Tumour Heterogeneity and Disease Infiltration as Paradigms of Glioblastoma Treatment Resistance
Simple Summary
Abstract
1. Introduction
2. Image-Guided Surgery to Reveal the GBM Infiltrative Margin
2.1. Glioma Stemness at the Infiltrative Margin
2.2. Infiltrative Margin: Photodynamic Therapy and Molecular Targets
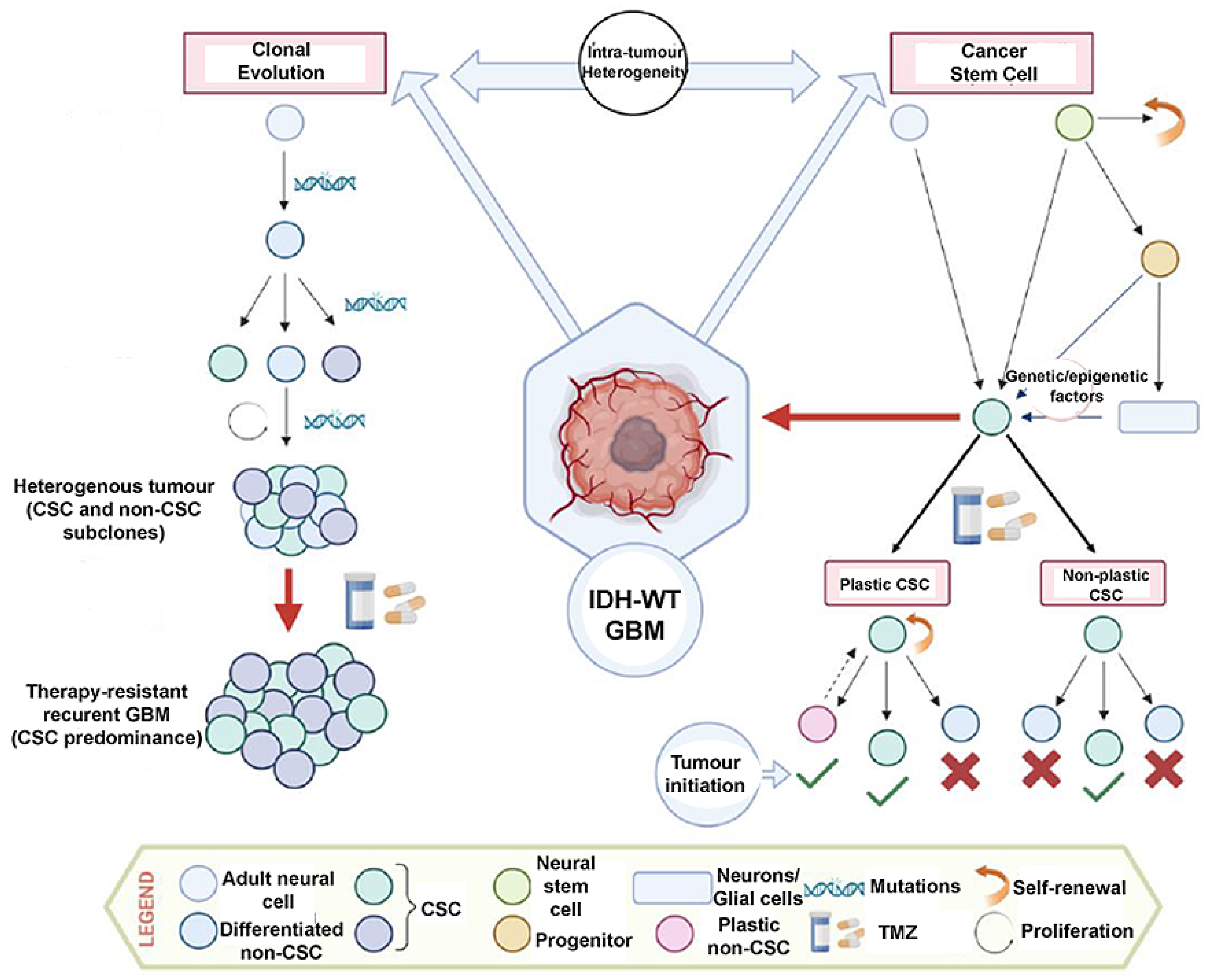
3. Intra-Tumour Heterogeneity and Cancer Stem Cells
3.1. Transformation of Neural Stem/Progenitor Cells
3.2. GSC Plasticity
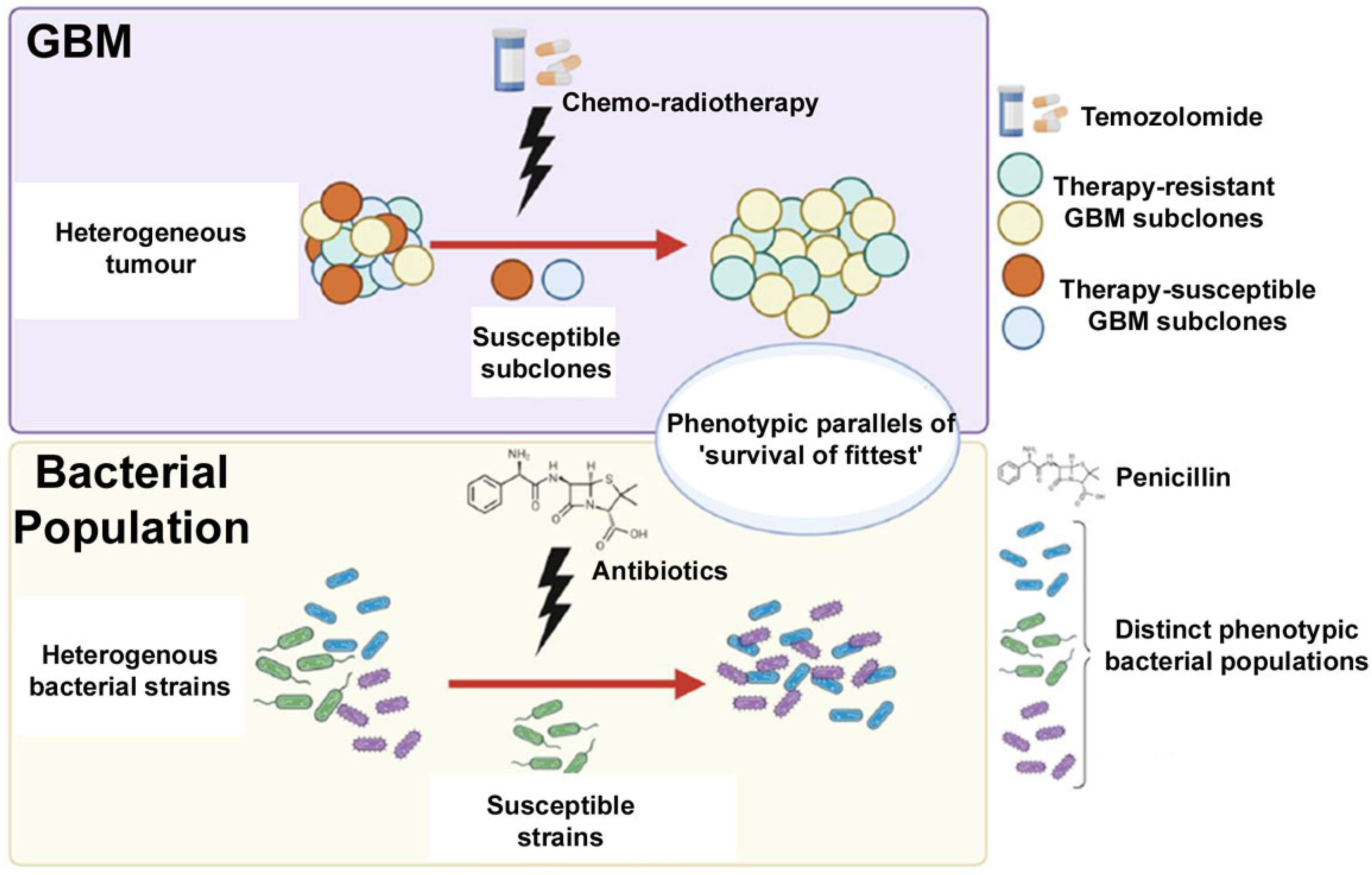
4. Comparison between CSCs and Antibiotic Resistance
5. Future Therapeutic Outlook
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef]
- Razavi, S.M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune Evasion Strategies of Glioblastoma. Front. Surg. 2016, 3, 11. [Google Scholar] [CrossRef]
- Fabbro-Peray, P.; Zouaoui, S.; Darlix, A.; Fabbro, M.; Pallud, J.; Rigau, V.; Mathieu-Daude, H.; Bessaoud, F.; Bauchet, F.; Riondel, A.; et al. Association of Patterns of Care, Prognostic Factors, and Use of Radiotherapy–Temozolomide Therapy with Survival in Patients with Newly Diagnosed Glioblastoma: A French National Population-Based Study. J. Neurooncol. 2019, 142, 91–101. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. New Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Altmann, C.; Keller, S.; Schmidt, M.H.H. The Role of Svz Stem Cells in Glioblastoma. Cancers 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.L.; Cooper, L.A.D.; Kong, J.; Gutman, D.; Williams, M.; Tucker-Burden, C.; McCrary, M.R.; Bouras, A.; Kaluzova, M.; Dunn, W.D.; et al. 5-Aminolevulinic Acid Guided Sampling of Glioblastoma Microenvironments Identifies Pro-Survival Signaling at Infiltrative Margins. Sci. Rep. 2017, 7, 15593. [Google Scholar] [CrossRef] [PubMed]
- Seker-Polat, F.; Degirmenci, N.P.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Montana, V.; Sontheimer, H. Bradykinin Promotes the Chemotactic Invasion of Primary Brain Tumors. J. Neurosci. 2011, 31, 4858–4867. [Google Scholar] [CrossRef]
- Diksin, M.; Smith, S.J.; Rahman, R. The Molecular and Phenotypic Basis of the Glioma Invasive Perivascular Niche. Int. J. Mol. Sci. 2017, 18, 2342. [Google Scholar] [CrossRef]
- Chédotal, A.; Kerjan, G.; Moreau-Fauvarque, C. The Brain within the Tumor: New Roles for Axon Guidance Molecules in Cancers. Cell Death Differ. 2005, 12, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- de Gooijer, M.C.; Guillén Navarro, M.; Bernards, R.; Wurdinger, T.; van Tellingen, O. An Experimenter’s Guide to Glioblastoma Invasion Pathways. Trends Mol. Med. 2018, 24, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Pinel, S.; Thomas, N.; Boura, C.; Barberi-Heyob, M. Approaches to Physical Stimulation of Metallic Nanoparticles for Glioblastoma Treatment. Adv. Drug Deliv. Rev. 2019, 138, 344–357. [Google Scholar] [CrossRef]
- Laperriere, N.; Zuraw, L.; Cairncross, G. Radiotherapy for Newly Diagnosed Malignant Glioma in Adults: A Systematic Review. Radiother. Oncol. 2002, 64, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Smith, S.J.; Diksin, M.; Chhaya, S.; Sairam, S.; Estevez-Cebrero, M.A.; Rahman, R. The Invasive Region of Glioblastoma Defined by 5ALA Guided Surgery Has an Altered Cancer Stem Cell Marker Profile Compared to Central Tumour. Int. J. Mol. Sci. 2017, 18, 2452. [Google Scholar] [CrossRef]
- Kis, D.; Szivos, L.; Rekecki, M.; Shukir, B.S.; Mate, A.; Hideghety, K.; Barzo, P. Predicting the True Extent of Glioblastoma Based on Probabilistic Tractography. Front. Neurosci. 2022, 16, 886465. [Google Scholar] [CrossRef]
- Lasocki, A.; Gaillard, F. Non-Contrast-Enhancing Tumor: A New Frontier in Glioblastoma Research. Am. J. Neuroradiol. 2019, 40, 758–765. [Google Scholar] [CrossRef]
- de Bonis, P.; Anile, C.; Pompucci, A.; Fiorentino, A.; Balducci, M.; Chiesa, S.; Lauriola, L.; Maira, G.; Mangiola, A. The Influence of Surgery on Recurrence Pattern of Glioblastoma. Clin. Neurol. Neurosurg. 2013, 115, 37–43. [Google Scholar] [CrossRef]
- Knudsen, A.M.; Halle, B.; Cédile, O.; Burton, M.; Baun, C.; Thisgaard, H.; Anand, A.; Hubert, C.; Thomassen, M.; Michaelsen, S.R.; et al. Surgical Resection of Glioblastomas Induces Pleiotrophin-Mediated Self-Renewal of Glioblastoma Stem Cells in Recurrent Tumors. Neuro Oncol. 2022, 24, 1074–1087. [Google Scholar] [CrossRef]
- Gupta, K.; Burns, T.C. Radiation-Induced Alterations in the Recurrent Glioblastoma Microenvironment: Therapeutic Implications. Front. Oncol. 2018, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Olzowy, B.; Hundt, C.S.; Stocker, S.; Bise, K.; Reulen, H.J.; Stummer, W. Photoirradiation Therapy of Experimental Malignant Glioma with 5-Aminolevulinic Acid. J. Neurosurg. 2002, 97, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Krammer, B.; Plaetzer, K. ALA and Its Clinical Impact, from Bench to Bedside. Photochem. Photobiol. Sci. 2008, 7, 283–289. [Google Scholar] [CrossRef]
- Etminan, N.; Peters, C.A.; Ficnar, J.; Anlasik, S.; Bünemann, E.; Slotty, P.J.; Hänggi, D.; Steiger, H.J.; Sorg, R.v.; Stummer, W. Modulation of Migratory Activity and Invasiveness of Human Glioma Spheroids Following 5-Aminolevulinic Acid-Based Photodynamic Treatment. J. Neurosurg. 2011, 115, 281–288. [Google Scholar] [CrossRef]
- Schimanski, A.; Ebbert, L.; Sabel, M.C.; Finocchiaro, G.; Lamszus, K.; Ewelt, C.; Etminan, N.; Fischer, J.C.; Sorg, R.V. Human Glioblastoma Stem-like Cells Accumulate Protoporphyrin IX When Subjected to Exogenous 5-Aminolaevulinic Acid, Rendering Them Sensitive to Photodynamic Treatment. J. Photochem. Photobiol. B 2016, 163, 203–210. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Two—Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagn. Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Andrieux, G.; Das, T.; Griffin, M.; Straehle, J.; Paine, S.M.L.; Beck, J.; Boerries, M.; Heiland, D.H.; Smith, S.J.; Rahman, R.; et al. Spatially Resolved Transcriptomic Profiles Reveal Unique Defining Molecular Features of Infiltrative 5ALA-Metabolizing Cells Associated with Glioblastoma Recurrence. Genome Med. 2023, 15, 48. [Google Scholar] [CrossRef]
- Smith, S.J.; Rowlinson, J.; Estevez-Cebrero, M.; Onion, D.; Ritchie, A.; Clarke, P.; Wood, K.; Diksin, M.; Lourdusamy, A.; Grundy, R.G.; et al. Metabolism-Based Isolation of Invasive Glioblastoma Cells with Specific Gene Signatures and Tumorigenic Potential. Neurooncol. Adv. 2020, 2, vdaa087. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Aubry, M.; de Tayrac, M.; Etcheverry, A.; Clavreul, A.; Saikali, S.; Menei, P.; Mosser, J. From the Core to beyond the Margin: A Genomic Picture of Glioblastoma Intratumor Heterogeneity. Oncotarget 2015, 6, 12094–12109. [Google Scholar] [CrossRef]
- Visvader, J.E. Cells of Origin in Cancer. Nature 2011, 469, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.; Brons, N.H.C.; Leite, S.; Sauvageot, N.; Sarkisjan, D.; et al. Stem Cell-Associated Heterogeneity in Glioblastoma Results from Intrinsic Tumor Plasticity Shaped by the Microenvironment. Nat. Commun. 2019, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- Nowell, P.C. The Clonal Evolution of Tumor Cell Populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Shackleton, M.; Quintana, E.; Fearon, E.R.; Morrison, S.J. Heterogeneity in Cancer: Cancer Stem Cells versus Clonal Evolution. Cell 2009, 138, 822–829. [Google Scholar] [CrossRef]
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.W.; Lee, J.H. Genetic Architectures and Cell-of-Origin in Glioblastoma. Front. Oncol. 2021, 10, 615400. [Google Scholar] [CrossRef]
- Ming, G.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Steed, T.C.; Treiber, J.M.; Taha, B.; Engin, H.B.; Carter, H.; Patel, K.S.; Dale, A.M.; Carter, B.S.; Chen, C.C. Glioblastomas Located in Proximity to the Subventricular Zone (SVZ) Exhibited Enrichment of Gene Expression Profiles Associated with the Cancer Stem Cell State. J. Neurooncol. 2020, 148, 455–462. [Google Scholar] [CrossRef]
- Pollen, A.A.; Nowakowski, T.J.; Chen, J.; Retallack, H.; Sandoval-Espinosa, C.; Nicholas, C.R.; Shuga, J.; Liu, S.J.; Oldham, M.C.; Diaz, A.; et al. Molecular Identity of Human Outer Radial Glia during Cortical Development. Cell 2015, 163, 55–67. [Google Scholar] [CrossRef]
- Matarredona, E.R.; Zarco, N.; Castro, C.; Guerrero-Cazares, H. Editorial: Neural Stem Cells of the Subventricular Zone: From Neurogenesis to Glioblastoma Origin. Front. Oncol. 2021, 11, 750116. [Google Scholar] [CrossRef]
- Verdugo, E.; Puerto, I.; Medina, M.Á. An Update on the Molecular Biology of Glioblastoma, with Clinical Implications and Progress in Its Treatment. Cancer Commun. 2022, 42, 1083–1111. [Google Scholar] [CrossRef] [PubMed]
- Bardella, C.; Al-Shammari, A.R.; Soares, L.; Tomlinson, I.; O’Neill, E.; Szele, F.G. The Role of Inflammation in Subventricular Zone Cancer. Prog. Neurobiol. 2018, 170, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Beiriger, J.; Habib, A.; Jovanovich, N.; Kodavali, C.V.; Edwards, L.; Amankulor, N.; Zinn, P.O. The Subventricular Zone in Glioblastoma: Genesis, Maintenance, and Modeling. Front. Oncol. 2022, 12, 790976. [Google Scholar] [CrossRef] [PubMed]
- Yabo, Y.A.; Niclou, S.P.; Golebiewska, A. Cancer Cell Heterogeneity and Plasticity: A Paradigm Shift in Glioblastoma. Neuro Oncol. 2022, 24, 669–682. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D.; Bushong, E.A.; Ke, E.; Soda, Y.; Marumoto, T.; Singer, O.; Ellisman, M.H.; Verma, I.M. Dedifferentiation of Neurons and Astrocytes by Oncogenes Can Induce Gliomas in Mice. Science 2012, 338, 1080–1084. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.-Y.; Kim, W.K.; Lee, J.-K.; Park, J.; et al. Human Glioblastoma Arises From Subventroicular Zone Cells with Low-Level Driver Mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef]
- Orzan, F.; de Bacco, F.; Crisafulli, G.; Pellegatta, S.; Mussolin, B.; Siravegna, G.; D’Ambrosio, A.; Comoglio, P.M.; Finocchiaro, G.; Boccaccio, C. Genetic Evolution of Glioblastoma Stem-Like Cells From Primary to Recurrent Tumor. Stem Cells 2017, 35, 2218–2228. [Google Scholar] [CrossRef]
- Auffinger, B.; Spencer, D.; Pytel, P.; Ahmed, A.U.; Lesniak, M.S. The Role of Glioma Stem Cells in Chemotherapy Resistance and Glioblastoma Multiforme Recurrence. Expert. Rev. Neurother. 2015, 15, 741–752. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood-Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef]
- Dymova, M.A.; Kuligina, E.V.; Richter, V.A. Molecular Mechanisms of Drug Resistance in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 6385. [Google Scholar] [CrossRef] [PubMed]
- Saalik, P.; Lingasamy, P.; Toome, K.; Mastandrea, I.; Rousso-Noori, L.; Tobu, A.; Simon-Garcia, L.; Hunt, H.; Paiste, P.; Kotamraju, V.R.; et al. Peptide-guided Nanoparticles for Glioblastoma Targeting. J. Control Release 2019, 308, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, Y.; Zhang, R.; Yang, F.; Zhang, D.; Huang, M.; Zhang, L.; Dorsey, J.F.; Binder, Z.A.; O’Rourke, D.M.; et al. Targeting PAK4 to Reprogram the Vascular Microenvironment and Improve CAR-T Immunotherapy for Glioblastoma. Nat. Cancer 2021, 2, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lu, T.; Li, Z.; Teng, K.-Y.; Mansour, A.G.; Yu, M.; Tian, L.; Xu, B.; Ma, S.; Zhang, J.; et al. An Oncolytic Virus Expressing IL15/IL15Rα Combined with Off-the-Shelf EGFR-CAR NK Cells Targets Glioblastoma. Cancer Res. 2021, 81, 3635–3648. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhotra, P.; Rahman, R. Tumour Heterogeneity and Disease Infiltration as Paradigms of Glioblastoma Treatment Resistance. Onco 2024, 4, 349-358. https://doi.org/10.3390/onco4040024
Malhotra P, Rahman R. Tumour Heterogeneity and Disease Infiltration as Paradigms of Glioblastoma Treatment Resistance. Onco. 2024; 4(4):349-358. https://doi.org/10.3390/onco4040024
Chicago/Turabian StyleMalhotra, Pulkit, and Ruman Rahman. 2024. "Tumour Heterogeneity and Disease Infiltration as Paradigms of Glioblastoma Treatment Resistance" Onco 4, no. 4: 349-358. https://doi.org/10.3390/onco4040024
APA StyleMalhotra, P., & Rahman, R. (2024). Tumour Heterogeneity and Disease Infiltration as Paradigms of Glioblastoma Treatment Resistance. Onco, 4(4), 349-358. https://doi.org/10.3390/onco4040024






