Halo Blight of Mungbean in Australia
Abstract
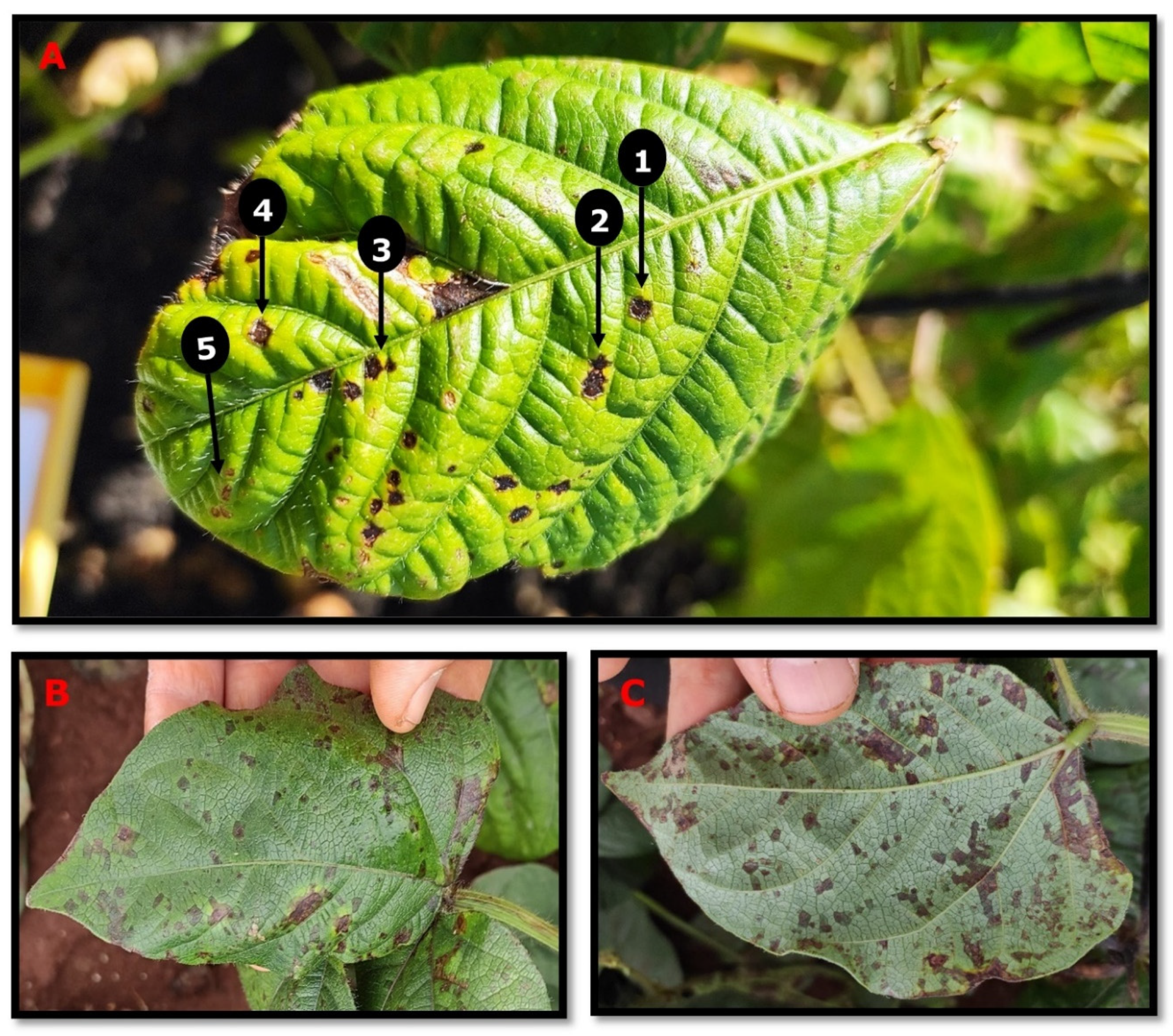
:1. The Pathogen and Disease Symptoms
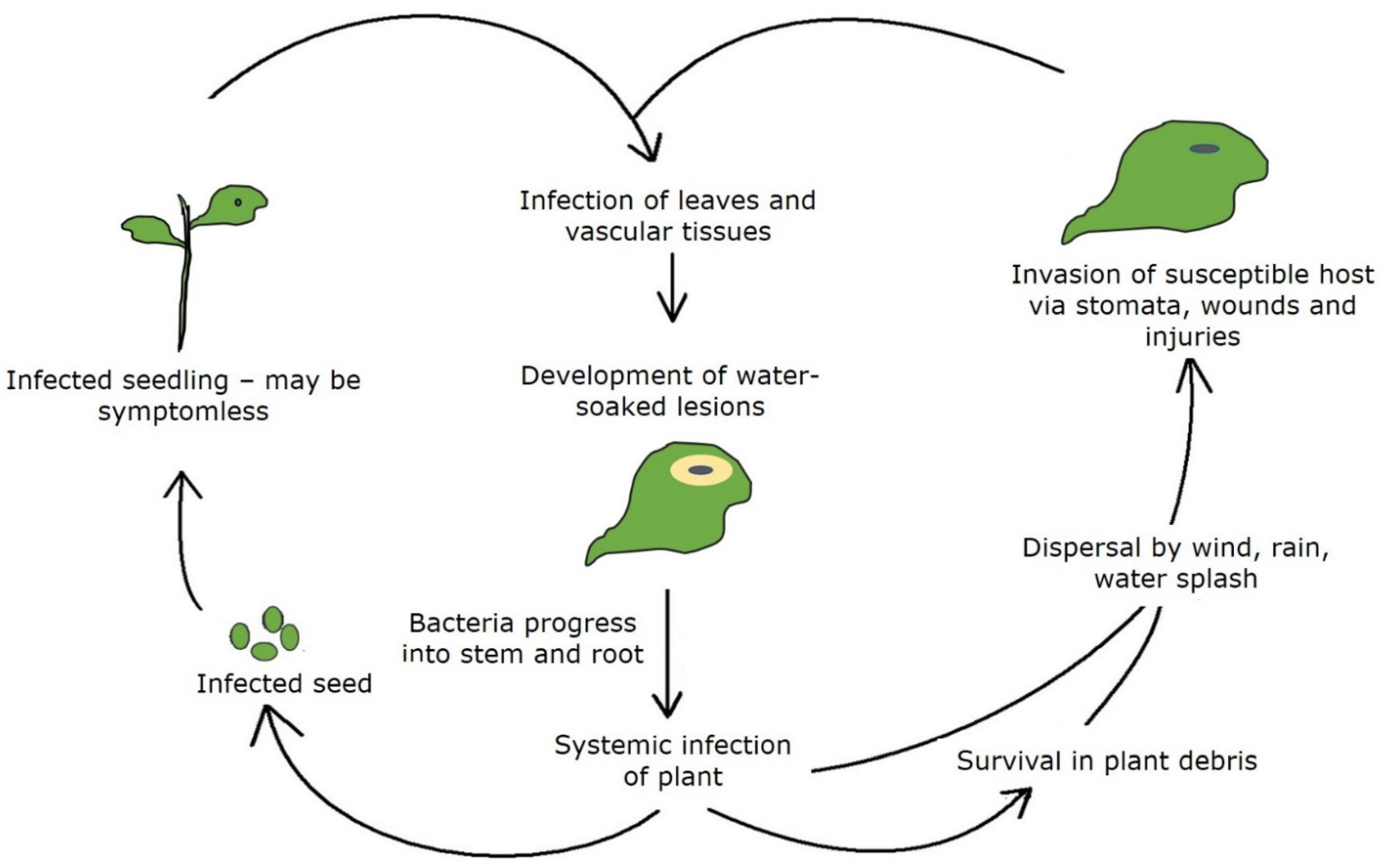
2. Pathogen Survival and Disease Spread
3. Management of Halo Blight
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noble, T.J.; Young, A.J.; Kelly, L.A.; Barrerro, R.A.; Douglas, C.A.; Long, H.; Williams, B.; Mundree, S. Characterisation of the Pseudomonas savastanoi pv. phaseolicola population found in Eastern Australia associated with halo blight disease in Vigna radiata. Australas. Plant Pathol. 2020, 49, 515–524. [Google Scholar] [CrossRef]
- Marques, A.S.d.A.; Samson, R. Population dynamics of Pseudomonas savastanoi pv. phaseolicola in bean, throughout the epiphytic and pathogenic phases. Pesqui. Agropecuária Bras. 2016, 51, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Noble, T.J.; Young, A.J.; Douglas, C.A.; Williams, B.; Mundree, S. Diagnosis and management of halo blight in Australian mungbeans: A review. Crop. Pasture Sci. 2019, 70, 195–203. [Google Scholar] [CrossRef]
- Allen, S.; Kochman, J. Eliminating seed-borne inoculum of Fusarium oxysporum f. sp. vasinfectum in cotton. In Proceedings of the Beltwide Cotton Conference, Australian Cotton Cooperative Research Centre, Narrabri, Australia, 9–13 January 2001; pp. 139–140. [Google Scholar]
- Bashan, Y. Mechanisms of symptom production by foliar bacterial pathogens. Phytoparasitica 1987, 15, 197–223. [Google Scholar] [CrossRef]
- Ryley, M.; Douglas, C.; Ryan, M.; Tatnell, J.; Martin, W.; King, K.; Keller, L. Integrated management of foliar pathogens of mungbean in Australia. In Proceedings of the Australian Summer Grains Conference, Gold Coast, Queensland, Australia, 21 June 2010; pp. 1–9. [Google Scholar]
- Taylor, J. The quantitative estimation of the infection of bean seed with Pseudomonas phaseolicola (Burkh.) Dowson. Ann. Appl. Biol. 1970, 66, 29–36. [Google Scholar] [CrossRef]
- Rico, A.; Lopez, R.; Asensio, C.; Aizpun, M.T.; Asensio-S-Manzanera, M.C.; Murillo, J. Nontoxigenic strains of Pseudomonas syringae pv. phaseolicola are a main cause of halo blight of beans in Spain and escape current detection methods. Phytopathology 2003, 93, 1553–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowicz, B.; Maćkowiak, A.; Pospieszny, H. Improved identification of Pseudomonas savastanoi pv. phaseolicola at the molecular level. EPPO Bull. 2002, 32, 467–469. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, A.S.; Douglas, C. Halo Blight of Mungbean in Australia. Crops 2021, 1, 3-7. https://doi.org/10.3390/crops1010002
Abdullah AS, Douglas C. Halo Blight of Mungbean in Australia. Crops. 2021; 1(1):3-7. https://doi.org/10.3390/crops1010002
Chicago/Turabian StyleAbdullah, Araz Sedqi, and Col Douglas. 2021. "Halo Blight of Mungbean in Australia" Crops 1, no. 1: 3-7. https://doi.org/10.3390/crops1010002
APA StyleAbdullah, A. S., & Douglas, C. (2021). Halo Blight of Mungbean in Australia. Crops, 1(1), 3-7. https://doi.org/10.3390/crops1010002




