Comparative Growth of Elephant Ear Taro (Alocasia macrorrhiza) and Giant Swamp Taro (Cyrtosperma merkusii) in Hawai‘i
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Varieties
- Alocasia macrorrhiza (L.) G. Don
- Cyrtosperma merkusii (Hassk.) Schott
2.2. Experimental Design, Growth Trials, and Agronomic Practices
2.3. Weather
2.4. Growth
2.5. The Total Yields of the Varieties
2.6. Statistical Analysis
3. Results
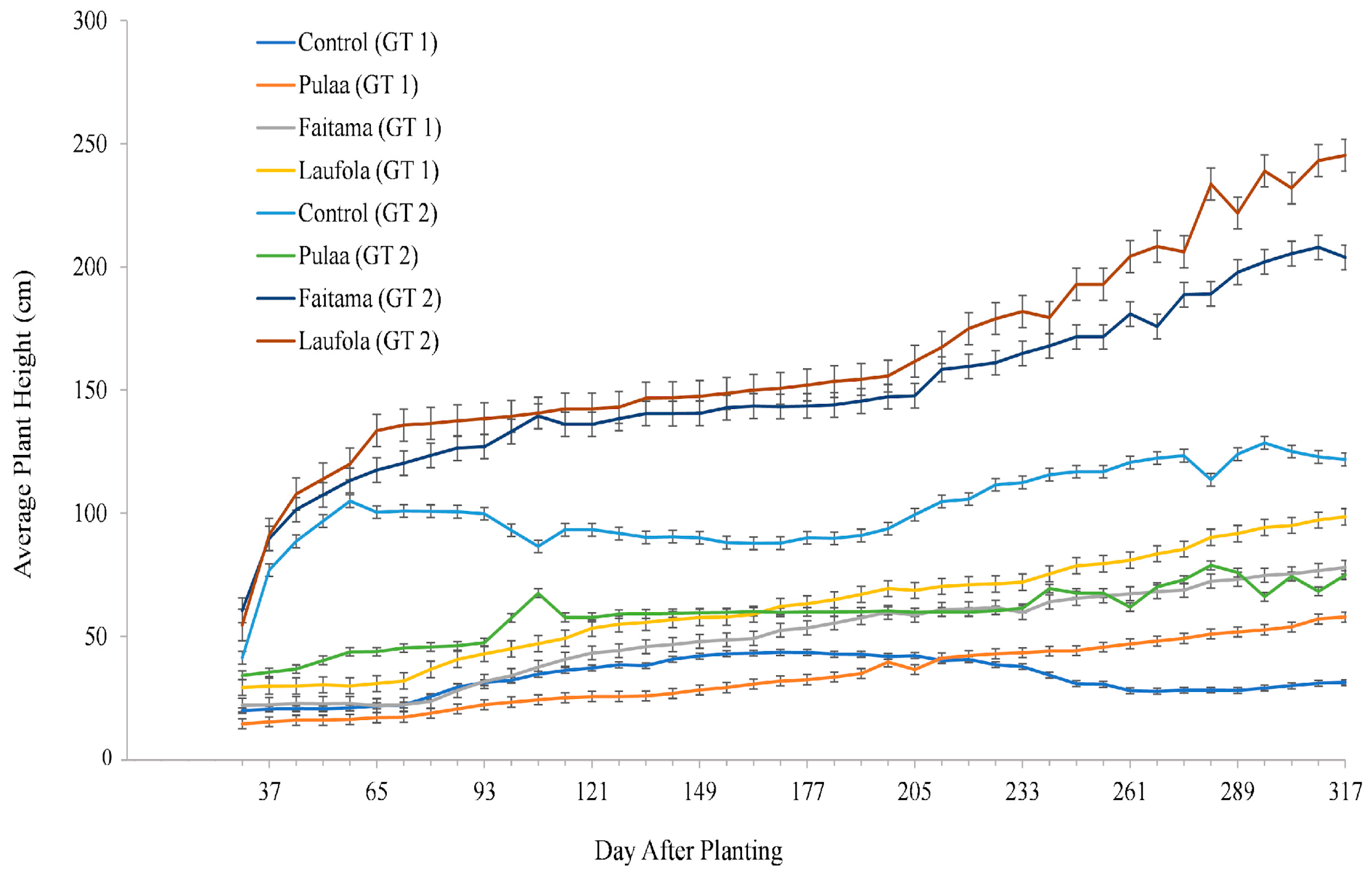
3.1. Average Plant Height
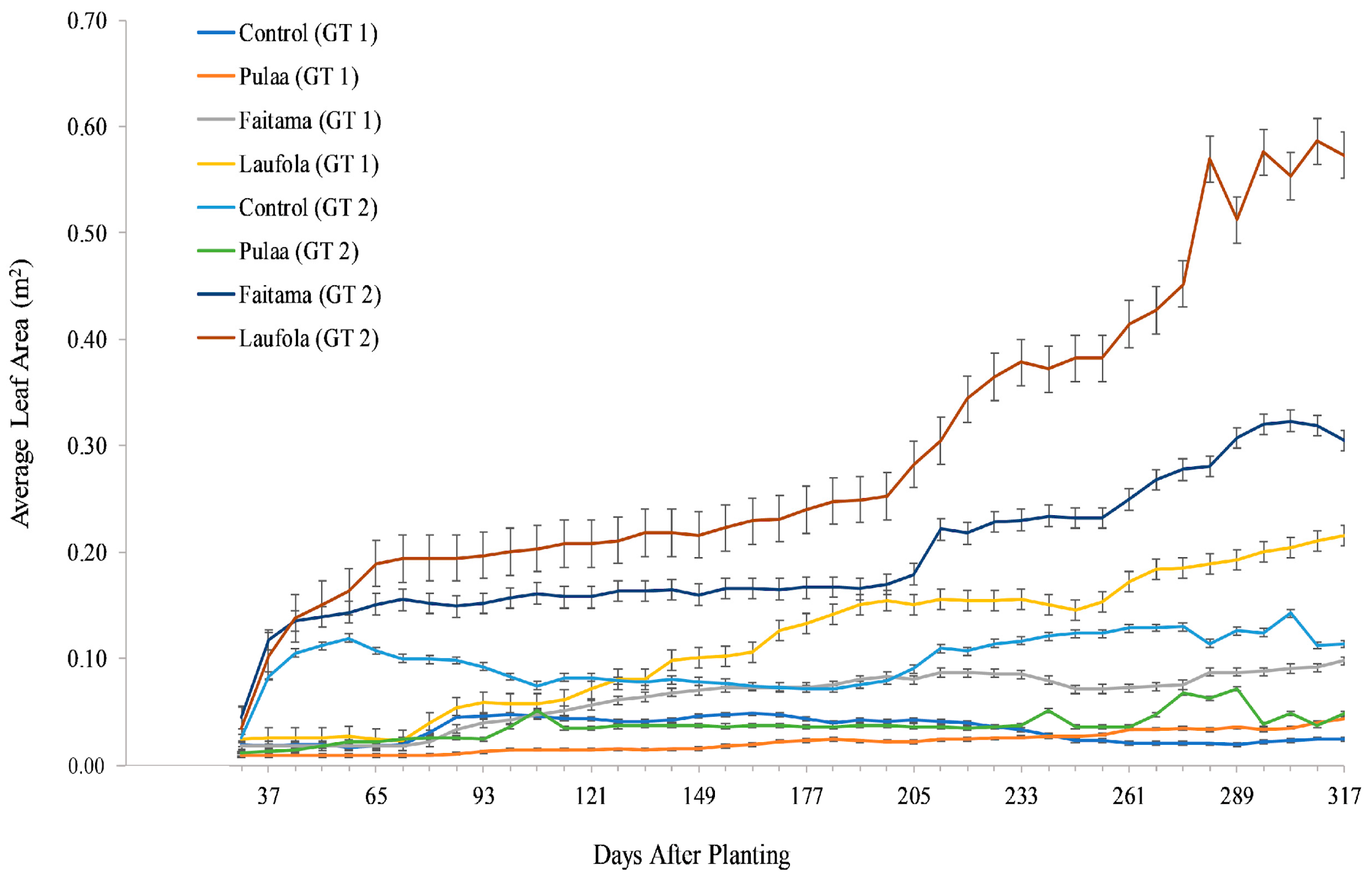
3.2. Average Leaf Area
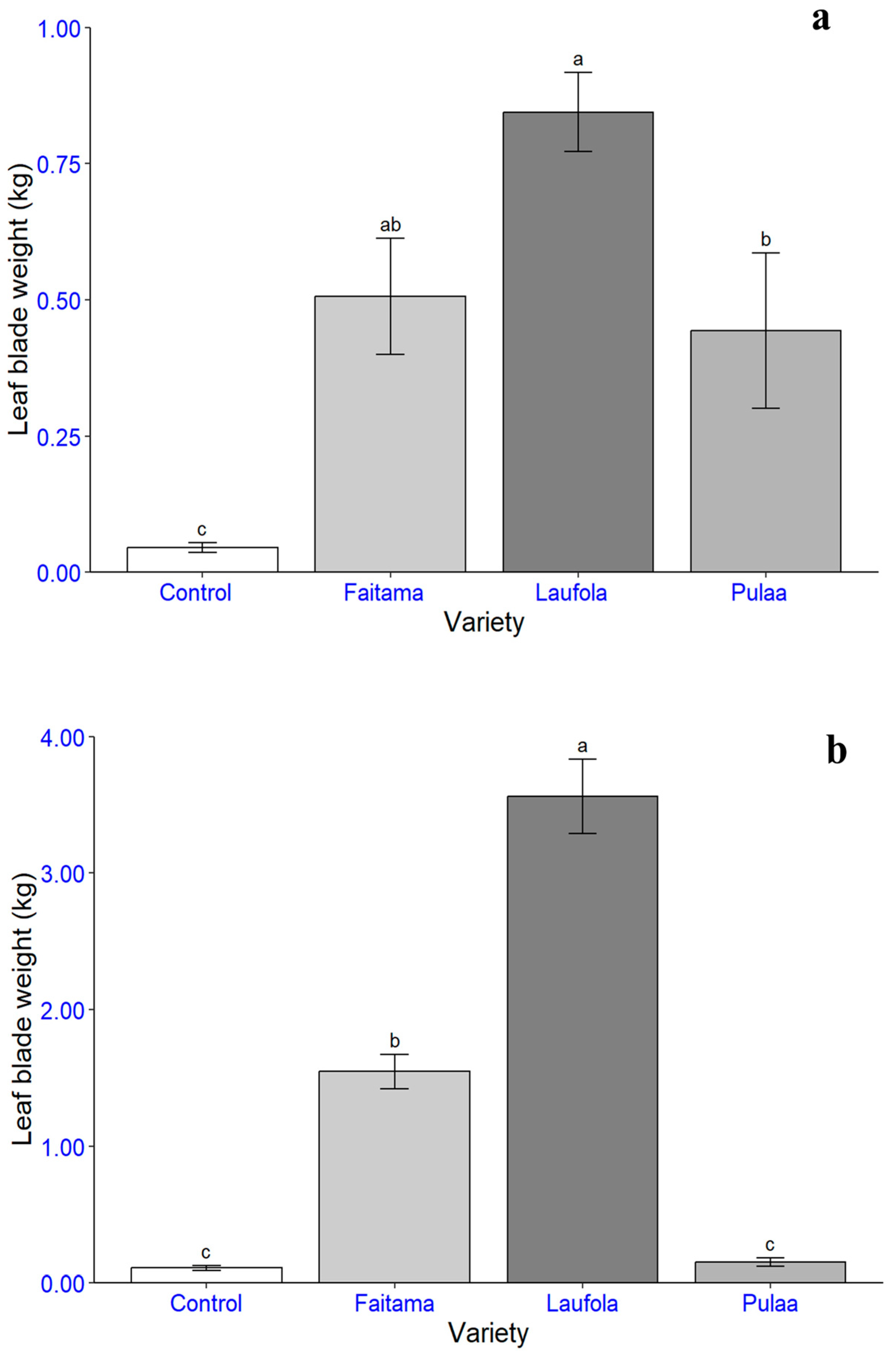
3.3. Statistical Analysis of Yields
3.4. The Total Yields of the Varieties
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishimura, C.H. Feed for Hawaii’s Livestock Industry: Some Problems and Prospects; Report No. 3; Legislative Reference Bureau: Honolulu, HI, USA, 1975; pp. 6, 37, 64–66, 77. Available online: https://library.lrb.hawaii.gov/cgi-bin/koha/opac-retrieve-file.pl?id=3293fb9391f30ab52d29424cafaef8d1 (accessed on 27 July 2021).
- Fujii-Oride, N. The Need for Feed. Hawai’i Business Magazine, 14 November 2016. Available online: https://www.hawaiibusiness.com/the-need-for-feed/ (accessed on 22 May 2021).
- Hugh, W.I.; Nolan, J.C., Jr.; Fox, L.K. The Livestock Industry in Hawaii. 1985, pp. 4, 8. Available online: http://hdl.handle.net/10125/58787 (accessed on 15 September 2021).
- Cox, L.J.; Bredhoff, S. The Hawaii Beef Industry: Situation and Outlook Update; LM-8; University of Hawaii at Manoa, College of Tropical Agriculture and Human Resources, Publication Livestock Management: Honolulu, HI, USA, 2003; Available online: http://hdl.handle.net/10125/12346 (accessed on 28 August 2021).
- Asem-Hiablie, S.; Rotz, C.A.; Sandlin, J.D.; M’Randa, R.S.; Stout, R.C. Management characteristics of beef cattle production in Hawaii. Prof. Anim. Sci. 2018, 34, 167–176. [Google Scholar] [CrossRef]
- Younge, O.R.; Ripperton, J.C. Nitrogen Fertilization of Pasture and Forage Grasses in Hawaii; Hawaii Agricultural Experiment Station, College of Tropical Agriculture, University of Hawaii: Hawaii, HI, USA, 1960. [Google Scholar]
- Whiteman, P.C.; Norton, B.W. Alternative uses for pigeonpea. In Proceedings of the International Workshop on Pigeonpeas, ICRISAT Center, Patancheru, India, 15–19 December 1980; Nene, Y.L., Kumble, V., Eds.; International Crops Research Institute for the Semi-Arid Tropics: Patancheruvu, India, 1981; Volume 1, pp. 365–377. Available online: http://oar.icrisat.org/id/eprint/1956 (accessed on 4 September 2023).
- DuPonte, M.W.; Cowell, K.; Jha, R. Banana silage: An alternative feed for swine. Livest. Manag. 2016, 31, 1–3, 6. Available online: http://www.ctahr.hawaii.edu/oc/freepubs/pdf/LM-31.pdf (accessed on 20 September 2023).
- Stevens, L.; DuPonte, M.W.; Jha, R. Nutritional Value of Agricultural By-Products of the Hawaiian Islands to Be Used as Animal Feeds. Livest. Manag. 2019, 34. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/LM-34.pdf (accessed on 20 September 2023).
- Tiwari, U.P.; Jha, R. Nutrient profile and in vitro digestibility of fresh and ensiled cassava in swine. In Proceedings of the 3rd International Seminar on Animal Industry, Bogor, Indonesia, 17–18 September 2015; pp. 252–253. Available online: https://www.researchgate.net/publication/282442822_Nutrient_profile_and_in_vitro_digestibility_of_fresh_and_ensiled_cassava_in_swine (accessed on 20 September 2023).
- Tiwari, U.P.; Jha, R. Nutrient profile and digestibility of tubers and agro-industrial coproducts determined using an in vitro model of swine. Anim. Nutr. 2016, 2, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Adrian, J.A.L.; Arancon, N.Q.; Mathews, B.W.; Carpenter, J.R. Mineral composition and soil-plant relationships for common guava (Psidium guajava L.) and yellow strawberry guava (Psidium cattleianum var. Lucidum) tree parts and fruits. Commun. Soil Sci. Plant Anal. 2015, 46, 1960–1979. [Google Scholar] [CrossRef]
- Neall, V.E. Volcanic soils. In Land Use, Land Cover and Soil Sciences: Soils and Soil Sciences–2; Verheye, W.H., Ed.; EOLSS Publ.: Oxford, UK, 2009; Volume VII, pp. 26–36. ISBN 9781848262416. [Google Scholar]
- Englberger, L.; Schierle, J.; Kraemer, K.; Aalbersberg, W.; Dolodolotawake, U.; Humphries, J.; Graham, R.; Reid, A.P.; Lorens, A.; Albert, K.; et al. Carotenoid and mineral content of Micronesian giant swamp taro (Cyrtosperma) cultivars. J. Food Compos. Anal. 2008, 21, 93–106. [Google Scholar] [CrossRef]
- Manner, H.I. Farm and forestry production and marketing profile for giant taro (Alocasia macrorrhiza). In Specialty Crops for Pacific Island Agroforestry; Elevitch, C.R., Ed.; Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2011; Available online: https://www.agroforestry.org/images/pdfs/Giant_taro_specialty_crop.pdf (accessed on 2 March 2021).
- Manner, H.I. Farm and forestry production and marketing profile for Giant Swamp Taro (Cyrtosperma chamissonis). In Specialty Crops for Pacific Island Agroforestry; Elevitch, C.R., Ed.; Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2011; Available online: https://www.agroforestry.org/images/pdfs/Giant_swamp_taro_specialty_crop.pdf (accessed on 10 March 2021).
- Foliaki, S.; Sakai, W.S.; Tongatule, S.T.; Tungata, U.; Ka’ipo, R.; Furutani, S.C.; Tsang, M.; Nielson, G.; Short, R. Potential for production of Alocasia, giant taro, on the Hamakua coast of the island of Hawai’i. In Proceedings of the Taking Taro into the 1990s: A Taro Conference, Hilo, HI, USA, 17 August 1989; Hollyer, J.R., Sato, D.M., Eds.; University of Hawaii: Honolulu, HI, USA, 1990; pp. 37–45. Available online: http://hdl.handle.net/10125/3121 (accessed on 28 August 2018).
- Sakai, W.S. Aroid Root Crops: Alocasia, Cyrtosperma, and Amorphophallus. In Handbook of Tropical Foods; Chan, H.T., Ed.; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland, 1983; pp. 29–53. ISBN 0-8247-1880-1. [Google Scholar]
- Rashid, M.M.; Daunicht, H.J. Chemical composition of nine edible aroid cultivars of Bangladesh. Sci. Hortic. 1979, 10, 127–134. [Google Scholar] [CrossRef]
- Plucknett, D.L. Giant swamp taro, a little known Asian Pacific food crop. In Proceedings of the Fourth Symposium of the International Society for Tropical Root Crops, CIAT, Cali, Colombia, 1–7 August 1976; Cock, J., MacIntyre, R., Graham, M., Eds.; IDRC: Ottawa, ON, Canada, 1977; pp. 36–40. [Google Scholar]
- Annual Rainfall, INTERACTIVE MAP [Rainfall by Month—Location 19.653° N, 155.049° W]. Evaportranspitaion of Hawaii, Geography Department–University of Hawai‘i at Mānoa. 2014. Available online: http://evapotranspiration.geography.hawaii.edu/interactivemap.html (accessed on 5 April 2021).
- Climate of Hawai‘i. Honolulu, HI, Weather Forcase Office. National Weather Service, National Oceanic and Atmospheric Administration. Available online: https://www.weather.gov/hfo/climate_summary (accessed on 29 March 2021).
- Giambelluca, T.W.; Shuai, X.; Barnes, M.L.; Alliss, R.J.; Longman, R.J.; Miura, T.; Chen, Q.; Frazier, A.G.; Mudd, R.G.; Cuo, L.; et al. Evapotranspiration of Hawai‘I; Final Report Submitted to the US Army Corps of Engineers—Honolulu District, and the Commission Water Resource Management, State of Hawaii: Hawaii, HI, USA, 2014; pp. 89–99. [Google Scholar]
- Barrau, J. Subsistence Agriculture in Polynesia and Micronesia. In Technical Bulletin of Bernice P. Bishop Museum No. 223; Bishop Museum Press: Hawaii, HI, USA, 1961; Volume 223, pp. 43–67. [Google Scholar]
- Migvar, L. How to Grow Taros, Yams, Cassava and Sweet Potatoes. In Agriculture Extension Bulletin No. 7; Literature Production Center: Saipan, USA, 1968; pp. 7–8. [Google Scholar]
- Englberger, L.; Levendusky, A.; Albert, K.; Johnson, E.; Paul, Y. Report on the Second Visit to the Mwoakilloa Atoll, Pohnpei, Federated States of Micronesia. October 30–November 7, 2004; An Island Food Community of Pohnpei Project in Conjunction with Pohnpei Agriculture of the Office of Economic Affairs and Pohnpei State Department of Health: Kolonia, Pohnepei, 2004; pp. 7, 16. [Google Scholar]
- Levendusky, A.; Englberger, L.; Teelander, R.; Mwarluck, O.; Seilo, H.; Sorryz, R.; Relech, S.; Salzman, J.; Mony, M.; Gustafson, A. Documentation of Mortlockese Giant Swamp Taro Cultivars and Other Local Foods on Ta, Moch, and Satowan; Island Food Community of Pohnpei: Pohnpei, Micronesia, 2006. [Google Scholar]
- Srivastava, S.K. Fertilizer requirement of alti (Alocasia indica). Indian Agric. 1972, 16, 105–106. [Google Scholar]
- Climate Data Online: Dataset Discovery [Daily Summaries from Station GHCND:USW00021515]. National Oceanic and Atmospheric Administration (NOAA). National Centers for Environmental Information. Available online: https://www.ncdc.noaa.gov/cdo-web/datasets#GHCN (accessed on 29 April 2021).
- Lewu, M.N.; Mulidzi, A.R.; Gerrano, A.S.; Adebola, P.O. Comparative growth and yield of taro (Colocasia esculenta) accessions cultivated in the Western Cape, South Africa. Int. J. Agric. Biol. 2017, 19, 589–594. [Google Scholar] [CrossRef]
- Montgomery, E.G. Correlation Studies in Corn; Annual Report 24; Nebraska Agriculture Experimental Stations: Lincoln, NE, USA, 1911; pp. 108–159. [Google Scholar]
- Paul, K.K.; Bari, M.A.; Debnath, S.C. Correlation and path coefficient analysis in Giant Taro (Alocasia macrorrhiza L.). Bangladesh J. Sci. Ind. Res. 2015, 50, 117–122. [Google Scholar] [CrossRef]
- SAS; Version 9.2; SAS Institute Inc.: Cary, NC, USA, 2010.
- RStudio Team, RStudio: Integrated Development for R; Version 2019; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 2 February 2020).
- Kularatna, T.D. Alocasia macrorrhiza, Colocasia esculenta, and Cyrtosperma growth and yield trials in Hilo, Hawaii. Developing Sustainable Tropical Crops for Marginal Lands (Data set of Plant Height, Leaf Area, Stem Yields, Petiole Yields, Leaf Blade Yields of the varieties). National Agricultural Library. United State Department of Agriculture. 2020; unpublished work. [Google Scholar]
- Abd El-Aal, M.M.M.; El-Anany, A.M.A.; Rizk, S.M. Rationalization of water consumption for taro plant through the rationing of irrigation and expand the plant ability to resist stress conditions. Int. J. Plant Soil Sci. 2019, 29, 1–6, 13, 18. [Google Scholar] [CrossRef]
- Wang, Y.; Janz, B.; Engedal, T.; De Neergaard, A. Effect of irrigation regimes and nitrogen rates on water use efficiency and nitrogen uptake in maize. Agric. Water Manag. 2017, 179, 271–276. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.B.; Park, H.J.; Cheon, C.G.; Choi, J.G.; Seo, J.H.; Im, J.S.; Park, Y.E.; Cho, J.H.; Chang, D.C. Effect of Plant Container Type on Seed Potato (Solanum tuberosum L.) Growth and Yield in Substrate Culture. Potato Res. 2021, 65, 105–117. [Google Scholar] [CrossRef]
- Nelson, S.; Brooks, F.; Teves, G. Taro leaf blight in Hawaii. In Plant Disease Bulletin; No. PD-71; University of Hawaii: Manoa, HI, USA, 2011; Available online: http://hdl.handle.net/10125/33293 (accessed on 13 May 2019).
- Harrington, C.A.; Radwan, M.A.; DeBell, D.S. Leaf characteristics reflect growth rates of 2-year-old Populus trees. Can. J. For. Res. 1997, 27, 1321–1325. [Google Scholar] [CrossRef]
- Rao, V.R.; Matthews, P.J.; Eyzaguirre, P.B.; Hunter, D. The Global Diversity of Taro: Ethnobotany and Conservation; Bioversity International: Rome, Italy, 2010; Available online: http://hdl.handle.net/10502/4766 (accessed on 10 August 2021).


| Month | Temperature/Month (°C) | Precipitation/Month (mm) | ||
|---|---|---|---|---|
| Max | Min | Average | ||
| November 2018 | 25.83 | 18.11 | 21.97 | 349.20 |
| December 2018 | 24.42 | 17.47 | 20.94 | 306.80 |
| January 2019 | 24.72 | 15.85 | 20.29 | 48.30 |
| February 2019 | 24.14 | 15.38 | 19.76 | 321.20 |
| March 2019 | 23.37 | 15.69 | 19.53 | 150.00 |
| April 2019 | 24.98 | 17.57 | 21.28 | 413.00 |
| May 2019 | 26.73 | 18.20 | 22.47 | 103.50 |
| June 2019 | 26.92 | 19.02 | 22.97 | 146.00 |
| July 2019 | 27.45 | 19.44 | 23.45 | 333.90 |
| August 2019 | 28.08 | 20.26 | 24.17 | 279.50 |
| September 2019 | 27.89 | 20.06 | 23.97 | 242.80 |
| October 2019 | 27.22 | 19.20 | 23.21 | 307.10 |
| November 2019 | 27.06 | 18.49 | 22.78 | 284.40 |
| December 2019 | 25.00 | 18.12 | 21.56 | 303.40 |
| January 2020 | 24.38 | 17.31 | 20.85 | 687.70 |
| February 2020 | 24.40 | 15.85 | 20.13 | 192.00 |
| March 2020 | 23.44 | 17.06 | 20.25 | 704.10 |
| April 2020 | 25.56 | 17.35 | 21.46 | 253.10 |
| May 2020 | 25.69 | 17.87 | 21.78 | 147.90 |
| June 2020 | 26.24 | 18.50 | 22.37 | 106.70 |
| July 2020 | 26.79 | 19.41 | 23.10 | 163.00 |
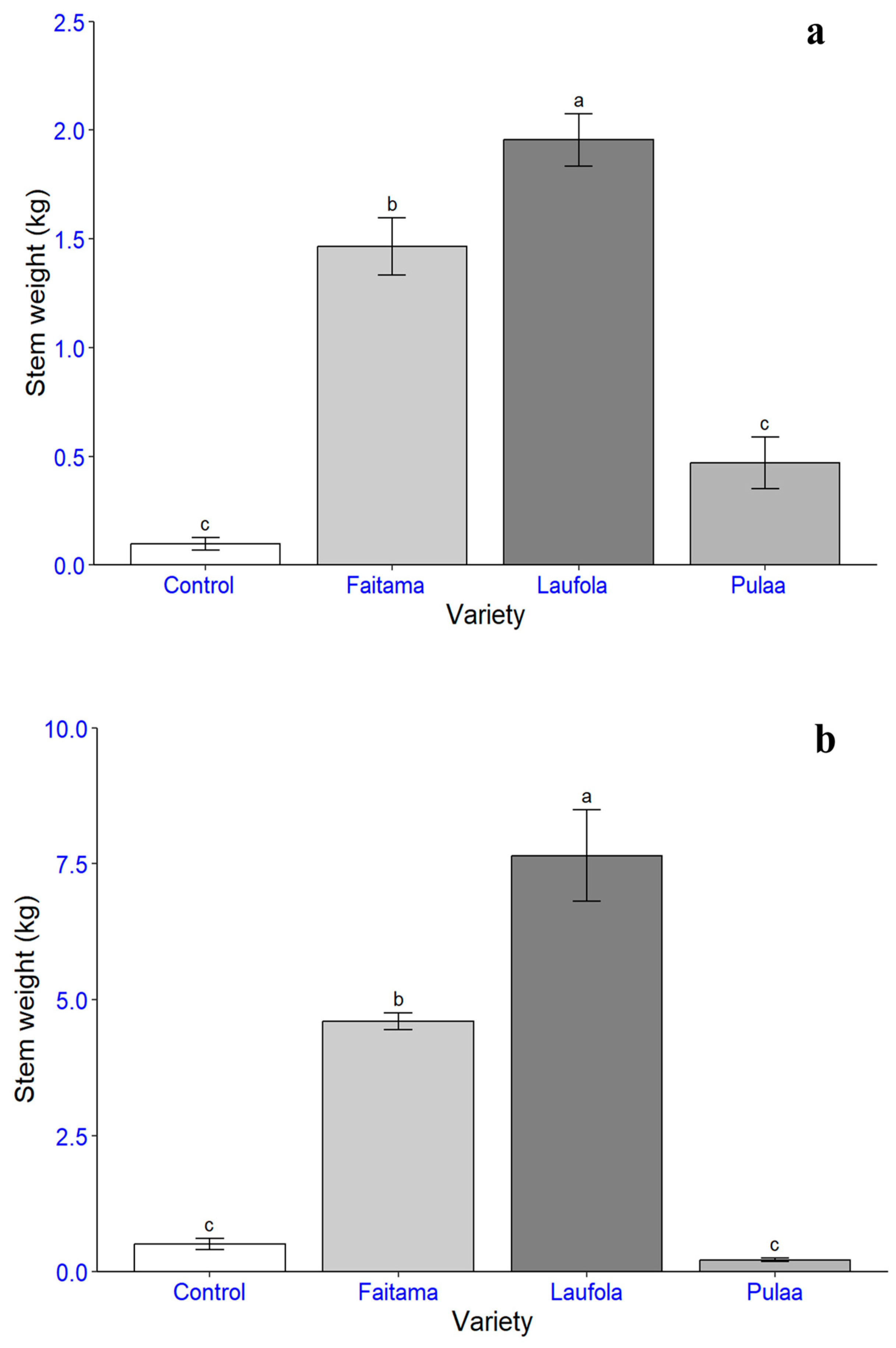
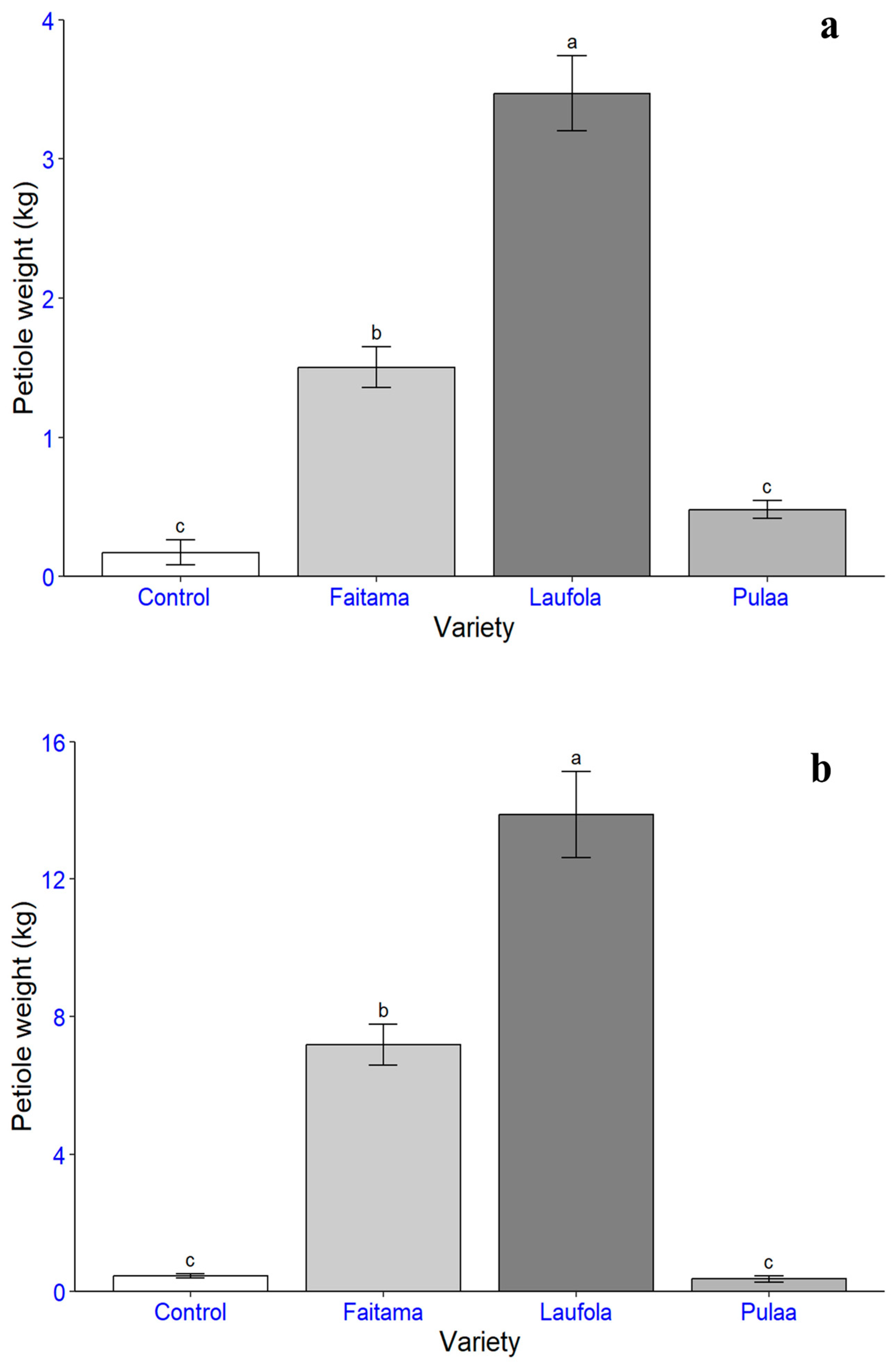
| Variety | Average Weight (kg/ha) | Total Weight (kg/ha) | |||
|---|---|---|---|---|---|
| Stem | Petiole | Leaf Blade | |||
| Trial 1 | |||||
| Laufola | 14,033.9 a | 24,908.5 a | 6061.2 a | 45,003.6 | |
| Faitama | 10,507.4 b | 10,796.5 b | 3635.4 ab | 24,939.3 | |
| Pula’a | 3370.0 c | 3462.1 c | 3180.4 b | 10,012.5 | |
| Control | 702.8 c | 1240.5 c | 327.4 c | 2270.8 | |
| Trial 2 | |||||
| Laufola | 54,895.9 a | 99,646.9 a | 25,563.3 a | 180,106.2 | |
| Faitama | 33,022.8 b | 51,527.7 b | 11,098.6 b | 95,649.1 | |
| Control | 3638.4 c | 3316.5 c | 774.3 c | 7729.2 | |
| Pula’a | 1555.7 c | 2683.8 c | 1096.8 c | 5336.4 | |
| Characters | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| F1 | 0.85 *** | 0.93 *** | 0.67 *** | −0.35 ** | 0.93 *** | 0.88 *** | 0.33 ** | |
| F2 | 0.96 *** | 0.68 *** | −0.37 ** | 0.96 *** | 0.88 *** | 0.28 * | ||
| F3 | 0.70 ** | −0.42 *** | 0.98 *** | 0.88 *** | 0.21 | |||
| F4 | −0.40 ** | 0.72 *** | 0.71 *** | 0.38 ** | ||||
| F5 | −0.41 *** | −0.34 ** | 0.28 * | |||||
| F6 | 0.92 *** | 0.27 * | ||||||
| F7 | 0.49 *** | |||||||
| F8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kularatna, T.D.; Arancon, N.Q.; Eiben, J.A. Comparative Growth of Elephant Ear Taro (Alocasia macrorrhiza) and Giant Swamp Taro (Cyrtosperma merkusii) in Hawai‘i. Crops 2024, 4, 55-71. https://doi.org/10.3390/crops4010005
Kularatna TD, Arancon NQ, Eiben JA. Comparative Growth of Elephant Ear Taro (Alocasia macrorrhiza) and Giant Swamp Taro (Cyrtosperma merkusii) in Hawai‘i. Crops. 2024; 4(1):55-71. https://doi.org/10.3390/crops4010005
Chicago/Turabian StyleKularatna, Thathmini D., Norman Q. Arancon, and Jesse A. Eiben. 2024. "Comparative Growth of Elephant Ear Taro (Alocasia macrorrhiza) and Giant Swamp Taro (Cyrtosperma merkusii) in Hawai‘i" Crops 4, no. 1: 55-71. https://doi.org/10.3390/crops4010005
APA StyleKularatna, T. D., Arancon, N. Q., & Eiben, J. A. (2024). Comparative Growth of Elephant Ear Taro (Alocasia macrorrhiza) and Giant Swamp Taro (Cyrtosperma merkusii) in Hawai‘i. Crops, 4(1), 55-71. https://doi.org/10.3390/crops4010005





