The Emerging Applications of Raman Spectroscopy in Clinical Oncology: A Narrative Review Focused on Circulating Tumor DNA Detection and Therapeutic Drug Monitoring
Abstract
Simple Summary
Abstract
1. Introduction
2. Description of Raman and SERS
3. The Potential Applications of SERS in Clinical Oncology
4. The Potential Role for SERS in TDM
5. The Potential Role for SERS in ctDNA Analysis
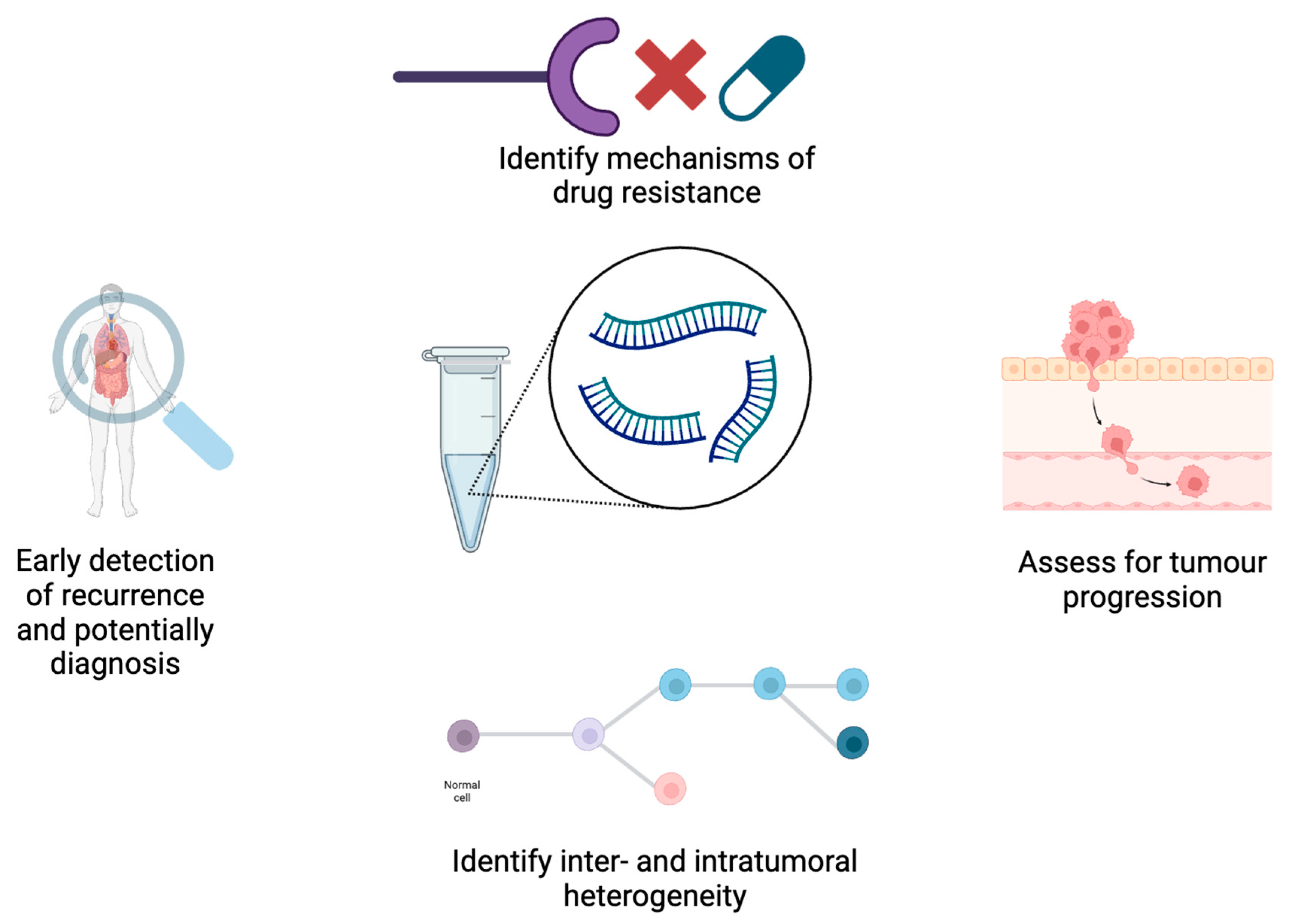
5.1. Applications for ctDNA Analysis in Oncology
5.2. Current Obstacles to Clinical Implementation of ctDNA Analysis in Oncology
5.3. The Role of SERS in ctDNA Detection
6. Conclusions and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783. [Google Scholar] [CrossRef]
- Vázquez-Iglesias, L.; Casagrande, G.M.S.; García-Lojo, D.; Leal, L.F.; Ngo, T.A.; Pérez-Juste, J.; Reis, R.M.; Kant, K.; Pastoriza-Santos, I. SERS sensing for cancer biomarker: Approaches and directions. Bioact. Mater. 2024, 34, 248–268. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131–140. [Google Scholar] [CrossRef]
- Zheng, X.S.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Label-free SERS in biological and biomedical applications: Recent progress, current challenges and opportunities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 197, 56–77. [Google Scholar] [CrossRef]
- Constantinou, M.; Hadjigeorgiou, K.; Abalde-Cela, S.; Andreou, C. Label-Free Sensing with Metal Nanostructure-Based Surface-Enhanced Raman Spectroscopy for Cancer Diagnosis. ACS Appl. Nano Mater. 2022, 5, 12276–12299. [Google Scholar] [CrossRef]
- Wang, J.; Koo, K.M.; Wee, E.J.; Wang, Y.; Trau, M. A nanoplasmonic label-free surface-enhanced Raman scattering strategy for non-invasive cancer genetic subtyping in patient samples. Nanoscale 2017, 9, 3496–3503. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, X.; Rong, Z.; Xiu, B.; Yang, X.; Wang, C.; Hao, W.; Zhang, Q.; Liu, Z.; Duan, C.; et al. Non-invasive detection of hepatocellular carcinoma serum metabolic profile through surface-enhanced Raman spectroscopy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2475–2484. [Google Scholar] [CrossRef]
- Wang, Y.; Schlücker, S. Rational design and synthesis of SERS labels. Analyst 2013, 138, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Rodal-Cedeira, S.; Vazquez-Arias, A.; Bodelon, G.; Skorikov, A.; Nunez-Sanchez, S.; Laporta, A.; Polavarapu, L.; Bals, S.; Liz-Marzan, L.M.; Perez-Juste, J. An expanded surface-enhanced Raman scattering tags library by combinatorial encapsulation of reporter molecules in metal nanoshells. ACS Nano 2020, 14, 14655–14664. [Google Scholar] [CrossRef]
- Fenn, M.B.; Xanthopoulos, P.; Pyrgiotakis, G.; Grobmyer, S.R.; Pardalos, P.M.; Hench, L.L. Raman spectroscopy for clinical oncology. Adv. Opt. Technol. 2011, 2011, 213783. [Google Scholar] [CrossRef]
- Koo, K.M.; Wang, J.; Richards, R.S.; Farrell, A.; Yaxley, J.W.; Samaratunga, H.; Teloken, P.E.; Roberts, M.J.; Coughlin, G.D.; Lavin, M.F. Design and clinical verification of surface-enhanced Raman spectroscopy diagnostic technology for individual cancer risk prediction. ACS Nano 2018, 12, 8362–8371. [Google Scholar] [CrossRef]
- Bai, X.-R.; Wang, L.-H.; Ren, J.-Q.; Bai, X.-W.; Zeng, L.-W.; Shen, A.-G.; Hu, J.-M. Accurate clinical diagnosis of liver cancer based on simultaneous detection of ternary specific antigens by magnetic induced mixing surface-enhanced Raman scattering emissions. Anal. Chem. 2019, 91, 2955–2963. [Google Scholar] [CrossRef]
- Lee, J.U.; Kim, W.H.; Lee, H.S.; Park, K.H.; Sim, S.J. Quantitative and Specific Detection of Exosomal miRNAs for Accurate Diagnosis of Breast Cancer Using a Surface-Enhanced Raman Scattering Sensor Based on Plasmonic Head-Flocked Gold Nanopillars. Small 2019, 15, e1804968. [Google Scholar] [CrossRef]
- Davis, R.M.; Kiss, B.; Trivedi, D.R.; Metzner, T.J.; Liao, J.C.; Gambhir, S.S. Surface-enhanced Raman scattering nanoparticles for multiplexed imaging of bladder cancer tissue permeability and molecular phenotype. ACS Nano 2018, 12, 9669–9679. [Google Scholar] [CrossRef]
- Dong, S.; He, D.; Zhang, Q.; Huang, C.; Hu, Z.; Zhang, C.; Nie, L.; Wang, K.; Luo, W.; Yu, J. Early cancer detection by serum biomolecular fingerprinting spectroscopy with machine learning. eLight 2023, 3, 17. [Google Scholar] [CrossRef]
- Allsbrook, W.C., Jr.; Mangold, K.A.; Johnson, M.H.; Lane, R.B.; Lane, C.G.; Epstein, J.I. Interobserver reproducibility of Gleason grading of prostatic carcinoma: General pathologist. Hum. Pathol. 2001, 32, 81–88. [Google Scholar] [CrossRef]
- Egevad, L.; Allsbrook, W.C.; Epstein, J.I. Current practice of Gleason grading among genitourinary pathologists. Hum. Pathol. 2005, 36, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ladekarl, M. Objective malignancy grading: A review emphasizing unbiased stereology applied to breast tumors. APMIS. Suppl. 1998, 79, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Kalyan Kumar, K.; Chowdary, M.; Mathew, S.; Rao, L.; Murali Krishna, C.; Kurien, J. Raman spectroscopic diagnosis of breast cancers: Evaluation of models. J. Raman Spectrosc. 2008, 39, 1276–1282. [Google Scholar] [CrossRef]
- Rehman, S.; Movasaghi, Z.; Tucker, A.T.; Joel, S.P.; Darr, J.A.; Ruban, A.V.; Rehman, I.U. Raman spectroscopic analysis of breast cancer tissues: Identifying differences between normal, invasive ductal carcinoma and ductal carcinoma in situ of the breast tissue. J. Raman Spectrosc. 2007, 38, 1345–1351. [Google Scholar] [CrossRef]
- Larraona-Puy, M.; Ghita, A.; Zoladek, A.; Perkins, W.; Varma, S.; Leach, I.H.; Koloydenko, A.A.; Williams, H.; Notingher, I. Development of Raman microspectroscopy for automated detection and imaging of basal cell carcinoma. J. Biomed. Opt. 2009, 14, 054031. [Google Scholar] [CrossRef]
- Beljebbar, A.; Dukic, S.; Amharref, N.; Manfait, M. Ex vivo and in vivo diagnosis of C6 glioblastoma development by Raman spectroscopy coupled to a microprobe. Anal. Bioanal. Chem. 2010, 398, 477–487. [Google Scholar] [CrossRef]
- Teh, S.; Zheng, W.; Ho, K.; Teh, M.; Yeoh, K.; Huang, Z. Near-infrared Raman spectroscopy for early diagnosis and typing of adenocarcinoma in the stomach. J. Br. Surg. 2010, 97, 550–557. [Google Scholar] [CrossRef]
- Lyng, F.M.; Faoláin, E.Ó.; Conroy, J.; Meade, A.; Knief, P.; Duffy, B.; Hunter, M.; Byrne, J.; Kelehan, P.; Byrne, H. Vibrational spectroscopy for cervical cancer pathology, from biochemical analysis to diagnostic tool. Exp. Mol. Pathol. 2007, 82, 121–129. [Google Scholar] [CrossRef]
- Jeevan, R.; Cromwell, D.; Trivella, M.; Lawrence, G.; Kearins, O.; Pereira, J.; Sheppard, C.; Caddy, C.; Van Der Meulen, J. Reoperation rates after breast conserving surgery for breast cancer among women in England: Retrospective study of hospital episode statistics. BMJ 2012, 345, e4505. [Google Scholar] [CrossRef]
- Short, M.A.; Lam, S.; McWilliams, A.; Zhao, J.; Lui, H.; Zeng, H. Development and preliminary results of an endoscopic Raman probe for potential in vivo diagnosis of lung cancers. Opt. Lett. 2008, 33, 711–713. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Zheng, W.; Lin, K.; Ho, K.Y.; Teh, M.; Yeoh, K.G.; So, J.B.Y.; Huang, Z. Combining near-infrared-excited autofluorescence and Raman spectroscopy improves in vivo diagnosis of gastric cancer. Biosens. Bioelectron. 2011, 26, 4104–4110. [Google Scholar] [CrossRef] [PubMed]
- Shipp, D.W.; Rakha, E.A.; Koloydenko, A.A.; Macmillan, R.D.; Ellis, I.O.; Notingher, I. Intra-operative spectroscopic assessment of surgical margins during breast conserving surgery. Breast Cancer Res. 2018, 20, 69. [Google Scholar] [CrossRef]
- Lin, Y.S.; Thummel, K.E.; Thompson, B.D.; Totah, R.A.; Cho, C.W. Sources of Interindividual Variability; Springer: New York, NY, USA, 2021; pp. 481–550. [Google Scholar]
- Ingelman-Sundberg, M. Pharmacogenetics: An opportunity for a safer and more efficient pharmacotherapy. J. Intern. Med. 2001, 250, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.A.; Chatelut, E.; Fotoohi, A.K.; Larson, R.A.; Martin, J.H.; Mathijssen, R.H.J.; Salamone, S.J. Therapeutic drug monitoring in oncology: International Association of Therapeutic Drug Monitoring and Clinical Toxicology consensus guidelines for imatinib therapy. Eur. J. Cancer 2021, 157, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Groenland, S.L.; Van Eerden, R.A.G.; Verheijen, R.B.; Koolen, S.L.W.; Moes, D.J.A.R.; Desar, I.M.E.; Reyners, A.K.L.; Gelderblom, H.J.; Van Erp, N.P.; Mathijssen, R.H.J.; et al. Therapeutic Drug Monitoring of Oral Anticancer Drugs: The Dutch Pharmacology Oncology Group–Therapeutic Drug Monitoring Protocol for a Prospective Study. Ther. Drug Monit. 2019, 41, 561–567. [Google Scholar] [CrossRef]
- Dasgupta, A.; Krasowski, M. Therapeutic Drug Monitoring Data: A Concise Guide; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Xu, Q.A.; Madden, T.L. Analytical Methods for Therapeutic Drug Monitoring and Toxicology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dougan, J.A.; Faulds, K. Surface enhanced Raman scattering for multiplexed detection. Analyst 2012, 137, 545–554. [Google Scholar] [CrossRef]
- Kämmer, E.; Olschewski, K.; Bocklitz, T.; Rösch, P.; Weber, K.; Cialla, D.; Popp, J. A new calibration concept for a reproducible quantitative detection based on SERS measurements in a microfluidic device demonstrated on the model analyte adenine. Phys. Chem. Chem. Phys. 2014, 16, 9056–9063. [Google Scholar] [CrossRef]
- Fang, Y.; Seong, N.-H.; Dlott, D.D. Measurement of the distribution of site enhancements in surface-enhanced Raman scattering. Science 2008, 321, 388–392. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, W.; Liu, J.; Nie, Y.; Li, P.; He, C. Surface-enhanced Raman scattering sensing of trace fenthion coupled with stable silver colloids and OH stretching band of water as an internal standard. J. Anal. Chem. 2016, 71, 891–895. [Google Scholar] [CrossRef]
- Bonifacio, A. Label-free surface-enhanced Raman scattering for clinical applications. In Principles and Clinical Diagnostic Applications of Surface-Enhanced Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 125–170. [Google Scholar]
- Jaworska, A.; Fornasaro, S.; Sergo, V.; Bonifacio, A. Potential of surface enhanced Raman spectroscopy (SERS) in therapeutic drug monitoring (TDM). A critical review. Biosensors 2016, 6, 47. [Google Scholar] [CrossRef]
- Beljebbar, A.; Sockalingum, G.; Angiboust, J.; Manfait, M. Comparative FT SERS, resonance Raman and SERRS studies of doxorubicin and its complex with DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 2083–2090. [Google Scholar] [CrossRef]
- Durucan, O.; Rindzevicius, T.; Schmidt, M.S.; Matteucci, M.; Boisen, A. Nanopillar filters for surface-enhanced Raman spectroscopy. ACS Sens. 2017, 2, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Ramírez-García, G.; Sidhik, S.; Lopez-Luke, T.; Rodriguez-Gonzalez, C.; Ciapara, I.H.; Castillo, P.S.; Camacho-Villegas, T.; De la Rosa, E. Ultrasensitive SERS substrate for label-free therapeutic-drug monitoring of paclitaxel and cyclophosphamide in blood serum. Anal. Chem. 2018, 91, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Panikar, S.S.; Banu, N.; Escobar, E.-R.; García, G.-R.; Cervantes-Martínez, J.; Villegas, T.-C.; Salas, P.; De la Rosa, E. Stealth modified bottom up SERS substrates for label-free therapeutic drug monitoring of doxorubicin in blood serum. Talanta 2020, 218, 121138. [Google Scholar] [CrossRef] [PubMed]
- Pannico, M.; Musto, P. SERS spectroscopy for the therapeutic drug monitoring of the anticancer drug 6-Mercaptopurine: Molecular and kinetic studies. Appl. Surf. Sci. 2021, 539, 148225. [Google Scholar] [CrossRef]
- Goksel, Y.; Dumont, E.; Slipets, R.; Rajendran, S.T.; Sarikaya, S.; Thamdrup, L.H.; Schmiegelow, K.; Rindzevicius, T.; Zor, K.; Boisen, A. Methotrexate detection in serum at clinically relevant levels with electrochemically assisted SERS on a benchtop, custom built Raman spectrometer. ACS Sens. 2022, 7, 2358–2369. [Google Scholar] [CrossRef]
- Vicario, A.; Sergo, V.; Toffoli, G.; Bonifacio, A. Surface-enhanced Raman spectroscopy of the anti-cancer drug irinotecan in presence of human serum albumin. Colloids Surf. B Biointerfaces 2015, 127, 41–46. [Google Scholar] [CrossRef]
- Zhang, W.-S.; Wang, Y.-N.; Wang, Y.; Xu, Z.-R. Highly reproducible and fast detection of 6-thioguanine in human serum using a droplet-based microfluidic SERS system. Sens. Actuators B Chem. 2019, 283, 532–537. [Google Scholar] [CrossRef]
- Mueller-Schoell, A.; Groenland, S.L.; Scherf-Clavel, O.; van Dyk, M.; Huisinga, W.; Michelet, R.; Jaehde, U.; Steeghs, N.; Huitema, A.D.; Kloft, C. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur. J. Clin. Pharmacol. 2021, 77, 441–464. [Google Scholar] [CrossRef]
- Fornasaro, S.; Bonifacio, A.; Marangon, E.; Buzzo, M.; Toffoli, G.; Rindzevicius, T.; Schmidt, M.S.; Sergo, V. Label-free quantification of anticancer drug imatinib in human plasma with surface enhanced Raman spectroscopy. Anal. Chem. 2018, 90, 12670–12677. [Google Scholar] [CrossRef]
- Pearce, C.M.; Resmini, M. Towards point of care systems for the therapeutic drug monitoring of imatinib. Anal. Bioanal. Chem. 2020, 412, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Sahu, A.; Gota, V.; Martínez-Torres, P.; Pichardo-Molina, J.; Murali Krishna, C. Raman spectroscopy for detection of imatinib in plasma: A proof of concept. J. Innov. Opt. Health Sci. 2015, 8, 1550019. [Google Scholar] [CrossRef]
- Litti, L.; Ramundo, A.; Biscaglia, F.; Toffoli, G.; Gobbo, M.; Meneghetti, M. A surface enhanced Raman scattering based colloid nanosensor for developing therapeutic drug monitoring. J. Colloid. Interface Sci. 2019, 533, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Litti, L.; Trivini, S.; Ferraro, D.; Reguera, J. 3D printed microfluidic device for magnetic trapping and SERS quantitative evaluation of environmental and biomedical analytes. ACS Appl. Mater. Interfaces 2021, 13, 34752–34761. [Google Scholar] [CrossRef] [PubMed]
- Zwart, T.C.; Gokoel, S.R.; van der Boog, P.J.; de Fijter, J.W.; Kweekel, D.M.; Swen, J.J.; Guchelaar, H.J.; Moes, D.J.A. Therapeutic drug monitoring of tacrolimus and mycophenolic acid in outpatient renal transplant recipients using a volumetric dried blood spot sampling device. Br. J. Clin. Pharmacol. 2018, 84, 2889–2902. [Google Scholar] [CrossRef]
- Martial, L.C.; Hoogtanders, K.E.; Schreuder, M.F.; Cornelissen, E.A.; van der Heijden, J.; Joore, M.A.; Van Maarseveen, E.M.; Burger, D.M.; Croes, S.; Brüggemann, R.J. Dried blood spot sampling for tacrolimus and mycophenolic acid in children: Analytical and clinical validation. Ther. Drug Monit. 2017, 39, 412–421. [Google Scholar] [CrossRef]
- Wilhelm, A.J.; den Burger, J.C.; Swart, E.L. Therapeutic drug monitoring by dried blood spot: Progress to date and future directions. Clin. Pharmacokinet. 2014, 53, 961–973. [Google Scholar] [CrossRef]
- Zailani, N.N.B.; Ho, P.C.-L. Dried blood spots—A platform for therapeutic drug monitoring (TDM) and drug/disease response monitoring (DRM). Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 467–494. [Google Scholar] [CrossRef]
- Narayanan, S.; Yuile, A.; Venkatesh, B.; McKay, M.; Itchins, M.; Pavlakis, N.; Wheeler, H.; Gray, L.; Wei, J.; Miller, S. Therapeutic drug monitoring of osimertinib in EGFR mutant non-small cell lung cancer by dried blood spot and plasma collection: A pilot study. Br. J. Clin. Pharmacol. 2024, 90, 1942–1951. [Google Scholar] [CrossRef]
- Yu, W.W.; White, I.M. Inkjet printed surface enhanced Raman spectroscopy array on cellulose paper. Anal. Chem. 2010, 82, 9626–9630. [Google Scholar] [CrossRef]
- Mandel, P.; Métais, P. Les acides nucléiques du plasma sanguin chez l’Homme. CR Seances Soc. Biol. Fil. 1948, 142, 241–243. [Google Scholar] [PubMed]
- Stejskal, P.; Goodarzi, H.; Srovnal, J.; Hajdúch, M.; Van’T Veer, L.J.; Magbanua, M.J.M. Circulating tumor nucleic acids: Biology, release mechanisms, and clinical relevance. Mol. Cancer 2023, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Mair, R.; Mouliere, F.; Smith, C.G.; Chandrananda, D.; Gale, D.; Marass, F.; Tsui, D.W.; Massie, C.E.; Wright, A.J.; Watts, C. Measurement of plasma cell-free mitochondrial tumor DNA improves detection of glioblastoma in patient-derived orthotopic xenograft models. Cancer Res. 2019, 79, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Q.; Raleigh, J.M.; Callahan, J.; Vergara, I.A.; Ftouni, S.; Hatzimihalis, A.; Colebatch, A.J.; Li, J.; Semple, T.; Doig, K. Circulating tumor DNA analysis and functional imaging provide complementary approaches for comprehensive disease monitoring in metastatic melanoma. Precis. Oncol. 2017, 1, 1–14. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Koldobskiy, M.A.; Göndör, A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016, 17, 284–299. [Google Scholar] [CrossRef]
- Gray, J.; Thompson, J.C.; Carpenter, E.L.; Elkhouly, E.; Aggarwal, C. Plasma cell-free DNA genotyping: From an emerging concept to a standard-of-care tool in metastatic non-small cell lung cancer. Oncologist 2021, 26, e1812. [Google Scholar] [CrossRef]
- Gremel, G.; Lee, R.J.; Girotti, M.R.; Mandal, A.K.; Valpione, S.; Garner, G.; Ayub, M.; Wood, S.; Rothwell, D.G.; Fusi, A. Distinct subclonal tumour responses to therapy revealed by circulating cell-free DNA. Ann. Oncol. 2016, 27, 1959–1965. [Google Scholar] [CrossRef]
- Parikh, A.R.; Van Seventer, E.E.; Boland, G.M.; Hartwig, A.; Jaimovich, A.; Raymond, V.M.; Talasaz, A.; Corcoran, R.B. A Plasma-Only Integrated Genomic and Epigenomic Circulating Tumor DNA (ctDNA) Assay to Inform Recurrence Risk in Colorectal Cancer (CRC); American Society of Clinical Oncology: Alexandria, VA, USA, 2019. [Google Scholar]
- Larribère, L.; Martens, U.M. Advantages and challenges of using ctDNA NGS to assess the presence of minimal residual disease (MRD) in solid tumors. Cancers 2021, 13, 5698. [Google Scholar] [CrossRef]
- Moding, E.J.; Nabet, B.Y.; Alizadeh, A.A.; Diehn, M. Detecting liquid remnants of solid tumors: Circulating tumor DNA minimal residual disease. Cancer Discov. 2021, 11, 2968–2986. [Google Scholar] [CrossRef]
- Powles, T.; Assaf, Z.J.; Davarpanah, N.; Banchereau, R.; Szabados, B.E.; Yuen, K.C.; Grivas, P.; Hussain, M.; Oudard, S.; Gschwend, J.E. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021, 595, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Cohen, J.; Lahouel, K.; Lo, S.N.; Wang, Y.; Wong, R.; Shapiro, J.D.; Harris, S.J.; Khattak, M.A.; Burge, M.E. Adjuvant Chemotherapy Guided by Circulating Tumor DNA Analysis in Stage II Colon Cancer: The Randomized DYNAMIC Trial; American Society of Clinical Oncology: Alexandria, VA, USA, 2022. [Google Scholar]
- Tannock, I.F.; Hickman, J.A. Limits to Personalized Cancer Medicine; Mass Medical Soc: Waltham, MA, USA, 2016; Volume 375, pp. 1289–1294. [Google Scholar]
- Bayle, A.; Peyraud, F.; Belcaid, L.; Brunet, M.; Aldea, M.; Clodion, R.; Dubos, P.; Vasseur, D.; Nicotra, C.; Geraud, A. Liquid versus tissue biopsy for detecting actionable alterations according to the ESMO Scale for Clinical Actionability of molecular Targets in patients with advanced cancer: A study from the French National Center for Precision Medicine (PRISM). Ann. Oncol. 2022, 33, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Abe, M.; Saeki, Y.; Kono, F.; Ono, Y.; Abe, H. Circulating tumor cells (CTC) and Cell-free DNA (cfDNA): Liquid biopsy for cancer diagnostics. Pers. Med. Universe 2020, 9, 59–63. [Google Scholar] [CrossRef]
- Turner, N.C.; Swift, C.; Jenkins, B.; Kilburn, L.; Coakley, M.; Beaney, M.; Fox, L.; Goddard, K.; Garcia-Murillas, I.; Proszek, P. Results of the c-TRAK TN trial: A clinical trial utilising ctDNA mutation tracking to detect molecular residual disease and trigger intervention in patients with moderate-and high-risk early-stage triple-negative breast cancer. Ann. Oncol. 2023, 34, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Scholes, D.G.; Carpenter, E.L.; Aggarwal, C. Molecular response assessment using circulating tumor DNA (ctDNA) in advanced solid tumors. Br. J. Cancer 2023, 129, 1893–1902. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.-Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.-S.; Ferrero, J.M. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- Xu, J.; Gao, H.; Guan, X.; Meng, J.; Ding, S.; Long, Q.; Yi, W. Circulating tumor DNA: From discovery to clinical application in breast cancer. Front. Immunol. 2024, 15, 1355887. [Google Scholar] [CrossRef]
- García-Pardo, M.; Czarnecka-Kujawa, K.; Law, J.H.; Salvarrey, A.M.; Fernandes, R.; Fan, Z.J.; Waddell, T.K.; Yasufuku, K.; Liu, G.; Donahoe, L.L. Association of circulating tumor DNA testing before tissue diagnosis with time to treatment among patients with suspected advanced lung cancer: The accelerate nonrandomized clinical trial. JAMA Netw. Open 2023, 6, e2325332. [Google Scholar] [CrossRef]
- Davidson, C.J.; Zeringer, E.; Champion, K.J.; Gauthier, M.-P.; Wang, F.; Boonyaratanakornkit, J.; Jones, J.R.; Schreiber, E. Improving the limit of detection for Sanger sequencing: A comparison of methodologies for KRAS variant detection. Biotechniques 2012, 53, 182–188. [Google Scholar] [CrossRef]
- Chin, R.-I.; Chen, K.; Usmani, A.; Chua, C.; Harris, P.K.; Binkley, M.S.; Azad, T.D.; Dudley, J.C.; Chaudhuri, A.A. Detection of solid tumor molecular residual disease (MRD) using circulating tumor DNA (ctDNA). Mol. Diagn. Ther. 2019, 23, 311–331. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Jackman, J.A.; Park, J.H.; Cho, N.-J.; Kim, D.-H. Nanoplasmonic sensors for detecting circulating cancer biomarkers. Adv. Drug Deliv. Rev. 2018, 125, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Irudayaraj, J. Surface-enhanced Raman spectroscopy at single-molecule scale and its implications in biology. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120026. [Google Scholar] [CrossRef] [PubMed]
- Gracie, K.; Correa, E.; Mabbott, S.; Dougan, J.A.; Graham, D.; Goodacre, R.; Faulds, K. Simultaneous detection and quantification of three bacterial meningitis pathogens by SERS. Chem. Sci. 2014, 5, 1030–1040. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, N.; Chen, H.; Dang, H.; Chen, L.; Choo, J. Performance evaluation of surface-enhanced Raman scattering–polymerase chain reaction sensors for future use in sensitive genetic assays. Anal. Chem. 2020, 92, 2628–2634. [Google Scholar] [CrossRef]
- Xu, L.-J.; Lei, Z.-C.; Li, J.; Zong, C.; Yang, C.J.; Ren, B. Label-free surface-enhanced Raman spectroscopy detection of DNA with single-base sensitivity. J. Am. Chem. Soc. 2015, 137, 5149–5154. [Google Scholar] [CrossRef]
- Lyu, N.; Hassanzadeh-Barforoushi, A.; Rey Gomez, L.M.; Zhang, W.; Wang, Y. SERS biosensors for liquid biopsy towards cancer diagnosis by detection of various circulating biomarkers: Current progress and perspectives. Nano Converg. 2024, 11, 22. [Google Scholar] [CrossRef]
- Li, Z.-N.; Zhao, L.; Yu, L.-F.; Wei, M.-J. BRAF and KRAS mutations in metastatic colorectal cancer: Future perspectives for personalized therapy. Gastroenterol. Rep. 2020, 8, 192–205. [Google Scholar] [CrossRef]
- Wee, E.J.; Wang, Y.; Tsao, S.C.-H.; Trau, M. Simple, sensitive and accurate multiplex detection of clinically important melanoma DNA mutations in circulating tumour DNA with SERS nanotags. Theranostics 2016, 6, 1506. [Google Scholar] [CrossRef]
- Lyu, N.; Rajendran, V.K.; Diefenbach, R.J.; Charles, K.; Clarke, S.J.; Engel, A.; Rizos, H.; Molloy, M.P.; Wang, Y. Multiplex detection of ctDNA mutations in plasma of colorectal cancer patients by PCR/SERS assay. Nanotheranostics 2020, 4, 224. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Cao, X.; Liu, Z.; Chen, B.; Du, Q.; Lu, X. Simple and ultrasensitive detection of glioma-related ctDNAs in mice serum by SERS-based catalytic hairpin assembly signal amplification coupled with magnetic aggregation. Int. J. Nanomed. 2023, 18, 3211–3230. [Google Scholar] [CrossRef]

| Cancer Type and Population | SERS Methodology | Mutations Detected | Detection Limit | Reference |
|---|---|---|---|---|
| Melanoma, humans | SERS/multiplex PCR | BRAF V600E, NRAS Q61K, c-kit L756P | <5 ng | Wee et al., 2016 [95] |
| Colorectal cancer, humans | SERS/multiplex PCR | KRAS G12V, G13D, BRAF V600E | 0.1% | Lyu et al., 2020 [96] |
| Glioblastoma, mouse model | SERS/catalytic hairpin assembly | BRAF V600E, IDH R132H | ~6 × 10−18 mol (6 aM) | Wang et al., 2023 [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, S.; Wang, Y.; Gurney, H. The Emerging Applications of Raman Spectroscopy in Clinical Oncology: A Narrative Review Focused on Circulating Tumor DNA Detection and Therapeutic Drug Monitoring. Onco 2024, 4, 335-348. https://doi.org/10.3390/onco4040023
Narayanan S, Wang Y, Gurney H. The Emerging Applications of Raman Spectroscopy in Clinical Oncology: A Narrative Review Focused on Circulating Tumor DNA Detection and Therapeutic Drug Monitoring. Onco. 2024; 4(4):335-348. https://doi.org/10.3390/onco4040023
Chicago/Turabian StyleNarayanan, Sathya, Yuling Wang, and Howard Gurney. 2024. "The Emerging Applications of Raman Spectroscopy in Clinical Oncology: A Narrative Review Focused on Circulating Tumor DNA Detection and Therapeutic Drug Monitoring" Onco 4, no. 4: 335-348. https://doi.org/10.3390/onco4040023
APA StyleNarayanan, S., Wang, Y., & Gurney, H. (2024). The Emerging Applications of Raman Spectroscopy in Clinical Oncology: A Narrative Review Focused on Circulating Tumor DNA Detection and Therapeutic Drug Monitoring. Onco, 4(4), 335-348. https://doi.org/10.3390/onco4040023







