IOTA Three-Step Strategy for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Data Sources and Searches
2.3. Study Selection and Data Collection
- Prospective cohort study including patients diagnosed as having at least one adnexal mass classified using the three-step strategy after transvaginal/transabdominal ultrasound assessment.
- Report of histologic diagnosis of the adnexal mass after surgical removal or conservative management, demonstrating spontaneous resolution or persistence in cases of benign appearing masses after 12 months of follow-up scan as the reference standard.
- Presence of data reported that would allow constructing a 2 × 2 table to estimate true positive, true negative, false positive, and false negative cases for the three steps strategy.
2.4. Risk of Bias in Individual Studies
2.5. Statistical Analysis
3. Results
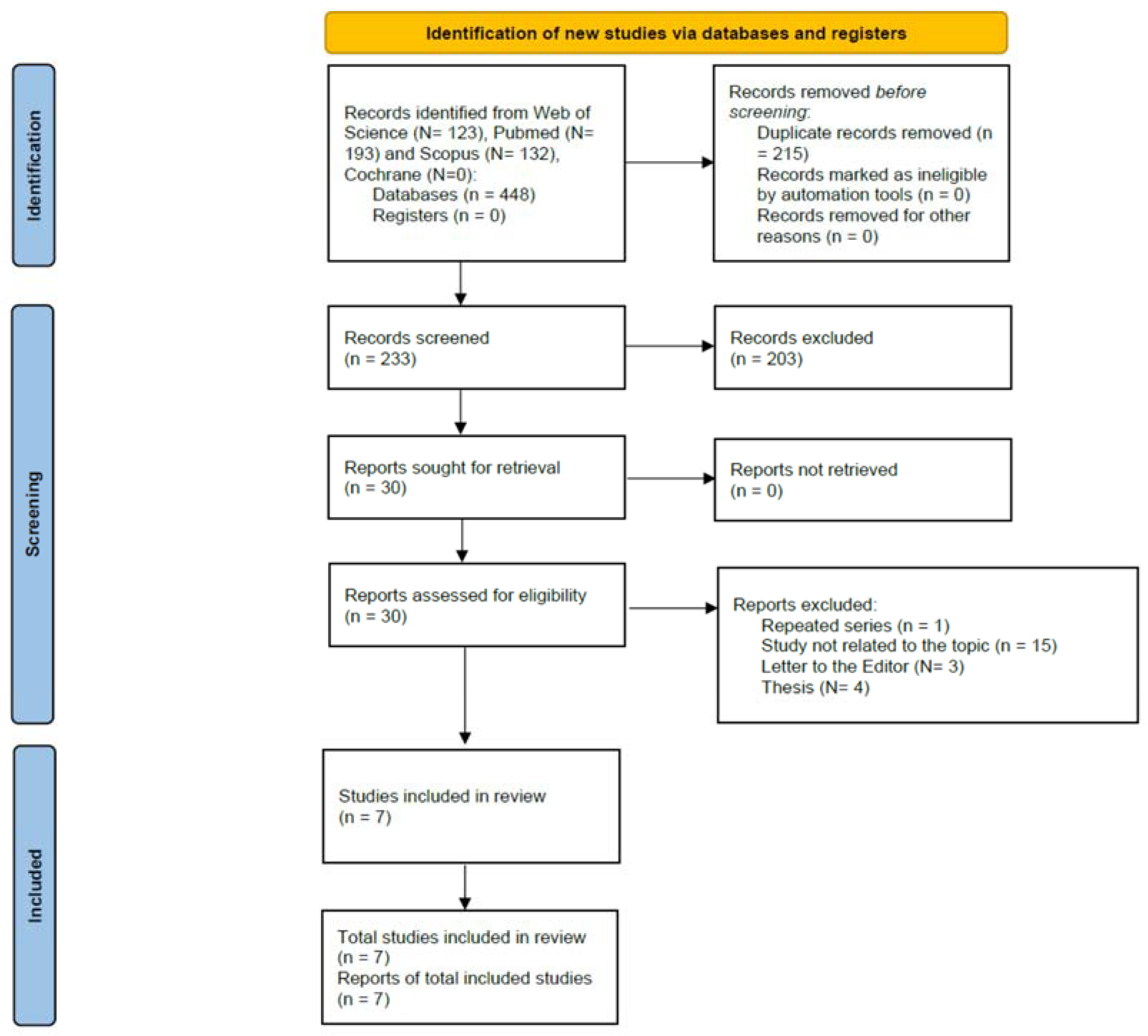
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Qualitative Synthesis
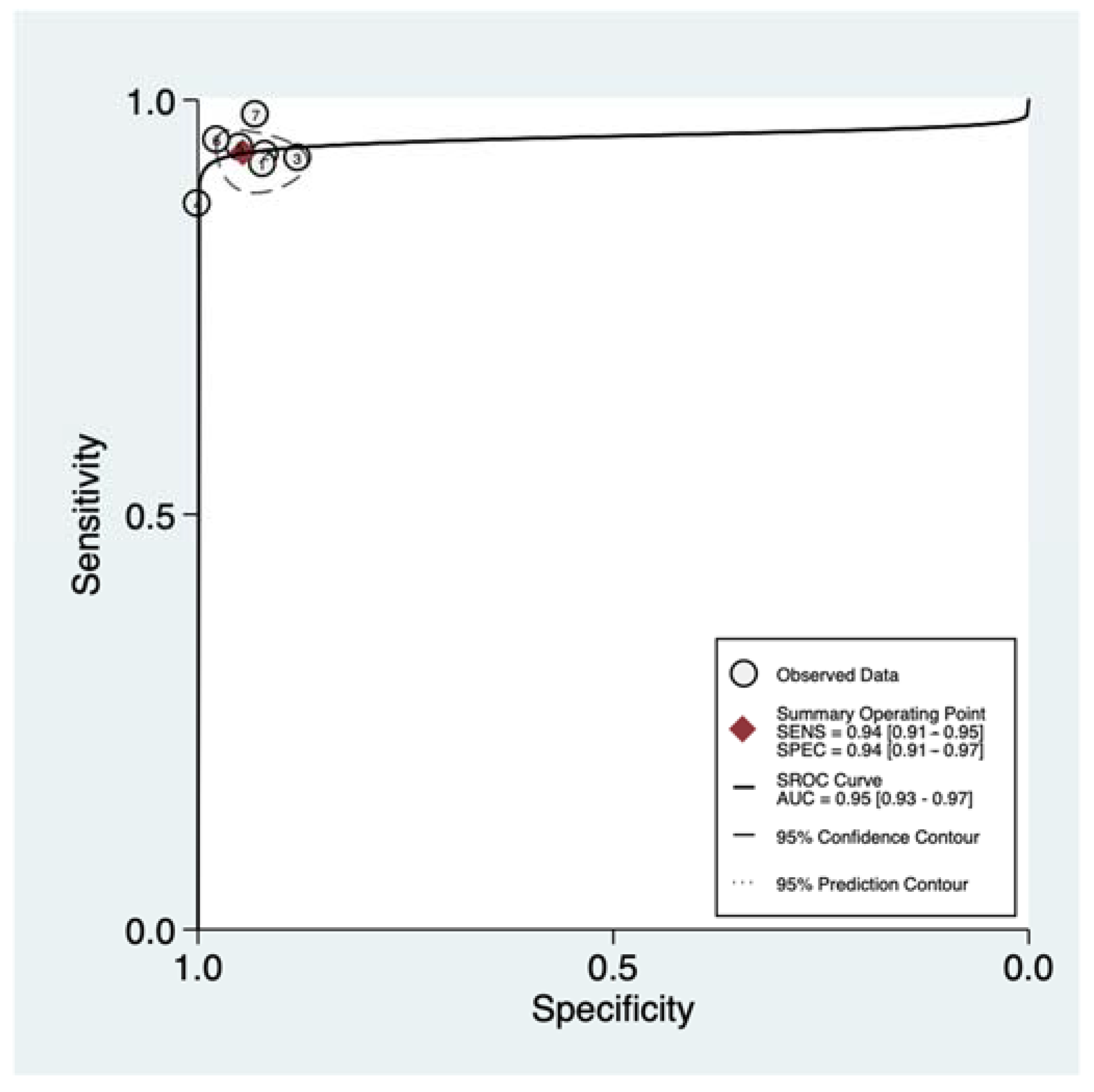
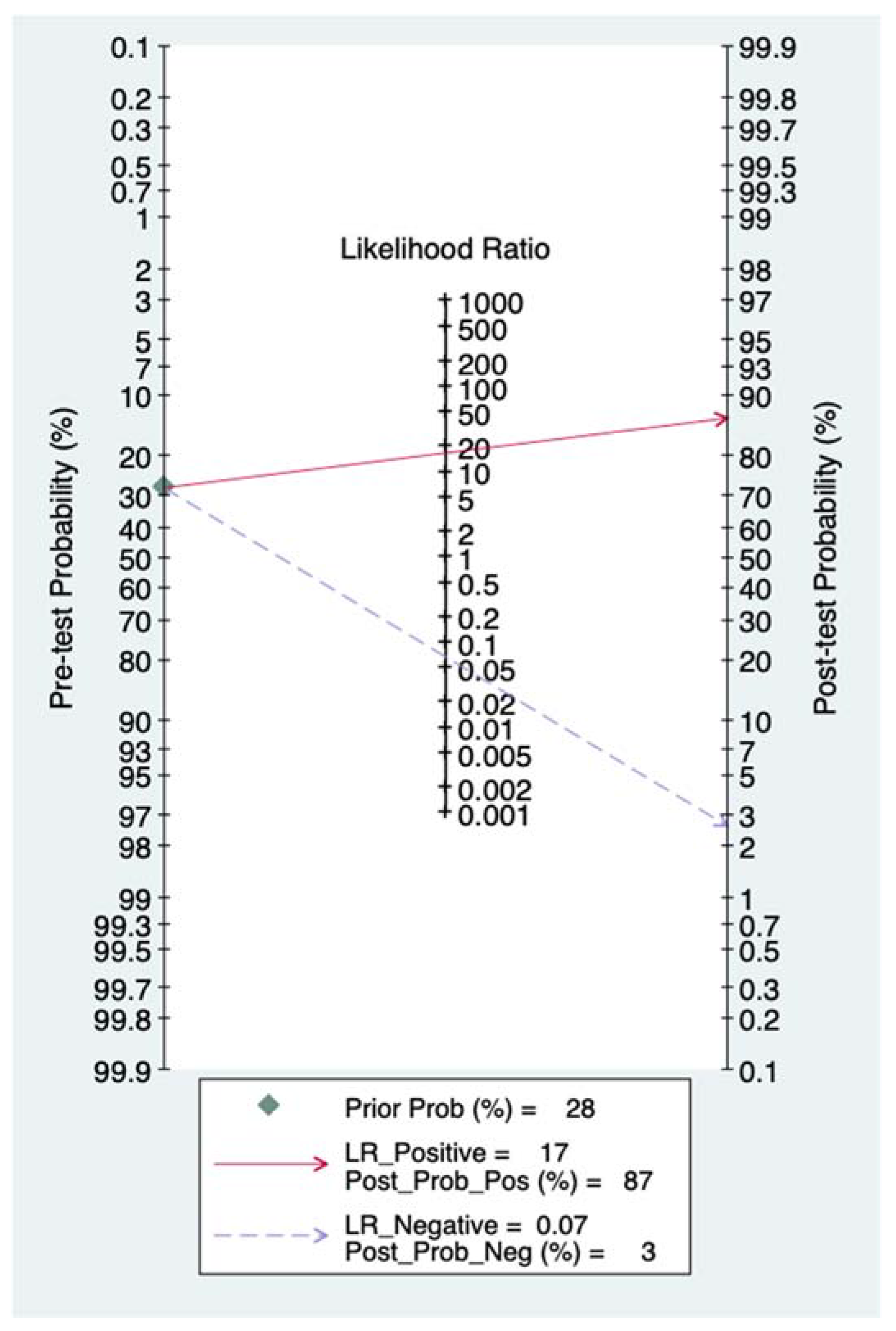
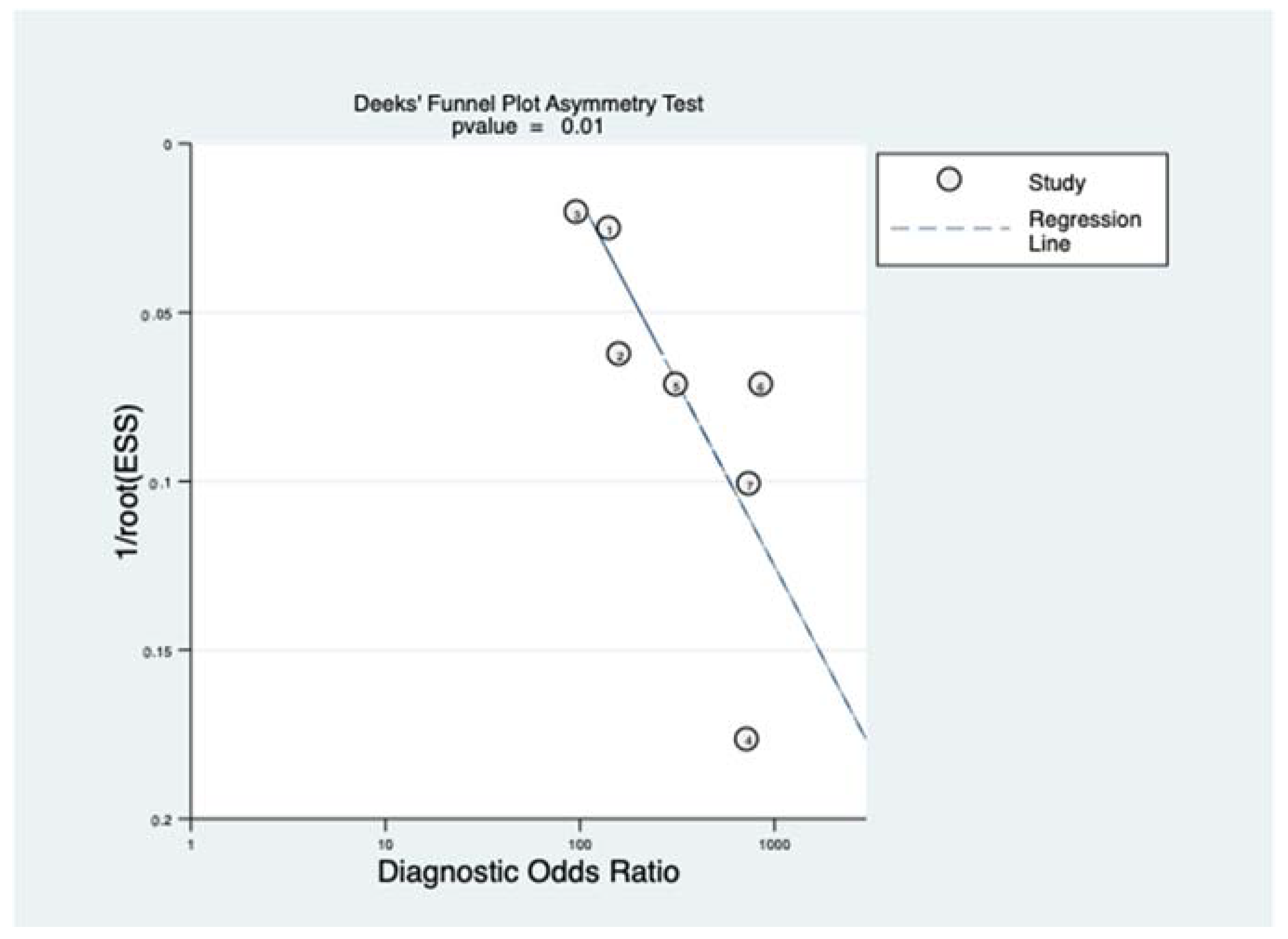
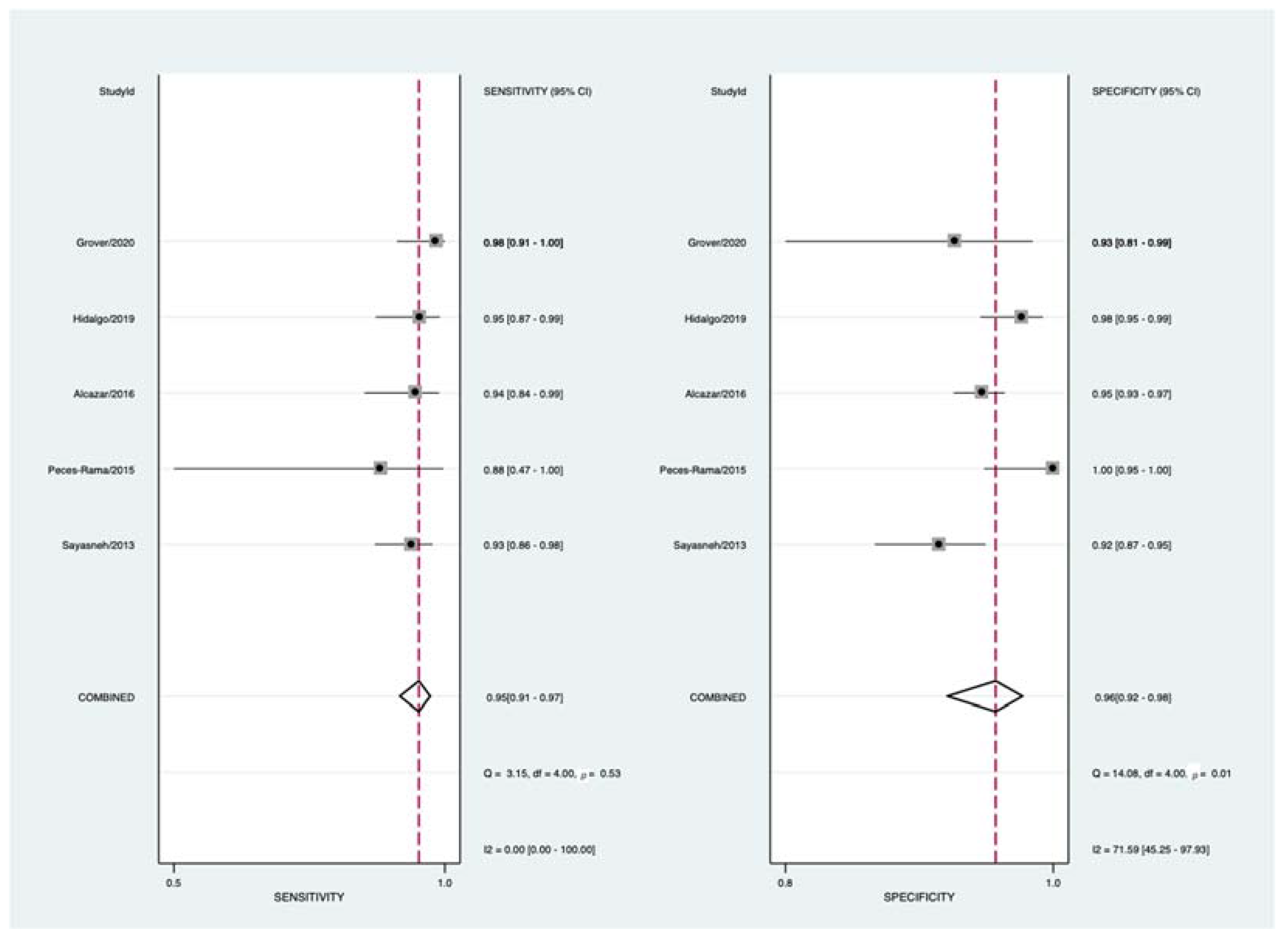
3.4. Quantitative Synthesis
4. Discussion
4.1. Summary of Evidence
4.2. Limitations and Strengths
4.3. Interpretation of Results
4.4. Future Research Agenda
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woo, Y.L.; Kyrgiou, M.; Bryant, A.; Everett, T.; Dickinson, H.O. Centralisation of services for gynaecological cancers—A Cochrane systematic review. Gynecol. Oncol. 2012, 126, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Valentin, L.; Bourne, T.H.; Collins, W.P.; Verrelst, H.; Vergote, I.; International Ovarian Tumor Analysis (IOTA) Group. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: A consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet. Gynecol. 2000, 16, 500–505. [Google Scholar] [CrossRef]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ferrazzi, E.; Ameye, L.; Konstantinovic, M.L.; Van Calster, B.; Collins, W.P.; Vergote, I.; Van Huffel, S.; et al. Logistic regression model to distinguish between the benign and malignant adnexal mass before surgery: A multicenter study by the International Ovarian Tumor Analysis Group. J. Clin. Oncol. 2005, 23, 8794–8801. [Google Scholar] [CrossRef]
- Timmerman, D.; Van Calster, B.; Testa, A.C.; Guerriero, S.; Fischerova, D.; Lissoni, A.A.; Van Holsbeke, C.; Fruscio, R.; Czekierdowski, A.; Jurkovic, D.; et al. Ovarian cancer prediction in adnexal masses using ultrasound-based logistic regression models: A temporal and external validation study by the IOTA group. Ultrasound Obstet. Gynecol. 2010, 36, 226–234. [Google Scholar] [CrossRef]
- Timmerman, D.; Testa, A.C.; Bourne, T.; Ameye, L.; Jurkovic, D.; Van Holsbeke, C.; Paladini, D.; Van Calster, B.; Vergote, I.; Van Huffel, S.; et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet. Gynecol. 2008, 31, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Ameye, L.; Timmerman, D.; Valentin, L.; Paladini, D.; Zhang, J.; Van Holsbeke, C.; Lissoni, A.A.; Savelli, L.; Veldman, J.; Testa, A.C.; et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet. Gynecol. 2012, 40, 582–591. [Google Scholar] [CrossRef]
- Van Calster, B.; Van Hoorde, K.; Valentin, L.; Testa, A.C.; Fischerova, D.; Van Holsbeke, C.; Savelli, L.; Franchi, D.; Epstein, E.; Kaijser, J.; et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ 2014, 349, g5920. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, R.F.; Timmerman, D.; Strachowski, L.M.; Froyman, W.; Benacerraf, B.R.; Bennett, G.L.; Bourne, T.; Brown, D.L.; Coleman, B.G.; Frates, M.C.; et al. O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology 2020, 294, 168–185. [Google Scholar] [CrossRef]
- Nunes, N.; Ambler, G.; Foo, X.; Naftalin, J.; Widschwendter, M.; Jurkovic, D. Use of IOTA simple rules for diagnosis of ovarian cancer: Meta-analysis. Ultrasound Obstet. Gynecol. 2014, 44, 503–514. [Google Scholar] [CrossRef]
- Kaijser, J.; Sayasneh, A.; Van Hoorde, K.; Ghaem-Maghami, S.; Bourne, T.; Timmerman, D.; Ben Van Calster, B. Presurgical diagnosis of adnexal tumours using mathematical models and scoring systems: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 449–462. [Google Scholar] [CrossRef]
- Meys, E.; Kaijser, J.; Kruitwagen, R.; Slangen, B.; Van Calster, B.; Aertgeerts, B.; Verbakel, J.; Timmerman, D.; Van Gorp, T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2016, 58, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Gareeballah, A.; Gameraddin, M.; Alshoabi, S.A.; Alsaedi, A.; Elzaki, M.; Alsharif, W.; Daoud, I.M.; Aldahery, S.; Alelyani, M.; AbdElrahim, E.; et al. The diagnostic performance of International Ovarian Tumor Analysis: Simple Rules for diagnosing ovarian tumors-a systematic review and meta-analysis. Front. Oncol. 2025, 14, 1474930. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Z.; Zhang, M.; Luo, H. Diagnostic Accuracy of the ADNEX Model for Ovarian Cancer at the 15% Cut-Off Value: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 684257. [Google Scholar] [CrossRef]
- Yue, X.; Zhong, L.; Wang, Y.; Zhang, C.; Chen, X.; Wang, S.; Hu, J.; Hu, J.; Wang, C.; Liu, X. Value of Assessment of Different Neoplasias in the Adnexa in the Differential Diagnosis of Malignant Ovarian Tumor and Benign Ovarian Tumor: A Meta-analysis. Ultrasound Med. Biol. 2022, 48, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, H.; Zhang, Y. Diagnostic Accuracies of the Ultrasound and Magnetic Resonance Imaging ADNEX Scoring Systems For Ovarian Adnexal Mass: Systematic Review and Meta-Analysis. Acad. Radiol. 2022, 29, 897–908. [Google Scholar] [CrossRef]
- Barreñada, L.; Ledger, A.; Dhiman, P.; Collins, G.; Wynants, L.; Verbakel, J.Y.; Timmerman, D.; Valentin, L.; Van Calster, B. ADNEX risk prediction model for diagnosis of ovarian cancer: Systematic review and meta-analysis of external validation studies. BMJ Med. 2024, 3, e000817. [Google Scholar] [CrossRef]
- Vara, J.; Manzour, N.; Chacón, E.; López-Picazo, A.; Linares, M.; Pascual, M.Á.; Guerriero, S.; Alcázar, J.L. Ovarian Adnexal Reporting Data System (O-RADS) for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 3151. [Google Scholar] [CrossRef]
- Zhang, Q.; Dai, X.; Li, W. Systematic Review and Meta-Analysis of O-RADS Ultrasound and O-RADS MRI for Risk Assessment of Ovarian and Adnexal Lesions. AJR Am. J. Roentgenol. 2023, 221, 21–33. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.E.; Hwang, J.A.; Shin, H. O-RADS US: A Systematic Review and Meta- Analysis of Category-specific Malignancy Rates. Radiology 2023, 308, e223269. [Google Scholar] [CrossRef]
- Han, J.; Wen, J.; Hu, W. Comparison of O-RADS with the ADNEX model and IOTA SR for risk stratification of adnexal lesions: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1354837. [Google Scholar] [CrossRef]
- Perez, M.; Messeguer, A.; Vara, J.; Vilches, J.C.; Brunel, I.; Lozano, M.; Orozco, R.; Alcazar, J.L. GI-RADS versus O-RADS in the differential diagnosis of adnexal masses: A systematic review and head-to-head meta-analysis. Ultrasonography 2024, 43, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Sotiriadis, A.; Papatheodorou, S.I.; Martins, W.P. Synthesizing Evidence from Diagnostic Accuracy TEsts: The SEDATE guideline. Ultrasound Obstet. Gynecol. 2016, 47, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Sayasneh, A.; Kaijser, J.; Preisler, J.; Johnson, S.; Stalder, C.; Husicka, R.; Guha, S.; Naji, O.; Abdallah, Y.; Raslan, F.; et al. A multicenter prospective external validation of the diagnostic performance of IOTA simple descriptors and rules to characterize ovarian masses. Gynecol. Oncol. 2013, 130, 140–146. [Google Scholar] [CrossRef]
- Testa, A.; Kaijser, J.; Wynants, L.; Fischerova, D.; Van Holsbeke, C.; Franchi, D.; Savelli, L.; Epstein, E.; Czekierdowski, A.; Guerriero, S.; et al. Strategies to diagnose ovarian cancer: New evidence from phase 3 of the multicentre international IOTA study. Br. J. Cancer 2014, 111, 680–688. [Google Scholar] [CrossRef]
- Rama, A.P.; Llanos, M.C.L.; Ferrer, M.L.S.; Zambrano, J.L.A.; Mendoza, A.M.; Díaz, A.N. Simple descriptors and simple rules of the International Ovarian Tumor Analysis (IOTA) Group: A prospective study of combined use for the description of adnexal masses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 7–11. [Google Scholar] [CrossRef]
- Alcázar, J.L.; Pascual, M.A.; Graupera, B.; Aubá, M.; Errasti, T.; Olartecoechea, B.; Ruiz-Zambrana, A.; Hereter, L.; Ajossa, S.; Guerriero, S. External validation of IOTA simple descriptors and simple rules for classifying adnexal masses. Ultrasound Obstet. Gynecol. 2016, 48, 397–402. [Google Scholar] [CrossRef]
- Hidalgo, J.J.; Ros, F.; Aubá, M.; Errasti, T.; Olartecoechea, B.; Ruiz-Zambrana, Á.; Alcázar, J.L. Prospective external validation of IOTA three-step strategy for characterizing and classifying adnexal masses and retrospective assessment of alternative two-step strategy using simple-rules risk. Ultrasound Obstet. Gynecol. 2019, 53, 693–700. [Google Scholar] [CrossRef]
- Grover, S.B.; Patra, S.; Grover, H.; Mittal, P.; Khanna, G. Prospective revalidation of IOTA “two-step”, “alternative two-step” and “three-step” strategies for characterization of adnexal masses—An Indian study focussing the radiology context. Indian J. Radiol. Imaging 2020, 30, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. ESGO/ISUOG/IOTA/ESGE Consensus Statement on preoperative diagnosis of ovarian tumors. Ultrasound Obstet. Gynecol. 2021, 58, 148–168. [Google Scholar] [CrossRef]
- Landolfo, C.; Bourne, T.; Froyman, W.; Van Calster, B.; Ceusters, J.; Testa, A.C.; Wynants, L.; Sladkevicius, P.; Van Holsbeke, C.; Domali, E.; et al. Benign descriptors and ADNEX in two-step strategy to estimate risk of malignancy in ovarian tumors: Retrospective validation in IOTA5 multicenter cohort. Ultrasound Obstet. Gynecol. 2023, 61, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Alcazar, J.L.; Pascual, M.A.; Ajossa, S.; Gerada, M.; Bargellini, R.; Virgilio, B.; Melis, G.B. Diagnosis of the most frequent benign ovarian cysts: Is ultrasonography accurate and reproducible? J. Womens Health 2009, 18, 519–527. [Google Scholar] [CrossRef]
- de Gauna, B.R.; Sanchez, P.; Pineda, L.; Utrilla-Layna, J.; Juez, L.; Alcázar, J.L. Interobserver agreement in describing adnexal masses using the International Ovarian Tumor Analysis simple rules in a real-time setting and using three- dimensional ultrasound volumes and digital clips. Ultrasound Obstet. Gynecol. 2014, 44, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, L.; Savelli, L.; Jokubkiene, L.; Di Legge, A.; Condous, G.; Testa, A.C.; Sladkevicius, P.; Valentin, L. Intra- and interobserver agreement with regard to describing adnexal masses using International Ovarian Tumor Analysis terminology: Reproducibility study involving seven observers. Ultrasound Obstet. Gynecol. 2014, 44, 100–108. [Google Scholar] [CrossRef]
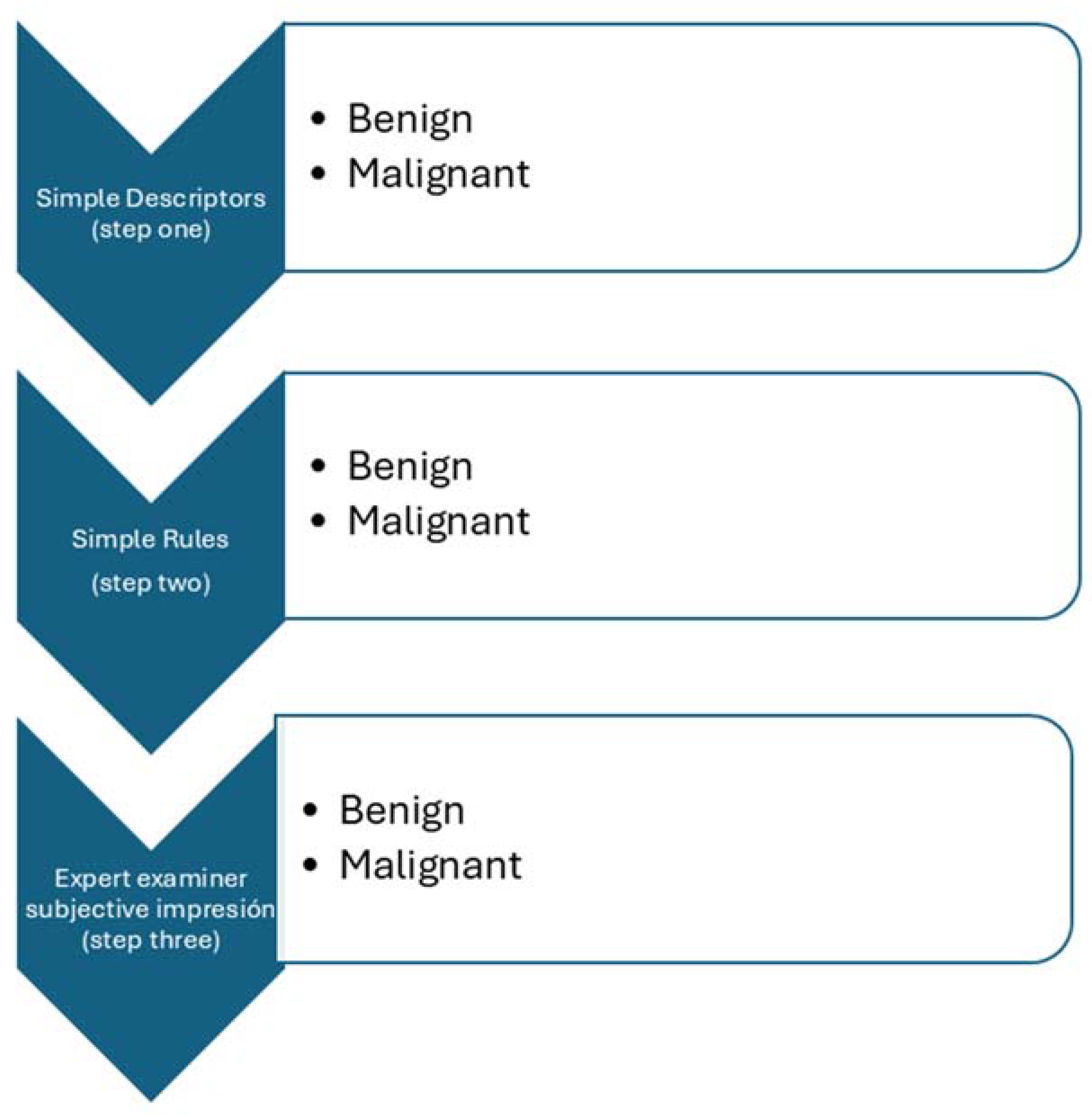

| Benign Descriptors | Malignant Descriptors |
|---|---|
| Unilocular tumor with ground glass echogenicity in premenopausal age. | Tumor with ascites and at least moderate color Doppler blood flow in postmenopausal women. |
| Unilocular tumor with mixed echogenicity and acoustic shadows in premenopausal age. Unilocular tumor anechoic tumor with regular walls and largest diameter of less than 100 mm. | Clinical: Women aged > 50 years and Laboratory: Serum CA-125 > 100. |
| Benign Features | Malignant Features |
|---|---|
| B1 Unilocular cyst B2 Solid components < 7 mm B3 Acoustic shadows B4 Smooth multilocular cyst < 100 mm B5 Color flow score 1 | M1 Irregular solid tumor M2 Ascites M3 At least 4 papillary projections M4 Irregular multilocular solid > 100 mm M5 Color flow score 4 |
| Author | Year | Country | Study Design | N | N Malignancy | N Centres | Index Test | N Observers | Reference Test | Flow and Timing *** |
|---|---|---|---|---|---|---|---|---|---|---|
| Ameye et al. [6] | 2012 | Belgium * | Prospective | 1938 | 951 | 21 | IOTA 3-step | Multiple ** | Histology | <120 days |
| Sayasneh et al. [26] | 2013 | UK | Prospective | 301 | 92 | 3 | IOTA 3-step | 36 | Histology | <120 days |
| Testa et al. [27] | 2014 | Italy * | Prospective | 2403 | 980 | 18 | IOTA 3-step | Multiple ** | Histology | <120 days |
| Peces-Rama et al. [28] | 2015 | Spain | Prospective | 81 | 7 | 1 | IOTA 3-step | 3 | Histology or FU | <120 days |
| Alcázar et al. [29] | 2016 | Spain | Prospective | 666 | 53 | 2 | IOTA 3-step | 9 | Histology or FU | <120 days |
| Hidalgo et al. [30] | 2019 | Spain | Prospective | 283 | 62 | 2 | IOTA 3-step | 6 | Histology or FU | <60 days |
| Grover et al. [31] | 2020 | India | Prospective | 100 | 57 | 1 | IOTA 3-step | 2 | Histology | N.A. |
| Study | Patient Selection | Index Test | Reference Test | Flow and Timing |
|---|---|---|---|---|
| Ameye [6] | Low | Low | Low | Low |
| Sayasneh [26] | Low | High | Low | Low |
| Testa [27] | Low | Low | Low | Low |
| Peces-Rama [28] | Low | Low | Low | Low |
| Alcázar [29] | Low | Low | Low | Low |
| Hidalgo [30] | Low | Low | Low | Low |
| Grover [31] | Low | Low | Low | Unclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcázar, J.L.; Vargas, F.; Boscá, G.; Salazar, B.; Aguilar, J.C.; Catalan, C.; Balazs, A.; Burky, D.; Pertkiewicz, M.; Vilches, J.C.; et al. IOTA Three-Step Strategy for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis. Onco 2025, 5, 22. https://doi.org/10.3390/onco5020022
Alcázar JL, Vargas F, Boscá G, Salazar B, Aguilar JC, Catalan C, Balazs A, Burky D, Pertkiewicz M, Vilches JC, et al. IOTA Three-Step Strategy for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis. Onco. 2025; 5(2):22. https://doi.org/10.3390/onco5020022
Chicago/Turabian StyleAlcázar, Juan Luis, Francisco Vargas, Guillem Boscá, Blanca Salazar, Juan Carlos Aguilar, Cynthia Catalan, Arleana Balazs, Daniela Burky, Magdalena Pertkiewicz, José Carlos Vilches, and et al. 2025. "IOTA Three-Step Strategy for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis" Onco 5, no. 2: 22. https://doi.org/10.3390/onco5020022
APA StyleAlcázar, J. L., Vargas, F., Boscá, G., Salazar, B., Aguilar, J. C., Catalan, C., Balazs, A., Burky, D., Pertkiewicz, M., Vilches, J. C., & Orozco, R. (2025). IOTA Three-Step Strategy for Classifying Adnexal Masses: A Systematic Review and Meta-Analysis. Onco, 5(2), 22. https://doi.org/10.3390/onco5020022






