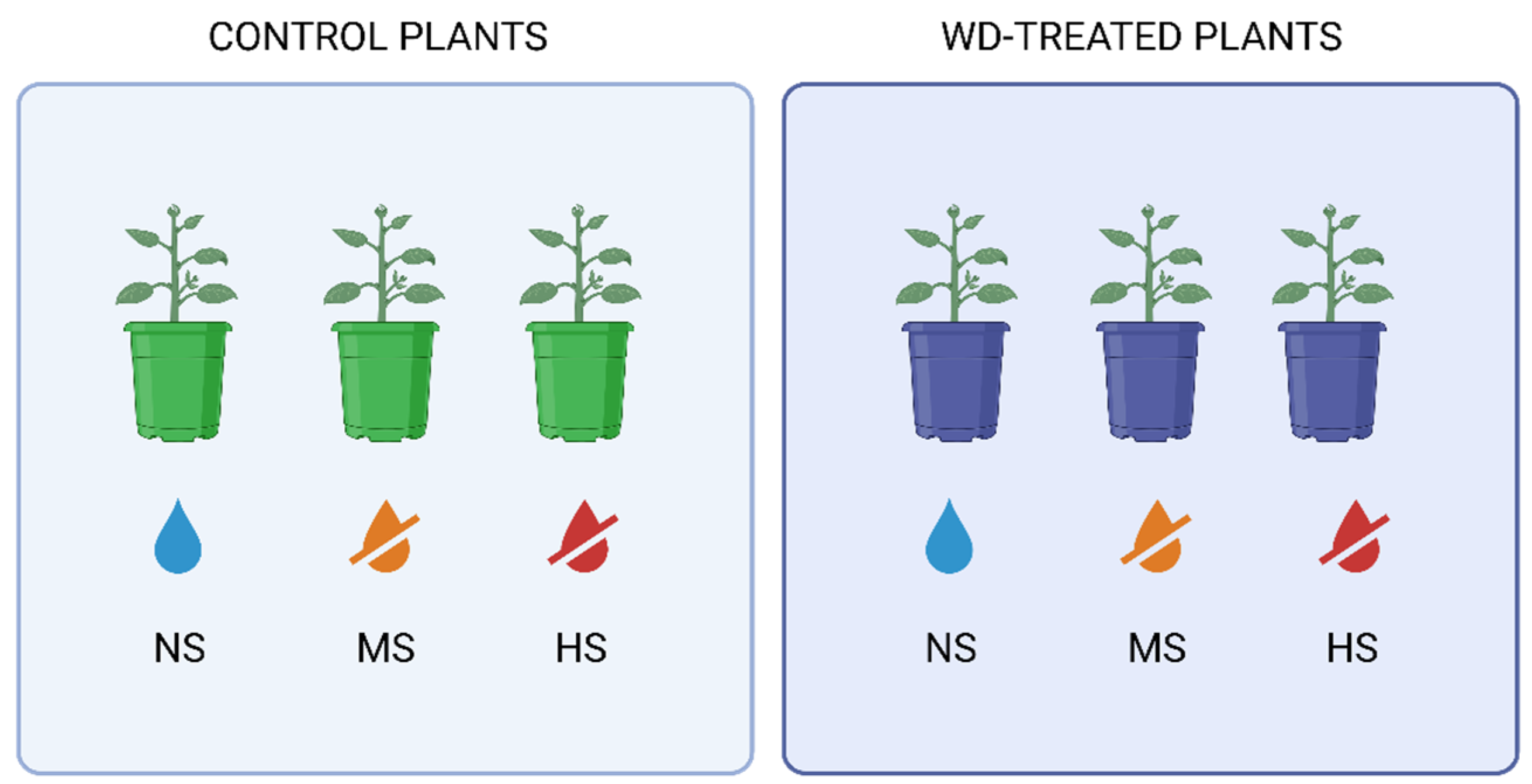
2.1. Experimental Design
2.2. Photosynthetic Parameters
2.3. Malondialdehyde
2.4. Proline
2.5. Antioxidant Compounds
2.6. Mineral Content
2.7. Statistical Analysis
3. Results
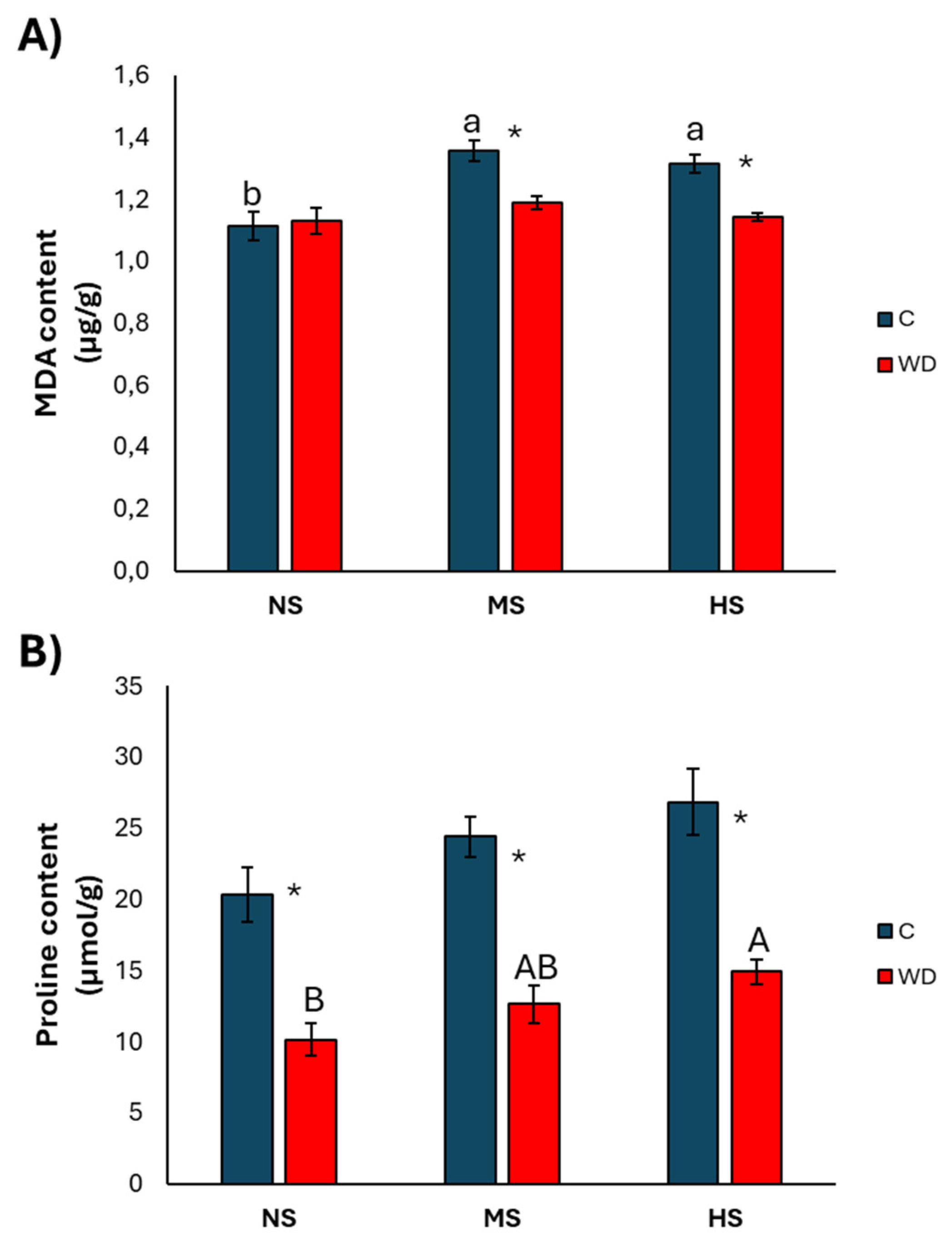
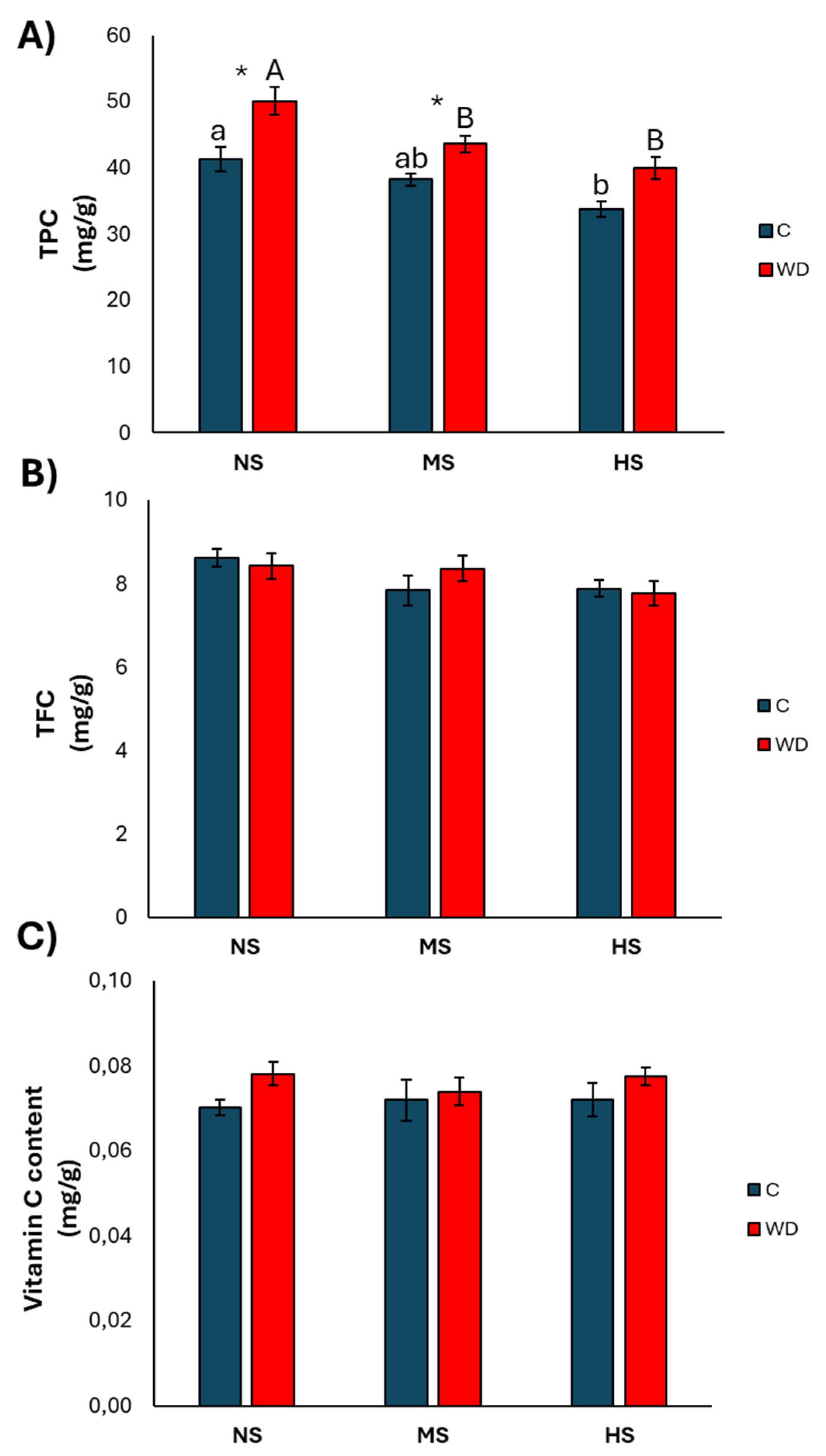
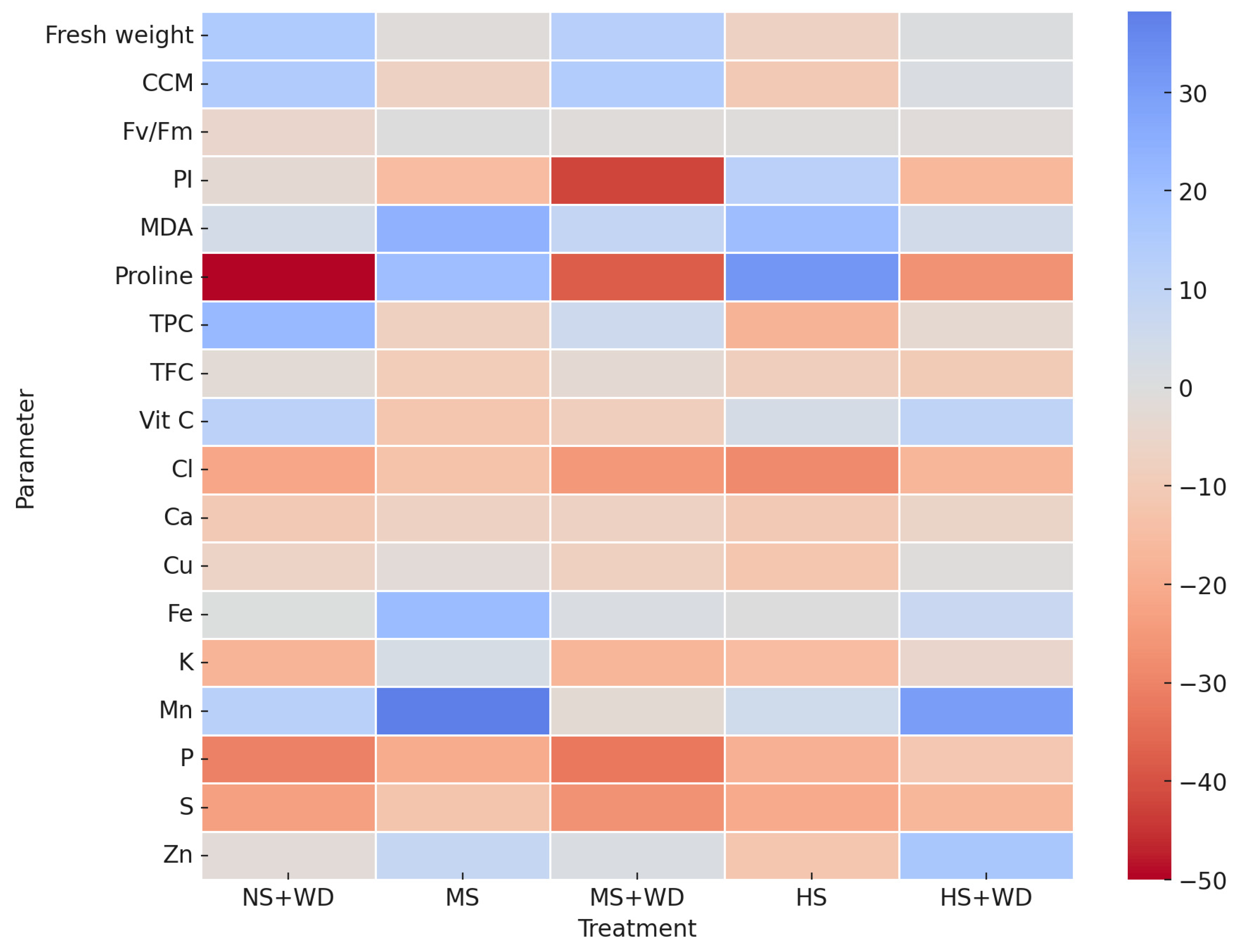
3.1. Effect of Water Deficiency
3.2. Effect of Treatment with Wood Distillate
4. Discussion
4.1. Effect of Water Deficiency
4.2. Effect of Treatment with Wood Distillate
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multi-Scalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index—SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Bhattacharya, A. Effect of soil water deficit on growth and development of plants: A review. In Soil Water Deficit and Physiological Issues in Plants; Springer: Singapore, 2021; pp. 393–488. [Google Scholar]
- Abbas, S.; Javed, M.T.; Ali, Q.; Azeem, M.; Ali, S. Nutrient deficiency stress and relation with plant growth and development. In Engineering Tolerance in Crop Plants Against Abiotic Stress; CRC Press: Boca Raton, FL, USA, 2021; pp. 239–262. [Google Scholar]
- Falkenmark, M. Growing water scarcity in agriculture: Future challenge to global water security. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20120410. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding Plant Responses to Drought: From Genes to the Whole Plant. Funct. Plant Biol. 2009, 36, 265–298. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Galmés, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid Variations of Mesophyll Conductance in Response to Changes in CO2 Concentration around Leaves. Plant Cell Environ. 2014, 27, 1285–1297. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms, and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Singh, V.P.; Liu, Y. Combined Use of Meteorological Drought Indices at Multi-Time Scales for Improving Hydrological Drought Detection. Sci. Total Environ. 2016, 571, 1058–1068. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and Concepts in Quantifying Resistance to Drought, Salt and Freezing, Abiotic Stresses that Affect Plant Water Status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Drought Resistance and Its Improvement. In Plant Breeding for Water-Limited Environments; Blum, A., Ed.; Springer: New York, NY, USA, 2011; pp. 53–92. [Google Scholar] [CrossRef]
- Lawlor, D.W. Genetic Engineering to Improve Plant Performance under Drought: Physiological Evaluation of Achievements, Limitations, and Possibilities. J. Exp. Bot. 2013, 64, 83–108. [Google Scholar] [CrossRef]
- Pinto, S.C.; Benvenutti, V.; Nothias, L.F.; Afonso, F.; Medeiros, M. Effects of Drought on Essential Oil Yield and Composition in Basil (Ocimum basilicum L.). J. Appl. Res. Med. Aromat. Plants 2018, 10, 33–39. [Google Scholar] [CrossRef]
- Avdouli, D.; Max, J.F.; Katsoulas, N.; Levizou, E. Basil as secondary crop in cascade hydroponics: Exploring salinity tolerance limits in terms of growth, amino acid profile, and nutrient composition. Horticulturae 2021, 7, 203. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of Extreme Weather Disasters on Global Crop Production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; López-Moreno, J.I.; Vicente-Serrano, S.M.; Lasanta-Martínez, T.; Beguería, S. Mediterranean Water Resources in a Global Change Scenario. Earth Sci. Rev. 2011, 105, 121–139. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The Relation between Climate Change in the Mediterranean Region and Global Warming. Reg. Environ. Change 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Tiilikkala, K.; Fagernäs, L.; Tiilikkala, J. History and Use of Wood Pyrolysis Liquids as Biocide and Plant Protection Product. Open Agric. J. 2010, 4, 111–118. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, Y.; Tang, J.; Xu, W. Wood Vinegar as a Soil Conditioner Improves Drought Tolerance in Soybean. Agric. Water Manag. 2016, 177, 243–249. [Google Scholar] [CrossRef]
- Ghorbani, M.; Azarnejad, N.; Carril, P.; Celletti, S.; Loppi, S. Boosting the Resilience to Drought of Crop Plants Using Wood Distillate: A Pilot Study with Lettuce (Lactuca sativa L.). Plant Stress 2024, 12, 100450. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Amoo, S.O. Bioactive Compounds and Antioxidant Activity of Hibiscus sabdariffa L. J. Med. Plants Res. 2012, 6, 5423–5430. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, M.; He, G.; Wu, F. Organic Acids in Wood Vinegar and Its Effects on Drought Resistance of Maize. J. Agric. Sci. Technol. 2019, 21, 129–138. [Google Scholar]
- Fedeli, R.; Celletti, S.; Loppi, S. Wood Distillate Promotes the Tolerance of Lettuce in Extreme Salt Stress Conditions. Plants 2024, 13, 1335. [Google Scholar] [CrossRef]
- Fedeli, R.; Celletti, S.; Alexandrov, D.; Nafikova, E.; Loppi, S. Biochar-mediated bioremediation: A sustainable strategy to increase Avena sativa L. tolerance to crude oil soil contamination. Environ. Sci. Pollut. Res. 2024, 31, 52774–52783. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Vannini, A.; Djatouf, N.; Celletti, S.; Loppi, S. Can Lettuce Plants Grow in Saline Soils Supplemented with Biochar? Heliyon 2024, 10, e12006. [Google Scholar] [CrossRef]
- Fedeli, R.; Cruz, C.; Loppi, S.; Munzi, S. Hormetic Effect of Wood Distillate on Hydroponically Grown Lettuce. Plants 2024, 13, 447. [Google Scholar] [CrossRef]
- Azarnejad, N.; Celletti, S.; Ghorbani, M.; Fedeli, R.; Loppi, S. Dose-Dependent Effects of a Corn Starch-Based Bioplastic on Basil (Ocimum basilicum L.): Implications for Growth, Biochemical Parameters, and Nutrient Content. Toxics 2024, 12, 80. [Google Scholar] [CrossRef]
- Borella, M.; Baghdadi, A.; Bertoldo, G.; Della Lucia, M.; Chiodi, C.; Celletti, S.; Deb, S.; Baglieri, A.; Zegada-Lizarazu, W.; Pagani, E.; et al. Transcriptomic and Physiological Approaches to Decipher Cold Stress Mitigation Exerted by Brown-Seaweed Extract (BSE) Application in Tomato. Front. Plant Sci. 2023, 14, 1232421. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Loppi, S.; Cruz, C.; Munzi, S. Evaluating Seawater and Wood Distillate for Sustainable Hydroponic Cultivation: Implications for Crop Growth and Nutritional Quality. Sustainability 2024, 16, 7186. [Google Scholar] [CrossRef]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Evaluation of Yield and Nutraceutical Traits of Orange-Fleshed Sweet Potato Storage Roots in Two Agro-Climatic Zones of Northern Ethiopia. Plants 2023, 12, 1319. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Mazza, I.; Perini, C.; Salerni, E.; Loppi, S. New Frontiers in the Cultivation of Edible Fungi: The Application of Biostimulants Enhances the Nutritional Characteristics of Pleurotus eryngii (DC.). Quél. Agric. 2024, 14, 1012. [Google Scholar] [CrossRef]
- Fedeli, R.; Di Lella, L.; Loppi, S. Suitability of XRF for Routine Analysis of Multi-Elemental Composition: A Multi-Standard Verification. Methods Protoc. 2024, 7, 53. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 12 November 2024).
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance; Ahmad, P., Wani, M.R., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 209–250. [Google Scholar] [CrossRef]
- Ahmad, P.; Jamsheed, S.; Hameed, A.; Rasool, S.; Sharma, I.; Azooz, M.M.; Hasanuzzaman, M. Drought Stress Induced Oxidative Damage and Antioxidants in Plants. In Oxidative Damage to Plants; Gill, S.S., Tuteja, N., Eds.; Academic Press: London, UK, 2014; pp. 345–367. [Google Scholar]
- Bukhari, S.A.H.; Peerzada, A.M.; Javed, M.H.; Dawood, M.; Hussain, N.; Ahmad, S. Growth and Development Dynamics in Agronomic Crops under Environmental Stress. In Agronomic Crops: Volume 1: Production Technologies; Ahmad, P., Wani, M.R., Eds.; Springer: Cham, Switzerland, 2019; pp. 83–114. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Carpentier, R. Effects of water stress on the photosynthetic efficiency of plants. In Chlorophyll a Fluorescence: A Signature of Photosynthesi; Springer: Dordrecht, The Netherlands, 2004; pp. 623–635. [Google Scholar] [CrossRef]
- Dehghani Bidgoli, R.; Dehghan, S.; Emam, Y. Drought Effects on Physiological and Biochemical Processes in Maize and Wheat. Plant Physiol. Biochem. 2018, 131, 134–142. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: Growth, photosynthesis, water relations and oxidative defence mechanism . J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.H.; Nam, S.Y. Changes in growth, visual qualities, and photosynthetic parameters in Peperomia species and cultivars under different color temperatures of white lighting conditions. J. Agric. Life Environ. Sci. 2023, 35, 307–321. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.; Nam, S.Y. Optimized concentrations of auxinic rooting promoters improve stem cutting propagation efficiency and morphophysiological characteristics in Hedera algeriensis cv. Gloire de Marengo. Hortic. Sci. Technol. 2025, 43, 1–16. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Role of Antioxidant Defense Systems in Mitigating Oxidative Stress in Plants Exposed to Drought. Agronomy 2019, 9, 427. [Google Scholar] [CrossRef]
- Fan, X.; Cui, Y.; Liu, X. Malondialdehyde as an Indicator of Drought-Induced Oxidative Damage in Plants: A Review. Environ. Exp. Bot. 2022, 195, 104788. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O. Differential Responses of Rice and Wheat to Drought Stress: A Focus on Proline Accumulation. J. Plant Physiol. 2019, 233, 57–65. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Basra, S.M. Improving Drought Tolerance in Rice and Wheat: The Role of Proline and Its Metabolic Pathways. Plant Physiol. Biochem. 2017, 115, 180–188. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Shen, Q.; Smith, S.J. Nutrient Uptake and Transport in Plants under Drought Conditions. Plant Cell Environ. 2010, 33, 496–509. [Google Scholar] [CrossRef]
- Saeed, B.; Rahman, M.U.; Saeed, N. Essential Nutrients’ Uptake and Translocation in Drought-Stressed Plants. Agric. Water Manag. 2020, 242, 106391. [Google Scholar] [CrossRef]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, Prospects, and Potential Application of Pyroligneous Acid in Agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root Proteomics Reveals the Effects of Wood Vinegar on Wheat Growth and Subsequent Tolerance to Drought Stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage, and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef]
- Talla, S.K.; Panigrahy, M.; Kappara, S.; Nirosha, P.; Neelamraju, S.; Ramanan, R. Cytokinin delays dark-induced senescence in rice by maintaining the chlorophyll cycle and photosynthetic complexes. J. Exp. Bot. 2016, 67, 1839–1851. [Google Scholar] [CrossRef]
- Wu, W.; Du, K.; Kang, X.; Wei, H. The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A. The role of abscisic acid in drought stress: How ABA helps plants to cope with drought stress. In Drought Stress Tolerance in Plants, Vol. 2: Molecular and Genetic Perspectives; Springer: Cham, Switzerland, 2016; pp. 123–151. [Google Scholar]
- Loo, A.Y.; Jain, K.; Darah, I. Antioxidant Activity of Compounds Isolated from the Pyroligneous Acid, Rhizophora apiculata. Food Chem. 2008, 107, 1151–1160. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of Seaweed Extract on the Growth, Yield, and Nutrient Uptake of Soybean (Glycine max) under Rainfed Conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Liang, D.; Xia, H.; Wu, Y.; Wang, C.; Ma, Y.; Li, X. Application of Biostimulants for Improving the Growth, Yield, and Bioactive Compounds of Medicinal Plants under Abiotic Stresses. Agronomy 2020, 10, 1285. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar Mitigates Drought Stress in Wheat Plants by Improving Water Relations and Nutrient Uptake. Soil Tillage Res. 2014, 140, 36–45. [Google Scholar] [CrossRef]
- Jindo, K.; Martinsen, V.; Becerra, V.; Soria, M.A. Application of Wood Vinegar as Biochar Derivative: Impact on Soil Properties and Crop Growth. Sci. Total Environ. 2020, 731, 138974. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, H.; Jiang, W. Zinc and Drought Stress: Impact on Plant Growth and Antioxidant Enzyme System. Plant Physiol. Biochem. 2020, 148, 174–182. [Google Scholar] [CrossRef]
| Fresh Weight | Chlorophyll | Fv/Fm | |||||
|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | |
| WS | 2 | 5.43 | 0.012 | 4.12 | 0.014 | 3.87 | 0.532 |
| WD | 1 | 6.25 | 0.021 | 7.02 | 0.018 | 5.1 | 0.487 |
| WS × WD | 1 | 0.89 | 0.041 | 1.1 | 0.033 | 0.75 | 0.478 |
| Piabs | MDA | Proline | |||||
| df | F | p | F | p | F | p | |
| WS | 2 | 2.96 | 0.874 | 6.3 | 0.024 | 5.68 | 0.451 |
| WD | 1 | 4.55 | 0.647 | 5.98 | 0.145 | 4.88 | 0.024 |
| WS × WD | 1 | 1.03 | 0.364 | 0.92 | 0.401 | 1.15 | 0.321 |
| TPC | TFC | Vitamin C | |||||
| df | F | p | F | p | F | p | |
| WS | 2 | 8.75 | 0.001 | 3.15 | 0.146 | 1.89 | 0.154 |
| WD | 1 | 9.5 | 0.004 | 4.32 | 0.198 | 2.65 | 0.109 |
| WS × WD | 1 | 0.68 | 0.049 | 0.82 | 0.443 | 1.05 | 0.351 |
| Al | Ca | Cu | |||||
| df | F | p | F | p | F | p | |
| WS | 2 | 4.21 | 0.02 | 6.35 | 0.31 | 3.77 | 0.35 |
| WD | 1 | 5.55 | 0.108 | 4.88 | 0.24 | 6.32 | 0.14 |
| WS × WD | 1 | 1.22 | 0.272 | 0.92 | 0.34 | 1.05 | 0.312 |
| Fe | K | Mn | |||||
| df | F | p | F | p | F | p | |
| WS | 2 | 5.12 | 0.24 | 7.42 | 0.29 | 4.88 | 0.27 |
| WD | 1 | 7.2 | 0.12 | 6.5 | 0.18 | 5.75 | 0.37 |
| WS × WD | 1 | 0.87 | 0.369 | 0.78 | 0.398 | 1.02 | 0.318 |
| P | S | Zn | |||||
| df | F | p | F | p | F | p | |
| WS | 2 | 6.1 | 0.4 | 3.95 | 0.28 | 5.7 | 0.41 |
| WD | 1 | 6.4 | 0.51 | 5.3 | 0.22 | 6.8 | 0.25 |
| WS × WD | 1 | 0.95 | 0.353 | 1.15 | 0.289 | 1.08 | 0.307 |
| NS | MS | HS | ||||
|---|---|---|---|---|---|---|
| C | WD | C | WD | C | WD | |
| Fresh weight (g) | 9.8 ± 0.5 * | 11.2 ± 0.3 | 9.7 ± 0.3 * | 11.0 ± 0.4 | 9.1 ± 0.4 | 9.8 ± 0.6 |
| Chl (mg m−2) | 295 ± 9 * | 369 ± 6 A | 297 ± 11 * | 338 ± 9 AB | 272 ± 8 * | 332 ± 11 B |
| FV/FM | 0.821 ± 0.003 | 0.815 ± 0.009 | 0.811 ± 0.009 | 0.820 ± 0.003 | 0.808 ± 0.010 | 0.818 ± 0.004 |
| PIABS | 2.82 ± 0.25 | 2.60 ± 0.21 | 2.57 ± 0.22 | 2.48 ± 0.23 | 2.75 ± 0.29 | 2.63 ± 0.14 |
| NS | MS | HS | ||||
|---|---|---|---|---|---|---|
| C | WD | C | WD | C | WD | |
| Al (mg/kg) | 475 ± 15 ab | 505 ± 20 | 582 ± 38 a | 514 ± 17 | 518 ± 24 b | 507 ± 30 |
| Ca (%) | 1.4 ± 0.1 | 1.3 ±0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Cu (mg/kg) | 3.4 ± 0.4 | 3.2 ± 0.3 | 3.4 ± 0.3 | 3.2 ± 0.2 | 3.0 ± 0.2 | 3.5 ± 0.3 |
| Fe (mg/kg) | 64 ± 6 | 64 ± 3 | 77 ± 4 | 65 ± 3 | 64 ± 3 | 69 ± 6 |
| K (%) | 2.3 ± 0.1 * | 1.9 ± 0.1 | 2.4 ± 0.2 * | 1.9 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.1 |
| Mn (mg/kg) | 49 ± 6 | 55 ± 5 | 68 ± 6 * | 49 ± 3 | 52 ± 3 | 64 ± 7 |
| P (mg/kg) | 6296 ± 309 * | 4382 ± 293 B | 5017 ± 496 | 4226 ± 260 B | 5106 ± 251 | 5569 ± 297 A |
| S (mg/kg) | 1674 ± 142 * | 1282 ± 64 | 1471 ± 145 | 1218 ± 74 | 1328 ± 83 | 1389 ± 99 |
| Zn (mg/kg) | 33 ± 2 | 32 ± 2 | 35 ± 1 | 33 ± 2 | 29 ± 1 * | 38 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedeli, R.; Zhatkanbayeva, Z.; Loppi, S. Wood Distillate as a Solution for Growing Crops Under Water Deficiency. Crops 2025, 5, 22. https://doi.org/10.3390/crops5020022
Fedeli R, Zhatkanbayeva Z, Loppi S. Wood Distillate as a Solution for Growing Crops Under Water Deficiency. Crops. 2025; 5(2):22. https://doi.org/10.3390/crops5020022
Chicago/Turabian StyleFedeli, Riccardo, Zhanna Zhatkanbayeva, and Stefano Loppi. 2025. "Wood Distillate as a Solution for Growing Crops Under Water Deficiency" Crops 5, no. 2: 22. https://doi.org/10.3390/crops5020022
APA StyleFedeli, R., Zhatkanbayeva, Z., & Loppi, S. (2025). Wood Distillate as a Solution for Growing Crops Under Water Deficiency. Crops, 5(2), 22. https://doi.org/10.3390/crops5020022








