Minimizing the Adverse Impacts of Soil Salinity on Maize and Tomato Growth and Productivity through the Application of Plant Growth-Promoting Rhizobacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Bacterial Strains and Culture Conditions
2.3. Experimental Layout and Treatments
2.4. Plant Harvest and Biometric Analysis
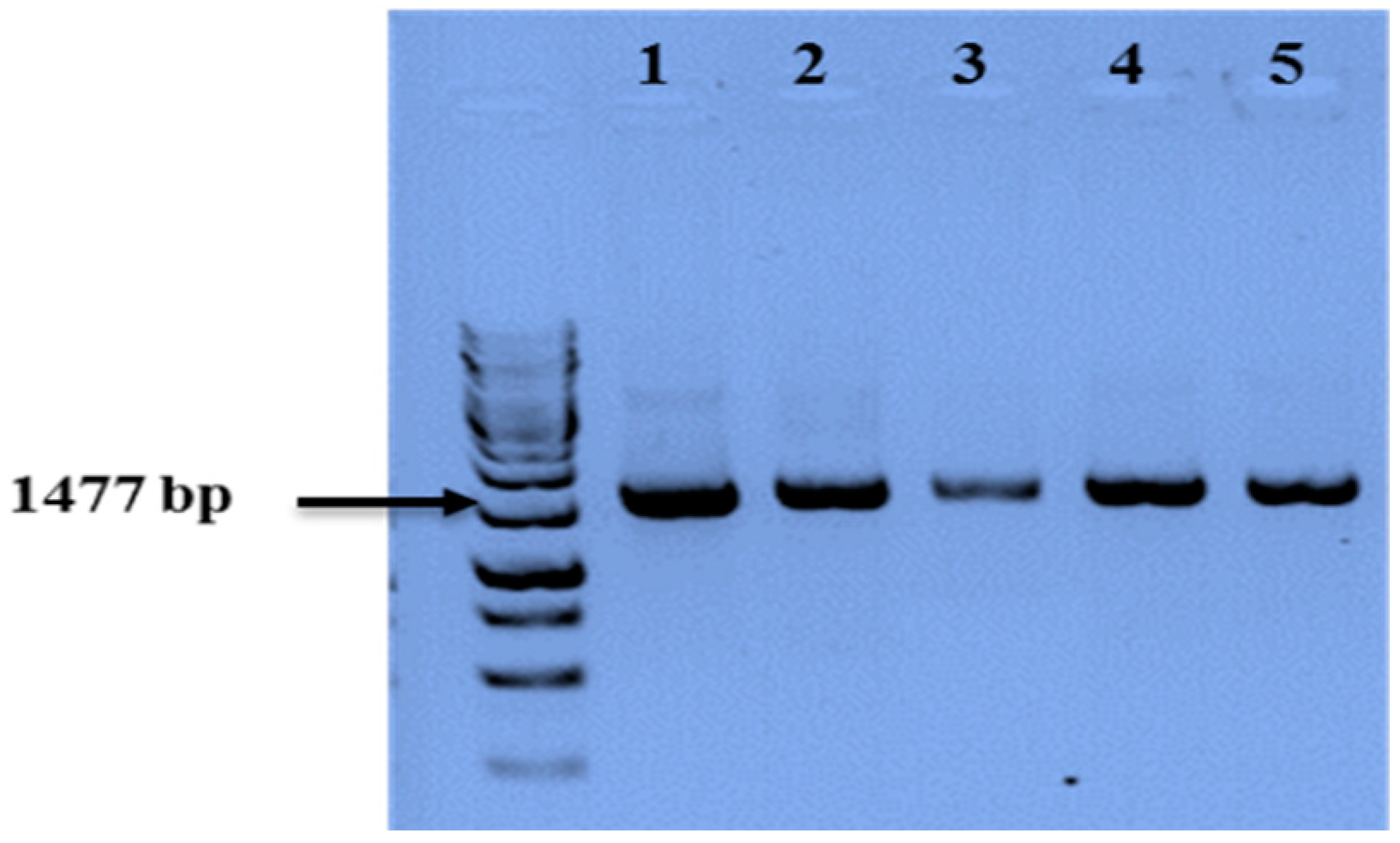
2.5. Molecular Identification of Selected Bacterial Strains
2.6. Data Analysis
3. Results
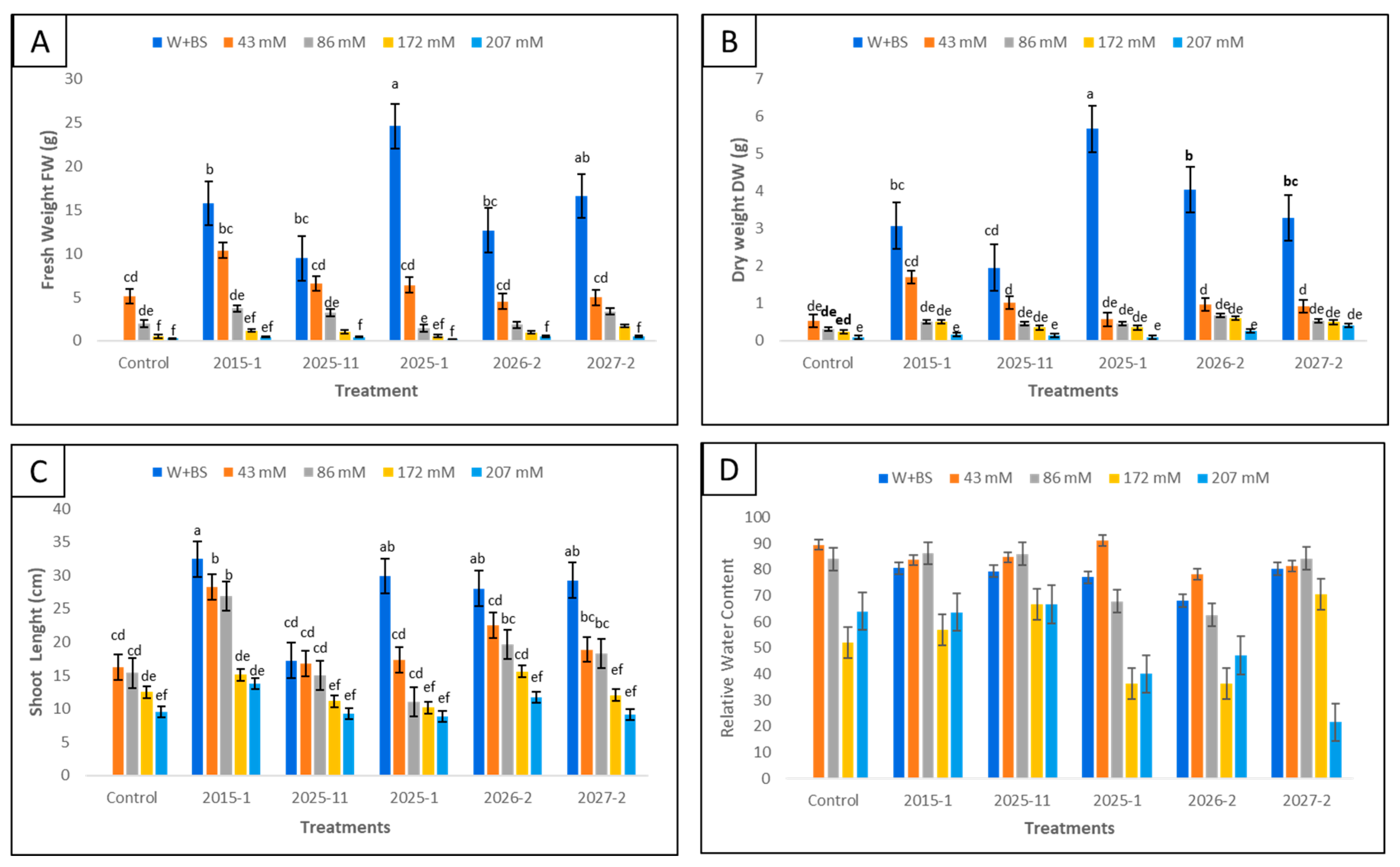
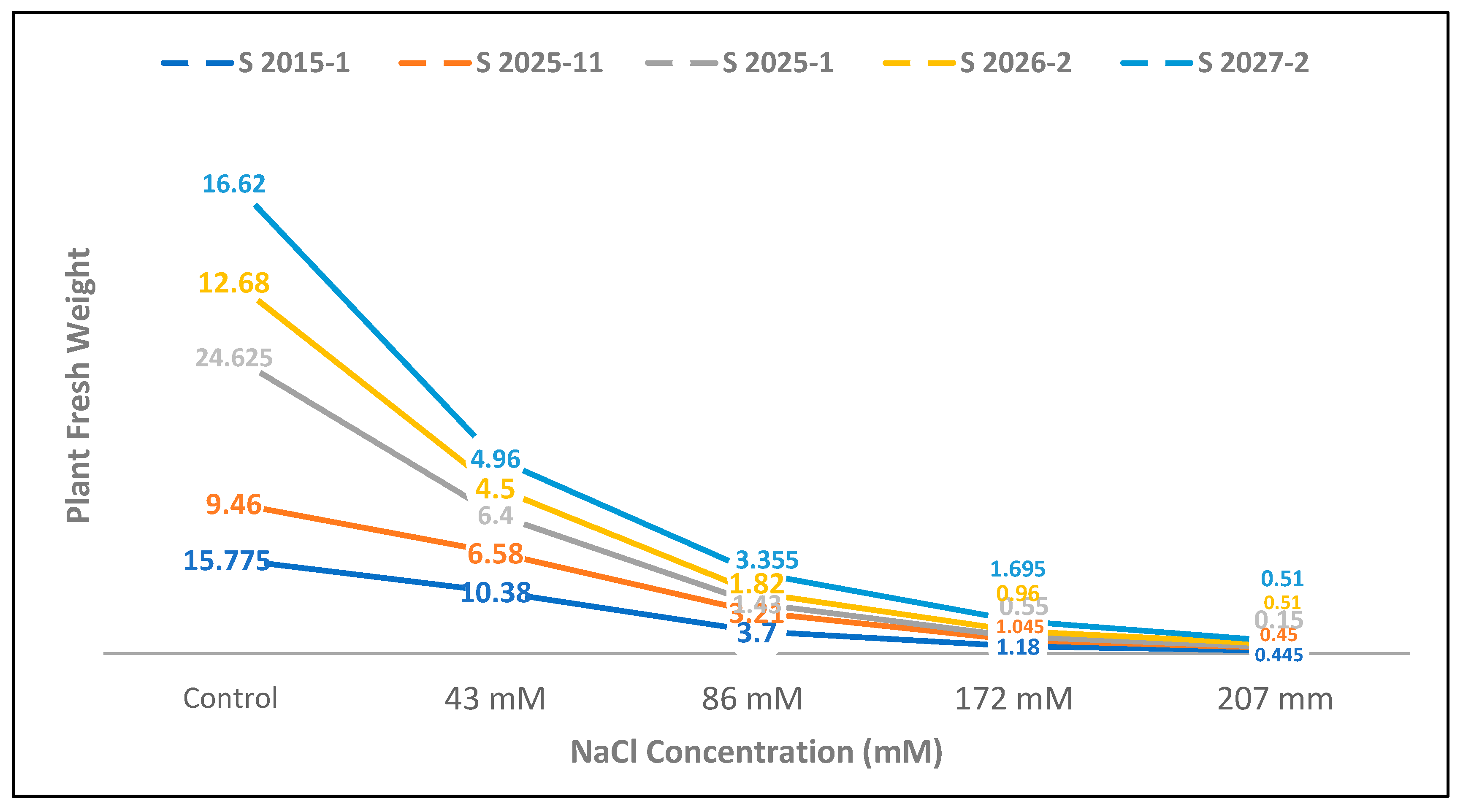
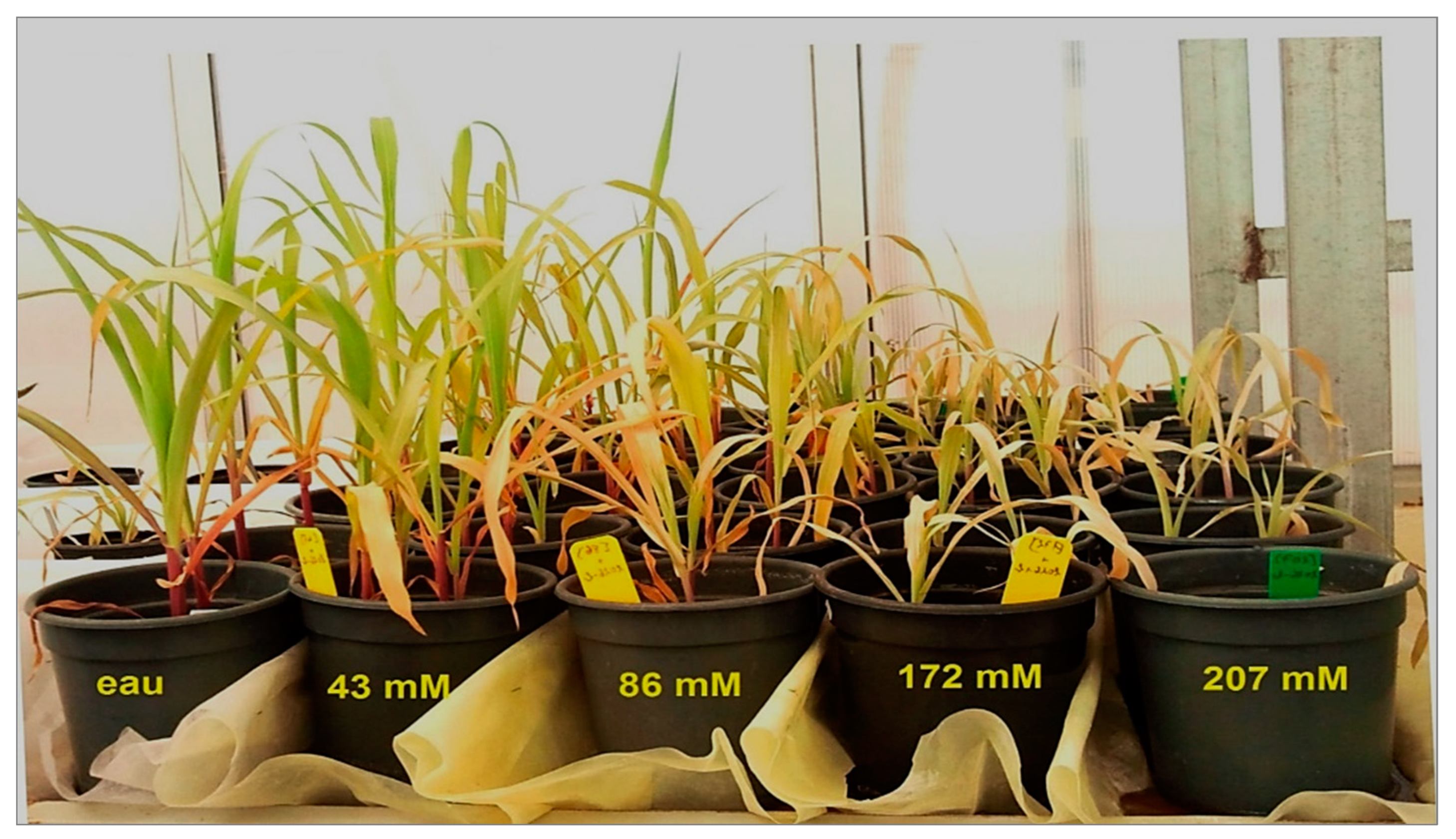
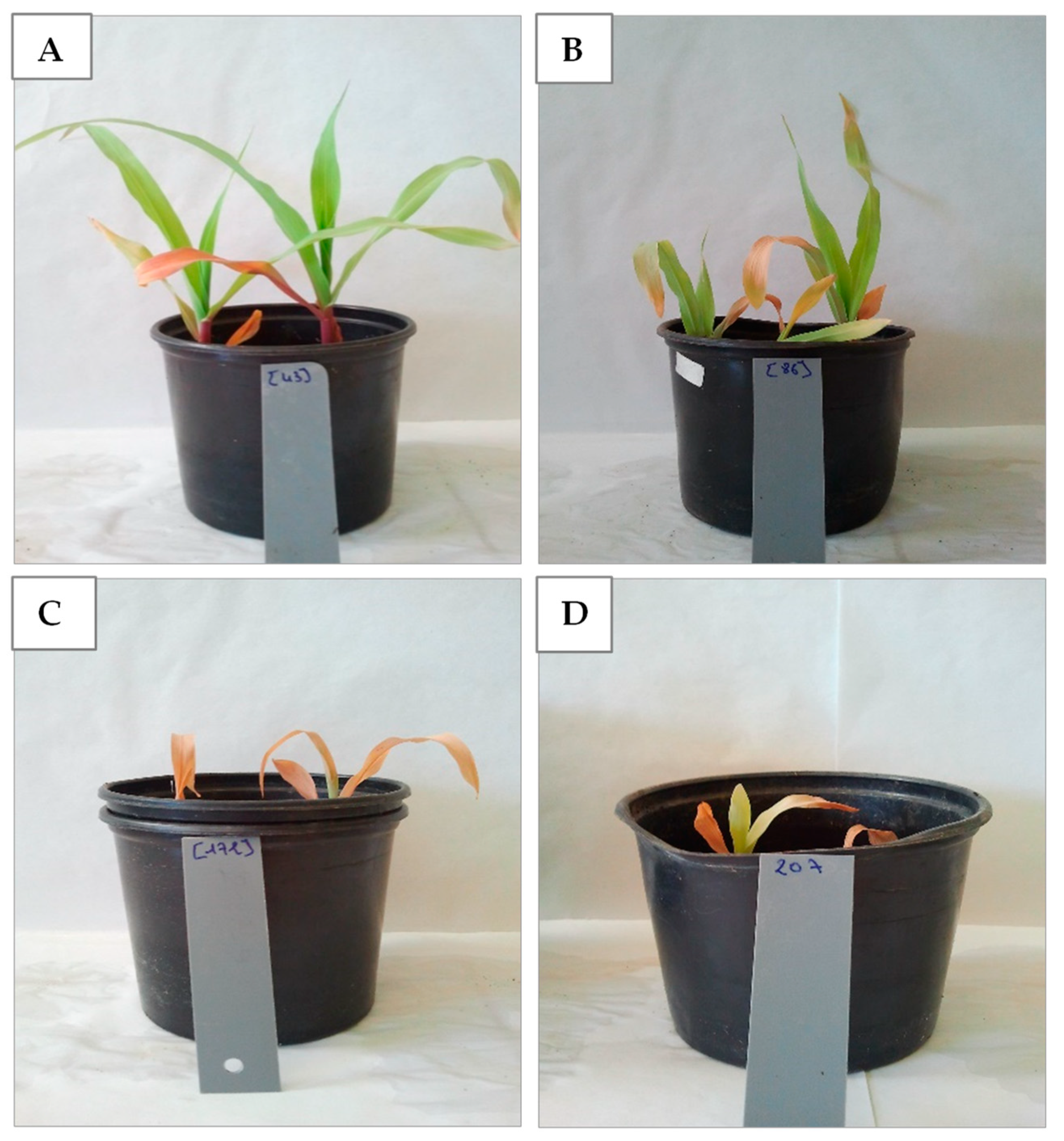
3.1. Differential Salt Tolerance in Tomato and Maize: Impact on Plant Growth and the Efficacy of Bacterial Inoculation
3.2. Impact of Salinity on Root and Stem Development: Efficacy of Bacterial Inoculation
3.3. Influence of Salt on Chlorophyll Content, Leaf Area, and Leaf Number in Tomato and Maize Plants
3.4. Molecular Identification of Selected PGP Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, G.; Xu, J.; Zhang, X.; Pan, X. Efficiency and Driving Factors of Agricultural Carbon Emissions: A Study in Chinese State Farms. Agriculture 2024, 14, 1454. [Google Scholar] [CrossRef]
- Hassani, H.; Huang, X.; Silva, E. Big data and climate change. Big Data Cogn. Comput. 2019, 3, 12. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Gaiser, T.; Ewert, F. Climate change impact and potential adaptation strategies under alternate climate scenarios for yam production in the sub-humid savannah zone of West Africa. Mitig. Adapt. Strat. Glob. Change 2016, 21, 955–968. [Google Scholar] [CrossRef]
- CSSRI, NAIP. Final Report of NAIP Sub-Project on: Strategies for Sustainable Management of Degraded Coastal Land and Water for Enhancing Livelihood Security of Farming Communities (Component 3, GEF Funded); Burnan, D., Mandal, S., Mahanta, K.K., Eds.; Central Soil Salinity Research Institute, Regional Research Station (CSSRI, RRS): Canning Town, India, 2014; p. 104. [Google Scholar]
- Aydınoğlu, A.U.; Özdemir, B.E. Green Deal: Review of history and academic research. Trakya Univ. Fac. Econ. Adm. Sci. E-J. 2022, 11, 107–121. [Google Scholar]
- Farooq, M.S.; Uzair, M.; Raza, A.; Habib, M.; Xu, Y.; Yousuf, M.; Ramzan Khan, M. Uncovering the research gaps to alleviate the negative impacts of climate change on food security: A review. Front. Plant Sci. 2022, 13, 927535. [Google Scholar] [CrossRef]
- Menberg, K.; Blum, P.; Kurylyk, B.L.; Bayer, P. Observed groundwater temperature response to recent climate change. Hydrol. Earth Syst. Sci. 2014, 18, 4453–4466. [Google Scholar] [CrossRef]
- Zhu, T.; Lund, J.R.; Jenkins, M.W.; Marques, G.F.; Ritzema, R.S. Climate change, urbanization, and optimal long-term floodplain protection. Water Resour. Res. 2007, 43, W06409. [Google Scholar] [CrossRef]
- Saidi, A.S.; Diouri, M. Food self-sufficiency under the Green-Morocco Plan. J. Exp. Biol. Agric. Sci. 2017, 5, 33–40. [Google Scholar] [CrossRef]
- Mrabet, R.; Moussadek, R.; Fadlaoui, A.; Van Ranst, E. Conservation agriculture in dry areas of Morocco. Field Crop. Res. 2012, 132, 84–94. [Google Scholar] [CrossRef]
- FAOStat. 2017. Available online: https://www.fao.org/faostat/fr/#home (accessed on 7 July 2020).
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Okon, O.G. Effect of salinity on physiological processes in plants. In Microorganisms Saline Environments: Strategies and Functions; Springer: Cham, Switzerland, 2019; pp. 237–262. [Google Scholar]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Wang, S.; Li, C.; Wang, X.; Chen, K.; Chen, L. Transcriptome sequencing revealed the genes and pathways involved in salinity stress of Chinese mitten crab, Eriocheir sinensis. Physiol. Genom. 2014, 46, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z.; Maurović, N.; Kondres, N.; Filipović, V.; Savić, R.; Romić, D. Growth and element uptake by salt-sensitive crops under combined NaCl and Cd stresses. Plants 2021, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yang, Z.F.; Shen, Z.Y.; Zhao, R. Environmental flows for the Yangtze Estuary based on salinity objectives. Commun. Nonlinear Sci. Numer. Simul. 2009, 14, 959–971. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.S.M.; Elrys, A.S.; Abd El-Mageed, T.A.; El-Tarabily, K.A. Halotolerant plant growth-promoting rhizobacteria improve soil fertility and plant salinity tolerance for sustainable agriculture—A review. Plant Stress. 2024, 12, 100482. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Bharti, C. Halo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Etesami, H.; Noori, F. Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Singapore, 2019; pp. 1–22. [Google Scholar]
- Sandhya, V.S.K.Z.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Nosheen, A.; Bano, A. Growth enrichment of Carthamus tinctorius (L) and reduction in dosage of chemical fertilizers with application of plant growth promoting rhizobacteria. Int. J. Agron. Agric. Res. 2014, 4, 75–84. [Google Scholar]
- Khan, N.; Bano, A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int. J. Phytoremed. 2016, 18, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Benabderrazik, K.; Kopainsky, B.; Monastyrnaya, E.; Thompson, W.; Tazi, L.; Joerin, J.; Six, J. Climate resilience and the human-water dynamics: The case of tomato production in Morocco. Sci. Total Environ. 2022, 849, 157597. [Google Scholar] [CrossRef] [PubMed]
- Achli, S.; Epule, T.E.; Dhiba, D.; Chehbouni, A.; Er-Raki, S. Vulnerability of barley, maize, and wheat yields to variations in growing season precipitation in Morocco. Appl. Sci. 2022, 12, 3407. [Google Scholar] [CrossRef]
- Inculet, C.-S.; Mihalache, G.; Sellitto, V.M.; Hlihor, R.-M.; Stoleru, V. The effects of a microorganisms-based commercial product on the morphological, biochemical, and yield of tomato plants under two different water regimes. Microorganisms 2019, 7, 706. [Google Scholar] [CrossRef] [PubMed]
- Hssaisoune, M.; Bouchaou, L.; Sifeddine, A.; Bouimetarhan, I.; Chehbouni, A. Moroccan groundwater resources and evolution with global climate changes. Geosciences 2020, 10, 81. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Rabie, G.H.; Almadini, A.M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotechnol. 2005, 4, 210. [Google Scholar]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef]
- Bonachela, S.; Fernández, M.D.; Cabrera-Corral, F.J.; Granados, M.R. Salt and irrigation management of soil-grown Mediterranean greenhouse tomato crops drip-irrigated with moderately saline water. Agric. Water Manag. 2022, 262, 107433. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. What are endophytes? In Microbial Root Endophytes; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–13. [Google Scholar]
- Vaishnav, A.; Shukla, A.K.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic bacteria in plant salt stress tolerance: Current and future prospects. J. Plant Growth Regul. 2019, 38, 650–668. [Google Scholar] [CrossRef]
- Ayaz, M.; Ali, Q.; Jiang, Q.; Wang, R.; Wang, Z.; Mu, G.; Gu, Q. Salt-tolerant Bacillus strains improve plant growth traits and regulation of phytohormones in wheat under salinity stress. Plants 2022, 11, 2769. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Chatterjee, P.; Biswas, S.; Biswas, A.K. Amelioration of salinity stress by NaCl pretreatment with reference to sugar metabolism in legumes Cajanas cajan L. and Vigna mungo L. Plant. Sci. Today 2017, 4, 28–40. [Google Scholar] [CrossRef]
- Ferreira, N.C.; Mazzuchelli, R.D.C.L.; Pacheco, A.C.; Araujo, F.F.D.; Antunes, J.E.L.; Araujo, A.S.F.D. Bacillus subtilis improves maize tolerance to salinity. Ciência Rural. 2018, 48, e20170910. [Google Scholar] [CrossRef]
- Song, P.; Zhao, B.; Sun, X.; Li, L.; Wang, Z.; Ma, C.; Zhang, J. Effects of Bacillus subtilis HS5B5 on maize seed germination and seedling growth under NaCl stress conditions. Agronomy 2023, 13, 1874. [Google Scholar] [CrossRef]
- Medeiros, P.R.; Duarte, S.N.; Silva, Ê.F. Efficiency of water use and fertilizer in fertirrigation of management in the tomato crop under conditions of soil salinity. Commun. Soil Sci. Plant Anal. 2012, 43, 258–264. [Google Scholar]
- De O Nunes, P.S.; De Medeiros, F.H.; De Oliveira, T.S.; De Almeida Zago, J.R.; Bettiol, W. Bacillus subtilis and Bacillus licheniformis promote tomato growth. Braz. J. Microbiol. 2023, 54, 397–406. [Google Scholar] [CrossRef]
- Popp, P.F.; Dotzler, M.; Radeck, J.; Bartels, J.; Mascher, T. The Bacillus BioBrick Box 2.0: Expanding the genetic toolbox for the standardized work with Bacillus subtilis. Sci. Rep. 2017, 7, 15058. [Google Scholar] [CrossRef]
- Cherif-Silini, H.; Thissera, B.; Bouket, A.C.; Saadaoui, N.; Silini, A.; Eshelli, M.; Belbahri, L. Durum wheat stress tolerance induced by endophyte Pantoea agglomerans with genes contributing to plant functions and secondary metabolite arsenal. Int. J. Mol. Sci. 2019, 20, 3989. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Jeong, M.H.; Min, B.H.; Kim, S.H.; Park, C.J. Survival rate and oxygen consumption patterns with respect to salinity changes in juvenile abalone Haliotis discus hannai. Anim. Cells Syst. 2014, 18, 380–386. [Google Scholar] [CrossRef]
- Giuffrida, F.; Scuderi, D.; Giurato, R.; Leonardi, C. Physiological response of broccoli and cauliflower as affected by NaCl salinity. In Proceedings of the VI International Symposium on Brassicas and XVIII Crucifer Genetics Workshop, Catania, Italy, 12–16 November 2012; pp. 435–441. [Google Scholar]
- Walitang, D.I.; Kim, C.G.; Kim, K.; Kang, Y.; Kim, Y.K.; Sa, T. The influence of host genotype and salt stress on the seed endophytic community of salt-sensitive and salt-tolerant rice cultivars. BMC Plant Biol. 2018, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lou, F.; Zhang, Y.; Song, N. Gill transcriptome sequencing and de novo annotation of Acanthogobius ommaturus in response to salinity stress. Genes 2020, 11, 631. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Wu, G.; Veronican Njeri, K.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Wu, F.; Wan, J.H.C.; Wu, S.; Wong, M. Effects of earthworms and plant growth-promoting rhizobacteria (PGPR) on availability of nitrogen, phosphorus, and potassium in soil. J. Plant Nutri. Soil Sci 2012, 175, 423–433. [Google Scholar] [CrossRef]
- Chakfe, N.; Riepe, G.; Dieval, F.; Le Magnen, J.F.; Wang, L.; Urban, E.; Beaufigeau, M.; Durand, B.; Imig, H.; Kretz, J.G. Longitudinal ruptures of polyester knitted vascular prostheses. J. Vasc. Surg. 2001, 33, 1015–1021. [Google Scholar] [CrossRef]
- Moustaine, M.; Elkahkahi, R.; Benbouazza, A.; Benkirane, R.; Achbani, E.H. Effect of plant growth-promoting rhizobacterial (PGPR) inoculation on growth in tomato (Solanum lycopersicum L.) and characterization for direct PGP abilities in Morocco. Int. J. Environ. Agric. Biotechnol. 2017, 2, 238708. [Google Scholar]
- Moustaine, M.; Kahkahi, R.E.; Benbouazza, A.; Benkirane, R.; Achbani, E.H. The role of plant growth-promoting rhizobacteria (PGPR) in stimulating the growth of wheat (Triticum aestivum L.) in Meknes region, Morocco. Plant Cell Biotechnol. Mol. Biol. 2016, 17, 363–373. [Google Scholar]
- Pholsen, S.; Higgs, D.E.B. Effects of potassium fertiliser and dolomite on the dry matter yield and forage quality of signal grass grown on Korat soil series in Northeast Thailand, University of Hertfordshire. Pensee J. 2014, 76, 253–260. [Google Scholar]
- Yadava, U.L. A rapid and nondestructive method to determine chlorophyll in intact leaves. HortScience 1986, 21, 1449–1450. [Google Scholar] [CrossRef]
- Campillo, E.; Gaddam, S.; Mettle-Amuah, D.; Heneks, J. A tale of two tissues: AtGH9C1 is an endo-β-1, 4-glucanase involved in root hair and endosperm development in Arabidopsis. PLoS ONE 2012, 7, e49363. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Qi, X.; Pan, F.; Wei, Q.; Shi, Y. Comparative proteomics analysis reveals important drought responsive proteins in the leaves of a potato variety tolerant to drought stress. Pak. J. Bot. 2020, 52, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Singh, A.K.; Singh, P.P.; Kumar, A. Interaction of plant growth promoting bacteria with tomato under abiotic stress: A review. Agric. Ecosyst. Environ. 2018, 267, 129–140. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Pappa, P.; Grillakis, M.G.; Varouchakis, E.A.; Tsanis, I.K. Modeling soil salinity in greenhouse cultivations under a changing climate with SALTMED: Model modification and application in Timpaki, Crete. Soil Sci. 2016, 181, 241–251. [Google Scholar] [CrossRef]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T. Beneficial soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Front. Plant. Sci. 2017, 8, 705. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef]
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Leontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Mellidou, I. Plant growth promoting rhizobacteria isolated from halophytes and drought-tolerant plants: Genomic characterisation and exploration of phyto-beneficial traits. Sci. Rep. 2020, 10, 14857. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Niu, Y.; Huo, R.; Gao, X. Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tobacco against bacterial wilt. Sci. Rep. 2017, 7, 40481. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jensen, M.M.; Goonesekera, E.M.; Yu, R.; Smets, B.F.; Valverde-Pérez, B.; Domingo-Félez, C. Denitrifying communities enriched with mixed nitrogen oxides preferentially reduce N2O under conditions of electron competition in wastewater. J. Chem. Eng. 2024, 498, 155292. [Google Scholar] [CrossRef]
- Din, B.U.; Sarfraz, S.; Xia, Y.; Kamran, M.A.; Javed, M.T.; Sultan, T.; Chaudhary, H.J. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 2019, 183, 109466. [Google Scholar]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Hirsch, A.M. Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef]
- Belaouni, H.A.; Bendaha, M.E.A.; Benattia, H.; Medouh, M.; Berini, K.I.; Ben Ahmed, S.; Zitouni, A. Alleviation of salt stress in winter wheat by Pantoea spp. endophytes isolated from spontaneous desert plants of the Sahara. Arch. Phytopathol. Plant Prot. 2022, 55, 2334–2355. [Google Scholar] [CrossRef]
- Bhise, K.K.; Dandge, P.B. Alleviation of salinity stress in rice plant by encapsulated salt tolerant plant growth promoting bacteria Pantoea agglomerans strain KL and its root colonization ability. Arch. Agron. Soil Sci. 2019, 65, 1955–1968. [Google Scholar] [CrossRef]
- Khan, W.U.D.; Aziz, T.; Maqsood, M.A.; Farooq, M.; Abdullah, Y.; Ramzani, P.M.A.; Bilal, H.M. Silicon nutrition mitigates salinity stress in maize by modulating ion accumulation, photosynthesis, and antioxidants. Photosynthetica 2018, 56, 1047–1057. [Google Scholar] [CrossRef]
- Bhise, K.K.; Dandge, P.B. Mitigation of salinity stress in plants using plant growth promoting bacteria. Symbiosis 2019, 79, 191–204. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). App. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Hussain, K.; Ashraf, M.; Ashraf, M.Y. Relationship between growth and ion relation in pearl millet (Pennisetum glaucum (L.) R. Br.) at different growth stages under salt stress. Afr. J. Plant Sci. 2008, 2, 23–27. [Google Scholar]
- González, L.; González-Vilar, M. Determination of Relative Water Content. In Handbook of Plant Ecophysiology Techniques; Reigosa Roger, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Garg, N.; Manchanda, G. Role of arbuscular mycorrhizae in the alleviation of ionic, osmotic and oxidative stresses induced by salinity in Cajanus cajan (L.) Millsp.(pigeonpea). J. Agron. Crop Sci. 2009, 195, 110–123. [Google Scholar] [CrossRef]




| Strain Code | Origin | Sampling Region |
|---|---|---|
| 2026-2 | Compost | Fez-Meknes |
| 2027-2 | Compost | Fez-Meknes |
| 2015-1 | Compost | Fez-Meknes |
| 2025-1 | Compost | Souss-Massa |
| 2025-11 | Compost | Souss-Massa |
| Roots Length | ||||
|---|---|---|---|---|
| Type III SS | ddl | D | Sig. | |
| Strain | 89.39 | 5 | 1.433 | 0.250 |
| Concentration | 398.01 ** | 1 | 31.908 | 0.000 |
| Error | 286.90 | 23 | - | - |
| Chlorophyll CCl | Stem Length | |||||||
|---|---|---|---|---|---|---|---|---|
| Type III SS | ddl | D | Sig. | Type III SS | ddl | D | Sig. | |
| Strain | 34.09 | 5 | 1.898 | 0.134 | 327.32 ** | 5 | 6.940 | 0.000 |
| Concentration | 83.61 ** | 1 | 23.281 | 0.000 | 853.08 ** | 1 | 90.433 | 0.000 |
| Error | 82.60 | 23 | - | - | 216.96 | 23 | - | - |
| Strain Code | Identified Species | Accession Number | |
|---|---|---|---|
| 1 | 2026-2 | Bacillus cereus | KR493006.1 |
| 2 | 2027-2 | Acinetobacter calcoaceticus | KP170504.1 |
| 3 | 2015-1 | Bacillus subtilis | KJ592619.2 |
| 4 | 2025-1 | Pantoea agglomerans | KJ781904.1 |
| 5 | 2025-11 | Paenibacillus brasiliensis | NR025106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahyaoui, H.; El Allaoui, N.; Aziz, A.; Hafidi, M.; Habbadi, K. Minimizing the Adverse Impacts of Soil Salinity on Maize and Tomato Growth and Productivity through the Application of Plant Growth-Promoting Rhizobacteria. Crops 2024, 4, 463-479. https://doi.org/10.3390/crops4040033
Yahyaoui H, El Allaoui N, Aziz A, Hafidi M, Habbadi K. Minimizing the Adverse Impacts of Soil Salinity on Maize and Tomato Growth and Productivity through the Application of Plant Growth-Promoting Rhizobacteria. Crops. 2024; 4(4):463-479. https://doi.org/10.3390/crops4040033
Chicago/Turabian StyleYahyaoui, Hiba, Nadia El Allaoui, Aziz Aziz, Majida Hafidi, and Khaoula Habbadi. 2024. "Minimizing the Adverse Impacts of Soil Salinity on Maize and Tomato Growth and Productivity through the Application of Plant Growth-Promoting Rhizobacteria" Crops 4, no. 4: 463-479. https://doi.org/10.3390/crops4040033
APA StyleYahyaoui, H., El Allaoui, N., Aziz, A., Hafidi, M., & Habbadi, K. (2024). Minimizing the Adverse Impacts of Soil Salinity on Maize and Tomato Growth and Productivity through the Application of Plant Growth-Promoting Rhizobacteria. Crops, 4(4), 463-479. https://doi.org/10.3390/crops4040033








