The Role of the Bone Marrow Microenvironment in Physical Function and Quality of Life in Patients with Multiple Myeloma After First-Line Treatment with Novel Agents and Autologous Transplantation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. 6 Min Walking Test
2.4. Handgrip Test
2.5. Isometric Strength
2.6. Maximal Aerobic Power
2.7. Body Composition
2.8. Health-Related Quality of Life
2.9. Blood Analysis
2.10. Flow Cytometry
2.11. Statistical Analysis
3. Results
3.1. Physical Function
3.2. Body Composition
3.3. Health-Related Quality of Life
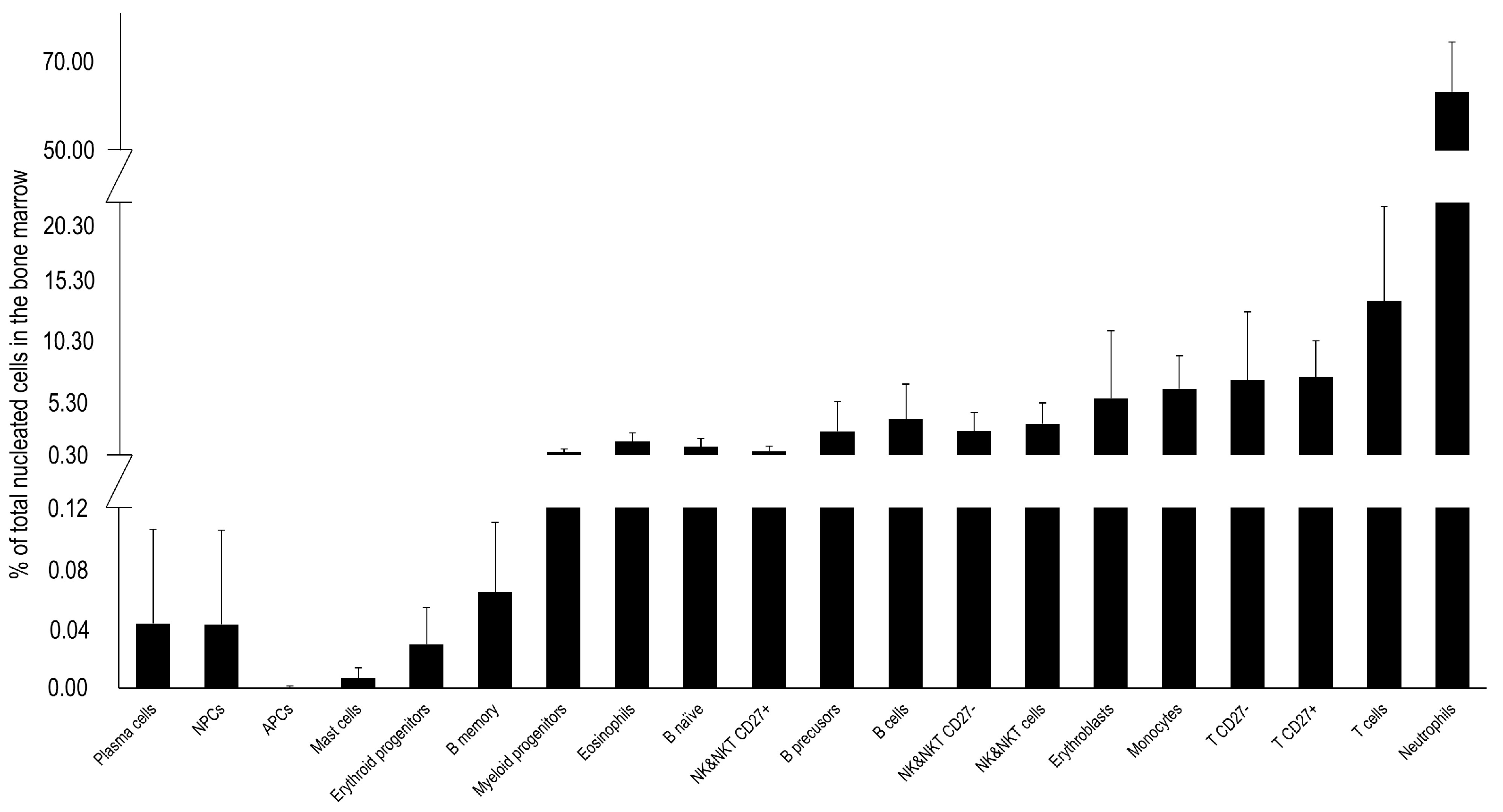
3.4. Bone Marrow Immune Cells
3.5. Peripheral Blood Cells
3.6. Correlations Between Bone Marrow Immune Cells and Physical Function
3.7. Correlations Between Bone Marrow Immune Cells and Health-Related Quality of Life
3.8. Correlations Between Bone Marrow Immune Cells and Body Composition
3.9. Correlations Among Peripheral Blood Cells, Physical Function, Health-Related Quality of Life, and Body Composition
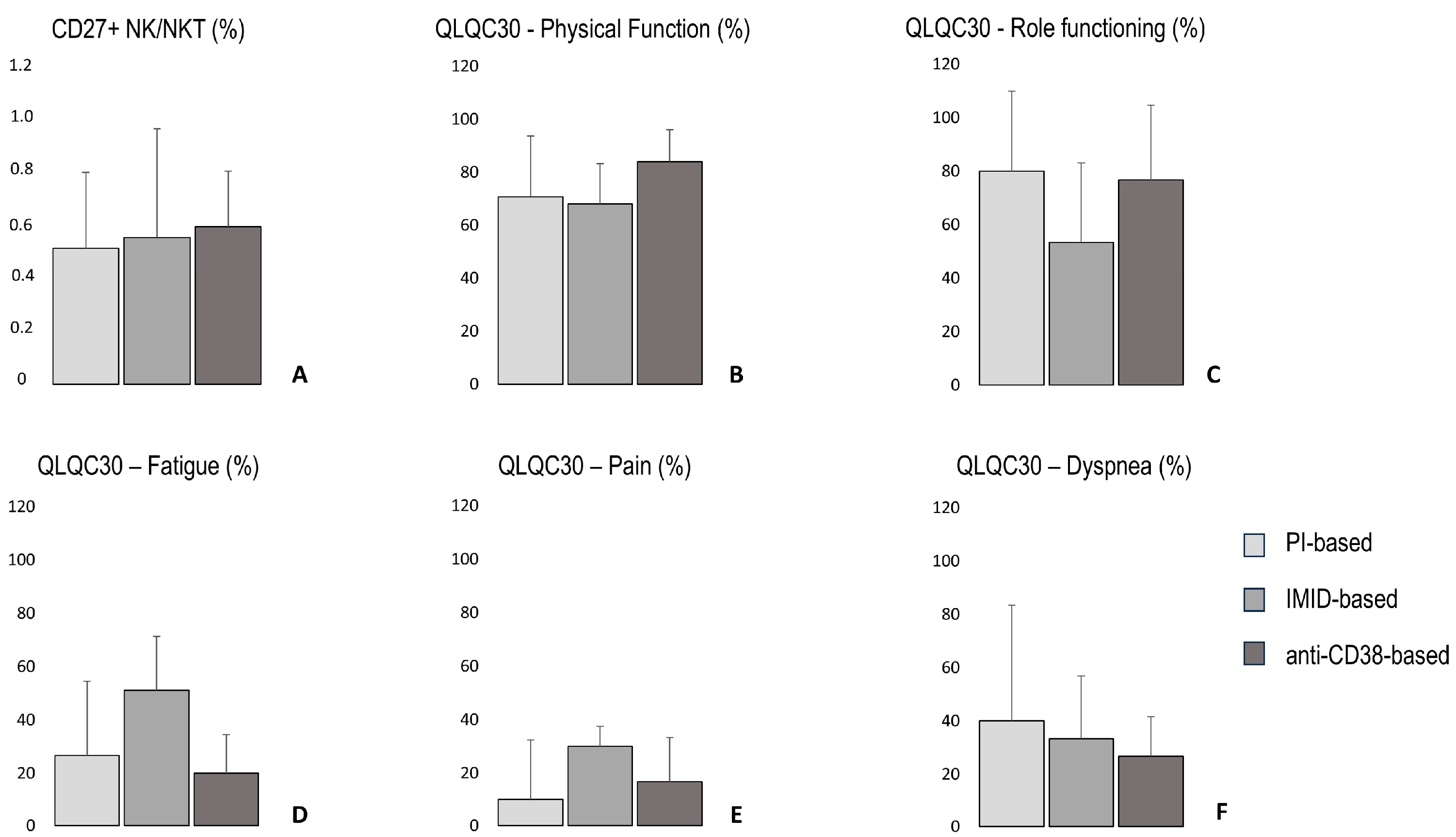
3.10. The Percentage of CD27+ NK/NKT Cells, Quality of Life Parameters, 6 Min Walking Distance, Total Handgrip Strength, Lean Body Mass, and Bone Density According to Treatment-Based Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Rajkumar, S.V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 20, 17046. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma: Diagnosis and treatment. Mayo Clin. Proc. 2016, 91, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Dadzie, T.G.; Green, A.C. The role of the bone microenvironment in regulating myeloma residual disease and treatment. Front. Oncol. 2022, 12, 999939. [Google Scholar] [CrossRef]
- Paiva, B.; Cedena, M.T.; Puig, N.; Arana, P.; Vidriales, M.B.; Cordon, L.; San Miguel, J.F. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood 2016, 127, 3165–3174. [Google Scholar] [CrossRef]
- Desantis, V.; Savino, F.D.; Scaringella, A.; Potenza, M.A.; Nacci, C.; Frassanito, M.A.; Vacca, A.; Montagnani, M. The Leading Role of the Immune Microenvironment in Multiple Myeloma: A New Target with a Great Prognostic and Clinical Value. J. Clin. Med. 2022, 11, 2513. [Google Scholar] [CrossRef]
- Raitakari, M.; Brown, R.D.; Gibson, J.; Joshua, D.E. T cells in myeloma. Hematol. Oncol. 2003, 21, 33–42. [Google Scholar] [CrossRef]
- Lopes, R.; Caetano, J.; Ferreira, B.; Barahona, F.; Carneiro, E.A.; João, C. The Immune Microenvironment in Multiple Myeloma: Friend or Foe? Cancers 2021, 13, 625. [Google Scholar] [CrossRef]
- Ponzetta, A.; Benigni, G.; Antonangeli, F.; Sciume, G.; Sanseviero, E.; Zingoni, A.; Bernardini, G. Multiple myeloma impairs bone marrow localization of effector natural killer cells by altering the chemokine microenvironment. Cancer Res. 2015, 75, 4766–4777. [Google Scholar] [CrossRef]
- Pessoa de Magalhães, R.J.; Vidriales, M.B.; Paiva, B.; Fernandez-Gimenez, C.; García-Sanz, R.; Mateos, M.V.; Gutierrez, N.C.; Lecrevisse, Q.; Blanco, J.F.; Hernández, J.; et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica 2013, 98, 79–86. [Google Scholar] [CrossRef]
- Jensen, C.E.; Vohra, S.N.; Nyrop, K.A.; Deal, A.M.; LeBlanc, M.R.; Grant, S.J.; Muss, H.B.; Lichtman, E.I.; Rubinstein, S.M.; Wood, W.A.; et al. Physical Function, Psychosocial Status, and Symptom Burden Among Adults with Plasma Cell Disorders and Associations with Quality of Life. Oncologist 2022, 27, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Gulbrandsen, N.; Hjermstad, M.J.; Wisløff, F.; Nordic Myeloma Study Group. Interpretation of quality of life scores in multiple myeloma by comparison with a reference population and assessment of the clinical importance of score differences. Eur. J. Haematol. 2004, 72, 172–180. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. Laboratory Submaximal Exercise Testing: YMCA Cycle Ergometer Test. In ACSM’s Health-Related Physical Fitness Assessment Manual, 2nd ed.; Wolters Kluwer: Philadelphia, PA, USA, 2008; pp. 112–116. [Google Scholar]
- Flores-Montero, J.; Sanoja- Flores, L.; Paiva, B. Next Generation Flow for Highly Sensitive and Standardized Detection of Minimal Residual Disease in Multiple Myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef]
- Kostopoulos, I.V.; Eleutherakis-Papaiakovou, E.; Rousakis, P.; Ntanasis-Stathopoulos, I.; Panteli, C.; Orologas-Stavrou, N.; Terpos, E. Aberrant plasma cell contamination of peripheral blood stem cell autografts, assessed by next-generation flow cytometry, is a negative predictor for deep response post autologous transplantation in multiple myeloma; a prospective study in 199 patients. Cancers 2021, 13, 4047. [Google Scholar] [CrossRef]
- Terpos, E.; Kostopoulos, I.V.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Migkou, M.; Rousakis, P.; Dimopoulos, M.A. Impact of minimal residual disease detection by next-generation flow cytometry in multiple myeloma patients with sustained complete remission after frontline therapy. HemaSphere 2019, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Obilor, E.I.; Amadi, E.C. Test for significance of Pearson’s correlation coefficient. IJIMSEP 2018, 6, 11–23. [Google Scholar]
- Dhodapkar, M.V.; Richter, J. Harnessing natural killer T (NKT) cells in human myeloma: Progress and challenges. Clin. Immunol. 2011, 140, 160–166. [Google Scholar] [CrossRef]
- Godfrey, J.; Benson, D.M., Jr. The role of natural killer cells in immunity against multiple myeloma. Leuk. Lymphoma 2012, 53, 1666–1676. [Google Scholar] [CrossRef]
- Silva, A.; Andrews, D.M.; Brooks, A.G.; Smyth, M.J.; Hayakawa, Y. Application of CD27 as a marker for distinguishing human NK cell subsets. Int. Immunol. 2008, 20, 625–630. [Google Scholar] [CrossRef]
- Vossen, M.; Matmati, M.; Hertoghs, K.M.; Baars, P.A.; Gent, M.R.; Leclercq, G.; van Lier, R.A. CD27 defines phenotypically and functionally different human NK cell subsets. J. Immunol. 2008, 180, 3739–3745. [Google Scholar] [CrossRef]
- Imanishi, T.; Saito, T. T cell co-stimulation and functional modulation by innate signals. Trends Immunol. 2020, 41, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S.J.; Kumar, P. Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef]
- Proskorovsky, I.; Lewis, P.; Williams, C.D.; Jordan, K.; Kyriakou, C.; Ishak, J.; Davies, F.E. Mapping EORTC QLQ-C30 and QLQ-MY20 to EQ-5D in patients with multiple myeloma. Health Qual. Life Outcomes 2014, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Christoulas, D.; Gavriatopoulou, M.; Dimopoulos, M.A. Mechanisms of bone destruction in multiple myeloma. Eur. J. Cancer Care 2017, 26, e12761. [Google Scholar] [CrossRef]
- Larsen, R.F.; Jarden, M.; Minet, L.R.; Frølund, U.C.; Hermann, A.P.; Breum, L.; Abildgaard, N. Exercise in newly diagnosed patients with multiple myeloma: A randomized controlled trial of effects on physical function, physical activity, lean body mass, bone mineral density, pain, and quality of life. Eur. J. Haematol. 2024, 113, 298–309. [Google Scholar] [CrossRef]
- Pomeroy, E.; Macintosh, A.; Wells, J.C.; Cole, T.J.; Stock, J.T. Relationship between body mass, lean mass, fat mass, and limb bone cross-sectional geometry: Implications for estimating body mass and physique from the skeleton. Am. J. Phys. Anthropol. 2018, 166, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. JCSM 2020, 11, 3–25. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Zamboni, M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Williams, A.; Baruah, D.; Patel, J.; Szabo, A.; Chhabra, S.; Dhakal, B.; D’Souza, A. Prevalence and significance of sarcopenia in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Bone Marrow Transpl. 2021, 56, 225–231. [Google Scholar] [CrossRef]
- Abdallah, N.H.; Nagayama, H.; Takahashi, N.; Gonsalves, W.; Fonder, A.; Dispenzieri, A.; Kumar, S.K. Muscle and fat composition in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2023, 13, 185. [Google Scholar] [CrossRef]
- Larsen, R.F.; Jarden, M.; Minet, L.R.; Frølund, U.C.; Möller, S.; Abildgaard, N. Physical function in patients newly diagnosed with multiple myeloma; a Danish cohort study. BMC Cancer 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, S.A.; Lane, A.; Hornsby, W.E.; Bishop, C.; Thomas, S.; Herndon II, J.E.; Jones, L.W. Quantitative measures of physical functioning after autologous hematopoietic stem cell transplantation in multiple myeloma: A feasibility study. Clin. Lymphoma Myeloma Leuk 2015, 15, 103–109. [Google Scholar] [CrossRef]
- Persoon, S.; Kersten, M.J.; Buffart, L.M.; Vander Slagmolen, G.; Baars, J.W.; Visser, O.; Chinapaw, M.J. Health-related physical fitness in patients with multiple myeloma or lymphoma recently treated with autologous stem cell transplantation. J. Sci. Med. Sport 2017, 20, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, K.; Alén, M.; Kraemer, W.J.; Gorostiaga, E.; Izquierdo, M.; Rusko, H.; Paavolainen, L. Neuromuscular adaptations during concurrent strength and endurance training versus strength training. Eur. J. Appl. Physiol. 2003, 89, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulou, P.; Zaras, N.; Methenitis, S.; Papadimas, G.; Papadopoulos, C.; Bogdanis, G.C.; Terzis, G. Effect of concurrent power training and high-intensity interval cycling on muscle morphology and performance. JSCR 2021, 35, 2464–2471. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Land, J.; Hackshaw, A.; Heinrich, M.; McCourt, O.; Beeken, R.J.; Yong, K.L. Fatigue, quality of life and physical fitness following an exercise intervention in multiple myeloma survivors (MASCOT): An exploratory randomised Phase 2 trial utilising a modified Zelen design. Br. J. Cancer 2020, 123, 187–195. [Google Scholar] [CrossRef]
- Groeneveldt, L.; Mein, G.; Garrod, R.; Jewell, A.P.; Someren, K.V.; Stephens, R.; Yong, K.L. A mixed exercise training programme is feasible and safe and may improve quality of life and muscle strength in multiple myeloma survivors. BMC Cancer 2013, 13, 31. [Google Scholar] [CrossRef]


| Anthropometrical Characteristics (Mean ± SD) | |
|---|---|
| Total mass (kg) | 81.4 ± 16.5 |
| Age (years) | 53.9 ± 7.7 |
| Height (m) | 1.73 ± 0.1 |
| Gender (N) | |
| Total participants | 21 |
| Women | 7 |
| Mem | 11 |
| Treatment (N) | |
| PI | 3 |
| IMID | 8 |
| PI + IMID | 4 |
| anti-CD38 + IMID | 1 |
| anti-CD38 + PI | 2 |
| anti-CD38 + IMID + PI | 3 |
| Treatment-Based Groups (N) | |
| PI—based | 7 |
| IMID—based | 8 |
| Anti-CD38—based | 6 |
| MRD (N) | |
| Total participants | 16 |
| MRD positive | 5 |
| MRD negative | 11 |
| % of Bone Marrow Nucleated Cells | 6MWT | Handgrip RH+LH | Isometric Strength UB | Isometric Strength LB | Maximal Aerobic Power |
|---|---|---|---|---|---|
| Plasma cells | −0.34 | −0.20 | 0.00 | −0.23 | 0.18 |
| NPCs | −0.34 | −0.19 | 0.00 | −0.23 | 0.17 |
| APCs | 0.38 | −0.17 | 0.03 | 0.27 | 0.20 |
| Mast cells | −0.28 | 0.01 | 0.18 | 0.00 | 0.39 |
| Myeloid progenitors | 0.08 | −0.34 | −0.10 | −0.13 | 0.09 |
| B cells | −0.12 | −0.35 | −0.40 | −0.56 | −0.07 |
| B cell precusors | −0.17 | −0.38 | −0.38 | −0.63 | −0.11 |
| Naïve B cells | 0.08 | −0.08 | −0.30 | −0.12 | 0.12 |
| Memory B cells | 0.32 | −0.18 | −0.24 | 0.28 | −0.03 |
| T cells | −0.08 | −0.22 | −0.09 | 0.17 | 0.09 |
| CD27− T cells | −0.08 | −0.26 | −0.15 | 0.12 | 0.08 |
| CD27+ T cells | 0.03 | −0.05 | 0.04 | 0.19 | 0.09 |
| NK/NKT cells | −0.23 | 0.04 | −0.08 | 0.29 | 0.15 |
| CD27− NK/NKT | −0.19 | 0.04 | −0.13 | 0.27 | 0.14 |
| CD27+ NK/NKT | −0.26 | 0.01 | 0.16 | 0.20 | 0.12 |
| Erythroblasts | 0.47 | −0.21 | −0.01 | 0.26 | 0.17 |
| Erythroid progenitors | 0.22 | 0.24 | 0.24 | 0.13 | 0.28 |
| Eosinophils | −0.06 | −0.11 | −0.14 | −0.48 | −0.12 |
| Monocytes | 0.39 | 0.27 | 0.24 | −0.09 | 0.31 |
| Neutrophils | −0.21 | 0.33 | 0.13 | −0.11 | −0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiliopoulou, P.; Rousakis, P.; Panteli, C.; Eleutherakis-Papaiakovou, E.; Migkou, M.; Kanellias, N.; Ntanasis-Stathopoulos, I.; Malandrakis, P.; Theodorakakou, F.; Fotiou, D.; et al. The Role of the Bone Marrow Microenvironment in Physical Function and Quality of Life in Patients with Multiple Myeloma After First-Line Treatment with Novel Agents and Autologous Transplantation. Onco 2025, 5, 21. https://doi.org/10.3390/onco5020021
Spiliopoulou P, Rousakis P, Panteli C, Eleutherakis-Papaiakovou E, Migkou M, Kanellias N, Ntanasis-Stathopoulos I, Malandrakis P, Theodorakakou F, Fotiou D, et al. The Role of the Bone Marrow Microenvironment in Physical Function and Quality of Life in Patients with Multiple Myeloma After First-Line Treatment with Novel Agents and Autologous Transplantation. Onco. 2025; 5(2):21. https://doi.org/10.3390/onco5020021
Chicago/Turabian StyleSpiliopoulou, Polyxeni, Pantelis Rousakis, Chrysanthi Panteli, Evangelos Eleutherakis-Papaiakovou, Magdalini Migkou, Nikolaos Kanellias, Ioannis Ntanasis-Stathopoulos, Panagiotis Malandrakis, Foteini Theodorakakou, Despina Fotiou, and et al. 2025. "The Role of the Bone Marrow Microenvironment in Physical Function and Quality of Life in Patients with Multiple Myeloma After First-Line Treatment with Novel Agents and Autologous Transplantation" Onco 5, no. 2: 21. https://doi.org/10.3390/onco5020021
APA StyleSpiliopoulou, P., Rousakis, P., Panteli, C., Eleutherakis-Papaiakovou, E., Migkou, M., Kanellias, N., Ntanasis-Stathopoulos, I., Malandrakis, P., Theodorakakou, F., Fotiou, D., Terpos, E., Myrianthopoulos, V., Gavriatopoulou, M., Tsitsilonis, O. E., Kastritis, E., Dimopoulos, M. A., & Terzis, G. (2025). The Role of the Bone Marrow Microenvironment in Physical Function and Quality of Life in Patients with Multiple Myeloma After First-Line Treatment with Novel Agents and Autologous Transplantation. Onco, 5(2), 21. https://doi.org/10.3390/onco5020021









