Targeting Pathways and Mechanisms in Gynecological Cancer with Antioxidant and Anti-Inflammatory Phytochemical Drugs
Simple Summary
Abstract
1. Introduction
2. Methodology: Aim, Review Process, Search Strategy, Data Extraction, and Analysis
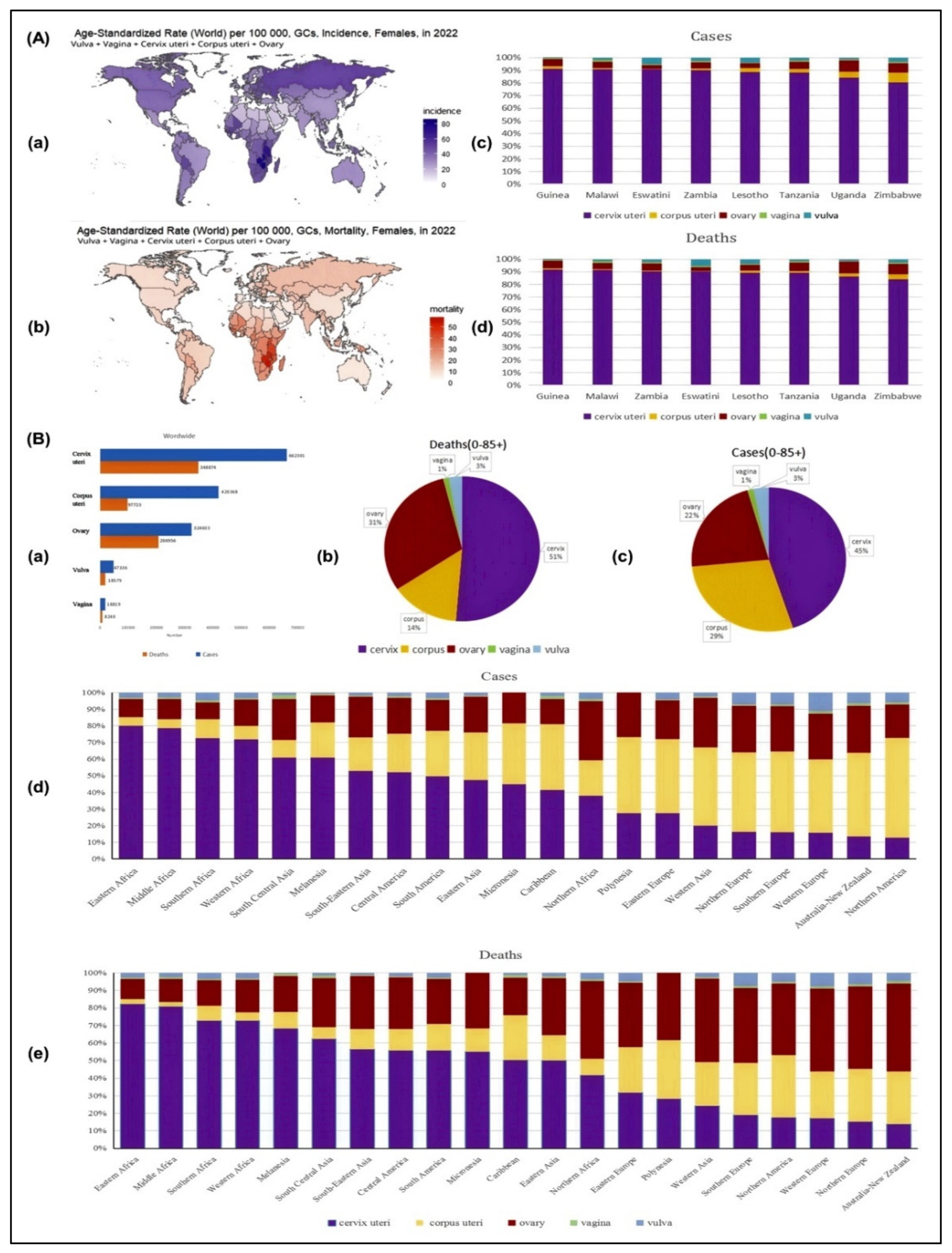
3. Clinical Manifestations of Gynecological Cancers
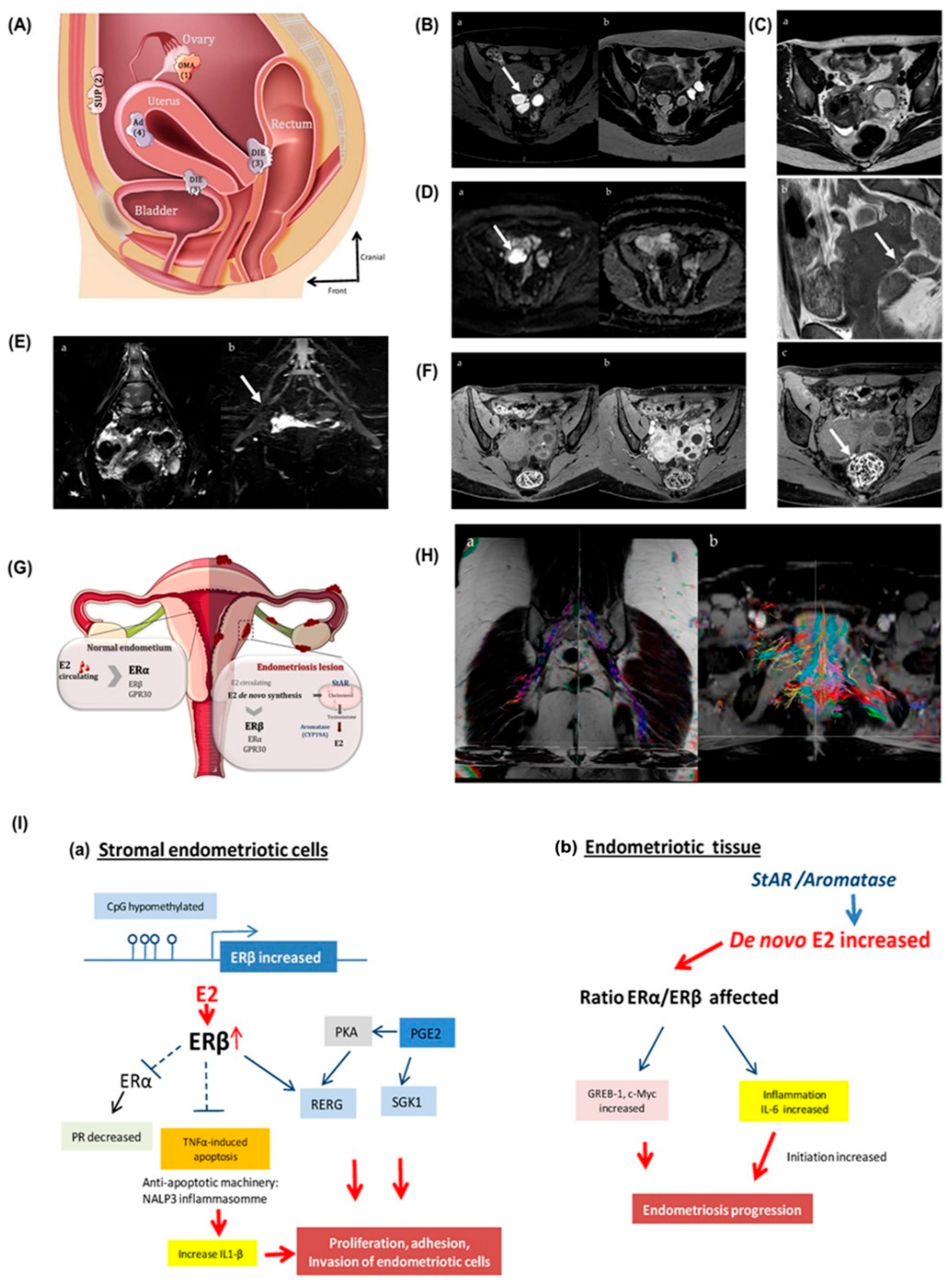
4. Clinical Diagnosis of Gynecological Cancer
5. Treatment of Gynecological Cancer
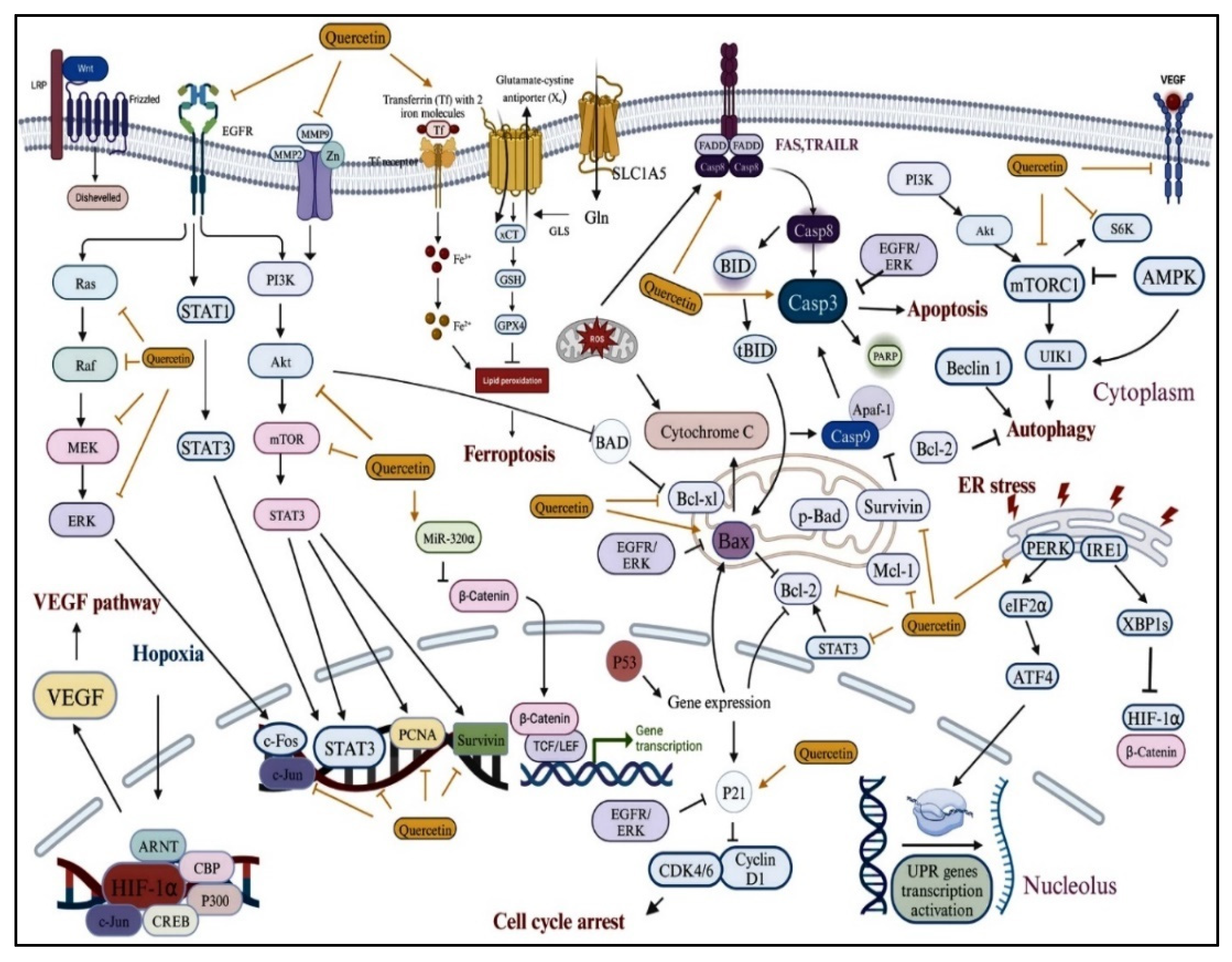
6. Molecular Pathways Targeted by Antioxidant and Anti-Inflammatory Drugs for Gynecological Cancer
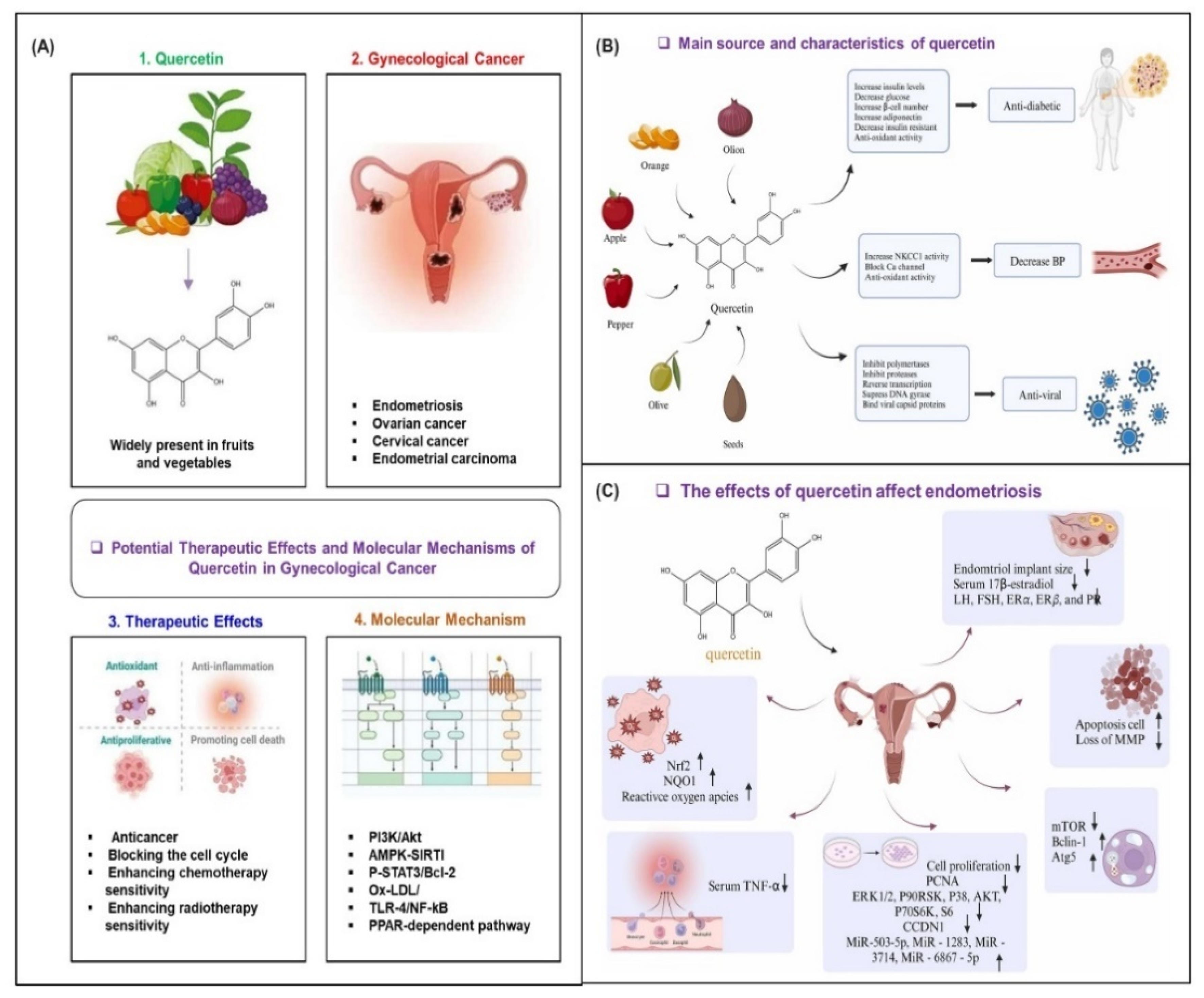
7. Therapeutic Mechanisms of Antioxidant and Anti-Inflammatory Drugs in Gynecological Cancer
8. Clinical Evidence for Treatment of Gynecological Cancer
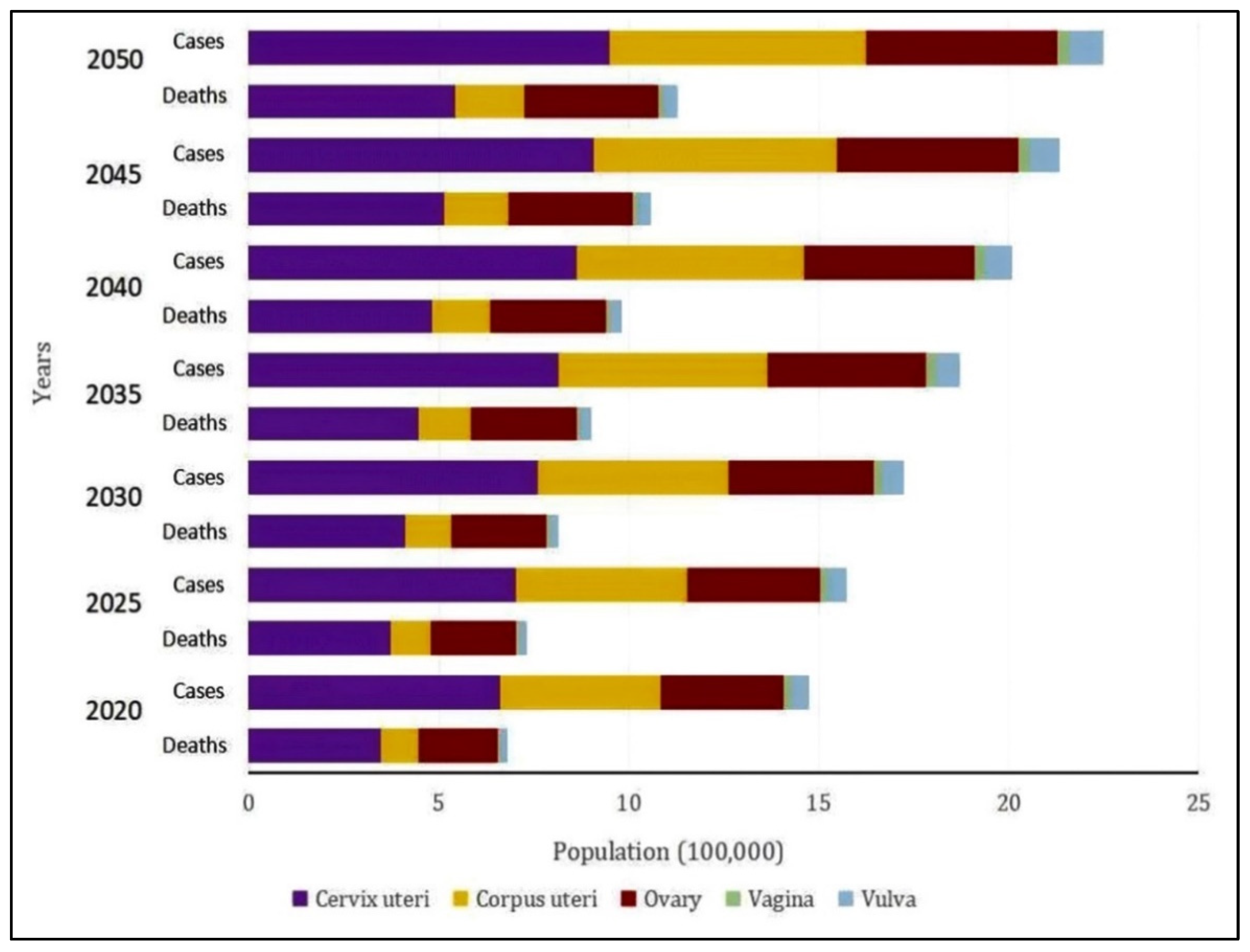
9. Future Directions for Treatment of Gynecological Cancer
| Phytochemical (Compound Class) | Botanical Name (Family) | Molecular Targets | Ref. |
|---|---|---|---|
| 6-Shogaol (phenylpropanoid) | Zingiber officinale (Roscoe) | Akt and STAT signaling pathway | [106,107] |
| Allicin (organosulfurs) | Allium sativum (Amaryllidaceae) | STAT3 signaling pathway | [108,109] |
| Alpinumisoflavone (pyranoisoflavone) | Derris eriocarpa (Leguminosae) | Nrf2, NQO-1, HO-1, miR-101, and Akt signaling | [110] |
| Andrographolide (diterpenoid) | Andrographis paniculata (Acanthaceae) | HIF-1α, VEGF, and PI3K pathway | [111] |
| Apigenin (flavonoid) | Petroselinum crispum (Apiaceae) | Intrinsic apoptosis pathway | [112] |
| Baicalein (flavonoid) | Scutellaria baicalensis (Lamiaceae) | MAPK, ERK, and p38 signaling pathways | [113,114] |
| Baicalin (flavonoid) | Scutellaria baicalensis (Lamiaceae) | MAPK, ERK, and p38 signaling pathways | [115] |
| Curcumin (phytopolyphenol) | Curcuma longa (Zingiberaceae) | Modulates cell signaling and gene expression | [116] |
| Decursin and Decursinol (coumarin) | Angelica gigas (Apiaceae) | Not mentioned | [117] |
| Dicumarol | Melilotus officinalis (Fabaceae) | Intrinsic apoptosis pathway | [118] |
| Epigallocatechin (flavonoid) | Camellia sinensis (Theaceae) | Inhibit cell proliferation and apoptosis | [119] |
| Emodin (resin) | Rheum palmatum L. (Polygonaceae) | PI3K/AKT and MAPK signaling pathways | [120,121,122] |
| Genistein (isoflavonoid) | Glycine max (Leguminosae) | WNT/β-catenin and Akt signaling pathway | [123,124] |
| Gingerol (polyphenol) | Zingiber officinale (Roscoe) | Intrinsic apoptosis pathway | [125,126] |
| Glycyrrhizin (triterpenes) | Glycyrrhiza glabra (Fabaceae) | TxA2 and JAK/STAT signaling pathway | [127] |
| Hispidulin (flavone) | Salvia involucrata (Lamiaceae) | Intrinsic apoptosis pathway | [128,129] |
| HS-1793 (stilbenoid) | Polygonum cuspidatum (Polygonaceae) | HIF-1α, VEGF, Ki-67, and CD31 | [130] |
| Licochalcone A (chalcone) | Glycyrrhiza glabra (Fabaceae) | Cyclins and CDKs | [131] |
| Nimbolide (triterpene) | Azadirachta indica (Meliaceae) | PI3K/AKT/mTOR and ERK signaling | [132] |
| Physapubescin B (steroid) | Physalis pubescens L. (Solanaceae) | Ki-67, Cdc25C, and PARP | [133] |
| Pterostilbene (polyphenol) | Polygonum cuspidatum (Polygonaceae) | Mitochondrial apoptosis; ERK and STAT3 signaling | [134,135,136] |
| Resveratrol (phenol) | Polygonum cuspidatum (Polygonaceae) | Regulating cell cycle and apoptosis pathways | [137] |
| Sulforaphane (organosulfur) | Brassica oleracea (Brassicaceae) | Cell cycle arrest and apoptosis (caspase-8, p21, hsp90) | [138] |
| Thymol (monoterpenoid) | Thymus vulgaris (Lamiaceae) | Mitochondrial mediated apoptosis | [139] |
| Thymoquinone (quinone) | Nigella sativa (Ranunculaceae) | STAT3 and associated proteins | [140,141] |
| Ursolic acid (triterpenoids) | Oldenlandia diffusa (Rubiaceae) | Ki-67, CD31, and miR-29a | [142,143] |
| Withaferin-A (phytosterols) | Withania somnifera (Solanaceae) | AKT/FOXO3a-Par-4 cell death, ERK, p38 | [144,145,146] |
| Phytochemical | Dose Range | Clinical Indication | Duration | Ref. |
|---|---|---|---|---|
| Curcumin | 6000 mg/day (oral) | Advanced/metastatic breast cancer (combined with docetaxel) | 7 days every 3 weeks | [147] |
| Curcumin | 6 g/day (oral) | Radiation dermatitis prevention during radiotherapy | Duration of radiotherapy | [148] |
| Curcumin | 300 mg (IV weekly) | Metastatic breast cancer (combined with paclitaxel) | 12 weeks | [148] |
| Polyphenon E (EGCG) | 400–800 mg twice daily | Chemoprevention in hormone receptor-negative breast cancer | 6 months | [149] |
| Indole-3-carbinol | 300–400 mg/day (oral) | Breast cancer prevention in high-risk women | 4–8 weeks | [150,151] |
| Diindolylmethane (BR-DIM) | 150 mg twice daily (oral) | Adjuvant therapy with tamoxifen for breast cancer | 12 months | [152] |
| Flaxseed lignans (SDG) | 25 g/day (dietary flaxseed) | Reduction in tumor biomarkers (Ki-67, c-erbB2) in postmenopausal breast cancer | ~32 days | [153] |
| Secoisolariciresinol diglucoside (SDG) | 50 mg/day (oral) | Chemoprevention in premenopausal women at high risk for breast cancer | 12 months | [154,155] |
| Scutellaria barbata (BZL101) | 40 g/day (oral extract) | Metastatic breast cancer (monotherapy) | Until progression | [155,156] |
| Grape seed proanthocyanidin (GSPE) | 100 mg three times daily (oral) | Management of radiation-induced breast induration | 6 months | [157] |
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASR | Age-standardized rate |
| GC | Gynecological cancer |
| AKT | Protein kinase B |
| EMS | Epithelial–mesenchymal transition |
| IL | Interleukin |
| MA | Myeloid-derived suppressor cells |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| ROS | Reactive oxygen species |
| TNF | Tumor necrosis factor |
| VEGFA | Vascular endothelial growth factor A |
References
- Zhang, L.; Bi, Q.; Deng, H.; Xu, J.; Chen, J.; Zhang, M.; Mu, X. Human papillomavirus infections among women with cervical lesions and cervical cancer in Eastern China: Genotype-specific prevalence and attribution. BMC Infect. Dis. 2017, 17, 107. [Google Scholar] [CrossRef]
- Polten, R.; Kutle, I.; Hachenberg, J.; Klapdor, R.; Morgan, M.; Schambach, A. Towards novel gene and cell therapy approaches for cervical cancer. Cancers 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Thanki, R.M.; Diwan, A. Artificial Intelligence for Early Detection and Diagnosis of Cervical Cancer; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Dong, H.; Chen, S.; Liang, X.; Cai, Q.; Zhang, X.; Xie, J.; Sun, Z. Vitamin D and its receptors in cervical cancer. J. Cancer 2024, 15, 926. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, X. Reactive oxygen species and DNA damage response in cancer. Int. Rev. Cell Mol. Biol. 2021, 364, 139–161. [Google Scholar] [PubMed]
- Xiao, Y.; Bi, M.; Guo, H.; Li, M. Multi-omics approaches for biomarker discovery in early ovarian cancer diagnosis. EBioMedicine 2022, 79, 104001. [Google Scholar] [CrossRef]
- Reid, F.; Bajwa, A. World Ovarian Cancer Coalition Atlas. 2020. In Global Trends in Incidence, Mortality, and Survival; World Ovarian Cancer Coalition: Toronto, ON, Canada, 2020; pp. 1–42. [Google Scholar]
- Zhang, J.; Yu, S.; Li, Q.; Wang, Q.; Zhang, J. Increased co-expression of MEST and BRCA1 is associated with worse prognosis and immune infiltration in ovarian cancer. Gynecol. Oncol. 2022, 164, 566–576. [Google Scholar] [CrossRef]
- Colditz, G.; Beers, C. Active cancer prevention. Cancer Control 2010, 45, 23. [Google Scholar]
- Gyllensten, U. Novel diagnostics for improved treatment of gynecological cancer. Upsala J. Med. Sci. 2025, 130, 10-48101. [Google Scholar] [CrossRef]
- Zhu, B.; Gu, H.; Mao, Z.; Beeraka, N.M.; Zhao, X.; Anand, M.P.; Zheng, Y.; Zhao, R.; Li, S.; Manogaran, P. Global burden of gynaecological cancers in 2022 and projections to 2050. J. Glob. Health 2024, 14, 04155. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Bisignano, C.; Hsu, J.M.; Bryazka, D.; Cao, S.; Bhattacharjee, N.V.; Dalton, B.E.; Lindstedt, P.A.; Smith, A.E.; Ababneh, H.S. Burden of disease scenarios by state in the USA, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 404, 2341–2370. [Google Scholar] [CrossRef]
- Goswami, B.; Rajappa, M.; Sharma, M.a.; Sharma, A. Inflammation: Its role and interplay in the development of cancer, with special focus on gynecological malignancies. Int. J. Gynecol. Cancer 2008, 18, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Milkovic, L.; Siems, W.; Siems, R.; Zarkovic, N. Oxidative stress and antioxidants in carcinogenesis and integrative therapy of cancer. Curr. Pharm. Des. 2014, 20, 6529–6542. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The role of oxidative stress in tumorigenesis and progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef]
- Ju, S.; Singh, M.K.; Han, S.; Ranbhise, J.; Ha, J.; Choe, W.; Yoon, K.-S.; Yeo, S.G.; Kim, S.S.; Kang, I. Oxidative Stress and Cancer Therapy: Controlling Cancer Cells Using Reactive Oxygen Species. Int. J. Mol. Sci. 2024, 25, 12387. [Google Scholar] [CrossRef]
- Closset, L.; Gultekin, O.; Salehi, S.; Sarhan, D.; Lehti, K.; Gonzalez-Molina, J. The extracellular matrix–immune microenvironment crosstalk in cancer therapy: Challenges and opportunities. Matrix Biol. 2023, 121, 217–228. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Bitsadze, V.; Slukhanchuk, E.; Solopova, A.; Khizroeva, J.K.; Yakubova, F.; Orudzhova, E.; Degtyareva, N.; Egorova, E.; Makatsariya, N.; Samburova, N. The role of the microenvironment in tumor growth and spreading. Obstet. Gynecol. Reprod. 2024, 18, 96–111. [Google Scholar] [CrossRef]
- De Nola, R.; Menga, A.; Castegna, A.; Loizzi, V.; Ranieri, G.; Cicinelli, E.; Cormio, G. The crowded crosstalk between cancer cells and stromal microenvironment in gynecological malignancies: Biological pathways and therapeutic implication. Int. J. Mol. Sci. 2019, 20, 2401. [Google Scholar] [CrossRef]
- Kan, X.; Zhou, Z.; Liu, L.; Aiskikaer, R.; Zou, Y. Significance of non-steroidal anti-inflammatory drugs in the prevention and treatment of cervical cancer. Heliyon 2025, 11, e42055. [Google Scholar] [CrossRef] [PubMed]
- Centini, G.; Schettini, G.; Pieri, E.; Giorgi, M.; Lazzeri, L.; Martire, F.G.; Mancini, V.; Raimondo, D.; Seracchioli, R.; Habib, N. Endometriosis-related ovarian cancer: Where are we now? A narrative review towards a pragmatic approach. J. Clin. Med. 2024, 13, 1933. [Google Scholar] [CrossRef]
- Conforti, R.A.; Delsouc, M.B.; Zorychta, E.; Telleria, C.M.; Casais, M. Copper in gynecological diseases. Int. J. Mol. Sci. 2023, 24, 17578. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Castilho, P.C.M.F.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Daglia, M.; Silva, A.S. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2020, 60, 2790–2800. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Khalaji, A.; Bahri Najafi, M.; Sadati, S.; Raisi, A.; Abolhassani, A.; Eshraghi, R.; Khaksary Mahabady, M.; Rahimian, N.; Mirzaei, H. NF-κB pathway and angiogenesis: Insights into colorectal cancer development and therapeutic targets. Eur. J. Med. Res. 2024, 29, 610. [Google Scholar] [CrossRef]
- Aldhahrani, A.; Alshaye, N.A.; Alshaya, D.S.; Mohamed, D.A.; Fayad, E.; Sophy, M.A.E.; Al-Ashger, N.A.; Salem, M.G.; Farouk, N.A. In Vitro biological evaluation of some hybrid molecules bearing 2-quinoline as anti-inflammatory and anti-cancer agents. J. Mol. Struct. 2025, 1326, 141060. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef]
- Ali Abdalla, Y.O.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Olson, S.H.; Mignone, L.; Nakraseive, C.; Caputo, T.; Barakat, R.; Harlap, S. Symptoms of ovarian cancer. Obstet. Gynecol. 2001, 98, 212–217. [Google Scholar] [CrossRef]
- Baines, P.A.; Allen, G.M. Pelvic pain and menstrual related illnesses. Emerg. Med. Clin. N. Am. 2001, 19, 763–780. [Google Scholar] [CrossRef]
- Melnyk, M.; Starczewski, A.; Nawrocka-Rutkowska, J.; Gorzko, A.; Melnyk, B.; Szydłowska, I. Giant Ovarian Tumors in Young Women: Diagnostic and Treatment Challenges—A Report of Two Cases and Narrative Review of the Recent Literature. J. Clin. Med. 2025, 14, 1236. [Google Scholar] [CrossRef]
- Naga, O. Last-Minute Review. In Pediatric Board Study Guide: A Last Minute Review; Springer Nature: Cham, Switzerland, 2024; pp. 1017–1126. [Google Scholar]
- McCluggage, W.G. Progress in the pathological arena of gynecological cancers. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 107–114. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Avesani, G.; Panico, C.; Manganaro, L.; Gui, B.; Lakhman, Y.; Andrieu, P.C.; Bharwani, N.; Rockall, A.; Thomassin-Naggara, I.; et al. Ovarian cancer staging and follow-up: Updated guidelines from the European Society of Urogenital Radiology female pelvic imaging working group. Eur. Radiol. 2025. [Google Scholar] [CrossRef]
- Addley, H.; Gregory, D.; Prewett, S.; Smith, J. Gynaecological Cancers. In Radiological Anatomy for Radiation and Particle Therapy; Springer Nature: Cham, Switzerland, 2025; pp. 271–295. [Google Scholar]
- Mayeri, D.G.; Kahasha, P.M.; Kibalama, I.B.; Mongane, J.; Louguè, M.; Birindwa, E.K.; Mwimangire, S.C.; Kikuru, C.K.; Materanya, J.M.; Bisimwa, Y.K.; et al. Cervical precancerous and cancerous lesions screening using Pap smear test at Provincial Referral Hospital of Bukavu, Eastern DR Congo: Profile and recommendations to stakeholders. Pan Afr. Med. J. 2024, 47, 57. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 8, Cd008587. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Walker, R.C. Advanced imaging (positron emission tomography and magnetic resonance imaging) and image-guided biopsy in initial staging and monitoring of therapy of lung cancer. Cancer J. 2013, 19, 208–216. [Google Scholar] [CrossRef]
- Choi, H.J.; Ju, W.; Myung, S.K.; Kim, Y. Diagnostic performance of computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with cervical cancer: Meta-analysis. Cancer Sci. 2010, 101, 1471–1479. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sharma, A.; Prasad, S.; Singh, K.; Kumar, M.; Sherawat, K.; Tuli, H.S.; Gupta, M. Novel therapeutic agents in clinical trials: Emerging approaches in cancer therapy. Discov. Oncol. 2024, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Salman, L.; Covens, A. Fertility Preservation in Cervical Cancer-Treatment Strategies and Indications. Curr. Oncol. 2024, 31, 296–306. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Alonzo, L.; Cannella, R.; Gullo, G.; Piombo, G.; Cicero, G.; Lopez, A.; Billone, V.; Andrisani, A.; Cucinella, G.; Lo Casto, A.; et al. Magnetic Resonance Imaging of Endometriosis: The Role of Advanced Techniques. J. Clin. Med. 2024, 13, 5783. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Fatima, D.N. Association of Peroxisomes, Reactive Oxygen Species (ROS) and Antioxidants: Insights from Preclinical and Clinical Evaluations. In The Metabolic Role of Peroxisome in Health and Disease; İla, H.B., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Orabi, S.A.; Abou-Hussein, S. Antioxidant defense mechanisms enhance oxidative stress tolerance in plants. A review. Curr. Sci. Int. 2019, 8, 565–576. [Google Scholar]
- Martínez, J.P.; Araya, H. Ascorbate–glutathione cycle: Enzymatic and non-enzymatic integrated mechanisms and its biomolecular regulation. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2010; pp. 303–322. [Google Scholar]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A.; Arguelles, S. Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des. 2018, 24, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Wang, R.X.; Zhou, M.; Ma, H.L.; Qiao, Y.B.; Li, Q.S. The role of chronic inflammation in various diseases and anti-inflammatory therapies containing natural products. ChemMedChem 2021, 16, 1576–1592. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-kappaB signaling pathways in neurological inflammation: A mini review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Escarcega, R.; Fuentes-Alexandro, S.; Garcia-Carrasco, M.; Gatica, A.; Zamora, A. The transcription factor nuclear factor-kappa B and cancer. Clin. Oncol. 2007, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers 2021, 13, 986. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Fang, J.; Lu, Y.; Zheng, J.; Jiang, X.; Shen, H.; Shang, X.; Lu, Y.; Fu, P. Exploring the crosstalk between endothelial cells, immune cells, and immune checkpoints in the tumor microenvironment: New insights and therapeutic implications. Cell Death Dis. 2023, 14, 586. [Google Scholar] [CrossRef]
- Jian, X.; Shi, C.; Luo, W.; Zhou, L.; Jiang, L.; Liu, K. Therapeutic effects and molecular mechanisms of quercetin in gynecological disorders. Biomed. Pharmacother. 2024, 173, 116418. [Google Scholar] [CrossRef]
- Mazumder, S.; Chanda, S.; Banerjee, S. Phytochemical Profile and Chemopreventive Properties of Potential Bioactive Ingredients. In Bioactive Ingredients for Healthcare Industry Volume 1: Extraction strategies, Stability and Medicinal Properties; Springer: Singapore, 2025; pp. 15–36. [Google Scholar]
- Sichetti, M.; Giuseffi, M.; Giglio, E.; Marino, G.; Mecca, M. Effect of Natural Polyphenols on Breast Cancer Chemoprevention and Treatment. Mol. Nutr. Food Res. 2025, e70055. [Google Scholar] [CrossRef]
- Woźniak, M.; Krajewski, R.; Makuch, S.; Agrawal, S. Phytochemicals in Gynecological Cancer Prevention. Int. J. Mol. Sci. 2021, 22, 1219. [Google Scholar] [CrossRef]
- Kumar, A.; Pradeep, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxidative Med. Cell Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Xiao, W.; Jiang, W.; Chen, Z.; Huang, Y.; Mao, J.; Zheng, W.; Hu, Y.; Shi, J. Advance in peptide-based drug development: Delivery platforms, therapeutics and vaccines. Signal Transduct. Target. Ther. 2025, 10, 74. [Google Scholar]
- Nisar, A.; Jagtap, S.; Vyavahare, S.; Deshpande, M.; Harsulkar, A.; Ranjekar, P.; Prakash, O. Phytochemicals in the treatment of inflammation-associated diseases: The journey from preclinical trials to clinical practice. Front. Pharmacol. 2023, 14, 1177050. [Google Scholar] [CrossRef]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The Anti-Inflammatory Properties of Phytochemicals and Their Effects on Epigenetic Mechanisms Involved in TLR4/NF-κB-Mediated Inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef]
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The role of cytokines in breast cancer development and progression. J. Interferon Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yao, Y.; Gao, H.; Hu, X. Mechanisms of angiogenesis in tumour. Front. Oncol. 2024, 14, 1359069. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Blagov, A.V.; Summerhill, V.I.; Sukhorukov, V.N.; Zhigmitova, E.B.; Postnov, A.Y.; Orekhov, A.N. Potential use of antioxidants for the treatment of chronic inflammatory diseases. Front. Pharmacol. 2024, 15, 1378335. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, R.O.S.; Pinheiro, J.L.S.; Rodrigues, L.H.M.; Gomes, R.C.; Duarte, A.B.S.; Emídio, J.J.; Diniz, L.R.L.; de Sousa, D.P. Anti-Inflammatory and Antioxidant Activities of Eugenol: An Update. Pharmaceuticals 2024, 17, 1505. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxidative Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Bastidas-Coral, A.P.; Bakker, A.D.; Zandieh-Doulabi, B.; Kleverlaan, C.J.; Bravenboer, N.; Forouzanfar, T.; Klein-Nulend, J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem Cells Int. 2016, 2016, 1318256. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Vincenzi, A.; Goettert, M.I.; Volken de Souza, C.F. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2021, 57, 27–38. [Google Scholar] [CrossRef]
- Yang, M.Q.; Zhang, S.L.; Sun, L.; Huang, L.T.; Yu, J.; Zhang, J.H.; Tian, Y.; Han, C.B.; Ma, J.T. Targeting mitochondria: Restoring the antitumor efficacy of exhausted T cells. Mol. Cancer 2024, 23, 260. [Google Scholar] [CrossRef]
- Kuo, C.L.; Ponneri Babuharisankar, A.; Lin, Y.C.; Lien, H.W.; Lo, Y.K.; Chou, H.Y.; Tangeda, V.; Cheng, L.C.; Cheng, A.N.; Lee, A.Y. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Nisar, S.; Masoodi, T.; Prabhu, K.S.; Kuttikrishnan, S.; Zarif, L.; Khatoon, S.; Ali, S.; Uddin, S.; Akil, A.A.-S.; Singh, M.; et al. Natural products as chemo-radiation therapy sensitizers in cancers. Biomed. Pharmacother. 2022, 154, 113610. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Kumar Mongre, R.; Sharma, N.; Singh Sodhi, S.; Ghosh, M.; Kumar Singh, A.; Kim, N.; Park, Y.H.; Shin, Y.G.; Kim, S.J.; Jiao Jiao, Z.; et al. Novel phyto-derivative BRM270 inhibits hepatocellular carcinoma cells proliferation by inducing G2/M phase cell cycle arrest and apoptosis in xenograft mice model. Biomed. Pharmacother. 2017, 87, 741–754. [Google Scholar] [CrossRef]
- Mongre, R.K.; Mishra, C.B.; Jung, S.; Lee, B.S.; Quynh, N.T.N.; Anh, N.H.; Myagmarjav, D.; Jo, T.; Lee, M.S. Exploring the Role of TRIP-Brs in Human Breast Cancer: An Investigation of Expression, Clinicopathological Significance, and Prognosis. Mol. Ther. Oncolytics 2020, 19, 105–126. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Darvish, M.; Tabatabaeian, J.; Fard, M.R.; Mottaghi, R.; Azadchehr, M.J.; Jahanshahi, M.; Sahebkar, A.; Mirzaei, H. Therapeutic role of curcumin and its novel formulations in gynecological cancers. J. Ovarian Res. 2020, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Yetkin-Arik, B.; Kastelein, A.W.; Klaassen, I.; Jansen, C.H.J.R.; Latul, Y.P.; Vittori, M.; Biri, A.; Kahraman, K.; Griffioen, A.W.; Amant, F.; et al. Angiogenesis in gynecological cancers and the options for anti-angiogenesis therapy. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2021, 1875, 188446. [Google Scholar] [CrossRef]
- Di Domenico, F.; Foppoli, C.; Coccia, R.; Perluigi, M. Antioxidants in cervical cancer: Chemopreventive and chemotherapeutic effects of polyphenols. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2012, 1822, 737–747. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021, 10, 100. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, Y.; Kumar, D. Unveiling the link between chronic inflammation and cancer. Metab. Open 2025, 25, 100347. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Sriwidodo, S.; Sofian, F.F.; Wilar, G.; Diantini, A. Nanoparticle-Based Antioxidants in Stress Signaling and Programmed Cell Death in Breast Cancer Treatment. Molecules 2023, 28, 5305. [Google Scholar] [CrossRef] [PubMed]
- Sidhic, J.; Aswathi, M.K.; Prasad, A.; Tom, A.; Mohan, P.; Sarbadhikary, P.; Narayanankutty, A.; George, S.; Abrahamse, H.; George, B.P. Advancements in metal and metal oxide nanoparticles for targeted cancer therapy and imaging: Mechanisms, applications, and safety concerns. J. Drug Deliv. Sci. Technol. 2025, 105, 106622. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, M.; Huang, H.; Jin, W.-L. Drug repurposing for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 92. [Google Scholar] [CrossRef]
- Duranti, E.; Cordani, N.; Villa, C. Edaravone: A Novel Possible Drug for Cancer Treatment? Int. J. Mol. Sci. 2024, 25, 1633. [Google Scholar] [CrossRef]
- Brasseur, K.; Gévry, N.; Asselin, E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget 2017, 8, 4008–4042. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Yan, M.; Xiang, Z. Gut and oral microbiota in gynecological cancers: Interaction, mechanism, and therapeutic value. npj Biofilms Microbiomes 2024, 10, 104. [Google Scholar] [CrossRef]
- Agnew, H.J.; Kitson, S.J.; Crosbie, E.J. Gynecological malignancies and obesity. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 88, 102337. [Google Scholar] [CrossRef]
- Islam, M.R.; Rauf, A.; Akash, S.; Trisha, S.I.; Nasim, A.H.; Akter, M.; Dhar, P.S.; Ogaly, H.A.; Hemeg, H.A.; Wilairatana, P.; et al. Targeted therapies of curcumin focus on its therapeutic benefits in cancers and human health: Molecular signaling pathway-based approaches and future perspectives. Biomed. Pharmacother. 2024, 170, 116034. [Google Scholar] [CrossRef]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Rudzińska, A.; Juchaniuk, P.; Oberda, J.; Wiśniewska, J.; Wojdan, W.; Szklener, K.; Mańdziuk, S. Phytochemicals in Cancer Treatment and Cancer Prevention-Review on Epidemiological Data and Clinical Trials. Nutrients 2023, 15, 1896. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Ren, J.; Yan, G.; Yang, L.; Kong, L.; Guan, Y.; Sun, H.; Liu, C.; Liu, L.; Han, Y.; Wang, X. Cancer chemoprevention: Signaling pathways and strategic approaches. Signal Transduct. Target. Ther. 2025, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Kim, M.O.; Lee, M.-H.; Oi, N.; Kim, S.-H.; Bae, K.B.; Huang, Z.; Kim, D.J.; Reddy, K.; Lee, S.-Y.; Park, S.J. [6]-Shogaol inhibits growth and induces apoptosis of non-small cell lung cancer cells by directly regulating Akt1/2. Carcinogenesis 2014, 35, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-κB signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef]
- Huang, L.; Song, Y.; Lian, J.; Wang, Z. Allicin inhibits the invasion of lung adenocarcinoma cells by altering tissue inhibitor of metalloproteinase/matrix metalloproteinase balance via reducing the activity of phosphoinositide 3-kinase/AKT signaling. Oncol. Lett. 2017, 14, 468–474. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Zhu, B.; Zhao, L.; Liu, Y.; Zhao, F.; Feng, J.; Jin, Y.; Sun, J.; Geng, R.; Wei, Y. Allicin inhibits proliferation and invasion in vitro and in vivo via SHP-1-mediated STAT3 signaling in cholangiocarcinoma. Cell. Physiol. Biochem. 2018, 47, 641–653. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Y.; Chu, L.; Wu, T.; You, J. Alpinumisoflavone suppresses tumour growth and metastasis of clear-cell renal cell carcinoma. Am. J. Cancer Res. 2017, 7, 999. [Google Scholar] [PubMed]
- Chang, J.-H.; Cheng, C.-W.; Yang, Y.-C.; Chen, W.-S.; Hung, W.-Y.; Chow, J.-M.; Chen, P.-S.; Hsiao, M.; Lee, W.-J.; Chien, M.-H. Downregulating CD26/DPPIV by apigenin modulates the interplay between Akt and Snail/Slug signaling to restrain metastasis of lung cancer with multiple EGFR statuses. J. Exp. Clin. Cancer Res. 2018, 37, 199. [Google Scholar] [CrossRef]
- Kangra, K.; Kakkar, S.; Mittal, V.; Kumar, V.; Aggarwal, N.; Chopra, H.; Malik, T.; Garg, V. Incredible use of plant-derived bioactives as anticancer agents. RSC adv. 2025, 15, 1721–1746. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.; Wang, Z.; Ma, L.; Peng, B.; Mao, K.; Li, C.; Su, M.; Zhou, C.; Peng, G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget 2018, 9, 20089–20102. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Harsha, C.; Banik, K.; Gupta, S.C.; Aggarwal, B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017, 131, 1781–1799. [Google Scholar] [CrossRef]
- Wu, W.; Tang, S.N.; Zhang, Y.; Puppala, M.; Cooper, T.K.; Xing, C.; Jiang, C.; Lü, J. Prostate Cancer Xenograft Inhibitory Activity and Pharmacokinetics of Decursinol, a Metabolite of Angelica gigas Pyranocoumarins, in Mouse Models. Am. J. Chin. Med. 2017, 45, 1773–1792. [Google Scholar] [CrossRef]
- Zhang, W.; Su, J.; Xu, H.; Yu, S.; Liu, Y.; Zhang, Y.; Sun, L.; Yue, Y.; Zhou, X. Dicumarol inhibits PDK1 and targets multiple malignant behaviors of ovarian cancer cells. PLoS ONE 2017, 12, e0179672. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Iwanowycz, S.; Wang, J.; Hodge, J.; Wang, Y.; Yu, F.; Fan, D. Emodin Inhibits Breast Cancer Growth by Blocking the Tumor-Promoting Feedforward Loop between Cancer Cells and Macrophages. Mol. Cancer Ther. 2016, 15, 1931–1942. [Google Scholar] [CrossRef]
- Lin, W.; Zhong, M.; Yin, H.; Chen, Y.; Cao, Q.; Wang, C.; Ling, C. Emodin induces hepatocellular carcinoma cell apoptosis through MAPK and PI3K/AKT signaling pathways in vitro and in vivo. Oncol. Rep. 2016, 36, 961–967. [Google Scholar] [CrossRef]
- Su, J.; Yan, Y.; Qu, J.; Xue, X.; Liu, Z.; Cai, H. Emodin induces apoptosis of lung cancer cells through ER stress and the TRIB3/NF-κB pathway. Oncol. Rep. 2017, 37, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Zhou, D.; Chen, H. Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of WNT/β-catenin signalling and reduces colon pre-neoplasia in rats. Br. J. Nutr. 2013, 109, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.C.; Peng, S.F.; Lai, K.C.; Liao, C.L.; Huang, Y.P.; Lin, C.C.; Lin, M.L.; Liu, K.C.; Tsai, C.C.; Ma, Y.S.; et al. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ. Toxicol. 2019, 34, 443–456. [Google Scholar] [CrossRef]
- Joo, J.H.; Hong, S.S.; Cho, Y.R.; Seo, D.W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38MAPK activity. Oncol. Rep. 2016, 35, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Fuzer, A.M.; Becceneri, A.B.; da Silva, J.A.; Tomasin, R.; Denoyer, D.; Kim, S.H.; McIntyre, K.A.; Pearson, H.B.; Yeo, B.; et al. [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo. Oncotarget 2017, 8, 72260–72271. [Google Scholar] [CrossRef]
- Deng, Q.P.; Wang, M.J.; Zeng, X.; Chen, G.G.; Huang, R.Y. Effects of Glycyrrhizin in a Mouse Model of Lung Adenocarcinoma. Cell Physiol. Biochem. 2017, 41, 1383–1392. [Google Scholar] [CrossRef]
- Chittasupho, C.; Samee, W.; Mangmool, S.; Karuna, N.; Anuchapreeda, S.; Okonogi, S.; Athikomkulchai, S. Phytochemical Characterization and Anticancer Activity of Clerodendrum chinense Leaf Extract Against Breast and Cervical Cancer Cells. Int. J. Mol. Sci. 2025, 26, 2729. [Google Scholar] [CrossRef]
- Chaudhry, G.E.; Zeenia; Sharifi-Rad, J.; Calina, D. Hispidulin: A promising anticancer agent and mechanistic breakthrough for targeted cancer therapy. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 1919–1934. [Google Scholar] [CrossRef]
- Kim, D.H.; Sung, B.; Kim, J.A.; Kang, Y.J.; Hwang, S.Y.; Hwang, N.L.; Suh, H.; Choi, Y.H.; Im, E.; Chung, H.Y.; et al. HS-1793, a resveratrol analogue, downregulates the expression of hypoxia-induced HIF-1 and VEGF and inhibits tumor growth of human breast cancer cells in a nude mouse xenograft model. Int. J. Oncol. 2017, 51, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Wu, G.J.; Chen, R.J.; Chang, C.C.; Lien, L.M.; Chiu, C.C.; Tseng, M.F.; Huang, L.T.; Lin, K.H. Licochalcone A attenuates glioma cell growth in vitro and in vivo through cell cycle arrest. Food Funct. 2018, 9, 4500–4507. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Gonzalez, E.; Arumugam, A.; Nandy, S.; Gonzalez, V.; Medel, J.; Camacho, F.; Ortega, A.; Bonkoungou, S.; Narayan, M.; et al. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition. Sci. Rep. 2016, 6, 19819. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Hu, Z.; Zhang, Z.; Ma, Q.; Tang, H.; Ma, Z. Physapubescin B Exhibits Potent Activity against Human Prostate Cancer In Vitro and In Vivo. J. Agric. Food Chem. 2015, 63, 9504–9512. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Y.; Fan, C.; Di, S.; Hu, W.; Jiang, S.; Li, T.; Ma, Z.; Chao, D.; Feng, X.; et al. Pterostilbene Inhibits the Growth of Human Esophageal Cancer Cells by Regulating Endoplasmic Reticulum Stress. Cell Physiol. Biochem. 2016, 38, 1226–1244. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, G.; Xu, Z.; Yang, G.; Li, B.; Wu, X.; Xiao, W.; Xie, B.; Hu, L.; Sun, X.; et al. Pterostilbene induces apoptosis and cell cycle arrest in diffuse large B-cell lymphoma cells. Sci. Rep. 2016, 6, 37417. [Google Scholar] [CrossRef]
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene, a natural phenolic compound, synergizes the antineoplastic effects of megestrol acetate in endometrial cancer. Sci. Rep. 2017, 7, 12754. [Google Scholar] [CrossRef]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002, 62, 4945–4954. [Google Scholar]
- Qazi, A.; Pal, J.; Maitah, M.; Fulciniti, M.; Pelluru, D.; Nanjappa, P.; Lee, S.; Batchu, R.B.; Prasad, M.; Bryant, C.S.; et al. Anticancer activity of a broccoli derivative, sulforaphane, in barrett adenocarcinoma: Potential use in chemoprevention and as adjuvant in chemotherapy. Transl. Oncol. 2010, 3, 389–399. [Google Scholar] [CrossRef]
- De La Chapa, J.J.; Singha, P.K.; Lee, D.R.; Gonzales, C.B. Thymol inhibits oral squamous cell carcinoma growth via mitochondria-mediated apoptosis. J. Oral Pathol. Med. 2018, 47, 674–682. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Wang, J.; Guo, X.F.; Liu, Z.; Dong, W.G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149–4159. [Google Scholar] [CrossRef] [PubMed]
- Odeh, L.H.; Talib, W.H.; Basheti, I.A. Synergistic effect of thymoquinone and melatonin against breast cancer implanted in mice. J. Cancer Res. Ther. 2018, 14 (Suppl. S2), S324–S330. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, V.R.; Sung, B.; Reuter, S.; Kannappan, R.; Deorukhkar, A.; Diagaradjane, P.; Wei, C.; Baladandayuthapani, V.; Krishnan, S.; et al. Ursolic acid inhibits growth and metastasis of human colorectal cancer in an orthotopic nude mouse model by targeting multiple cell signaling pathways: Chemosensitization with capecitabine. Clin. Cancer Res. 2012, 18, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Shi, H.; Chen, H.; Tao, J.; Shen, R.; Wang, T. Ursolic acid enhances the therapeutic effects of oxaliplatin in colorectal cancer by inhibition of drug resistance. Cancer Sci. 2018, 109, 94–102. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, B.W. Withaferin-A Inhibits Colon Cancer Cell Growth by Blocking STAT3 Transcriptional Activity. J. Cancer Prev. 2015, 20, 185–192. [Google Scholar] [CrossRef]
- Suman, S.; Das, T.P.; Sirimulla, S.; Alatassi, H.; Ankem, M.K.; Damodaran, C. Withaferin-A suppress AKT induced tumor growth in colorectal cancer cells. Oncotarget 2016, 7, 13854–13864. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Nagalingam, A.; Muniraj, N.; Saxena, N.K.; Sharma, D. Concomitant activation of ETS-like transcription factor-1 and Death Receptor-5 via extracellular signal-regulated kinase in withaferin A-mediated inhibition of hepatocarcinogenesis in mice. Sci. Rep. 2017, 7, 17943. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Crew, K.D.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.; Hudis, C.; McArthur, H.L.; Chang, J.; Rimawi, M.; Vornik, L.; et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev. Res. 2012, 5, 1144–1154. [Google Scholar] [CrossRef]
- Reed, G.A.; Peterson, K.S.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. A phase I study of indole-3-carbinol in women: Tolerability and effects. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Bradlow, L.; Sepkovic, D.; Mehl, S.; Mailman, J.; Osborne, M.P. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J. Cell Biochem. Suppl. 1997, 28–29, 111–116. [Google Scholar] [CrossRef]
- Thomson, C.A.; Chow, H.H.S.; Wertheim, B.C.; Roe, D.J.; Stopeck, A.; Maskarinec, G.; Altbach, M.; Chalasani, P.; Huang, C.; Strom, M.B.; et al. A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat. 2017, 165, 97–107. [Google Scholar] [CrossRef]
- Thompson, L.U.; Chen, J.M.; Li, T.; Strasser-Weippl, K.; Goss, P.E. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin. Cancer Res. 2005, 11, 3828–3835. [Google Scholar] [CrossRef]
- Fabian, C.J.; Khan, S.A.; Garber, J.E.; Dooley, W.C.; Yee, L.D.; Klemp, J.R.; Nydegger, J.L.; Powers, K.R.; Kreutzjans, A.L.; Zalles, C.M.; et al. Randomized Phase IIB Trial of the Lignan Secoisolariciresinol Diglucoside in Premenopausal Women at Increased Risk for Development of Breast Cancer. Cancer Prev. Res. 2020, 13, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.; Shtivelman, E.; Perez, A.; Vogel, C.; Franco, S.; Tan Chiu, E.; Melisko, M.; Tagliaferri, M.; Cohen, I.; Shoemaker, M.; et al. Phase I trial and antitumor effects of BZL101 for patients with advanced breast cancer. Breast Cancer Res. Treat. 2007, 105, 17–28. [Google Scholar] [CrossRef]
- Perez, A.T.; Arun, B.; Tripathy, D.; Tagliaferri, M.A.; Shaw, H.S.; Kimmick, G.G.; Cohen, I.; Shtivelman, E.; Caygill, K.A.; Grady, D.; et al. A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res. Treat. 2010, 120, 111–118. [Google Scholar] [CrossRef]
- Brooker, S.; Martin, S.; Pearson, A.; Bagchi, D.; Earl, J.; Gothard, L.; Hall, E.; Porter, L.; Yarnold, J. Double-blind, placebo-controlled, randomised phase II trial of IH636 grape seed proanthocyanidin extract (GSPE) in patients with radiation-induced breast induration. Radiother. Oncol. 2006, 79, 45–51. [Google Scholar] [CrossRef]
- Tannous, S.; Haykal, T.; Dhaini, J.; Hodroj, M.H.; Rizk, S. The anti-cancer effect of flaxseed lignan derivatives on different acute myeloid leukemia cancer cells. Biomed. Pharmacother. 2020, 132, 110884. [Google Scholar] [CrossRef]
- Delman, D.M.; Fabian, C.J.; Kimler, B.F.; Yeh, H.; Petroff, B.K. Effects of Flaxseed Lignan Secoisolariciresinol Diglucosideon Preneoplastic Biomarkers of Cancer Progression in a Model of Simultaneous Breast and Ovarian Cancer Development. Nutr. Cancer 2015, 67, 857–864. [Google Scholar] [CrossRef]
- Weth, F.R.; Hoggarth, G.B.; Weth, A.F.; Paterson, E.; White, M.P.J.; Tan, S.T.; Peng, L.; Gray, C. Unlocking hidden potential: Advancements, approaches, and obstacles in repurposing drugs for cancer therapy. Br. J. Cancer 2024, 130, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Zhang, Y.; Hu, D.; Tang, L.; Zhou, B.; Yang, L. Landscape of small nucleic acid therapeutics: Moving from the bench to the clinic as next-generation medicines. Signal Transduct. Target. Ther. 2025, 10, 73. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, S.; Shukla, A.K.; Ray, N.; Upadhyay, A.M.; Fahad, F.I.; Dutta, S.D.; Nagappan, A.; Mongre, R.K. Targeting Pathways and Mechanisms in Gynecological Cancer with Antioxidant and Anti-Inflammatory Phytochemical Drugs. Onco 2025, 5, 24. https://doi.org/10.3390/onco5020024
Shukla S, Shukla AK, Ray N, Upadhyay AM, Fahad FI, Dutta SD, Nagappan A, Mongre RK. Targeting Pathways and Mechanisms in Gynecological Cancer with Antioxidant and Anti-Inflammatory Phytochemical Drugs. Onco. 2025; 5(2):24. https://doi.org/10.3390/onco5020024
Chicago/Turabian StyleShukla, Sandhya, Arvind Kumar Shukla, Navin Ray, Adarsha Mahendra Upadhyay, Fowzul Islam Fahad, Sayan Deb Dutta, Arulkumar Nagappan, and Raj Kumar Mongre. 2025. "Targeting Pathways and Mechanisms in Gynecological Cancer with Antioxidant and Anti-Inflammatory Phytochemical Drugs" Onco 5, no. 2: 24. https://doi.org/10.3390/onco5020024
APA StyleShukla, S., Shukla, A. K., Ray, N., Upadhyay, A. M., Fahad, F. I., Dutta, S. D., Nagappan, A., & Mongre, R. K. (2025). Targeting Pathways and Mechanisms in Gynecological Cancer with Antioxidant and Anti-Inflammatory Phytochemical Drugs. Onco, 5(2), 24. https://doi.org/10.3390/onco5020024











