Advancements in Breast Cancer Detection: A Review of Global Trends, Risk Factors, Imaging Modalities, Machine Learning, and Deep Learning Approaches
Abstract
1. Introduction
1.1. Motivation
- A1.
- Breast cancer affects millions annually, with increasing prevalence across diverse age groups. Traditional diagnostic techniques such as mammography are often inadequate, particularly in dense breast tissues, leading to missed or delayed diagnoses.
- A2.
- DL techniques have revolutionized medical imaging by enabling precise analysis of complex, high-dimensional datasets. These methods reduce human error, increase diagnostic consistency, and improve efficiency.
- A3.
- Existing screening tools have inherent strengths and weaknesses. Investigating these methods to identify the most effective approaches for breast cancer detection remains critical for advancing diagnostic quality.
- A4.
- Innovations such as transfer learning, hybrid DL models, and explainable AI have enhanced diagnostic accuracy while addressing trust and interpretability concerns, essential factors for clinical integration.
- A5.
- By integrating advanced technologies into clinical practice, we can increase early detection rates, potentially saving countless lives and transforming breast cancer treatment outcomes.
1.2. Contributions
- A1.
- The global BC trends and risk factors are examined to inform preventive strategies.
- A2.
- We present a visualization of current trends in BC research, review the impacts of early detection and technology on mortality rates, and highlight the potential of lifestyle changes to reduce the incidence of breast cancer in high-risk groups.
- A3.
- This review examines various BC imaging techniques, including mammograms, ultrasound, MRI, CT scans, histopathology, and thermal imaging. We discuss their advantages and disadvantages and present examples of specific cases.
- A4.
- We explore the use of structured data (CSV) for BC detection, focusing on feature engineering, preprocessing, and algorithmic approaches.
- A5.
- The study presents a summary of hybrid frameworks combining deep feature extraction and machine learning classifiers to enhance diagnostic accuracy.
- A6.
- We report the level of accuracy achieved to date by various models using different datasets.
2. Background Study
3. Research Methodology
3.1. Study Selection Strategy
3.1.1. Search Strategy
- SS1.
- Conducted searches across major academic databases including IEEE Xplore, SpringerLink, ScienceDirect, Nature, Wiley Online Library, and MDPI.
- SS2.
- Used keywords: (“breast cancer detection” OR “breast cancer diagnosis”) AND (“machine learning” OR “deep learning” OR “artificial intelligence”) combined with modality-specific terms (“mammography”, “ultrasound”, “MRI”, “CT”, “Histopathological”, “Thermal”, etc.).
3.1.2. Inclusion Criteria
- IC1.
- Primary focus on studies published between 2023 and 2024.
- IC2.
- Included a few foundational papers from 2019 to 2022 that introduced significant methodological advances.
- IC3.
- Included studies were required to report quantitative performance metrics such as accuracy, AUC, sensitivity, and specificity.
3.1.3. Exclusion Criteria
- EC1.
- Non-English publications, conference abstracts, and editorials.
- EC2.
- Duplicate or overlapping studies.
3.2. Research Questions
- Q1.
- What are the incidence and death rates for breast cancer across various categories?
- Q2.
- What diagnostic methods are currently used for breast cancer detection?
- Q3.
- What are the key risk factors for breast cancer?
- Q4.
- How can lifestyle changes help reduce the risk of breast cancer?
- Q5.
- Which breast cancer datasets have been used in the development of machine and deep learning models?
- Q6.
- What are the reasons for adopting machine and deep learning techniques in breast cancer detection?
- Q7.
- What are the different medical imaging modalities used for breast cancer classification, and what is the highest accuracy achieved by various models on these modalities to date?
- Q8.
- What are the various CSV datasets employed for breast cancer classification, and what is the best accuracy attained by different models on these datasets so far?
- Q9.
- What are the strengths, weaknesses, and applications of imaging modalities in breast cancer detection techniques?
- Q10.
- What are the benefits, drawbacks, and use cases of breast cancer detection techniques based on CSV datasets?
4. Overview of Breast Cancer Datasets
5. Machine Learning Techniques
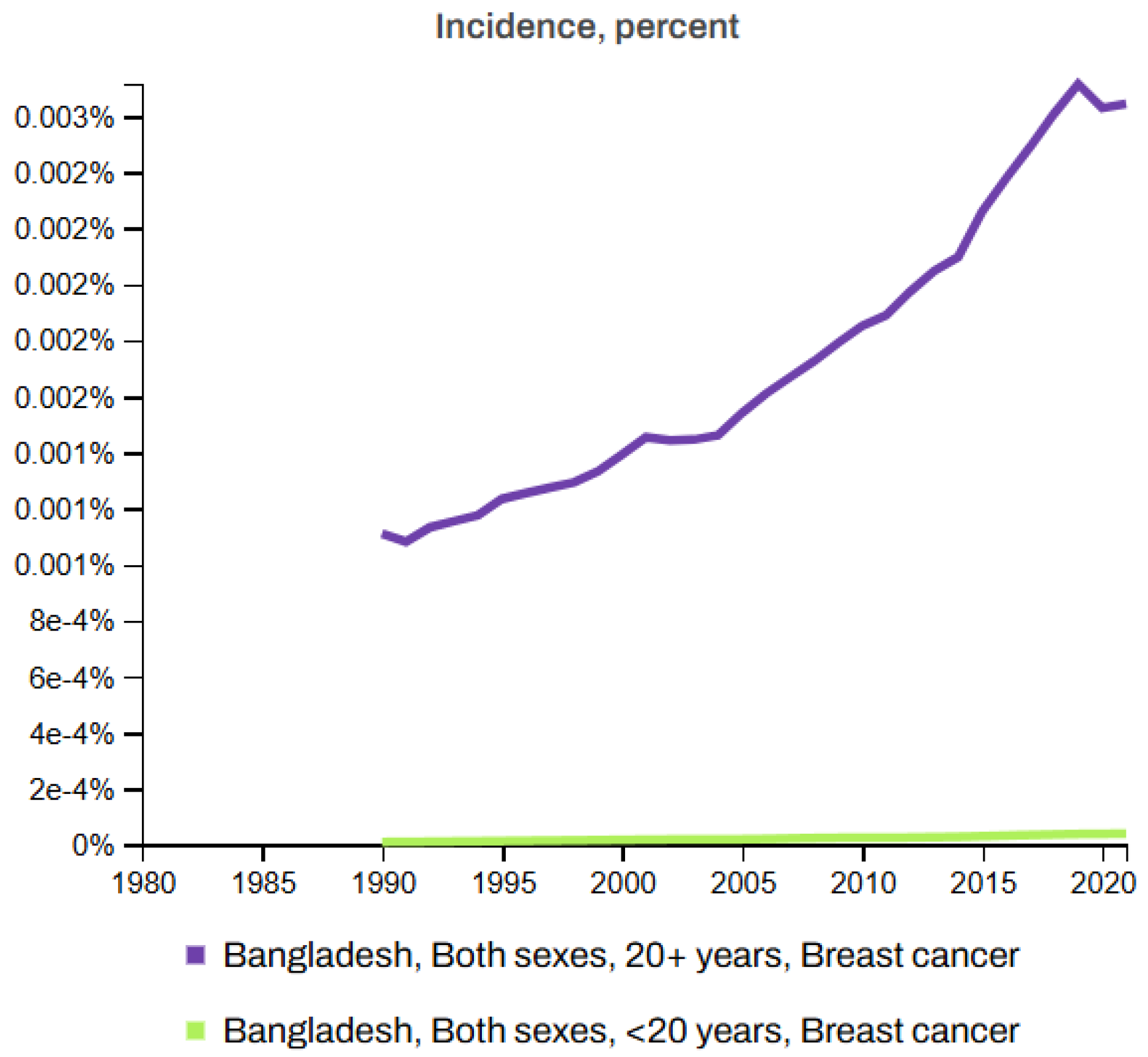
6. Cancer Statistics and Incidence and MortalityRates
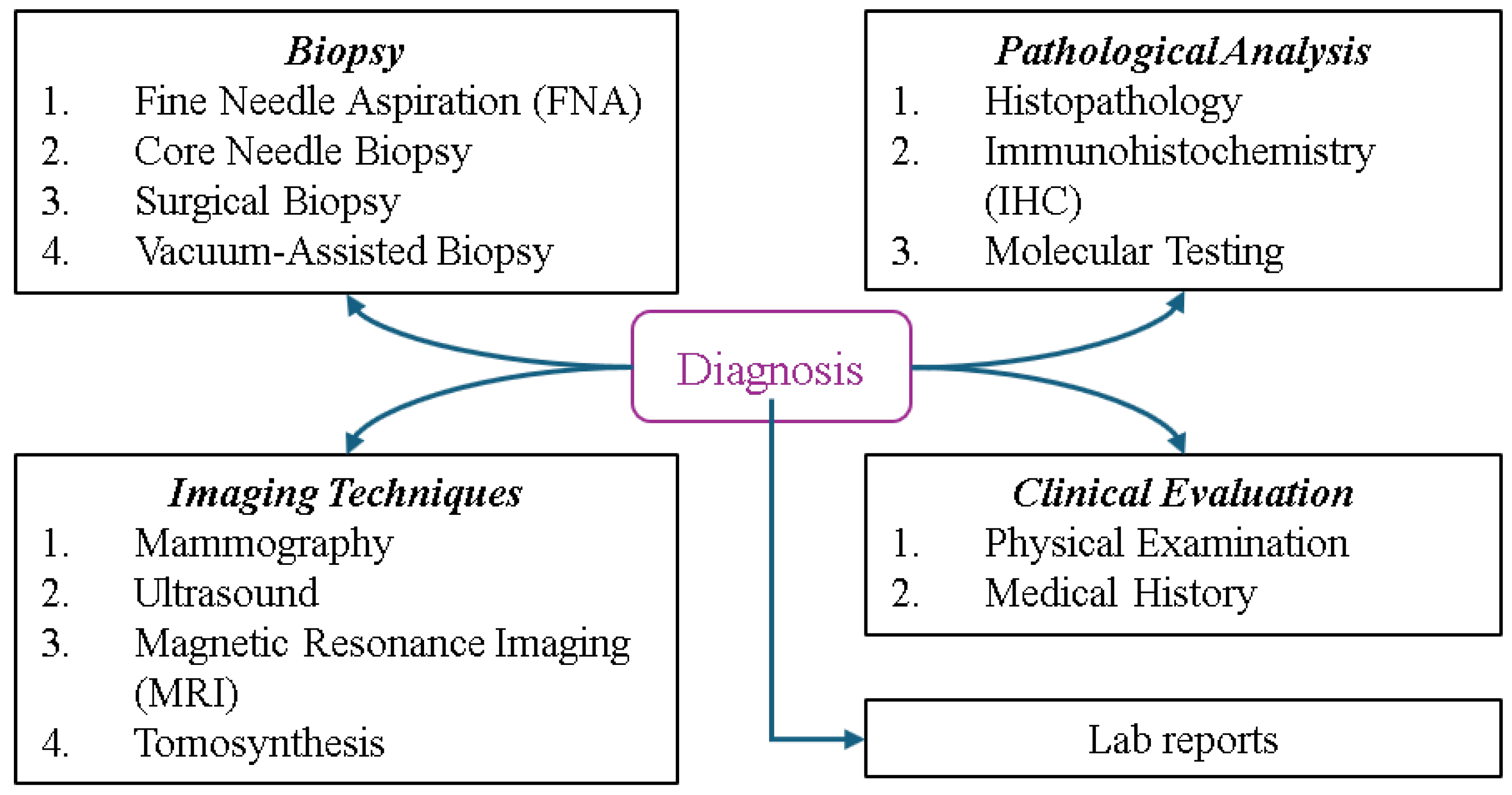
7. Breast Cancer Diagnosis and Classification
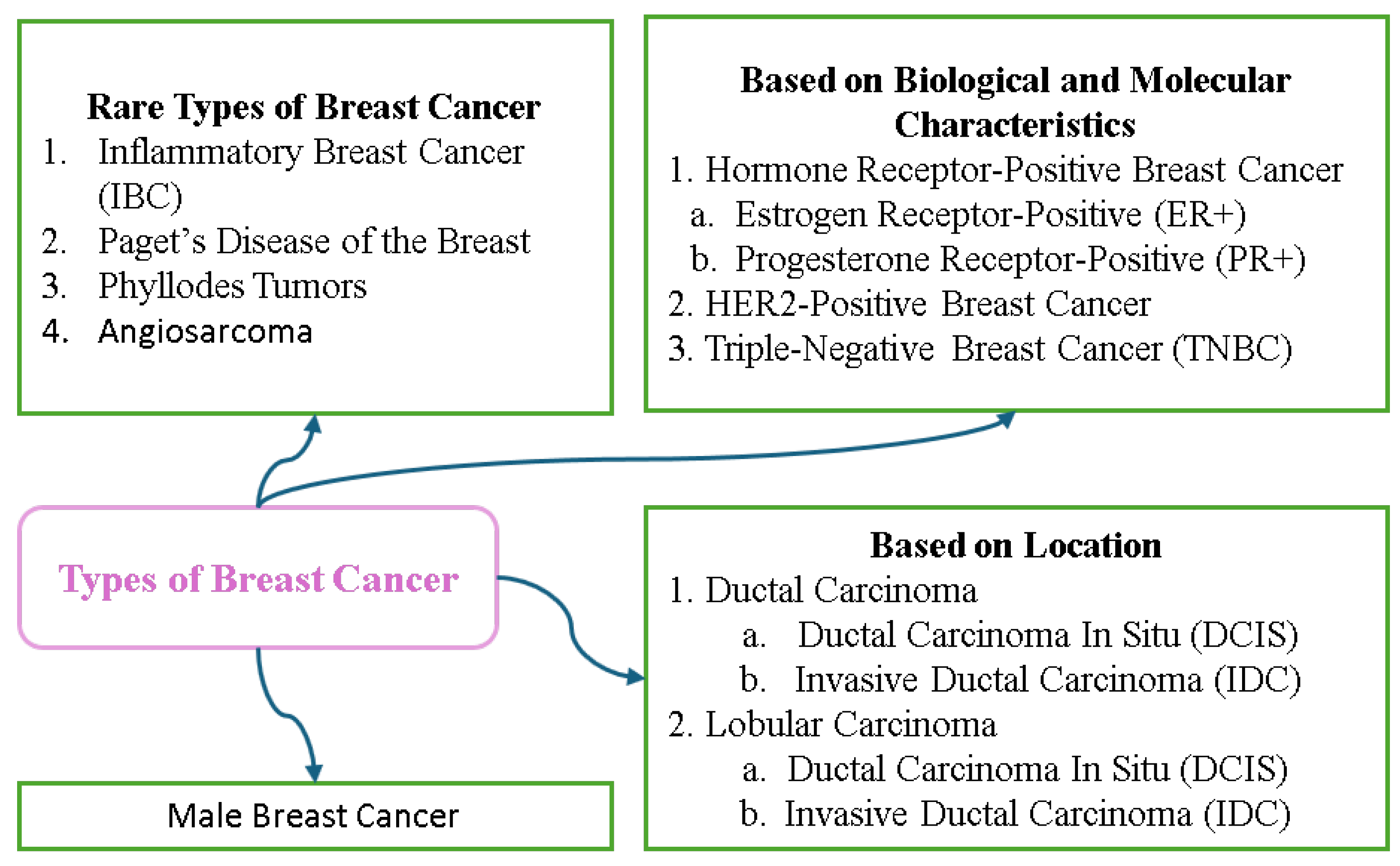
7.1. Breast Cancer Variants
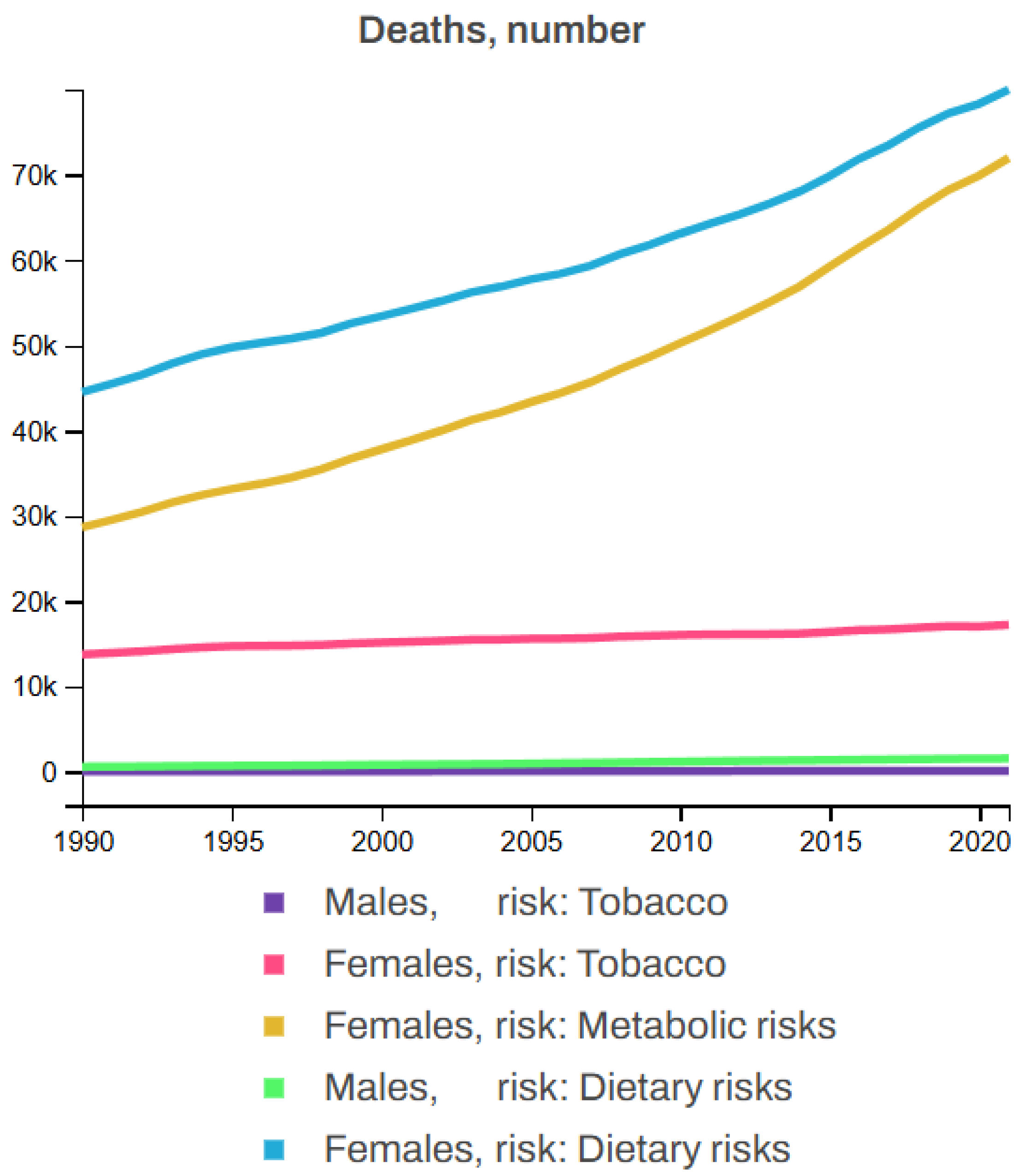
7.2. Key Risk Factors Associated with Breast Cancer
8. Breast Cancer Detection Approaches
8.1. Mammogram Image-Based Breast Cancer Detection
8.1.1. Model Performance on Mammogram Image: Pros, Cons, and Future Directions
8.1.2. Summary
8.1.3. Analysis
8.1.4. Critique
8.1.5. Comparison
8.2. Ultrasound Image-Based Breast Cancer Detection
8.2.1. Model Performance on Ultrasound Image: Pros, Cons, and Future Directions
8.2.2. Summary
8.2.3. Analysis
8.2.4. Critique
8.2.5. Comparison
8.3. Breast Cancer Detection Based on Magnetic Resonance Imaging (MRI) Images
8.3.1. Model Performance on MRI Image: Pros, Cons, and Future Directions
8.3.2. Summary
8.3.3. Analysis
8.3.4. Critique
8.3.5. Comparison
8.4. Breast Cancer Detection Using Computed Tomography (CT) Scan Images
8.4.1. Model Performance on CT Image: Pros, Cons, and Future Directions
8.4.2. Summary
8.4.3. Analysis
8.4.4. Critique
8.4.5. Comparison
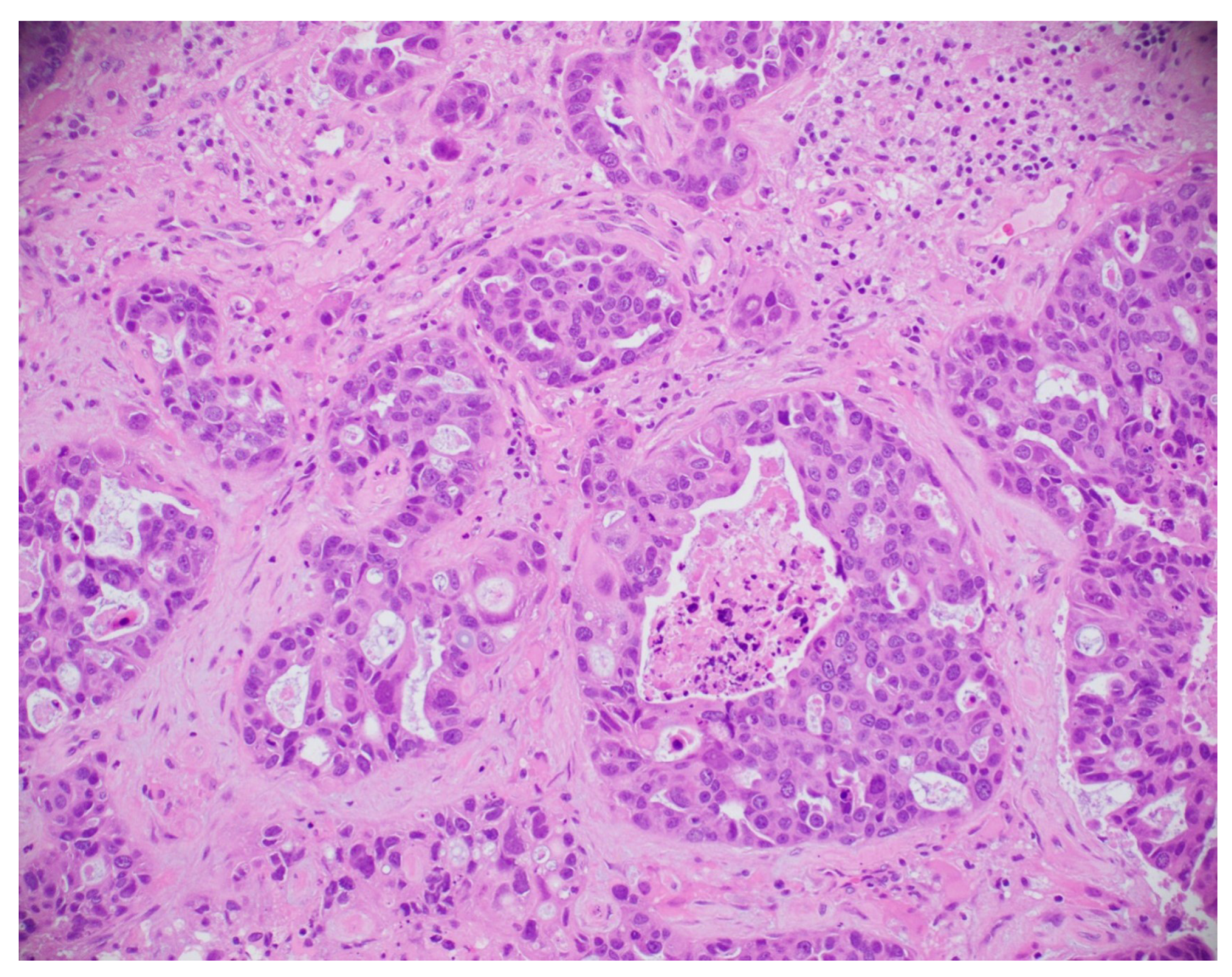
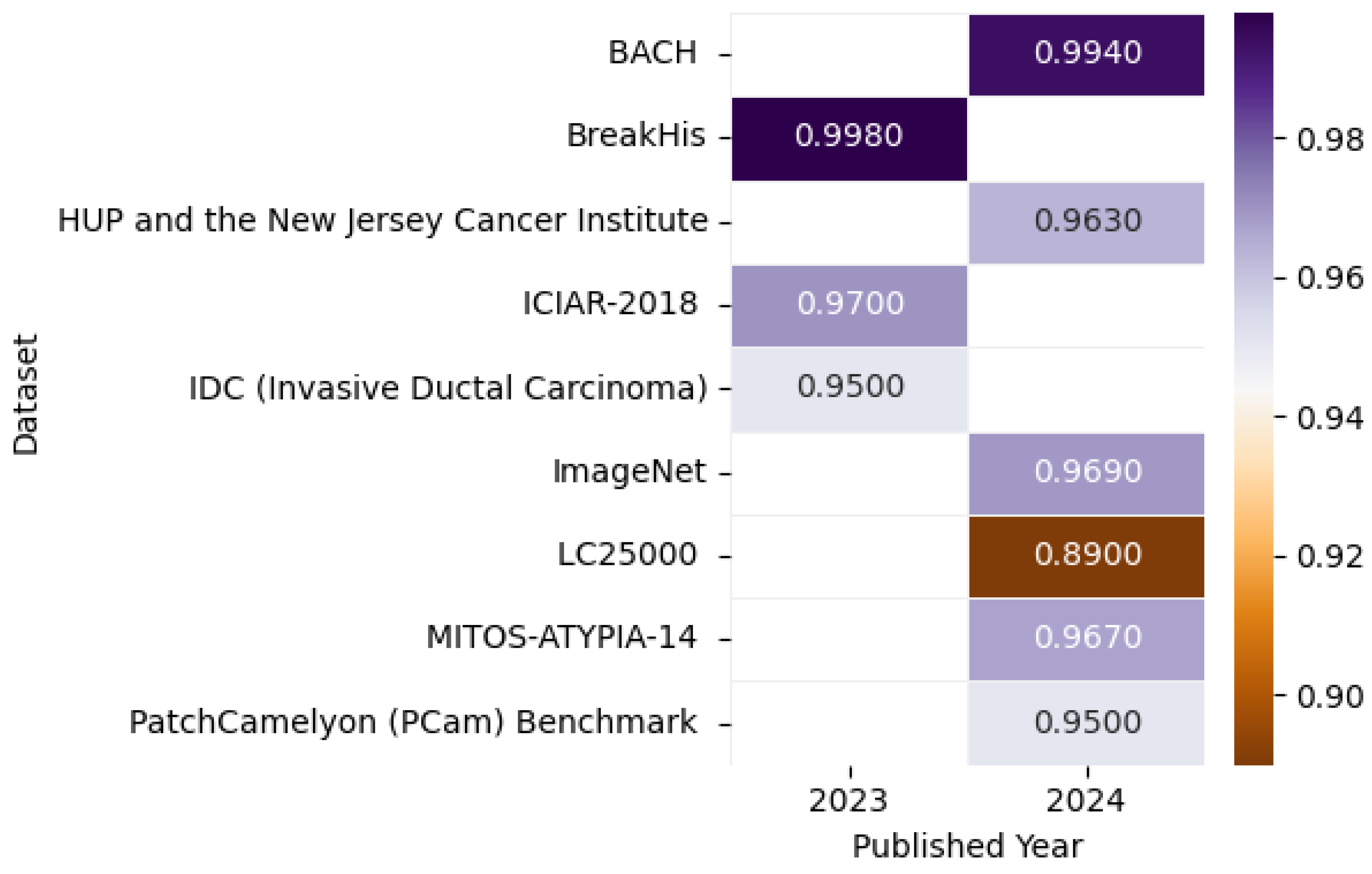
8.5. Histopathological Image-Based Breast Cancer Detection
8.5.1. Model Performance on Histopathological Image: Pros, Cons, and Future Directions
8.5.2. Summary
8.5.3. Analysis
8.5.4. Critique
8.5.5. Comparison
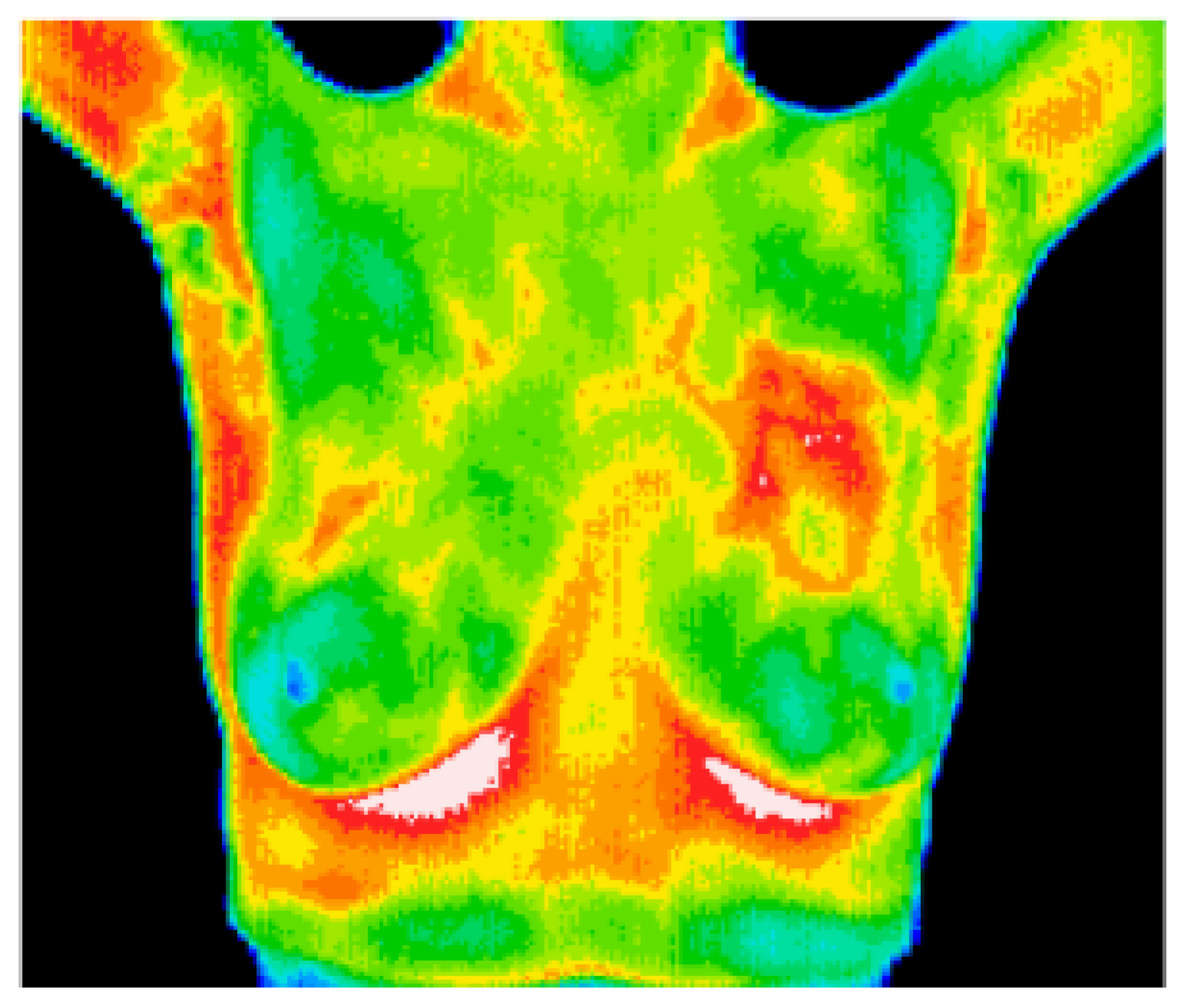
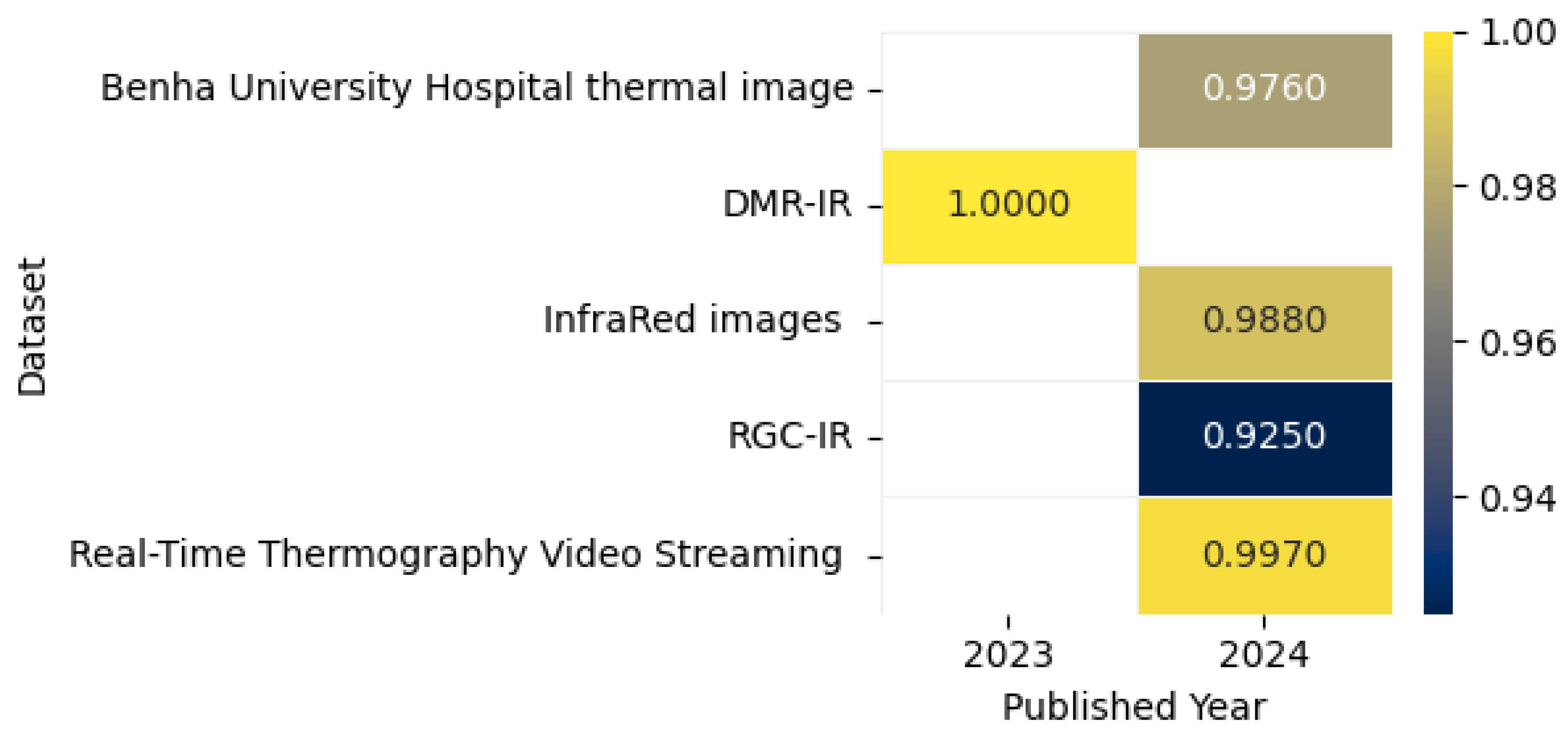
8.6. Breast Cancer Detection Using Thermal Imaging
8.6.1. Model Performance on Thermal Image: Pros, Cons, and Future Directions
8.6.2. Summary
8.6.3. Analysis
8.6.4. Critique
8.6.5. Comparison
8.7. CSV Dataset-Based Breast Cancer Detection
8.7.1. Model Performance on Tabular Data: Pros, Cons, and Future Directions
8.7.2. Summary
8.7.3. Analysis
8.7.4. Critique
8.7.5. Comparison
9. Imaging Techniques and Data in Breast Cancer Detection: Applications, Strengths, and Weaknesses
Model Interpretability in Clinical Practice
10. Trends in Breast Cancer Detection: ML, Statistical, and DL Techniques Across Journals
11. Conclusions
12. Future Research Directions
- Exploring advanced data augmentation techniques remains essential to address the challenge of limited medical imaging data in breast cancer detection. While traditional augmentation methods can enhance dataset diversity, they may compromise data integrity, potentially affecting model reliability. Future research should focus on developing and refining augmentation strategies, such as implicit data augmentation and novel generative approaches, to ensure both diversity and authenticity in the augmented data. Additionally, integrating these methods with deep learning models, including CNNs and transfer learning architectures, can further improve diagnostic accuracy and robustness, ultimately aiding in more reliable breast cancer detection [175,176].
- Develop deep learning models that are trained on diverse, high-quality datasets to mitigate biases and improve generalizability across various clinical settings. This will ensure that models perform consistently across different populations and healthcare systems.
- Prioritize XAI techniques (e.g., counterfactual explanations, concept activation vectors) to align model decisions with clinical reasoning. For example, integrating radiologist feedback loops during training could improve trust in CNN-based mammography systems [78].
- Design user-friendly tools and interfaces that seamlessly integrate deep learning models into existing healthcare practices. Adequate training for clinicians on AI-driven tools will be essential to ensure their adoption and practical utility.
- Focus on early breast cancer diagnosis by integrating longitudinal and multi-modal data to predict risk trajectories and enable preventive measures, thereby allowing for timely interventions.
- Foster collaboration among researchers, healthcare institutions, and data scientists with the goal of sharing datasets. Data should be collected from multiple sources to avoid biases and be properly formatted for ease of analysis, ensuring that deep learning models are trained on diverse and high-quality data.
- Develop advanced image preprocessing techniques for noise reduction, contrast enhancement, and image normalization. These techniques will improve image quality, leading to more accurate detection and classification.
- Innovate new segmentation architectures to improve the extraction of the region of interest (ROI) in medical images. Better segmentation will enhance the overall system performance and accuracy in identifying cancerous regions.
- Integrate various medical image modalities such as mammography, ultrasound, MRI, CT scans, histopathology, and thermal imaging to improve the accuracy and reliability of breast cancer detection. The synthesis of these modalities can provide a more comprehensive view of the disease.
- Incorporate non-imaging data such as cancer history, age, and other health issues alongside imaging data. This combination will allow for earlier and more accurate diagnosis, particularly for high-risk individuals.
- Conduct rigorous validation of deep learning models in real-world clinical settings, with the involvement of medical professionals. This ensures that the models are relevant, applicable, and safe for clinical use.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chugh, G.; Kumar, S.; Singh, N. TransNet: A Comparative Study on Breast Carcinoma Diagnosis with Classical Machine Learning and Transfer Learning Paradigm. Multimed. Tools Appl. 2024, 83, 33855–33877. [Google Scholar] [CrossRef]
- World Health Organization. Breast Cancer—who.int. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 23 November 2024).
- World Health Organization International Agency for Research on Cancer. Cancer Today—gco.iarc.who.int. 2022. Available online: https://gco.iarc.who.int/today/en/dataviz (accessed on 13 December 2024).
- Sekine, C.; Horiguchi, J. Current status and prospects of breast cancer imaging-based diagnosis using artificial intelligence. Int. J. Clin. Oncol. 2024, 29, 1641–1647. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation (IHME). The Breast Cancer Record. Available online: https://vizhub.healthdata.org/gbd-compare/cancer (accessed on 22 November 2024).
- Saroğlu, H.E.; Shayea, I.; Saoud, B.; Azmi, M.H.; El-Saleh, A.A.; Saad, S.A.; Alnakhli, M. Machine learning, IoT and 5G technologies for breast cancer studies: A review. Alex. Eng. J. 2024, 89, 210–223. [Google Scholar] [CrossRef]
- Amgad, M.; Hodge, J.M.; Elsebaie, M.A.; Bodelon, C.; Puvanesarajah, S.; Gutman, D.A.; Siziopikou, K.P.; Goldstein, J.A.; Gaudet, M.M.; Teras, L.R.; et al. A population-level digital histologic biomarker for enhanced prognosis of invasive breast cancer. Nat. Med. 2024, 30, 85–97. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef]
- Shahidi, F.; Daud, S.M.; Abas, H.; Ahmad, N.A.; Maarop, N. Breast cancer classification using deep learning approaches and histopathology image: A comparison study. IEEE Access 2020, 8, 187531–187552. [Google Scholar] [CrossRef]
- Alom, M.Z.; Yakopcic, C.; Nasrin, M.S.; Taha, T.M.; Asari, V.K. Breast cancer classification from histopathological images with inception recurrent residual convolutional neural network. J. Digit. Imaging 2019, 32, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Dey, A.; Singh, P.K.; Sarkar, R. DRDA-Net: Dense residual dual-shuffle attention network for breast cancer classification using histopathological images. Comput. Biol. Med. 2022, 145, 105437. [Google Scholar] [CrossRef] [PubMed]
- Ukwuoma, C.C.; Hossain, M.A.; Jackson, J.K.; Nneji, G.U.; Monday, H.N.; Qin, Z. Multi-classification of breast cancer lesions in histopathological images using DEEP_Pachi: Multiple self-attention head. Diagnostics 2022, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
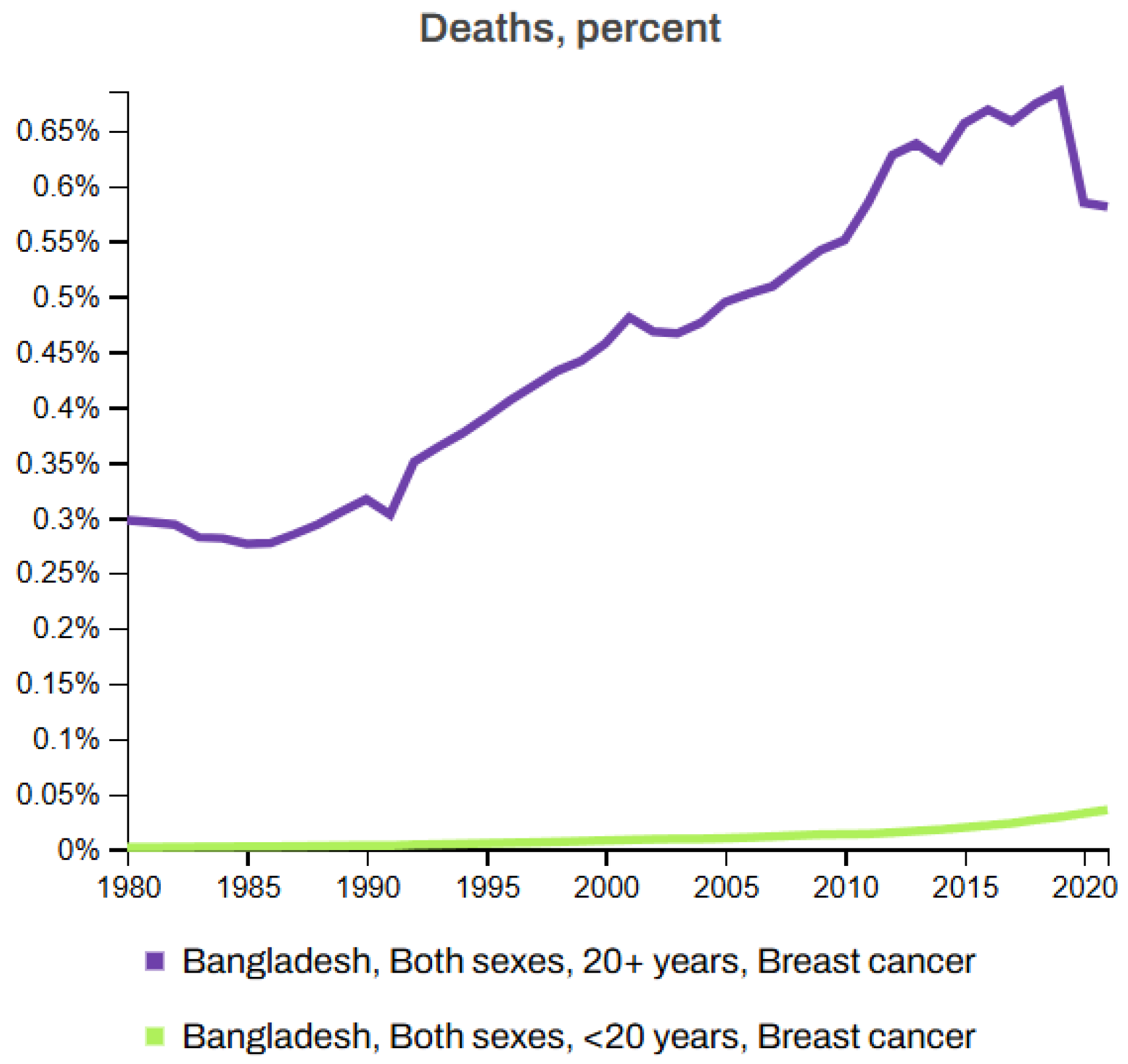
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global trend of breast cancer mortality rate: A 25-year study. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 2015. [Google Scholar] [CrossRef]
- Rautela, K.; Kumar, D.; Kumar, V. A comprehensive review on computational techniques for breast cancer: Past, present, and future. Multimed. Tools Appl. 2024, 83, 76267–76300. [Google Scholar] [CrossRef]
- WHO. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All|Knowledge Action Portal on NCDs—knowledge-action-portal.com. 6 February 2020. Available online: https://www.knowledge-action-portal.com/en/content/who-report-cancer-setting-priorities-investing-wisely-and-providing-care-all (accessed on 22 November 2024).
- Karthikeyan, A.; Priyakumar, U.D. Artificial intelligence: Machine learning for chemical sciences. J. Chem. Sci. 2022, 134, 2. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.P.; Hartley, R.L.; Temple-Oberle, C. Breast cancer in transgender patients: A systematic review. Part 2: Female to Male. Eur. J. Surg. Oncol. 2018, 44, 1463–1468. [Google Scholar] [CrossRef]
- Chokshi, M.; Morgan, O.; Carroll, E.F.; Fraker, J.L.; Holligan, H.; Kling, J.M. Disparities in Study Inclusion and Screening Rates in Breast Cancer Screening Rates among Transgender People: A Systematic Review. J. Am. Coll. Radiol. 2024, 21, 1430–1443. [Google Scholar] [CrossRef]
- Wahlström, E.; Audisio, R.A.; Selvaggi, G. Aspects to consider regarding breast cancer risk in trans men: A systematic review and risk management approach. PLoS ONE 2024, 19, e0299333. [Google Scholar] [CrossRef] [PubMed]
- Abhisheka, B.; Biswas, S.K.; Purkayastha, B. A comprehensive review on breast cancer detection, classification and segmentation using deep learning. Arch. Comput. Methods Eng. 2023, 30, 5023–5052. [Google Scholar] [CrossRef]
- Nasser, M.; Yusof, U.K. Deep learning based methods for breast cancer diagnosis: A systematic review and future direction. Diagnostics 2023, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Kumar, P.; Kumar, A. A systematic review of machine and deep learning techniques for the identification and classification of breast cancer through medical image modalities. Multimed. Tools Appl. 2024, 83, 35849–35942. [Google Scholar] [CrossRef]
- Sharafaddini, A.M.; Esfahani, K.K.; Mansouri, N. Deep learning approaches to detect breast cancer: A comprehensive review. Multimed. Tools Appl. 2024, 84, 24079–24190. [Google Scholar] [CrossRef]
- Sushanki, S.; Bhandari, A.K.; Singh, A.K. A review on computational methods for breast cancer detection in ultrasound images using multi-image modalities. Arch. Comput. Methods Eng. 2024, 31, 1277–1296. [Google Scholar] [CrossRef]
- Cui, C.; Li, L.; Cai, H.; Fan, Z.; Zhang, L.; Dan, T.; Li, J.; Wang, J. The Chinese Mammography Database (CMMD): An online mammography database with biopsy confirmed types for machine diagnosis of breast. Cancer Imaging Arch. 2021. [CrossRef]
- Suckling, J.; Boggis, C.R.M.; Hutt, I.; Astley, S.; Betal, D.; Cerneaz, N.; Dance, D.R.; Kok, S.L.; Parker, J.; Ricketts, I.; et al. The Mammographic Image Analysis Society Digital Mammogram Database. Online, 1994. Exerpta Medica. International Congress Series 1069. pp. 375–378. Available online: http://peipa.essex.ac.uk/info/mias.html (accessed on 20 April 2025).
- Heath, M.; Bowyer, K.; Kopans, D.; Moore, R.; Kegelmeyer, W.P. The Digital Database for Screening Mammography. In Proceedings of the Fifth International Workshop on Digital Mammography; Yaffe, M., Ed.; Medical Physics Publishing: Madison, WI, USA, 2001; pp. 212–218. [Google Scholar]
- Sawyer-Lee, R.; Gimenez, F.; Hoogi, A.; Rubin, D. Curated Breast Imaging Subset of Digital Database for Screening Mammography (CBIS-DDSM). 2016. Available online: https://www.cancerimagingarchive.net/collection/cbis-ddsm/ (accessed on 20 April 2025).
- Moreira, I.C.; Amaral, I.; Domingues, I.; Cardoso, A.; Cardoso, M.J.; Cardoso, J.S. Inbreast: Toward a full-field digital mammographic database. Acad. Radiol. 2012, 19, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Prapavesis, S.; Fornage, B.; Palko, A.; Weismann, C.F.; Zoumpoulis, P. Breast Ultrasound and US-Guided Interventional Techniques: A Multimedia Teaching File, 2003. CD-ROM Multimedia Teaching File. Available online: https://www.auntminnie.com/clinical-news/article/15565901/breast-ultrasound-and-us-guided-interventional-techniques-a-multimedia-teaching-file (accessed on 20 April 2025).
- Yap, M.H.; Pons, G.; Marti, J.; Ganau, S.; Sentis, M.; Zwiggelaar, R.; Davison, A.K.; Marti, R. Automated Breast Ultrasound Lesions Detection Using Convolutional Neural Networks. IEEE J. Biomed. Health Inform. 2018, 22, 1218–1226. [Google Scholar] [CrossRef]
- Dar, R.A.; Rasool, M.; Assad, A. Breast cancer detection using deep learning: Datasets, methods, and challenges ahead. Comput. Biol. Med. 2022, 149, 106073. [Google Scholar] [CrossRef]
- Al-Dhabyani, W.; Gomaa, M.; Khaled, H.; Fahmy, A. Dataset of breast ultrasound images. Data Brief 2020, 28, 104863. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, M.M.; Ali, M.S.; Nafi, A.A.N.; Alam, M.S.; Godder, T.K.; Miah, M.S.; Islam, M.K. Enhancing breast cancer segmentation and classification: An Ensemble Deep Convolutional Neural Network and U-net approach on ultrasound images. Mach. Learn. Appl. 2024, 16, 100555. [Google Scholar] [CrossRef]
- Rehman, N.U.; Wang, J.; Weiyan, H.; Ali, I.; Akbar, A.; Assam, M.; Ghadi, Y.Y.; Algarni, A. Edge of discovery: Enhancing breast tumor MRI analysis with boundary-driven deep learning. Biomed. Signal Process. Control 2024, 95, 106291. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. The Cancer Genome Atlas Program. Available online: https://www.cancer.gov/ccg/research/genome-sequencing/tcga (accessed on 12 April 2025).
- Li, X.; Abramson, R.G.; Arlinghaus, L.R.; Chakravarthy, A.B.; Abramson, V.G.; Sanders, M.; Yankeelov, T.E. Data From QIN-BREAST (Version 2). 2016. Available online: https://www.cancerimagingarchive.net/collection/qin-breast/ (accessed on 21 April 2025).
- Aresta, G.; Araújo, T.; Campilho, A.; Eloy, C.; Polónia, A.; Aguiar, P. BACH: Grand Challenge on Breast Cancer Histology Images. Med. Image Anal. 2019, 56, 122–139. [Google Scholar] [CrossRef]
- Spanhol, F.A.; Oliveira, L.S.; Petitjean, C.; Heutte, L. A Dataset for Breast Cancer Histopathological Image Classification. IEEE Trans. Biomed. Eng. 2016, 63, 1455–1462. [Google Scholar] [CrossRef]
- Bhowmik, M.K.; Gogoi, U.R.; Majumdar, G.; Datta, D.; Ghosh, A.K.; Bhattacharjee, D. DBT-TU-JU Breast Thermogram Dataset. 2018. Available online: https://www.mkbhowmik.in/dbtTu.aspx (accessed on 21 April 2025).
- Gupta, T.; Agrawal, R.; Sangal, R.; Rao, S.A. Performance Evaluation of Thermography-Based Computer-Aided Diagnostic Systems for Detecting Breast Cancer: An Empirical Study. ACM Trans. Comput. Healthc. 2024, 5, 1–30. [Google Scholar] [CrossRef]
- Street, W.; Wolberg, W.; Mangasarian, O. Nuclear feature extraction for breast tumor diagnosis. IS T/SPIE 1993 Int. Symp. Electron. Imaging Sci. Technol. 1993, 1905, 861–870. [Google Scholar]
- Teng, J. SEER Breast Cancer Data, 2019. Dataset Derived from the November 2017 Update of the SEER Program of the National Cancer Institute, Encompassing 4024 Female Patients Diagnosed with Infiltrating Duct and Lobular Carcinoma Breast Cancer Between 2006 and 2010. Available online: https://ieee-dataport.org/open-access/seer-breast-cancer-data (accessed on 21 April 2025).
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.A.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Elter, M.; Horsch, A.; Nauck, D.; Spies, M. Mammographic Mass Dataset. UCI Machine Learning Repository. 2007. Available online: https://archive.ics.uci.edu/dataset/161/mammographic+mass (accessed on 22 April 2025).
- Alnuaimi, A.F.; Albaldawi, T.H. An overview of machine learning classification techniques. In Proceedings of the BIO Web of Conferences. EDP Sciences, Copenhagen, Denmark, 25–30 August 2024; Volume 97, p. 00133. [Google Scholar]
- Mendonça, M.O.; Netto, S.L.; Diniz, P.S.; Theodoridis, S. Machine learning: Review and trends. In Signal Processing and Machine Learning Theory; Elsevier: Amsterdam, The Netherlands, 2024; pp. 869–959. [Google Scholar]
- Kronberger, G.; Burlacu, B.; Kommenda, M.; Winkler, S.M.; Affenzeller, M. Symbolic Regression; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Shmueli, G.; Polak, J. Practical Time Series Forecasting with r: A Hands-On Guide; Axelrod Schnall Publishers: Green Cove Springs, FL, USA, 2024. [Google Scholar]
- Zhou, Z.H. Ensemble Methods: Foundations and Algorithms; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar]
- Veeranjaneyulu, K.; Lakshmi, M.; Janakiraman, S. Swarm Intelligent Metaheuristic Optimization Algorithms-Based Artificial Neural Network Models for Breast Cancer Diagnosis: Emerging Trends, Challenges and Future Research Directions. Arch. Comput. Methods Eng. 2025, 32, 381–398. [Google Scholar] [CrossRef]
- Patil, P.; Fulkar, B.; Sharma, M.; Rewatkar, R. Detecting Breast Cancer: A Comparative Study of Various Machine Learning Models. In Proceedings of the 2024 Parul International Conference on Engineering and Technology (PICET), Vadodara, India, 3–4 May 2024; pp. 1–5. [Google Scholar]
- Barnils, N.P.; Schüz, B. Identifying intersectional groups at risk for missing breast cancer screening: Comparing regression-and decision tree-based approaches. Ssm-Popul. Health 2025, 29, 101736. [Google Scholar] [CrossRef] [PubMed]
- Win, N.S.S.; Li, G.; Lin, L. Revolutionizing early breast cancer screening: Advanced multi-spectral transmission imaging classification with improved Otsu’s method and K-means clustering. Comput. Biol. Med. 2025, 184, 109373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Liang, R.; He, X.; Wang, D.; Sun, L.; Yu, S.; Su, W.; Zhang, W.; Zhou, Q.; et al. An Effective Ultrasound Features-Based Diagnostic Model via Principal Component Analysis Facilitated Differentiating Subtypes of Mucinous Breast Cancer From Fibroadenomas. Clin. Breast Cancer 2024, 24, e583–e592. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lam, P.D.; Tinh, V.P.; Le, D.D.; Nam, N.H.; Khoa, T.A. Joint Federated Learning Using Deep Segmentation and the Gaussian Mixture Model for Breast Cancer Tumors. IEEE Access 2024, 12, 94231–94249. [Google Scholar] [CrossRef]
- Dimri, S.C.; Indu, R.; Negi, H.S.; Panwar, N.; Sarda, M. Hidden Markov Model-Applications, Strengths, and Weaknesses. In Proceedings of the 2024 2nd International Conference on Device Intelligence, Computing and Communication Technologies (DICCT), Dehradun, India, 15–16 March 2024; pp. 300–305. [Google Scholar]
- Costanzo, S.; Flores, A. Reinforcement Learning to Enhanced Microwave Imaging for Accurate Tumor Detection in Breast Images. In Proceedings of the 2024 IEEE International Conference on Metrology for eXtended Reality, Artificial Intelligence and Neural Engineering (MetroXRAINE), St Albans, UK, 21–23 October 2024; pp. 101–106. [Google Scholar]
- Jayakrishnan, R.; Meera, S. Multiclass Classification of Leukemia Cancer Subtypes using Gene Expression Data and Optimized Dueling Double Deep Q-Network. Chemom. Intell. Lab. Syst. 2025, 262, 105402. [Google Scholar] [CrossRef]
- Wang, Q.; Chang, C. Automating the optimization of proton PBS treatment planning for head and neck cancers using policy gradient-based deep reinforcement learning. Med. Phys. 2025, 52, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation (IHME). GBD Cancer Compare—vizhub.healthdata.org. Available online: https://vizhub.healthdata.org/gbd-compare/cancer (accessed on 22 November 2024).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021) Results, 2022. Available from the GBD Results Tool. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 23 April 2025).
- Ivanova, M.; Pescia, C.; Trapani, D.; Venetis, K.; Frascarelli, C.; Mane, E.; Cursano, G.; Sajjadi, E.; Scatena, C.; Cerbelli, B.; et al. Early Breast Cancer Risk Assessment: Integrating Histopathology with Artificial Intelligence. Cancers 2024, 16, 1981. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, M.; Naseem, U.; Nezhadmoghadam, F.; Jatoi, M.A.; Gulliver, T.A.; Tamez-Peña, J.G. Breast cancer risk prediction using machine learning: A systematic review. Front. Oncol. 2024, 14, 1343627. [Google Scholar] [CrossRef]
- IBM. What Is Machine Learning (ML)?|IBM—ibm.com. 2023. Available online: https://www.ibm.com/cloud/learn/machine-learning (accessed on 23 November 2024).
- Hristov, D.; Stojanov, D. In silico report on five high-risk Protein C pathogenic variants: G403R, P405S, S421N, C238S, and I243T. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2025, 831, 111907. [Google Scholar] [CrossRef]
- Spek, C.A.; Arruda, V.R. The protein C pathway in cancer metastasis. Thromb. Res. 2012, 129, S80–S84. [Google Scholar] [CrossRef]
- Lee, R.S.; Gimenez, F.; Hoogi, A.; Miyake, K.K.; Gorovoy, M.; Rubin, D.L. A Curated Mammography Data Set for Use in Computer-Aided Detection and Diagnosis Research. Sci. Data 2017, 4, 170177. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.; Bowyer, K.; Kopans, D.; Kegelmeyer, P., Jr.; Moore, R.; Chang, K.; Munishkumaran, S. Current Status of the Digital Database for Screening Mammography. In Digital Mammography; Karssemeijer, N., Thijssen, M., Hendriks, J., van Erning, L., Eds.; Computational Imaging and Vision; Springer: Dordrecht, The Netherlands, 1998; Volume 13. [Google Scholar] [CrossRef]
- Suckling, J.; Parker, J.; Dance, D.; Astley, S.; Hutt, I.; Boggis, C.; Ricketts, I.; Stamatakis, E.; Cerneaz, N.; Kok, S.; et al. Mammographic Image Analysis Society (MIAS) Database v1.21 2015. Available online: https://www.repository.cam.ac.uk/handle/1810/250394 (accessed on 23 April 2025).
- Kiros, H. Doctors Using AI Catch Breast Cancer More Often than Either Does Alone—technologyreview.com. 2022. Available online: https://www.technologyreview.com/2022/07/11/1055677/ai-diagnose-breast-cancer-mammograms/ (accessed on 30 November 2024).
- Qu, J.; Zhao, X.; Chen, P.; Wang, Z.; Liu, Z.; Yang, B.; Li, H. Deep learning on digital mammography for expert-level diagnosis accuracy in breast cancer detection. Multimed. Syst. 2022, 28, 1263–1274. [Google Scholar] [CrossRef]
- Jamil, R.; Dong, M.; Bano, S.; Javed, A.; Abdullah, M. Precancerous Change Detection Technique on Mammography Breast Cancer Images based on Mean Ratio and Log Ratio using Fuzzy c Mean Classification with Gabor Filter. Curr. Med. Imaging 2024, 20, e18749445290351. [Google Scholar] [CrossRef]
- Nemade, V.; Pathak, S.; Dubey, A.K. Deep learning-based ensemble model for classification of breast cancer. Microsyst. Technol. 2024, 30, 513–527. [Google Scholar] [CrossRef]
- Avcı, H.; Karakaya, J. A novel medical image enhancement algorithm for breast cancer detection on mammography images using machine learning. Diagnostics 2023, 13, 348. [Google Scholar] [CrossRef]
- Yaqub, M.; Jinchao, F.; Aijaz, N.; Ahmed, S.; Mehmood, A.; Jiang, H.; He, L. Intelligent breast cancer diagnosis with two-stage using mammogram images. Sci. Rep. 2024, 14, 16672. [Google Scholar] [CrossRef] [PubMed]
- Dada, E.G.; Oyewola, D.O.; Misra, S. Computer-aided diagnosis of breast cancer from mammogram images using deep learning algorithms. J. Electr. Syst. Inf. Technol. 2024, 11, 38. [Google Scholar] [CrossRef]
- Mudeng, V.; Jeong, J.w.; Choe, S.w. Simply Fine-Tuned Deep Learning-Based Classification for Breast Cancer with Mammograms. Comput. Mater. Contin. 2022, 73, 4677. [Google Scholar] [CrossRef]
- Sait, A.R.W.; Nagaraj, R. An Enhanced LightGBM-Based Breast Cancer Detection Technique Using Mammography Images. Diagnostics 2024, 14, 227. [Google Scholar] [CrossRef]
- Raaj, R.S. Breast cancer detection and diagnosis using hybrid deep learning architecture. Biomed. Signal Process. Control 2023, 82, 104558. [Google Scholar] [CrossRef]
- Mohammed, A.D.; Ekmekci, D. Breast Cancer Diagnosis Using YOLO-Based Multiscale Parallel CNN and Flattened Threshold Swish. Appl. Sci. 2024, 14, 2680. [Google Scholar] [CrossRef]
- Islam, R.; Tarique, M. Artificial Intelligence (AI) and Nuclear Features from the Fine Needle Aspirated (FNA) Tissue Samples to Recognize Breast Cancer. J. Imaging 2024, 10, 201. [Google Scholar] [CrossRef]
- Sakaida, M.; Yoshimura, T.; Tang, M.; Ichikawa, S.; Sugimori, H.; Hirata, K.; Kudo, K. The Effectiveness of Semi-Supervised Learning Techniques in Identifying Calcifications in X-ray Mammography and the Impact of Different Classification Probabilities. Appl. Sci. 2024, 14, 5968. [Google Scholar] [CrossRef]
- Trang, N.T.H.; Long, K.Q.; An, P.L.; Dang, T.N. Development of an artificial intelligence-based breast cancer detection model by combining mammograms and medical health records. Diagnostics 2023, 13, 346. [Google Scholar] [CrossRef]
- Dehghan Rouzi, M.; Moshiri, B.; Khoshnevisan, M.; Akhaee, M.A.; Jaryani, F.; Salehi Nasab, S.; Lee, M. Breast cancer detection with an ensemble of deep learning networks using a consensus-adaptive weighting method. J. Imaging 2023, 9, 247. [Google Scholar] [CrossRef]
- Jafari, Z.; Karami, E. Breast cancer detection in mammography images: A CNN-based approach with feature selection. Information 2023, 14, 410. [Google Scholar] [CrossRef]
- Anas, M.; Haq, I.U.; Husnain, G.; Jaffery, S.A.F. Advancing Breast Cancer Detection: Enhancing YOLOv5 Network for Accurate Classification in Mammogram Images. IEEE Access 2024, 12, 16474–16488. [Google Scholar] [CrossRef]
- Gómez-Flores, W.; Gregorio-Calas, M.J.; Coelho de Albuquerque Pereira, W. BUS-BRA: A breast ultrasound dataset for assessing computer-aided diagnosis systems. Med. Phys. 2024, 51, 3110–3123. [Google Scholar] [CrossRef] [PubMed]
- Vallez, N.; Bueno, G.; Deniz, O.; Rienda, M.A.; Pastor, C. BUS-UCLM: Breast Ultrasound Lesion Segmentation Dataset. Mendeley Data, V1, 2024. Available online: https://data.mendeley.com/datasets/7fvgj4jsp7/1 (accessed on 23 April 2025).
- Pawłowska, A.; Ćwierz Pieńkowska, A.; Domalik, A.; Jaguś, D.; Kasprzak, P.; Matkowski, R.; Fura, Ł.; Nowicki, A.; Zolek, N. A Curated Benchmark Dataset for Ultrasound-Based Breast Lesion Analysis. Sci. Data 2024, 11, 148. [Google Scholar] [CrossRef]
- Fisher, P.R. Breast Cancer Ultrasonography: Practice Essentials, Role of Ultrasonography in Screening, Breast Imaging Reporting and Data System—emedicine.medscape.com. 2021. Available online: https://emedicine.medscape.com/article/346725-overview (accessed on 30 November 2024).
- Rodrigues, P.S. Breast Ultrasound Image. Mendeley Data, V1, 2017. Available online: https://data.mendeley.com/datasets/wmy84gzngw/1 (accessed on 24 April 2025).
- Masud, M.; Hossain, M.S.; Alhumyani, H.; Alshamrani, S.S.; Cheikhrouhou, O.; Ibrahim, S.; Muhammad, G.; Rashed, A.E.E.; Gupta, B. Pre-trained convolutional neural networks for breast cancer detection using ultrasound images. ACM Trans. Internet Technol. (TOIT) 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Raza, A.; Ullah, N.; Khan, J.A.; Assam, M.; Guzzo, A.; Aljuaid, H. DeepBreastCancerNet: A novel deep learning model for breast cancer detection using ultrasound images. Appl. Sci. 2023, 13, 2082. [Google Scholar] [CrossRef]
- Yu, F.H.; Miao, S.M.; Li, C.Y.; Hang, J.; Deng, J.; Ye, X.H.; Liu, Y. Pretreatment ultrasound-based deep learning radiomics model for the early prediction of pathologic response to neoadjuvant chemotherapy in breast cancer. Eur. Radiol. 2023, 33, 5634–5644. [Google Scholar] [CrossRef]
- Mo, Y.; Han, C.; Liu, Y.; Liu, M.; Shi, Z.; Lin, J.; Zhao, B.; Huang, C.; Qiu, B.; Cui, Y.; et al. Hover-trans: Anatomy-aware hover-transformer for roi-free breast cancer diagnosis in ultrasound images. IEEE Trans. Med. Imaging 2023, 42, 1696–1706. [Google Scholar] [CrossRef]
- Shah, A. Breast Ultrasound Images Dataset. Kaggle. 2021. Available online: https://www.kaggle.com/datasets/aryashah2k/breast-ultrasound-images-dataset (accessed on 24 April 2025).
- Lanjewar, M.G.; Panchbhai, K.G.; Patle, L.B. Fusion of transfer learning models with LSTM for detection of breast cancer using ultrasound images. Comput. Biol. Med. 2024, 169, 107914. [Google Scholar] [CrossRef]
- Rao, K.S.; Terlapu, P.V.; Jayaram, D.; Raju, K.K.; Kumar, G.K.; Pemula, R.; Gopalachari, V.; Rakesh, S. Intelligent ultrasound imaging for enhanced breast cancer diagnosis: Ensemble transfer learning strategies. IEEE Access 2024, 12, 22243–22263. [Google Scholar] [CrossRef]
- Ellis, J.; Appiah, K.; Amankwaa-Frempong, E.; Kwok, S.C. Classification of 2D Ultrasound Breast Cancer Images with Deep Learning. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Seattle, WA, USA, 16–22 June 2024; pp. 5167–5173. [Google Scholar]
- Muduli, D.; Kumar, R.R.; Pradhan, J.; Kumar, A. An empirical evaluation of extreme learning machine uncertainty quantification for automated breast cancer detection. Neural Comput. Appl. 2023, 37, 7909–7924. [Google Scholar] [CrossRef]
- Sahu, A.; Das, P.K.; Meher, S. An efficient deep learning scheme to detect breast cancer using mammogram and ultrasound breast images. Biomed. Signal Process. Control 2024, 87, 105377. [Google Scholar] [CrossRef]
- Himel, M.H.A.M.H.; Chowdhury, P.; Hasan, M.A.M. A robust encoder decoder based weighted segmentation and dual staged feature fusion based meta classification for breast cancer utilizing ultrasound imaging. Intell. Syst. Appl. 2024, 22, 200367. [Google Scholar] [CrossRef]
- Umer, M.J.; Sharif, M.; Raza, M. A Multi-attention Triple Decoder Deep Convolution Network for Breast Cancer Segmentation Using Ultrasound Images. Cogn. Comput. 2024, 16, 581–594. [Google Scholar] [CrossRef]
- Guo, D.; Lu, C.; Chen, D.; Yuan, J.; Duan, Q.; Xue, Z.; Liu, S.; Huang, Y. A multimodal breast cancer diagnosis method based on Knowledge-Augmented Deep Learning. Biomed. Signal Process. Control 2024, 90, 105843. [Google Scholar] [CrossRef]
- Admass, W.S.; Munaye, Y.Y.; Salau, A.O. Integration of feature enhancement technique in Google inception network for breast cancer detection and classification. J. Big Data 2024, 11, 78. [Google Scholar] [CrossRef]
- Ru, J.; Zhu, Z.; Shi, J. Spatial and geometric learning for classification of breast tumors from multi-center ultrasound images: A hybrid learning approach. Bmc Med. Imaging 2024, 24, 133. [Google Scholar] [CrossRef]
- Karunanayake, N.; Makhanov, S.S. When deep learning is not enough: Artificial life as a supplementary tool for segmentation of ultrasound images of breast cancer. Med. Biol. Eng. Comput. 2024, 63, 2497–2520. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agrawal, S.; Singh, S.K.; Kumar, S. A novel transfer learning-based model for ultrasound breast cancer image classification. In Computational Vision and Bio-Inspired Computing: Proceedings of ICCVBIC 2022; Springer: Berlin/Heidelberg, Germany, 2023; pp. 511–523. [Google Scholar]
- Zakareya, S.; Izadkhah, H.; Karimpour, J. A new deep-learning-based model for breast cancer diagnosis from medical images. Diagnostics 2023, 13, 1944. [Google Scholar] [CrossRef]
- Zhang, B.; Vakanski, A.; Xian, M. BI-RADS-NET-V2: A Composite Multi-Task Neural Network for Computer-Aided Diagnosis of Breast Cancer in Ultrasound Images With Semantic and Quantitative Explanations. IEEE Access 2023, 11, 79480–79494. [Google Scholar] [CrossRef]
- Işık, G.; Paçal, İ. Few-shot classification of ultrasound breast cancer images using meta-learning algorithms. Neural Comput. Appl. 2024, 36, 12047–12059. [Google Scholar] [CrossRef]
- Hossain, S.; Azam, S.; Montaha, S.; Karim, A.; Chowa, S.S.; Mondol, C.; Hasan, M.Z.; Jonkman, M. Automated breast tumor ultrasound image segmentation with hybrid UNet and classification using fine-tuned CNN model. Heliyon 2023, 9, e21369. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mohammadi, S.; Hadizadeh, H.; Olfati, M.; Moradi, F.; Tanzifi, G.; Ghaderi, S. Brain metastases from breast cancer using magnetic resonance imaging: A systematic review. J. Med. Radiat. Sci. 2024, 71, 133–141. [Google Scholar] [CrossRef]
- Berg, W. Breast MRI|DenseBreast-info, Inc.—densebreast-info.org. Available online: https://densebreast-info.org/screening-technologies/breast-mri/ (accessed on 1 December 2024).
- Zhang, Z.; Lan, H.; Zhao, S. Analysis of the Value of Quantitative Features in Multimodal MRI Images to Construct a Radio-Omics Model for Breast Cancer Diagnosis. Breast Cancer Targets Ther. 2024, 16, 305–318. [Google Scholar] [CrossRef]
- Liu, M.Z.; Swintelski, C.; Sun, S.; Siddique, M.; Desperito, E.; Jambawalikar, S.; Ha, R. Weakly supervised deep learning approach to breast MRI assessment. Acad. Radiol. 2022, 29, S166–S172. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, J.; Dou, Y.; Rubert, N.; Wang, Y.; Deng, J. A deep learning fusion model with evidence-based confidence level analysis for differentiation of malignant and benign breast tumors using dynamic contrast enhanced MRI. Biomed. Signal Process. Control 2022, 72, 103319. [Google Scholar] [CrossRef]
- Yue, W.Y.; Zhang, H.T.; Gao, S.; Li, G.; Sun, Z.Y.; Tang, Z.; Cai, J.M.; Tian, N.; Zhou, J.; Dong, J.H.; et al. Predicting breast cancer subtypes using magnetic resonance imaging based radiomics with automatic segmentation. J. Comput. Assist. Tomogr. 2023, 47, 729–737. [Google Scholar] [CrossRef]
- Qian, H.; Ren, X.; Xu, M.; Fang, Z.; Zhang, R.; Bu, Y.; Zhou, C. Magnetic resonance imaging-based radiomics was used to evaluate the level of prognosis-related immune cell infiltration in breast cancer tumor microenvironment. Bmc Med. Imaging 2024, 24, 31. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, X.; Tang, W.; Chen, S.; Wang, M.; Fan, Y.; Lin, C.; Hu, W.; Yang, J.; Xiang, J.; et al. Noninvasive identification of HER2-low-positive status by MRI-based deep learning radiomics predicts the disease-free survival of patients with breast cancer. Eur. Radiol. 2024, 34, 899–913. [Google Scholar] [CrossRef]
- Cong, C.; Li, X.; Zhang, C.; Zhang, J.; Sun, K.; Liu, L.; Ambale-Venkatesh, B.; Chen, X.; Wang, Y. MRI-based breast cancer classification and localization by multiparametric feature extraction and combination using deep learning. J. Magn. Reson. Imaging 2024, 59, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Li, F.; Yao, J.; Tong, F.; Hua, M.; Liu, J.; Shi, C.; Sui, L.; Lu, H. Predictive value of MRI-based deep learning model for lymphovascular invasion status in node-negative invasive breast cancer. Sci. Rep. 2024, 14, 16204. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Mahto, M.; Srivastava, S. Breast Cancer Detection and Localization Using a Novel Multi Modal Approach. IEEE Trans. Instrum. Meas. 2024, 74, 4000413. [Google Scholar]
- Hasan, A.M.; Al-Waely, N.K.; Aljobouri, H.K.; Jalab, H.A.; Ibrahim, R.W.; Meziane, F. Molecular subtypes classification of breast cancer in DCE-MRI using deep features. Expert Syst. Appl. 2024, 236, 121371. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y. Deep Learning-Based Modified YOLACT Algorithm on Magnetic Resonance Imaging Images for Screening Common and Difficult Samples of Breast Cancer. Diagnostics 2023, 13, 1582. [Google Scholar] [CrossRef]
- Vivancos Bargalló, H.; Stick, L.B.; Korreman, S.S.; Kronborg, C.; Nielsen, M.M.; Borgen, A.C.; Offersen, B.V.; Nørrevang, O.; Kallehauge, J.F. Classification of laterality and mastectomy/lumpectomy for breast cancer patients for improved performance of deep learning auto segmentation. Acta Oncol. 2023, 62, 1546–1550. [Google Scholar] [CrossRef]
- Koh, J.; Yoon, Y.; Kim, S.; Han, K.; Kim, E.K. Deep learning for the detection of breast cancers on chest computed tomography. Clin. Breast Cancer 2022, 22, 26–31. [Google Scholar] [CrossRef]
- Yasaka, K.; Sato, C.; Hirakawa, H.; Fujita, N.; Kurokawa, M.; Watanabe, Y.; Kubo, T.; Abe, O. Impact of deep learning on radiologists and radiology residents in detecting breast cancer on CT: A cross-vendor test study. Clin. Radiol. 2024, 79, e41–e47. [Google Scholar] [CrossRef] [PubMed]
- Jalalian, A.; Mashohor, S.; Mahmud, R.; Karasfi, B.; Iqbal Saripan, M.; Ramli, A.R. Computer-assisted diagnosis system for breast cancer in computed tomography laser mammography (CTLM). J. Digit. Imaging 2017, 30, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dong, Y.h.; Xu, L.Y.; Shen, Y.Y.; Qin, G.; Zhang, Z.B. Deep bone oncology Diagnostics: Computed tomography based Machine learning for detection of bone tumors from breast cancer metastasis. J. Bone Oncol. 2024, 48, 100638. [Google Scholar] [CrossRef]
- Desperito, E.; Schwartz, L.; Capaccione, K.M.; Collins, B.T.; Jamabawalikar, S.; Peng, B.; Patrizio, R.; Salvatore, M.M. Chest CT for breast cancer diagnosis. Life 2022, 12, 1699. [Google Scholar] [CrossRef]
- Shehzad, I.; Zafar, A. Breast Cancer CT-Scan Image Classification Using Transfer Learning. Sn Comput. Sci. 2023, 4, 789. [Google Scholar] [CrossRef]
- Rakha, E.; Tozbikian, G. Invasive Breast Carcinoma of No Special Type and Variants Invasive Breast Cancer of No Special Type (NST). 2024. Available online: https://www.pathologyoutlines.com/topic/breastmalignantductalnos.html (accessed on 9 December 2024).
- Hava Muntean, C.; Chowkkar, M. Breast cancer detection from histopathological images using deep learning and transfer learning. In Proceedings of the 2022 7th International Conference on Machine Learning Technologies, Rome, Italy, 11–13 March 2022; pp. 164–169. [Google Scholar]
- Bhowal, P.; Sen, S.; Velasquez, J.D.; Sarkar, R. Fuzzy ensemble of deep learning models using choquet fuzzy integral, coalition game and information theory for breast cancer histology classification. Expert Syst. Appl. 2022, 190, 116167. [Google Scholar] [CrossRef]
- Yang, J.; Ju, J.; Guo, L.; Ji, B.; Shi, S.; Yang, Z.; Gao, S.; Yuan, X.; Tian, G.; Liang, Y.; et al. Prediction of HER2-positive breast cancer recurrence and metastasis risk from histopathological images and clinical information via multimodal deep learning. Comput. Struct. Biotechnol. J. 2022, 20, 333–342. [Google Scholar] [CrossRef]
- Maleki, A.; Raahemi, M.; Nasiri, H. Breast cancer diagnosis from histopathology images using deep neural network and XGBoost. Biomed. Signal Process. Control 2023, 86, 105152. [Google Scholar] [CrossRef]
- Majumdar, S.; Pramanik, P.; Sarkar, R. Gamma function based ensemble of CNN models for breast cancer detection in histopathology images. Expert Syst. Appl. 2023, 213, 119022. [Google Scholar] [CrossRef]
- Ray, R.K.; Linkon, A.A.; Bhuiyan, M.S.; Jewel, R.M.; Anjum, N.; Ghosh, B.P.; Mia, M.T.; Badruddowza; Sarker, M.S.U.; Shaima, M. Transforming Breast Cancer Identification: An In-Depth Examination of Advanced Machine Learning Models Applied to Histopathological Images. J. Comput. Sci. Technol. Stud. 2024, 6, 155–161. [Google Scholar] [CrossRef]
- Rajkumar, R.; Gopalakrishnan, S.; Praveena, K.; Venkatesan, M.; Ramamoorthy, K.; Hephzipah, J.J. Darknet-53 convolutional neural network-based image processing for breast cancer detection. Mesop. J. Artif. Intell. Healthc. 2024, 2024, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, A.M. Enhanced pre-processing technique for histopathological image stain normalization and cancer detection. Multimed. Tools Appl. 2024, 84, 29733–29761. [Google Scholar] [CrossRef]
- Addo, D.; Zhou, S.; Sarpong, K.; Nartey, O.T.; Abdullah, M.A.; Ukwuoma, C.C.; Al-antari, M.A. A hybrid lightweight breast cancer classification framework using the histopathological images. Biocybern. Biomed. Eng. 2024, 44, 31–54. [Google Scholar] [CrossRef]
- Sampath, N.; Srinath, N. Breast cancer detection from histopathological image dataset using hybrid convolution neural network. Int. J. Model. Simul. Sci. Comput. 2024, 15, 2441003. [Google Scholar] [CrossRef]
- Yu, D.; Lin, J.; Cao, T.; Chen, Y.; Li, M.; Zhang, X. SECS: An effective CNN joint construction strategy for breast cancer histopathological image classification. J. King Saud Univ.-Comput. Inf. Sci. 2023, 35, 810–820. [Google Scholar] [CrossRef]
- Zarif, S.; Abdulkader, H.; Elaraby, I.; Alharbi, A.; Elkilani, W.S.; Pławiak, P. Using hybrid pre-trained models for breast cancer detection. PLoS ONE 2024, 19, e0296912. [Google Scholar] [CrossRef]
- Maheshwari, N.U.; SatheesKumaran, S. Automatic Mitosis and Nuclear Atypia Detection for Breast Cancer Grading in Histopathological Images using Hybrid Machine Learning Technique. Multimed. Tools Appl. 2024, 83, 90105–90132. [Google Scholar] [CrossRef]
- Naz, A.; Khan, H.; Din, I.U.; Ali, A.; Husain, M. An Efficient Optimization System for Early Breast Cancer Diagnosis based on Internet of Medical Things and Deep Learning. Eng. Technol. Appl. Sci. Res. 2024, 14, 15957–15962. [Google Scholar] [CrossRef]
- Sharmin, S.; Ahammad, T.; Talukder, M.A.; Ghose, P. A hybrid dependable deep feature extraction and ensemble-based machine learning approach for breast cancer detection. IEEE Access 2023, 11, 87694–87708. [Google Scholar] [CrossRef]
- Tsietso, D.; Yahya, A.; Samikannu, R.; Tariq, M.U.; Babar, M.; Qureshi, B.; Koubaa, A. Multi-input deep learning approach for breast cancer screening using thermal infrared imaging and clinical data. IEEE Access 2023, 11, 52101–52116. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biswas, S.; Majee, A.; Sen, S.; Oliva, D.; Sarkar, R. Breast cancer detection from thermal images using a Grunwald-Letnikov-aided Dragonfly algorithm-based deep feature selection method. Comput. Biol. Med. 2022, 141, 105027. [Google Scholar] [CrossRef] [PubMed]
- Roubidoux, M.A.; Sabel, M.S.; Bailey, J.E.; Kleer, C.G.; Klein, K.A.; Helvie, M.A. Breast Scans—Dynamic Thermal Imaging—nydti.com. Available online: https://www.nydti.com/services/breast-scans/ (accessed on 9 December 2024).
- Civilibal, S.; Cevik, K.K.; Bozkurt, A. A deep learning approach for automatic detection, segmentation and classification of breast lesions from thermal images. Expert Syst. Appl. 2023, 212, 118774. [Google Scholar] [CrossRef]
- Ikechukwu, A.V.; Bhimshetty, S.; Deepu, R.; Mala, M. Advances in Thermal Imaging: A Convolutional Neural Network Approach for Improved Breast Cancer Diagnosis. In Proceedings of the 2024 International Conference on Distributed Computing and Optimization Techniques (ICDCOT), Bengaluru, India, 15–16 March 2024; pp. 1–7. [Google Scholar]
- Mahoro, E.; Akhloufi, M.A. Breast cancer classification on thermograms using deep CNN and transformers. Quant. Infrared Thermogr. J. 2024, 21, 30–49. [Google Scholar] [CrossRef]
- Al Husaini, M.A.; Habaebi, M.H.; Elsheikh, E.A.; Islam, M.R.; Suliman, F.; Husaini, Y.N.A. Evaluating the Effect of Noisy Thermal Images On the Detection of Early Breast Cancer Using Deep Learning. Res. Sq. 2024, 5, 3923–3953. [Google Scholar]
- Al Husaini, M.A.S.; Habaebi, M.H.; Islam, M.R. Real-time thermography for breast cancer detection with deep learning. Discov. Artif. Intell. 2024, 4, 57. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Sherif, F.F.; Abdallah, M.S.; Cho, Y.I.; ElMetwally, S.M. An Innovative Thermal Imaging Prototype for Precise Breast Cancer Detection: Integrating Compression Techniques and Classification Methods. Bioengineering 2024, 11, 764. [Google Scholar] [CrossRef]
- Pramanik, R.; Pramanik, P.; Sarkar, R. Breast cancer detection in thermograms using a hybrid of GA and GWO based deep feature selection method. Expert Syst. Appl. 2023, 219, 119643. [Google Scholar] [CrossRef]
- Moayedi, S.M.Z.; Rezai, A.; Hamidpour, S.S.F. Toward Effective Breast Cancer Detection in Thermal Images Using Efficient Feature Selection Algorithm and Feature Extraction Methods. Biomed. Eng. Appl. Basis Commun. 2024, 36, 2450007. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hamada, M.; Sharmin, S.; Rimi, T.A.; Talukder, A.S.; Imran, N.; Kobra, K.; Ahmed, M.R.; Rabbi, M.; Matin, M.M.H.; et al. Enhancing Early Breast Cancer Detection through Advanced Data Analysis. IEEE Access 2024, 12, 161941–161953. [Google Scholar] [CrossRef]
- Manikandan, P.; Durga, U.; Ponnuraja, C. An integrative machine learning framework for classifying SEER breast cancer. Sci. Rep. 2023, 13, 5362. [Google Scholar] [CrossRef]
- Albadr, M.A.A.; AL-Dhief, F.T.; Man, L.; Arram, A.; Abbas, A.H.; Homod, R.Z. Online sequential extreme learning machine approach for breast cancer diagnosis. Neural Comput. Appl. 2024, 36, 10413–10429. [Google Scholar] [CrossRef]
- Islam, M.R.; Islam, M.S.; Majumder, S. Breast Cancer Prediction: A Fusion of Genetic Algorithm, Chemical Reaction Optimization, and Machine Learning Techniques. Appl. Comput. Intell. Soft Comput. 2024, 2024, 7221343. [Google Scholar] [CrossRef]
- Valencia-Moreno, J.M.; Gonzalez-Fraga, J.A.; Gutierrez-Lopez, E.; Estrada-Senti, V.; Cantero-Ronquillo, H.A.; Kober, V. Breast cancer risk estimation with intelligent algorithms and risk factors for Cuban women. Comput. Biol. Med. 2024, 179, 108818. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J. Breast Cancer: A Multidisciplinary Approach; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Jochelson, M. Advanced imaging techniques for the detection of breast cancer. Am. Soc. Clin. Oncol. Educ. Book 2012, 32, 65–69. [Google Scholar] [CrossRef]
- Basurto-Hurtado, J.A.; Cruz-Albarran, I.A.; Toledano-Ayala, M.; Ibarra-Manzano, M.A.; Morales-Hernandez, L.A.; Perez-Ramirez, C.A. Diagnostic Strategies for Breast Cancer Detection: From Image Generation to Classification Strategies Using Artificial Intelligence Algorithms. Cancers 2022, 14, 3442. [Google Scholar] [CrossRef]
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D.; Musa, A.E. A review of various modalities in breast imaging: Technical aspects and clinical outcomes. Egypt. J. Radiol. Nucl. Med. 2020, 51, 57. [Google Scholar] [CrossRef]
- Caballo, M.; Pangallo, D.R.; Mann, R.M.; Sechopoulos, I. Deep learning-based segmentation of breast masses in dedicated breast CT imaging: Radiomic feature stability between radiologists and artificial intelligence. Comput. Biol. Med. 2020, 118, 103629. [Google Scholar] [CrossRef] [PubMed]
- Roslidar, R.; Rahman, A.; Muharar, R.; Syahputra, M.R.; Arnia, F.; Syukri, M.; Pradhan, B.; Munadi, K. A review on recent progress in thermal imaging and deep learning approaches for breast cancer detection. IEEE Access 2020, 8, 116176–116194. [Google Scholar] [CrossRef]
- Rasheed, M.E.H.; Youseffi, M. Breast Cancer and Medical Imaging; IOP Publishing: Bristol, UK, 2024. [Google Scholar] [CrossRef]
- Al Jarroudi, O.; El Bairi, K.; Curigliano, G. Breast Cancer Research and Treatment: Innovative Concepts; Springer Nature: Berlin, Germany, 2024; Volume 188. [Google Scholar]
- Darakh, A.; Shah, A.; Oza, P. Exploring the Benefits of Data Augmentation for Breast Cancer Classification using Transfer Learning. In Proceedings of the World Conference on Information Systems for Business Management, Bangkok, Thailand, 7–8 September; Springer: Berlin/Heidelberg, Germany, 2023; pp. 509–520. [Google Scholar]
- Adama, S.; dite Soukoura, D.F.; Ismaël, K.; Lahsen, B. Reliable Medical Data Augmentation for Deep Learning: A Case Study on Breast Cancer Prediction. In Proceedings of the 2024 Sixth International Conference on Intelligent Computing in Data Sciences (ICDS), Marrakech, Morocco, 23–24 October 2024; pp. 1–10. [Google Scholar]
- Talwar, B.; Arora, A.; Bharany, S. An energy efficient agent aware proactive fault tolerance for preventing deterioration of virtual machines within cloud environment. In Proceedings of the 2021 9th International Conference on Reliability, Infocom Technologies and Optimization (Trends and Future Directions) (ICRITO), Noida, India, 3–4 September 2021; pp. 1–7. [Google Scholar]
- Emmanuel, A.A.; Awokola, J.A.; Alam, S.; Bharany, S.; Agboola, P.; Shuaib, M.; Ahmed, R. A hybrid framework of blockchain and IoT technology in the pharmaceutical industry: A comprehensive study. Mob. Inf. Syst. 2023, 2023, 3265310. [Google Scholar] [CrossRef]
- Sundas, A.; Badotra, S.; Shahi, G.S.; Verma, A.; Bharany, S.; Ibrahim, A.O.; Abulfaraj, A.W.; Binzagr, F. Smart patient monitoring and recommendation (SPMR) using cloud analytics and deep learning. IEEE Access 2024, 12, 54238–54255. [Google Scholar] [CrossRef]


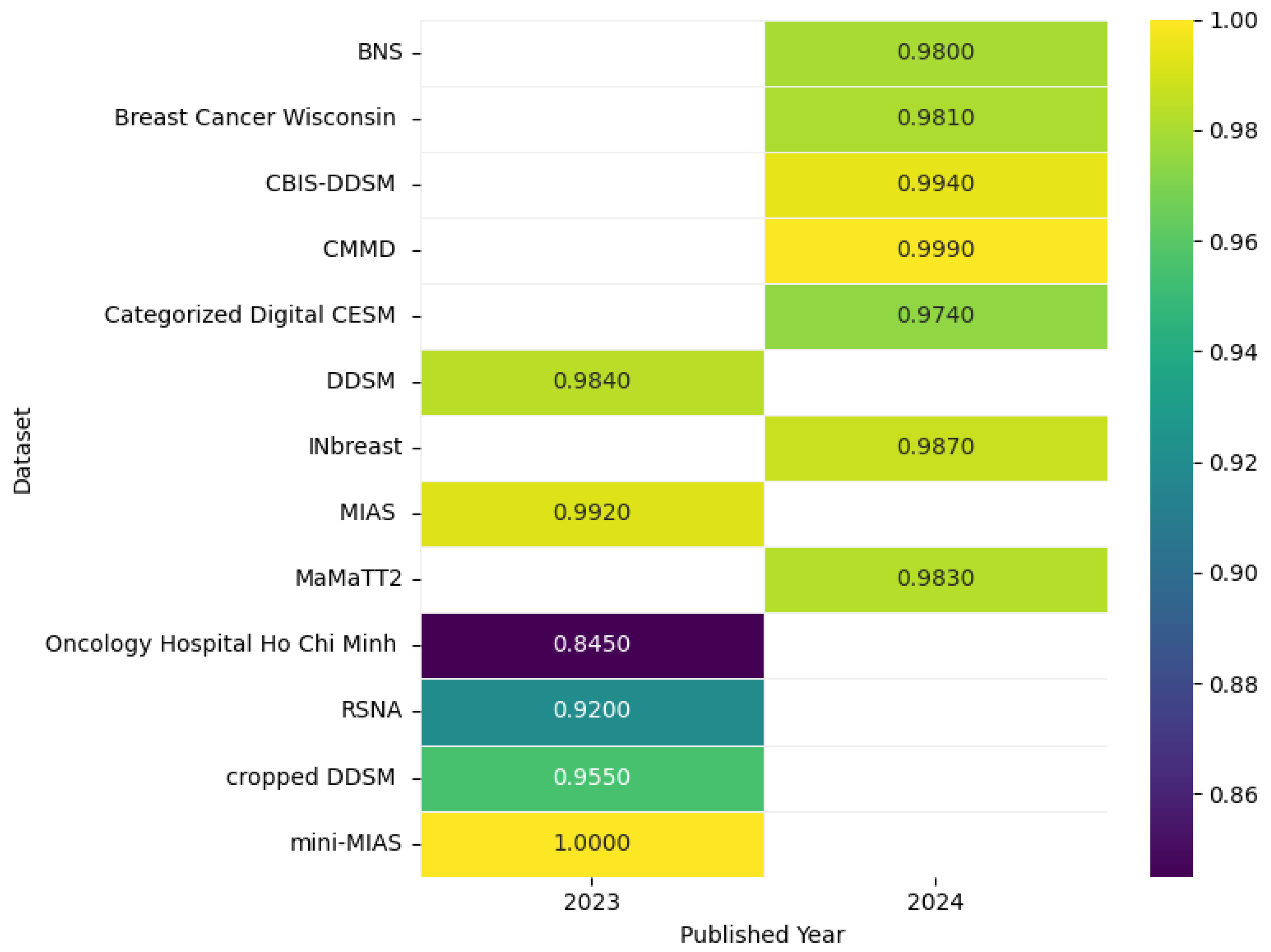
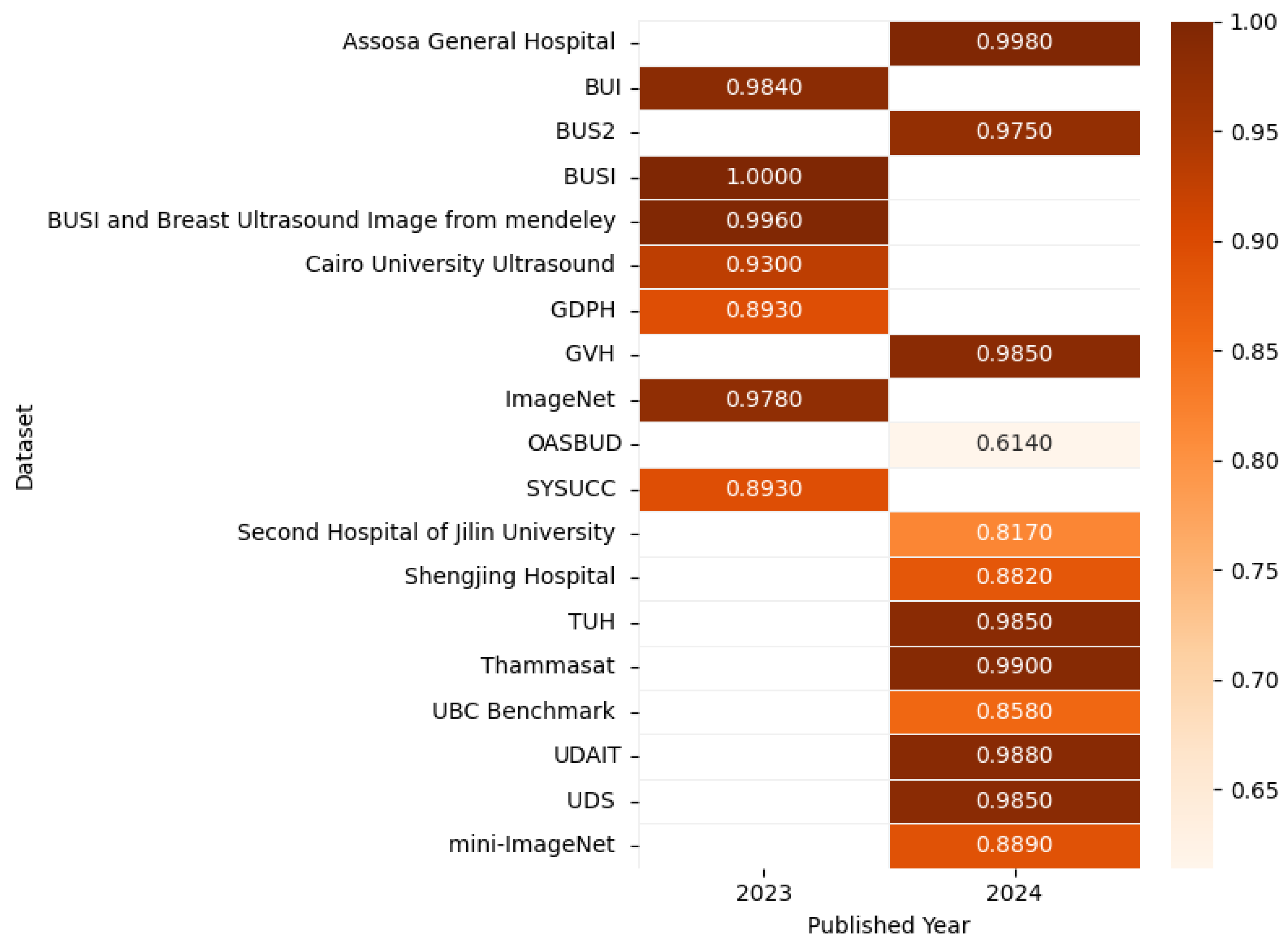


| Review Papers | Year | Research Questions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | ||
| Abhisheka et al. [21] | 2023 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Nasser et al. [22] | 2023 | ✓ | ✓ | ✓ | ✓ | ||||||
| Thakur et al. [23] | 2024 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Rautela et al. [15] | 2024 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Sharafaddini et al. [24] | 2024 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Sushanki et al. [25] | 2024 | ✓ | |||||||||
| Our review | 2025 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ref. | Dataset | Description | Source | Modality |
|---|---|---|---|---|
| [26] | The Chinese Mammography Database (CMMD) | This dataset contains 3728 mammograms from 1775 patients (China, 2012–2016), with biopsy-confirmed diagnoses and molecular subtypes (Luminal A/B, HER2+, Triple-negative) for 749 cases. | The dataset is publicly available at https://www.cancerimagingarchive.net/collection/cmmd/ (accessed on 5 April 2025) | X-Ray |
| [27] | Mammographic Image Analysis Society (MIAS) | The MIAS (Mammographic Image Analysis Society) database contains 322 digitized mammogram images from the UK National Breast Screening Program. Stored on a 2.3 GB 8 mm (Exabyte) tape, all images are clipped, padded, and resized to 1024 × 1024 pixels with a 200-micron resolution. It includes annotations on breast density, abnormality type, location, and severity, and is widely used for breast cancer detection research. | The dataset is publicly available at https://www.kaggle.com/datasets/kmader/mias-mammography (accessed on 5 April 2025) | X-Ray |
| [28] | Digital Database for Screening Mammography (DDSM) | The Digital Database for Screening Mammography (DDSM) is a publicly accessible resource created to aid research in mammographic image analysis and breast cancer detection. It consists of 2620 digitized mammography cases, which include a variety of benign, benign without callback, typical, and cancer cases carefully selected for the database. Each case includes mammographic images of both breasts, along with detailed annotations specifying lesion types, locations, and pathology outcomes. | The DDSM is hosted by the University of South Florida and available at http://www.mammoimage.org/databases/ (accessed on 5 April 2025) | X-Ray |
| [29] | Curated Breast Imaging Subset of DDSM (CBIS-DDSM) | The CBIS-DDSM dataset comprises 1566 standardized mammography studies selected from the original DDSM database of 2620 cases. It features DICOM-formatted images, precise lesion annotations, and verified pathology data to support CAD research. | The dataset is publicly available for academic use through The Cancer Imaging Archive: https://www.cancerimagingarchive.net/collection/cbis-ddsm/ (accessed on 5 April 2025) | X-Ray |
| [30] | INbreast | The INbreast dataset is a public digital mammography database containing 115 cases (410 images), including 90 bilateral cases (4 images each) and 25 mastectomy cases (2 images each). It features diverse breast lesions (masses, calcifications, asymmetries, distortions) with precise radiologist annotations in XML format. | The INbreast dataset was developed at Centro Hospitalar de São João in Porto, Portugal, with ethical approval from the Portuguese National Data Protection Commission and the Hospital Ethics Committee, using Siemens MammoNovation full-field digital mammography systems. | X-Ray |
| [31] | Breast Ultrasound and US-Guided Interventional Techniques: A Multimedia Teaching File | This specialized collection contains 306 breast ultrasound images with an average resolution of 377 × 396 pixels, extracted from 51 clinical cases. The images cover various breast conditions and were originally compiled for professional medical education purposes. | The “Breast Ultrasound and US-Guided Interventional Techniques” dataset was commercially distributed as a CD-ROM (2003) through BreastUltrasound.com (Thessaloniki, Greece). | Ultrasound |
| [32] | Dataset B | This dataset contains 163 breast ultrasound images (size 760 × 570 pixels) collected in 2012 at UDIAT Diagnostic Center, Parc Taulí Corporation (Sabadell, Spain). It includes 53 malignant and 110 benign lesion cases from different patients. | The breast cancer imaging dataset can be accessed online at goo.gl/SJmoti for research and development purposes. | Ultrasound |
| [33] | Open Access Series of Breast Ultrasound Data (OASBUD) | The Open Access Series of Breast Ultrasound Data (OASBUD) contains raw radiofrequency ultrasound scans from 78 female patients, featuring 98 breast lesions (52 malignant and 46 benign). | Researchers may access the publicly available dataset at https://zenodo.org/records/545928#.XYp6mShKi9w (accessed on 6 April 2025) | Ultrasound |
| [34] | Breast Ultrasound Dataset | This breast ultrasound dataset contains 780 images (500 × 500 pixels) from 600 female patients (aged 25–75), collected in 2018 at Baheya Hospital. It includes 437 benign, 210 malignant, and 133 normal cases | The dataset is publicly available for research purposes and can be accessed at https://www.kaggle.com/datasets/aryashah2k/breast-ultrasound-images-dataset (accessed on 6 April 2025) | Ultrasound |
| [35] | UDAIT | The UDAIT breast ultrasound collection contains 163 images (110 benign, 53 malignant masses) from the UDAIT Diagnostic Centre (Parc Taulí, Spain). Captured via Siemens ACUSON scanners, each image depicts a single mass. | The dataset is publicly available at https://www.kaggle.com/datasets/jarintasnim090/udiat-data (accessed on 6 April 2025) | Ultrasound |
| [36] | Reference Image Database to Evaluate Therapy Response (RIDER) | The RIDER Breast MRI dataset contains 500 dynamic contrast-enhanced MRI examinations stored in The Cancer Imaging Archive, with each 288 × 288 pixel scan comprising 60 slices and corresponding expert-delineated tumor contours. | The dataset is publicly available at https://www.cancerimagingarchive.net/collection/rider-breast-mri/ (accessed on 11 April 2025) | MRI |
| [37] | The Cancer Genome Atlas (TCGA) | The Cancer Genome Atlas (TCGA) is a landmark NCI-led initiative that molecularly characterized over 20,000 primary tumors and matched normal samples across 33 cancer types, generating 2.5 petabytes of multi-omics data (genomics, transcriptomics, epigenomics, proteomics) from 2006 to 2018. | Researchers may obtain the dataset from the public repository at https://www.cancer.gov/ccg/research/genome-sequencing/tcga (accessed on 11 April 2025) | MRI |
| [38] | QIN-BREAST | The QIN-BREAST dataset provides longitudinal PET/CT and MRI scans (DWI, DCE, T1-mapping) acquired during neoadjuvant breast cancer therapy. Collected using standardized protocols (GE PET/CT, Philips 3T MRI), it enables quantitative treatment response assessment. | The data, including multi-center extension QIN-BREAST-02, is available at https://www.cancerimagingarchive.net/collection/qin-breast/ (accessed on 11 April 2025) | MRI |
| [39] | BreAst Cancer Histology (BACH) | The dataset comprises 400 microscopy images (TIFF format, 2048 × 1536 pixels, 0.42 µm/pixel) equally distributed across four classes: normal (100), benign (100), in situ carcinoma (100), and invasive carcinoma (100). Each RGB image (10–20 MB) includes image-wise labels. | The dataset is publicly available at https://iciar2018-challenge.grand-challenge.org/Dataset/ (accessed on 13 April 2025) | Histopathology |
| [40] | BreakHis | The BreakHis dataset contains 9109 breast tumor histopathology images (700 × 460 pixels, RGB, PNG) from 82 patients, including 2480 benign and 5429 malignant samples at four magnifications (40×, 100×, 200×, 400×). | The dataset is openly accessible at https://web.inf.ufpr.br/vri/databases/breast-cancer-histopathological-database-breakhis/ (accessed on 13 April 2025) | Histopathology |
| [41] | DBT-TU-JU | The DBT-TU-JU Breast Thermogram dataset contains 1100 thermal images from 100 patients, collected at GBP Hospital, Tripura. It includes 6 views per patient (frontal, lateral, oblique, supine) with 45 normal, 49 abnormal (33 benign, 13 malignant), and 6 unknown cases, validated via mammography/FNAC reports. Expert-annotated hotspot regions are provided for abnormal cases. | Internal patient databases are only accessible to the individual patients and their treating physicians. | Thermography |
| [42] | Digital Mammography and Radiology – Infrared (DMR-IR) | The DMR-IR dataset contains 3534 infrared (IR) breast images from 141 patients, collected at Hospital Universitário Antônio Pedro (Brazil). | The images are stored in the Database for Research Mastology with Infrared Image—DMR-IR, accessible on the website https://visual.ic.uff.br/dmi/ (accessed on 13 April 2025) | Thermography |
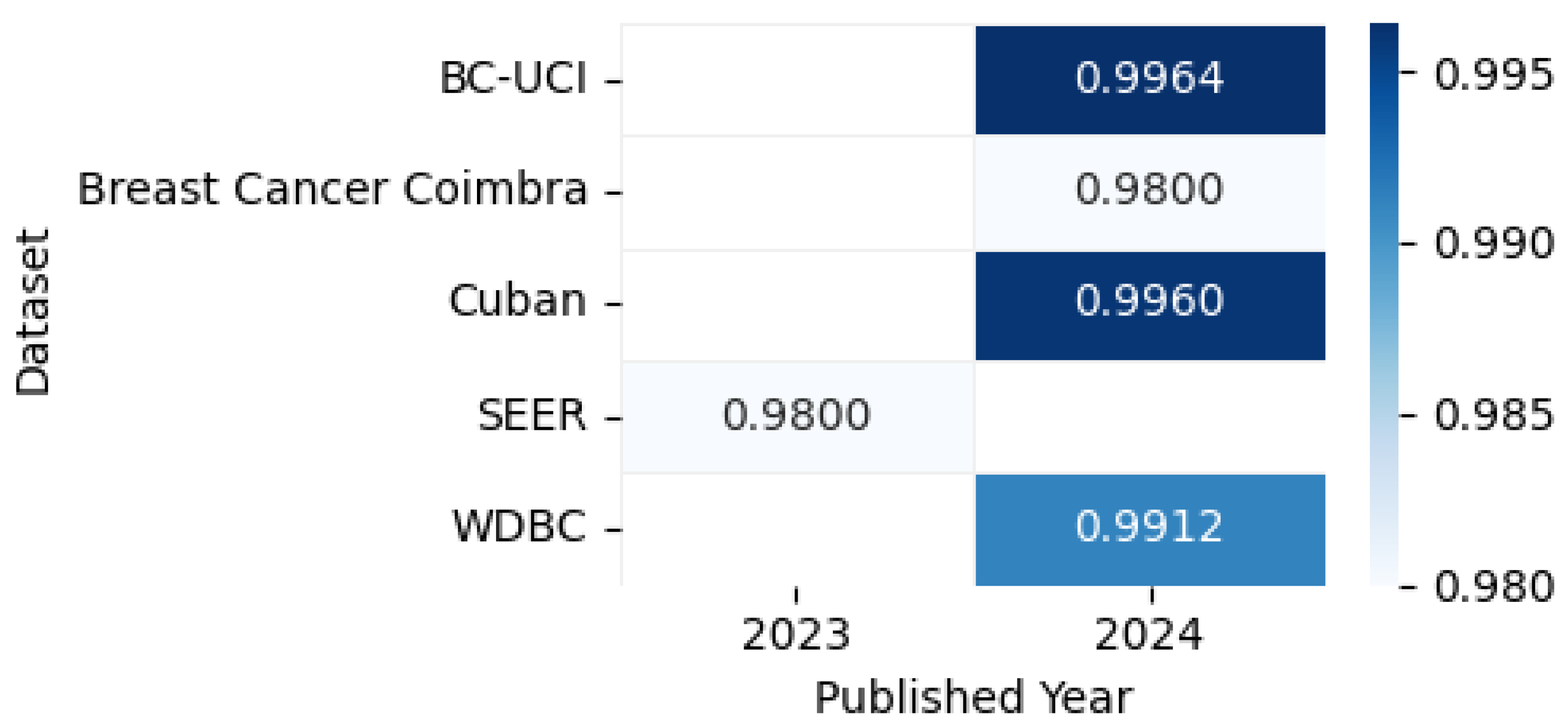
| [43] | Wisconsin Diagnostic Breast Cancer (WDBC) | The Wisconsin Diagnostic Breast Cancer (WDBC) dataset contains 569 samples (357 benign, 212 malignant) with 30 numerical features derived from cell nuclei characteristics | The data is available at https://www.kaggle.com/datasets/mohaiminul101/wisconsin-diagnostic-breast-cancer-wdbc (accessed on 13 April 2025) | CSV |
| [44] | SEER | The SEER Breast Cancer Dataset contains clinical records of 4024 female patients diagnosed with infiltrating ductal/lobular carcinoma (2006–2010), curated from the NCI’s SEER Program (2017 update). | The dataset is available at https://ieee-dataport.org/open-access/seer-breast-cancer-data (accessed on 13 April 2025) | CSV |
| [45] | METABRIC | The METABRIC (Molecular Taxonomy of Breast Cancer International Consortium) dataset is a widely used breast cancer resource containing clinical, genomic, and transcriptomic data from 2000 patients. It includes tumor characteristics, survival outcomes, gene expression, and copy number alterations, helping researchers study molecular subtypes, biomarkers, and treatment responses in breast cancer. | The dataset is available at https://ega-archive.org/studies/EGAS00000000083 (accessed on 13 April 2025) | CSV |
| [46] | Mammographic Mass Dataset (MM-Dataset) | The Mammographic Mass dataset contains 961 cases (516 benign, 445 malignant) with BI-RADS scores (1–5) and patient age. It helps evaluate CAD systems in reducing unnecessary biopsies ( 70% false positives) by predicting lesion severity from mammograms. | The dataset is available at https://archive.ics.uci.edu/dataset/161/mammographic+mass (accessed on 13 April 2025) | CSV |
| Type & Ref. | Categorization | Approach | Advantage | Limitation |
|---|---|---|---|---|
| Supervised Learning | ||||
| Classification & [47] | Discrete Output | Assigns input to predefined labels | Effective for clear categories; works well with algorithms like SVM, Decision Trees | Struggles with ambiguous cases; requires balanced datasets |
| Binary Classification & [47] | Two-class output (Yes/No) | Simple models like Logistic Regression | Easy to implement; computationally efficient | Cannot handle multi-class problems |
| Multiclass Classification & [48] | Multiple classes (>2) | Algorithms like Random Forest, Neural Networks. | Solves complex real-world problems with many categories | Requires more data and computational power |
| Multilabel Classification & [48] | Single instance → Multiple labels | Specialized algorithms like ML-kNN | Useful for text tagging, medical diagnosis | Complex evaluation; harder to train |
| Regression & [49] | Continuous Output | Predicts numerical values | Provides interpretable results | Sensitive to outliers; assumes linear relationships |
| Linear Regression & [49] | Linear modeling | Fits a straight line to data | Simple and fast | Poor performance on non-linear data |
| Non-linear Regression & [49] | Non-linear modeling (polynomials, kernels) | Captures complex patterns | Flexible and powerful for curved trends | Prone to overfitting; harder to tune |
| Ordinal Regression & [49] | Ordered categories (e.g., ratings 1–5) | Treats labels as ranked | Preserves order information | Difficult to model unequal intervals between ranks |
| Time Series Forecasting & [50] | Sequential data prediction | Models like ARIMA, LSTM | Critical for stock prices, weather forecasting | Sensitive to missing data; requires careful feature engineering |
| Ensemble Methods & [51] | Combines multiple models (e.g., Bagging, Boosting) | Random Forest, XGBoost | Reduces variance; improves accuracy | Computationally intensive; less interpretable |
| Stacking & [51] | Meta-Ensemble | Combines multiple models via a meta-learner (e.g., predictions of base models become input to a final model) | Often outperforms single models; leverages strengths of diverse algorithms | Computationally expensive; risk of overfitting if not properly tuned |
| Neural Networks & [52] | Deep Learning (CNN, RNN) | Learns hierarchical features | State-of-the-art for images, text, and complex data | Requires huge datasets; “black box” nature |
| Naïve Bayes & [53] | Probabilistic (Gaussian, Multinomial) | Based on Bayes’ theorem | Fast training; works well with small data | Assumes feature independence (often untrue) |
| Decision Trees & [54] | Predictive (Discrete/Continuous) | Rule-based splits | Easy to visualize and interpret | Prone to overfitting; unstable with small data changes |
| Unsupervised Learning | ||||
| K-means & [55] | Clustering | Groups data into k clusters | Simple and scalable | Sensitive to outliers; requires predefined k |
| PCA (Principal Component Analysis) & [56] | Dimensionality Reduction | Transforms data into uncorrelated components | Reduces noise and overfitting | Loses interpretability of original features |
| Gaussian Mixture Models (GMM) & [57] | Probabilistic clustering | Soft clustering (data points can belong to multiple clusters) | Flexible cluster shapes | Slow convergence; assumes Gaussian distribution |
| Hidden Markov Model (HMM) & [58] | Sequential data modeling | Models time-dependent processes | Powerful for speech recognition, genomics | Complex parameter tuning |
| Reinforcement Learning | ||||
| Q-Learning & [59] | Model-Free | Learns action–value function (Q-table) | Works in environments with unknown dynamics | Struggles with large state spaces |
| Deep Q Network (DQN) & [60] | Combines Q-Learning + Deep Learning | Uses neural networks to approximate Q-values | Handles high-dimensional states | Requires massive computational resources |
| Policy Gradient & [61] | Direct policy optimization | Updates policy parameters via gradient ascent | Can handle continuous action spaces | High variance; sample-inefficient |
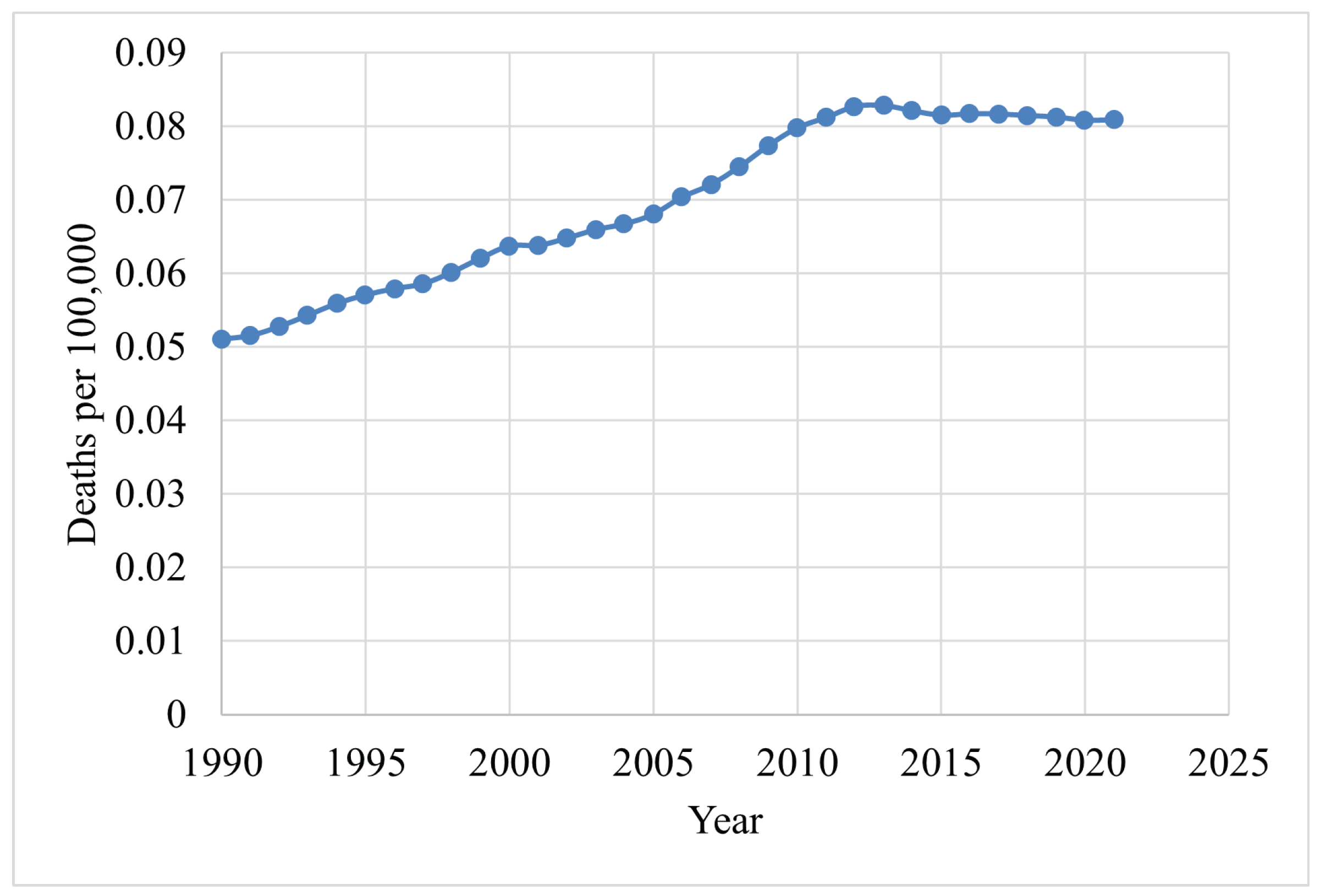
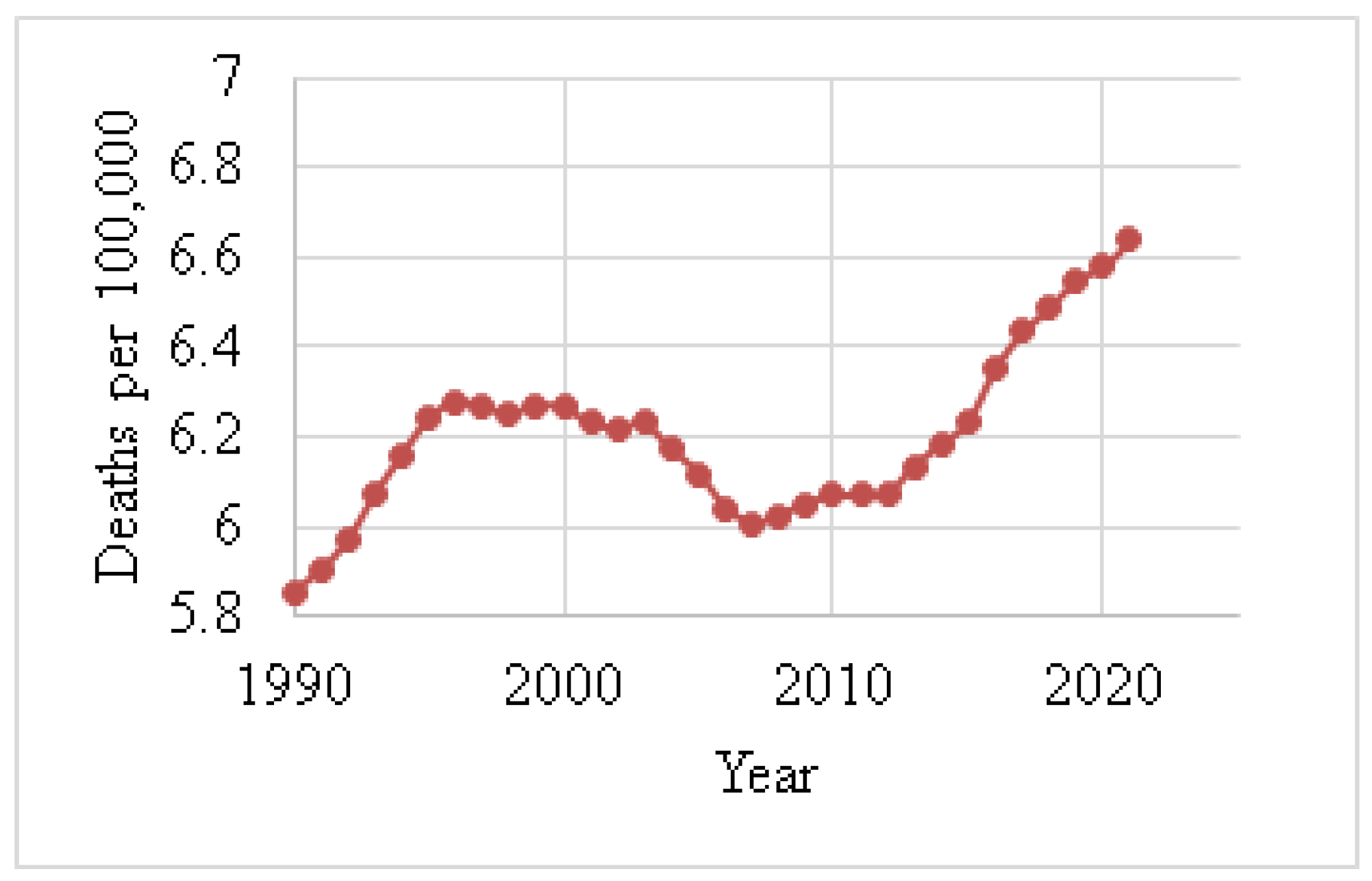
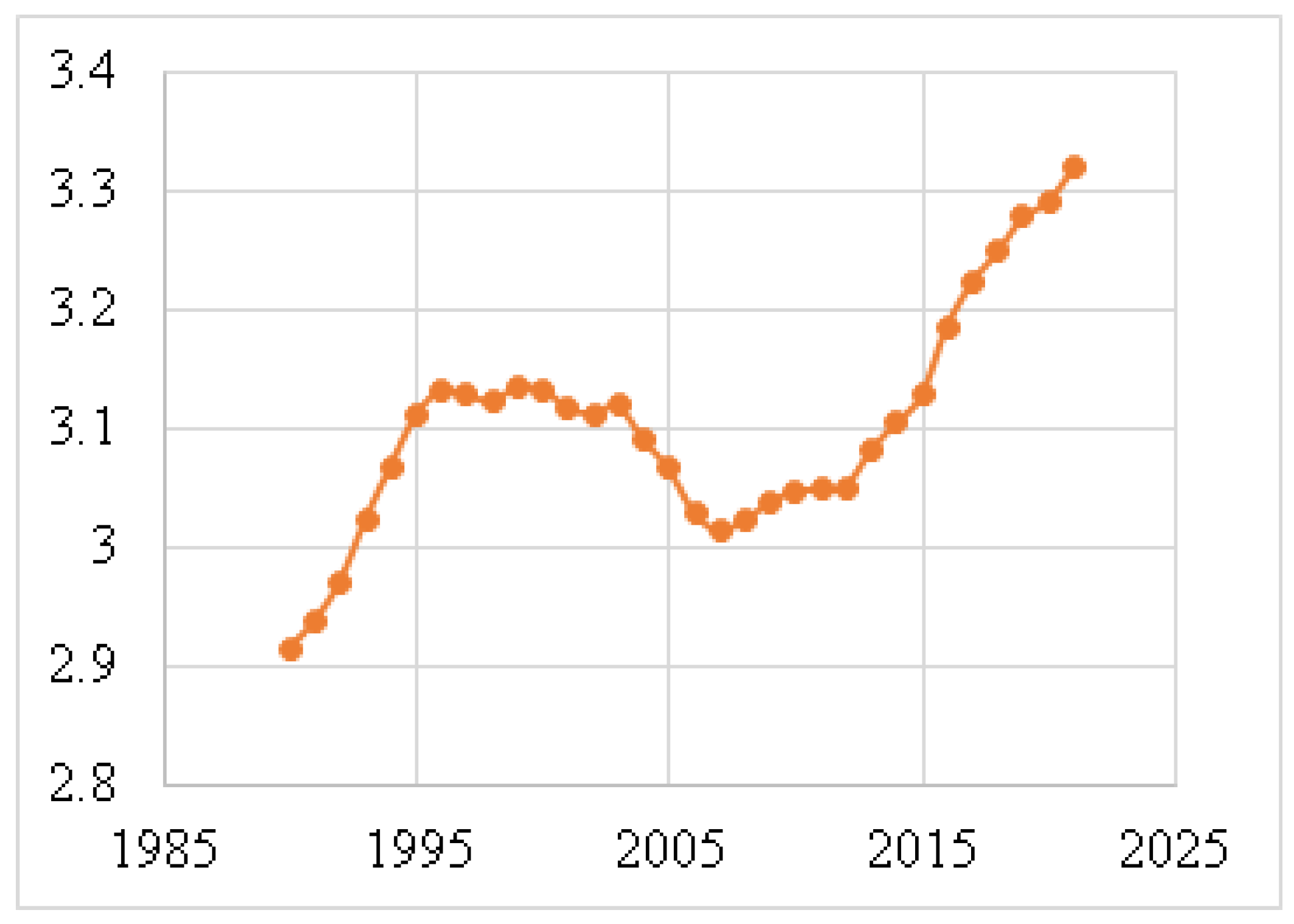
| Year | Breast | Lung | Stomach | Cervical | Colorectal | Brain |
|---|---|---|---|---|---|---|
| 1990 | 3 | 2 | 1 | 4 | 5 | 6 |
| 2000 | 3 | 1 | 2 | 4 | 5 | 6 |
| 2010 | 2 | 1 | 3 | 4 | 5 | 6 |
| 2011 | 1 | 2 | 3 | 4 | 5 | 6 |
| 2015 | 1 | 2 | 3 | 4 | 5 | 6 |
| 2018 | 1 | 2 | 3 | 4 | 5 | 6 |
| 2021 | 1 | 2 | 5 | 3 | 4 | 6 |
| Ref. | Year | Dataset | Image Size | Proposed Model | Proposed Approach | Results |
|---|---|---|---|---|---|---|
| [77] | 2024 | MIAS mammography & CBIS-DDSM | 1024 × 1024 & Non-fixed | MML-EOO-ACA-ATRUNet-MDN | Two-stage system: ACA-ATRUNet for segmentation, ACA-AMDN for cancer detection, hyperparameter optimization with MML-EOO. | MIAS mammography Accuracy: 0.8913 Precision: 0.8226 Specificity: 0.8937 F1-Score: 0.8536 CBIS-DDSM Accuracy: 0.8906 Precision: 0.8035 F1-Score: 0.8442 |
| [78] | 2024 | MaMaTT2 | 224 × 224 | EfficientNet | Data augmentation, EfficientNet. | Accuracy: 0.9829 |
| [74] | 2024 | MIAS breast cancer mammography images | 1024 × 1024 | Mammogram Breast Cancer Image Change Detection (MBCICD) | Log Ratio + Gabor Filter + Fuzzy c-means (FCM) | Accuracy: 0.9719 |
| [75] | 2024 | DDSM | Ensemble Model 2 | Ensemble model with VGG16, InceptionV3, VGG19 as base learners, and ANN for classification. | Accuracy: 0.9810 Sensitivity: 0.9701 Specificity: 0.9912 | |
| [76] | 2023 | mini-MIAS | 1024 × 1024 | SVM, ANN | CLAHE and USM preprocessing, SVM and ANN classifiers. | Performance metrics: Accuracy, Sensitivity, Specificity, and F1-Score all = 1.0 |
| [79] | 2022 | DDSM | 299 × 299 | CNN | CNN classifier with different fine-tuning. | Accuracy: 0.9996 Sensitivity: 1.0 Precision: 0.9992 F1-Score: 0.9996 |
| [73] | 2022 | Digital mammography | 1024 × 832 | DenseNet-121 | Dataset preparation, label extraction, BI-RADS score extraction, model training with DenseNet-121, and performance evaluation were performed. | Setting BI-RADS score 1–5 CV AUC: 0.953 Test AUC: 0.94 Specificity: 0.85 Sensitivity: 0.9 Setting BI-RADS score 4–5 CV AUC: 0.932 Test AUC: 0.927 Setting BI-RADS score 0 CV AUC: 0.758 Test AUC: 0.787 |
| [10] | 2019 | BreakHis & BC Classification Challenge 2015 | ×460 (RGB) & 2040 × 1536 | IRRCNN + Aug | Data augmentation, Inception Recurrent Residual Convolutional Neural Network (IRRCNN). | BreakHis dataset Accuracy: 0.9676 ± 0.0111 (Factor × 40) BC Classification Challenge 2015 Accuracy: 0.9711 Sensitivity: 0.9371 Specificity: 0.9809 AUC: 0.9905 |
| Ref. | Advantages | Limitations | Future Work Recommendations |
|---|---|---|---|
| [78] | EfficientNet outperformed CNN and Inception models on the MaMaTT2 dataset, achieving 98.29% overall accuracy (99.27% benign, 98.46% malign, 97.13% normal) using an augmented dataset of 22,500 images. | The original dataset was small (408 images), which may affect model generalizability. Legal and privacy concerns limit access to large, real-world medical datasets needed for broader validation. | Future work should expand datasets and incorporate more pre-trained models to boost accuracy. Overcoming data privacy challenges will be key for clinical implementation. |
| [74] | The method achieved 97.19–97.38% PCC on MIAS, outperforming FDA-RMG (94.81%) using Mean/Log Ratio, Gabor Filter, and FCM clustering. | Reliance on limited MIAS images may reduce real-world applicability, while high false negatives (93.31%) suggest missed subtle changes. | Larger real-patient datasets and improved algorithms could reduce false negatives, while exploring advanced feature extraction may boost accuracy further. |
| [75] | Ensemble Model 2 (neural net) achieved 98.10% accuracy on DDSM/CBIS-DDSM, outperforming InceptionV3 (95.71%). Combining VGG16/InceptionV3/VGG19 boosted specificity (99.12%) and sensitivity (97.01%). | The study relied on pre-curated, fixed-size images, limiting generalizability to diverse clinical settings. High computational resources were required for training ensemble models, which may hinder real-time deployment in resource-constrained environments. | Expanding validation to diverse datasets (e.g., INBreast or MIAS) could enhance robustness. Exploring lightweight meta-learners (e.g., SVM or Random Forest) may improve efficiency without compromising accuracy. |
| [79] | The proposed modified ResNet50 model, trained on the DDSM dataset with 13,128 images (5970 benign and 7158 malignant), achieved excellent classification performance, with test accuracy up to 99.96%, AUC of 1.0000, and F1-score of 0.9996 using SGD and 100% fine-tuning. | The training time for a single model was around 3.7 h, indicating high computational cost. Additionally, selecting optimal hyperparameters remains a challenge due to the exhaustive trial-and-error process. | Future research should focus on reducing computational time and developing methods for automated hyperparameter tuning to enhance efficiency and scalability. |
| Aspect | Details |
|---|---|
| Datasets | CBIS-DDSM, DDSM, MIAS, INbreast, mini-MIAS, MaMaTT2, BreakHis, etc. |
| Models | DenseNet, VGG16, AlexNet, InceptionV3, EfficientNet, IRRCNN, etc. |
| Preprocessing | CLAHE, USM, Log Ratio, Gabor Filter, Fuzzy C-Means (FCM) |
| Highest Accuracy | 99.96% (CNN with fine-tuning on DDSM) |
| Key Techniques | Ensemble models, hybrid systems, hyperparameter optimization |
| Technique | Impact |
|---|---|
| Deep Learning Models | High accuracy and specificity; handles complex patterns in mammograms. |
| Preprocessing | Enhances image quality, leading to improved model performance. |
| Ensemble Models | Combines strengths of multiple models, improving robustness and accuracy. |
| Hybrid Systems | Integrates traditional and deep learning methods for better performance. |
| Hyperparameter Optimization | Improves model tuning, leading to higher accuracy and efficiency. |
| Limitation | Details |
|---|---|
| Interpretability | Lack of explainability in model predictions hinders clinical adoption. |
| Generalization | Models may not perform well on datasets from different populations or devices. |
| Dataset Diversity | Limited diversity in datasets may lead to overfitting. |
| Real-World Applicability | Performance in clinical settings may differ from experimental results. |
| Study | Dataset | Model/Technique | Accuracy | Key Findings |
|---|---|---|---|---|
| Yaqub et al. (2024) [77] | MIAS, CBIS-DDSM | ACA-ATRUNet + ACA-AMDN + MML-EOO | 89.13% | Two-stage system with hyperparameter optimization. |
| Nemade et al. (2024) [75] | DDSM | Ensemble (VGG16, InceptionV3, VGG19) + ANN | 98.10% | High specificity and sensitivity. |
| Avci et al. (2023) [76] | mini-MIAS | CLAHE + USM + SVM/ANN | 100% | Preprocessing significantly improves metrics. |
| Dada et al. (2024) [78] | MaMaTT2 | EfficientNet | 98.29% | Outperforms other models on MaMaTT2. |
| Mudeng et al. (2022) [79] | DDSM | CNN with fine-tuning | 99.96% | Highest reported accuracy on DDSM. |
| Ref. | Year | Dataset | Image Size | Proposed Model | Proposed Approach | Results |
|---|---|---|---|---|---|---|
| [94] | 2021 | Breast Ultrasound Image | 57 × 57 & 161 × 199 | DenseNet201, ResNet50, VGG16 | Grad-CAM, occlusion mapping for feature extraction, DenseNet201, ResNet50 (Adam, RMSprop optimizers), VGG16 (Stochastic Gradient Descent). | Accuracy: 100% |
| [95] | 2023 | Initial Dataset [34] & Second Dataset [93] | 500 × 500 & Non-Fixed | DeepBreastCancerNet | DeepBreastCancerNet model, 24 layers, 6 convolutional layers, 9 inception modules, ReLU, batch normalization | Initial Dataset Accuracy: 0.9935 Second Dataset Accuracy: 0.9963 Precision: 0.9950 Recall: 1.0 F1-Score: 0.9950 |
| [96] | 2023 | Ultrasound dataset collected from three institutions | 224 × 224 | integrated DLR model | Developed an integrated DLR model with clinical–pathological variables | AUC: 0.962 (training) And 0.939 (validation) |
| [97] | 2023 | GDPH& SYSUCC | 844 × 627 | HoVer-Trans | An ROI-free model with HoVer-Transformer to capture tumor spatial relationships for breast cancer diagnosis | AUC: 0.924 Accuracy: 0.893 Specificity: 0.836 Sensitivity: 0.926 |
| [99] | 2024 | Breast Ultrasound Images Dataset | 500 × 500 | VGG16-LSTM | VGG16 with LSTM for feature extraction, SMOTETomek for feature balancing, and Grad-CAM for visualization and LIME for interpretability. | F1-score: 0.99 MCC & Kappa: 0.989 AUC: 1.0 |
| [100] | 2024 | ultrasound breast cancer (USBC) image | 500 × 500 | Inception V3 + Stacking | Inception V3 transfer learning + ensemble stacking (MLP, SVM with RBF and polynomial kernels) | Accuracy: 0.858 AUC: 0.947 Precision: 0.8579 Recall: 0.858 F1-score: 0.857 |
| [101] | 2024 | NYU dataset | 665 × 603 | deep learning classifier | Deep learning with transfer learning for breast cancer detection using ultrasound images on mobile devices. | Accuracy: 78% |
| Ref. | Advantages | Limitations | Future Work Recommendations |
|---|---|---|---|
| [94] | The model proposed in the reference article applies to the Breast Ultrasound Image dataset, achieves 100% accuracy in breast cancer detection using efficient pre-trained CNNs and provides interpretable results through Grad-CAM visualizations. | Its small dataset of 250 images limits generalizability, while high computational needs may restrict clinical deployment. | Expanding datasets, testing multi-class classification, and conducting clinical validations will enhance real-world applicability. |
| [95] | DeepBreastCancerNet applies to the second dataset [93], achieves 99.63% accuracy using an efficient 24-layer CNN with specialized activation functions, outperforming existing models while maintaining computational efficiency. | Limited by small dataset size (1030 images) and lack of clinical validation, with potential deployment challenges in resource-constrained environments. | Expand datasets through multi-center collaborations and optimize the model for clinical deployment, followed by rigorous real-world testing. |
| [107] | The proposed model applies to the dataset obtained from Assosa General Hospital, integrating a feature enhancement technique (LPP) into the Google Inception network, achieving high accuracy (99.80%) for breast cancer detection and classification. It also employs transfer learning to address dataset limitations, improving performance on ultrasound images. | The model was evaluated on a limited dataset of 1578 images, which may affect generalizability to diverse populations. Additionally, the computational complexity of LPP and the Inception network could pose challenges for real-time deployment in clinical settings. | Future work should validate the model on larger and more diverse datasets to enhance robustness. Incorporating domain knowledge from radiologists could further improve interpretability and clinical relevance. |
| [109] | The proposed DL-AL hybrid model significantly outperformed 14 state-of-the-art algorithms on 1264 ultrasound images, achieving up to 96% Dice coefficient and 0.26% H3 distance on simple cases. It also maintained strong generalization, achieving an accurate segmentation ratio (ASR) of 98.40% even for the most complex cases (), and handled 68% unseen data with a Dice score of 0.80. | The model relies heavily on manually engineered artificial life rules and genetic algorithms, which can increase design complexity and tuning effort. Moreover, the computational cost is relatively high, requiring up to 1.5 min and 0.5–1 million agents for segmentation on standard PCs, which may not be practical in time-constrained clinical workflows. | Future work should focus on optimizing the algorithm’s computational efficiency to ensure real-time deployment, especially by improving agent dynamics and parallelization. Expanding the model to include multi-modal imaging and evaluating it on larger, more diverse datasets could further enhance robustness and clinical applicability. |
| Aspect | Details |
|---|---|
| Datasets | BUSI, BUS-BRA, BUS-UCLM, Breast-Lesions-USG, NYU, GDPH&SYSUCC, etc. |
| Models | DeepBreastCancerNet, HoVer-Transformer, VGG16-LSTM, ResNet50, DenseNet201 |
| Techniques | Transfer learning, ensemble stacking, interpretability (Grad-CAM, LIME) |
| Highest Accuracy | 100% (DenseNet201, ResNet50, VGG16 on Breast Ultrasound Image dataset) |
| Key Challenges | Dataset diversity, real-world deployment, interpretability, overfitting |
| Technique | Impact |
|---|---|
| Deep Learning Models | High accuracy and AUC scores; effective for complex pattern recognition. |
| Transfer Learning | Reduces overfitting and improves performance on small datasets. |
| Ensemble Models | Combines strengths of multiple models, improving robustness and accuracy. |
| Interpretability | Techniques like Grad-CAM and LIME enhance clinical trust and applicability. |
| Clinical Integration | Combining imaging data with clinical variables improves predictive power. |
| Limitation | Details |
|---|---|
| Cross-Dataset Validation | Lack of testing on diverse datasets limits generalizability. |
| Interpretability | Grad-CAM and LIME provide insights but are insufficient for clinical trust. |
| Dataset Diversity | Limited diversity in datasets may lead to overfitting and reduced robustness. |
| Real-World Deployment | Challenges in deploying models in resource-limited settings remain unresolved. |
| Standardization | Inconsistent imaging protocols affect the model performance and applicability. |
| Study | Dataset | Model/Technique | Accuracy | Key Findings |
|---|---|---|---|---|
| Masud et al. (2021) [94] | Breast Ultrasound | DenseNet201, ResNet50, VGG16 | 100% | Fine-tuning with Adam, RMSprop, and SGD. |
| Raza et al. (2023) [95] | BUSI, Secondary | DeepBreastCancerNet | 99.63% | Robust performance with ReLU and batch normalization. |
| Yu et al. (2023) [96] | Multi-Institution | Integrated DLR Model | AUC 0.962 | Combined imaging and clinical variables. |
| Mo et al. (2023) [97] | GDPH&SYSUCC | HoVer-Transformer | AUC 0.924 | ROI-free model with spatial relationship capture. |
| Lanjewar et al. (2024) [99] | BUSI | VGG16-LSTM | F1-score 99% | Feature balancing with SMOTETomek. |
| Rao et al. (2024) [100] | USBC | Inception V3 + Stacking | AUC 0.947 | Ensemble stacking with MLP and SVM. |
| Appiah et al. (2024) [101] | NYU | Mobile DL Classifier | 78% | Focus on low-resource settings. |
| Ref. | Year | Dataset | Proposed Model | Proposed Approach | Results |
|---|---|---|---|---|---|
| [117] | 2024 | Private | Radiomics | Used multimodal MRI (T1WI, T2WI, DWI, ADC, DCE), feature extraction (plain, enhanced, diffuse), deep learning for classification | AUC: 0.927 Accuracy: 0.9015 Sensitivity: 0.9631 Sensitivity: 0.9548 |
| [118] | 2022 | Inter MRI dataset + I-SPY TRIAL | Weakly supervised network based on the ResNet-101 architecture | ResNet-101-based weakly supervised framework, Adam optimizer, SoftMax threshold (0.5) | AUC: 0.92 (±0.03) Accuracy: 94.2% (±3.4) Sensitivity: 74.4% (±8.5) Specificity: 95.3% (±3.3) |
| [119] | 2022 | Private | CNN | Uses a CNN with DCE-MRI images, combining tumor features and confidence analysis via prediction probability, heatmaps, and scan timing | Accuracy: 0.877 Precision: 0.912 Sensitivity: 0.861 AUC: 0.912 (±4.0%) |
| [120] | 2023 | Private | 3D UNet-based CNN | 3D UNet-based CNN for automatic segmentation cancer regions, extracting radiomics features, applying cross-combination approaches with feature optimization and then classification | AUC: 86.23% Accuracy: 65.96% Sensitivity: 63.83% Specificity: 87.75% |
| [121] | 2024 | TCGA | MRI-based radiomics model | integrates CIBERSORT analysis and an MRI-based radiomics model with LASSO, identifying M2 macrophages as a key prognostic factor for BC | Accuracy: 0.773 Sensitivity: 0.727 Specificity: 0.818 |
| [122] | 2024 | Private | Deep learning radiomics (DLR) model | Radiomics and deep semantic segmentation features combined for HER2 status prediction | HER2-negative vs. HER2-overexpressing Training AUC: 0.868 Validation AUC: 0.763 HER2-low-positive vs. HER2-zero Training AUC: 0.855 Validation AUC: 0.750 |
| [123] | 2024 | Private | CNN and LSTM Cascaded Network | lesion classification and localization using mpMRI sequences, with histopathology as the ground truth | Lesion classification Internal cohort: AUC = 0.98, Sensitivity = 0.96 External cohort: AUC = 0.91, Sensitivity = 0.83 DL method vs. radiologists (without DCE-MRI): AUC = 0.96 vs. 0.90 |
| [124] | 2024 | Private | MLP-radiomics | Combines radiomics and clinicoradiological features via MLP for lymphovascular invasion (LVI) prediction in BC | AUC: 0.896 |
| Ref. | Advantages | Limitations | Future Work Recommendations |
|---|---|---|---|
| [117] | The study developed a radiomics model using multimodal MRI data (T1WI, T2WI, DWI, ADC, DCE) from 95 patients, achieving high diagnostic performance with an AUC of 0.927, accuracy of 90.15%, and sensitivity of 96.31% for the combined (plain scan + enhanced + diffuse) model. | The dataset was relatively small (n = 95), and only mass lesions were analyzed, excluding non-mass enhancements. Additionally, only intratumoral radiomics features were extracted, omitting valuable peritumoral information. | Future studies should include larger datasets, incorporate peritumoral features, and integrate clinical and genetic data for more comprehensive modeling. Enhancing AI algorithms and fostering interdisciplinary research are also recommended to improve diagnostic precision and individualized treatment. |
| [126] | Using a dataset of 1359 DCE-MRI images, the proposed CNN-SVM hybrid model achieved 99.80% accuracy and 100% AUC in classifying molecular subtypes of breast cancer with low computational complexity. | The study relied on a manually annotated dataset, which may limit scalability, and it used only the first post-contrast DCE-MRI, potentially excluding valuable dynamic information. | Future research should explore combining pre- and post-contrast MRI dynamics to enhance classification performance and validate results on larger, multi-center datasets for broader clinical applicability. |
| [127] | The modified YOLACT model, trained on the Rider Breast MRI dataset with 6000 preprocessed images (4400 normal and 1600 difficult), achieved a classification accuracy of 98.5% for common samples and 93.6% for difficult samples, outperforming standard YOLACT and offering faster performance than Mask R-CNN. | The model was trained and validated using data only from the Rider Breast MRI dataset, which may limit its generalizability. Additionally, comparisons were limited to YOLACT and Mask R-CNN, without evaluation against broader advanced models. | Future research should involve multi-source MRI datasets, compare the model with other advanced segmentation algorithms, and evaluate its diagnostic performance against histopathological results for real-world clinical applicability. |
| Aspect | Details |
|---|---|
| Datasets | Private datasets, TCGA, RIDER, DCE-MRI, etc. |
| MRI Modalities | T1WI, T2WI, DWI, ADC, DCE-MRI |
| Models | ResNet-101, CNN, 3D UNet, CNN-LSTM, MLP-radiomics |
| Techniques | Multimodal MRI, weakly supervised learning, radiomics, deep semantic segmentation |
| Highest AUC | 0.98 (CNN-LSTM cascaded network) |
| Key Challenges | Generalizability, dataset variability, computational complexity |
| Technique | Impact |
|---|---|
| Multimodal MRI | Combines complementary features from different MRI sequences for improved accuracy. |
| Weakly Supervised Learning | Effective in scenarios with limited annotated data, reducing labeling costs. |
| CNN Architectures | High accuracy in classification and segmentation tasks. |
| Radiomics | Extracts quantitative features from medical images, enhancing predictive power. |
| Immune Composition Integration | Enables prognosis prediction and personalized treatment strategies. |
| Limitation | Details |
|---|---|
| Generalizability | Models often fail to perform consistently across external datasets. |
| Dataset Dependency | Reliance on large annotated datasets limits scalability. |
| Computational Complexity | High resource requirements hinder real-world deployment. |
| Interpretability | Lack of transparency in model decisions reduces clinical trust. |
| Clinical Integration | Challenges in integrating AI-driven decisions into clinical workflows. |
| Study | Dataset | Model/Technique | AUC | Key Findings |
|---|---|---|---|---|
| Zhang et al. (2024) [117] | Private | Multimodal MRI + DL | 0.927 | Combines T1WI, T2WI, DWI, ADC, and DCE-MRI. |
| Liu et al. (2022) [118] | Inter MRI + I-SPY | ResNet-101 (weakly supervised) | 0.92 | Effective with limited annotated data. |
| Wu et al. (2022) [119] | Private | CNN + DCE-MRI | 0.912 | Combines tumor features and confidence analysis. |
| Yue et al. (2023) [120] | Private | 3D UNet + Radiomics | 0.8623 | Predicts molecular subtypes with high specificity. |
| Qian et al. (2024) [121] | TCGA | MRI-based Radiomics + CIBERSORT | 0.773 | Identifies M2 macrophages as a prognostic factor. |
| Guo et al. (2024) [122] | Private | DLR (Radiomics + Deep Semantic Segmentation) | 0.868 | Predicts HER2 status with high accuracy. |
| Chao et al. (2024) [123] | Private | CNN-LSTM Cascaded Network | 0.98 | Outperforms radiologists in lesion classification. |
| Liang et al. (2024) [124] | Private | MLP-Radiomics | 0.896 | Predicts lymphovascular invasion effectively. |
| Ref. | Year | Dataset | Image Size | Proposed Model | Approach | Results |
|---|---|---|---|---|---|---|
| [128] | 2023 | Private | - | CNN-Unet with Random Forest | Automatic segmentation, normal tissues, laterality classification, target volume delineation, procedural classification. | Accuracy: 0.9375 AUC: 0.993 |
| [129] | 2022 | Private | 512 × 512 | RetinaNet | Augmented chest CT images with bounding boxes and RetinaNet for breast cancer detection | Internal Test Set Sensitivity: 96.5% with 13.5 false positives per case (FPs/case) External Test Set Sensitivity: 96.1% with 15.6 FPs/case |
| [130] | 2024 | Private | 512 × 512 | Deep Learning | Enhanced contrast of chest CT images and the applied deep learning model | AUC on Validation set: 0.976 and Test set: 0.967 |
| [128] | 2023 | Private | - | Cnn-U-Net with Stochastic Gradient Descent | Automatic contouring of normal tissues, classification of laterality, automatic delineation of target volumes, and classification | Accuracy: 0.9375 AUC: 0.93 |
| Ref. | Advantages | Limitations | Future Work Recommendations |
|---|---|---|---|
| [133] | Chest CT achieved high diagnostic performance with 84.21% sensitivity, 99.3% specificity, and 98.70% accuracy, outperforming mammography (78.95%, 93.78%, and 93.16%, respectively). It also reduced unnecessary biopsies and eliminated the need for patient recall. | The study was retrospective and used experienced radiologists, so results may not generalize to less experienced readers. Breast imaging was incomplete in 132 cases, and standard-dose CT was used rather than optimized low-dose protocols. | Prospective studies with low-dose CT and standardized imaging protocols are needed. Integration of AI algorithms and the use of skin markers for consistent breast positioning could further improve diagnostic accuracy and reproducibility. |
| [131] | The proposed CAD system achieved high performance metrics with 95.2% accuracy, 92.4% sensitivity, and 98.1% specificity on CTLM images, demonstrating robust detection of breast cancer lesions. The 3D Fuzzy C-means segmentation and MLPNN classifier effectively analyzed angiogenesis patterns, with an AUROC of 0.98, outperforming traditional methods. | The study was limited by a small dataset (180 patients) and lacked diverse angiogenesis shapes, potentially reducing generalizability. Additionally, the reliance on TIFF instead of DICOM format may have impacted image quality and processing efficiency. | Expanding the dataset to include more angiogenesis variants (e.g., ringed or polypoid shapes) could improve model robustness. Integrating DICOM-formatted images and testing in multi-center clinical trials would enhance clinical applicability. |
| Aspect | Details |
|---|---|
| Datasets | Private datasets, Breast Wellness Centre, GE Healthcare, etc. |
| Models | CNN-Unet, RetinaNet, Random Forest, Deep Learning |
| Techniques | Automatic segmentation, image augmentation, contrast enhancement |
| Highest Accuracy | 93.75% (CNN-Unet + Random Forest) |
| Key Challenges | Dataset dependency, computational complexity, generalizability |
| Technique | Impact |
|---|---|
| Automatic Segmentation | Reduces human error and improves workflow efficiency. |
| Image Augmentation | Enhances model performance by increasing dataset variability. |
| Contrast Enhancement | Improves lesion visibility and detection accuracy. |
| Ensemble Models | Combines strengths of multiple models, improving robustness and accuracy. |
| Weakly Supervised Learning | Effective in scenarios with limited annotated data, reducing labeling costs. |
| Limitation | Details |
|---|---|
| Dataset Dependency | Reliance on high-quality, annotated datasets limits scalability. |
| Generalizability | Models often fail to perform consistently across external datasets. |
| Computational Complexity | High resource requirements hinder real-world deployment. |
| Workflow Efficiency | Automatic segmentation methods require refinement for clinical integration. |
| Clinical Integration | Challenges in integrating AI-driven decisions into clinical workflows. |
| Study | Dataset | Model/Technique | Accuracy /AUC | Key Findings |
|---|---|---|---|---|
| Bargalló et al. (2023) [128] | Private | CNN-Unet + Random Forest | 93.75% | Reduces target delineation errors. |
| Koh et al. (2022) [129] | Private | RetinaNet | 96.5% (internal) | Robust performance across datasets. |
| Yasak et al. (2024) [130] | Private | Deep Learning + Contrast Enhancement | AUC 0.976 | Improves radiologists’ performance. |
| Ref. | Year | Dataset | Image Size | Proposed Model | Approach | Results |
|---|---|---|---|---|---|---|
| [136] | 2022 | BreaKHis | 128 × 128 64 × 64 32 × 32 | CNN and DenseNet121 | The CNN used tuning, and DenseNet121 applied transfer learning, with magnification, scaling, and rotation effects | CNN: Accuracy: 90.9% DenseNet121: Accuracy: 86.6% |
| [137] | 2022 | ICIAR 2018 | 512 × 512 | Choquet Integral-based Fusion Model | DCNN feature extraction, and model fusion (VGG16, Xception, Inception, InceptionResNet V2) using Choquet Integral and Coalition Game Theory | Two-Class (Accuracy: 96%, Precision and Recall: 0.96) Four-Class (Accuracy: 95%, Precision & Recall: 0.95) |
| [138] | 2022 | H&E images from Cancer Hospital of the Chinese Academy of Medical Sciences, and TCGA | 512 × 512 | Multimodal deep learning model | Integrates whole slide H&E images with clinical data, DCNN for feature extraction, classification | AUC: 0.76 CV: 0.72 in the TCGA testing set |
| [139] | 2023 | BreakHis | 128 × 128 64 × 64 32 × 32 | Extreme Gradient Boosting | DenseNet201 networks to extract features, classification using XGBoost | Accuracy: 93.6% (40×) 91.3% (200×) 93.8% (400×) |
| [140] | 2023 | BreakHis, ICIAR-2018 | 128 × 128 512 × 512 | Ensemble technique | Rank-based ensemble of GoogleNet, VGG11, and MobileNetV3_Small | Accuracy: 99.16% (40X) on BreakHis, and 96.95% on ICIAR-2018 |
| [141] | 2024 | Breast Cancer Histopathology | - | ResNet50 | Transfer learning models (ResNet50, ResNet101, VGG16, VGG19) | Accuracy: 0.922 AUC: 0.91 Recall: 0.957 |
| [142] | 2024 | Private | - | Darknet-53 CNN | CLAHE for image enhancement and HGLCM for feature extraction. The Darknet-53 CNN for classification | Accuracy: 95.6% |
| [143] | 2024 | BreakHis and LC25000 | 128 × 128 | Ensemble max-voting | QCAHE reduced noise, SCAN optimized stain preservation, and Arithmetic Optimization improved SCAN parameters. | Accuracy: 89.03% Precision: 88.56% Sensitivity: 88.09% F1-score: 88.7% Specificity: 89.08% |
| [144] | 2024 | BreaKHis and BACH | 128 × 128 512 × 512 | BCHI-CovNet | Uses multiscale depth-wise separable convolution, pooling, and self-attention | 99.38% accuracy on BACH and 99.22% on BreaKHis |
| Ref. | Advantages | Limitations | Future Work Recommendations |
|---|---|---|---|
| [144] | The proposed BCHI-CovNet model integrates depth-wise separable convolution, second-order pooling, and multi-head self-attention, achieving high classification accuracy with fewer parameters and low FLOPs. It attained 99.15% (40×), 99.08% (100×), 99.22% (200×), and 98.87% (400×) accuracy on the BreaKHis dataset, and 99.38% on the BACH dataset, demonstrating superior performance and lightweight design. | The model’s performance is affected by color variations in histopathological images across BreaKHis and BACH datasets. This color inconsistency limits feature extraction quality and impacts generalizability. | Future work should include advanced color normalization during preprocessing to handle image variability. Additionally, expanding the model evaluation to more diverse and multi-center datasets is suggested to enhance robustness. |
| [145] | The proposed Hybrid CNN model, integrating the Sine Cosine Algorithm (SCA) and transfer learning, achieves a high accuracy of 96.9%, outperforming traditional models like K-NN, SVM, and standard CNN. The model utilizes VGG16 pre-trained on ImageNet and fine-tunes the last three convolutional layers, enhancing performance while preventing overfitting. | The study lacks evaluation on publicly available breast cancer datasets such as BreaKHis or BACH, limiting comparison with existing works. Also, performance across varied imaging modalities and patient demographics remains unexplored. | Future work should validate the model on benchmark datasets like BreaKHis and BACH to assess generalizability. Incorporating multi-modal data and real-time blockchain integration for secure deployment in clinical settings is also recommended. |
| [137] | The proposed Choquet Integral-based ensemble achieved 96% (2-class) and 95% (4-class) accuracy on the BACH dataset, outperforming all single models. It effectively combines features from five DCNNs using a novel fuzzy measure method. | The fuzzy measure calculation is computationally expensive and complex. The method was tested only on the BACH dataset, limiting generalizability. | Future work should reduce computational cost and test on larger datasets. Adding more models and improving stain robustness is also recommended. |
| Aspect | Details |
|---|---|
| Datasets | BreakHis, BACH, ICIAR-2018, TCGA, LC25000, etc. |
| Models | CNN, DenseNet, ResNet, XGBoost, ensemble methods |
| Techniques | Transfer learning, feature fusion, multimodal analysis, preprocessing |
| Highest Accuracy | 99.38% (BCHI-CovNet on BACH dataset) |
| Key Challenges | Dataset dependency, computational complexity, generalizability |
| Technique | Impact |
|---|---|
| CNN Architectures | High accuracy in classification tasks, effective feature extraction. |
| Transfer Learning | Improves performance on small datasets, reduces training time. |
| Ensemble Methods | Combines strengths of multiple models, improving robustness and accuracy. |
| Multimodal Approaches | Integrates imaging and clinical data for enhanced predictive power. |
| Preprocessing | Techniques like CLAHE improve image quality and model performance. |
| Limitation | Details |
|---|---|
| Dataset Dependency | Reliance on high-quality, annotated datasets limits scalability. |
| Generalizability | Models often fail to perform consistently across external datasets. |
| Computational Complexity | High resource requirements hinder real-world deployment. |
| Interpretability | Lack of transparency in model decisions reduces clinical trust. |
| Clinical Integration | Challenges in integrating AI-driven decisions into clinical workflows. |
| Study | Dataset | Model/Technique | Accuracy/ AUC | Key Findings |
|---|---|---|---|---|
| Muntean & Chowkkar (2022) [136] | BreakHis | CNN, DenseNet121 | 90.9% | Transfer learning improves performance. |
| Bhowal et al. (2022) [137] | BACH | Fusion Model (VGG16, Xception, InceptionResNet V2) | 95.6% | Combines multiple models for high accuracy. |
| Yang et al. (2022) [138] | TCGA | Multimodal DL model | AUC 0.76 | Integrates imaging and clinical data. |
| Majumdar et al. (2023) [140] | BreakHis, ICIAR-2018 | Ensemble method (GoogleNet, VGG11, MobileNetV3) | 99.16% | Robust performance across datasets. |
| Addo et al. (2024) [144] | BACH, BreakHis | BCHI-CovNet | 99.38% | Lightweight model with high accuracy. |
| Ref. | Year | Dataset | Image Size | Proposed Model | Approach | Results |
|---|---|---|---|---|---|---|
| [151] | 2023 | MIAS Thermogram | 224 × 224 | Transfer learning | Multi-view thermograms, clinical data integration, ROI extraction, transfer learning | Accuracy: 90.48% Sensitivity: 93.33% AUC: 0.94 |
| [152] | 2022 | DMR-IR | 640 × 480 | VGG16 + Memory-Based Dragonfly Algorithm | Two-stage framework: (1) VGG16 for feature extraction, (2) GL-enhanced DA for feature selection | Accuracy: 100% with 82% fewer features than VGG16 |
| [154] | 2023 | COCO | - | Mask R-CNN with ResNet-50 and ResNet-101 backbones | DL model detects, segments, and classifies breast tissues, assigning bounding boxes and tumor borders. | ResNet-50: 97.1% accuracy, mAP of 0.921, overlap score of 0.868 |
| [155] | 2024 | DMR | 640 × 480 | Custom CNN model | Augmentation, resizing, filtering, and HOG feature extraction; custom CNN for classification | Accuracy rates of 95.7% to 98.5% |
| [156] | 2024 | Not mentioned | 224 × 224 | TransUNet | TransUNet for segmentation, followed by classification | Accuracy: 97.26% Sensitivity: 97.26% |
| [157] | 2024 | Not mentioned | 224 × 224 | Inception MV4 | Noise mitigation and preprocessing techniques, followed by classification. | Accuracy: 99.748% Sensitivity: 0.996 Specificity: 1.0 Precision: 1.0 AUC: 0.998 |
| Ref. | Advantages | Limitations | Future Work Recommendations |
|---|---|---|---|
| [160] | The proposed hybrid model using Genetic Algorithm (GA) and Grey Wolf Optimizer (GWO) achieves 100% accuracy on the DMR-IR dataset, utilizing only 3% of the 1000 deep features extracted by the lightweight SqueezeNet 1.1 model. This significantly reduces computational time, storage, and processing overhead while maintaining state-of-the-art performance. | Despite the promising results, the model is evaluated on a single primary dataset (DMR-IR), which limits the generalizability across diverse clinical settings. Moreover, the study does not consider real-time deployment scenarios or the variability in thermal imaging conditions and patient demographics. | Future work should focus on validating the approach across multiple and more diverse datasets, including real-world clinical environments, to ensure robustness and scalability. Additionally, integrating multi-modal data such as clinical reports or other imaging types (e.g., mammography, ultrasound) may enhance diagnostic accuracy and applicability. |
| [158] | The proposed real-time thermography framework using deep learning models—Inception v3, Inception v4, and modified Inception Mv4—achieved high detection accuracy, with Inception Mv4 reaching 99.748%, outperforming Inception v4 (99.712%) and Inception v3 (96.8%) on a dataset of 1000 thermal images (700 normal, 300 abnormal) captured via FLIR One Pro thermal camera. Incorporating in situ cooling enhanced detection accuracy by up to 11%, particularly for small and deep tumors. | The detection performance is sensitive to external conditions like room temperature and patient movement, which can affect thermal image quality and classification results. Moreover, smaller or deeper tumors (e.g., <2 V and depth T9) remained difficult to detect even with cooling, and patient discomfort during cooling procedures may limit widespread applicability. | Future work should integrate thermal video clips into the dataset and explore higher-resolution thermal cameras to improve small tumor detection. Additionally, using alternative or advanced deep learning models (e.g., spiking neural networks) and standardizing situ-cooling protocols can further enhance real-time breast cancer diagnostic precision and reproducibility. |
| [161] | The proposed intelligent method, using infrared breast thermograms from the DMR-IR dataset (200 subjects), achieved high accuracy in malignancy detection—98.8% with SVM, outperforming kNN and D-Tree classifiers. Features extracted using Local Binary Pattern (LBP) and Gray Level Co-occurrence Matrix (GLCM), followed by Firefly feature selection, enabled effective classification of healthy vs. abnormal breast tissue. | Despite strong performance with SVM, kNN (81.5%) and D-Tree (94.5%) yielded lower accuracy, showing model performance is sensitive to classifier choice and may not generalize equally. Additionally, the small dataset size (200 IR images) limits the robustness of the model across larger, real-world clinical settings. | Future studies should expand testing on larger and more diverse IR thermogram datasets to improve model reliability. Integrating deep learning approaches or hybrid models could further enhance feature extraction and classification accuracy from infrared breast images. |
| Aspect | Details |
|---|---|
| Datasets | MIAS Thermogram, DMR-IR, COCO, DMR, etc. |
| Models | VGG16, Mask R-CNN, ResNet, TransUNet, Inception MV4 |
| Techniques | Multi-view thermograms, transfer learning, feature selection, noise mitigation |
| Highest Accuracy | 100% (Chatterjee et al., 2022 [152] |
| Key Challenges | Dataset variability, computational complexity, noise reduction |
| Technique | Impact |
|---|---|
| Multi-View Thermograms | Provides a more comprehensive analysis compared to single-view approaches. |
| Transfer Learning | Improves performance on small datasets, reduces training time. |
| Feature Selection | Reduces feature dimensionality while maintaining accuracy. |
| Noise Mitigation | Enhances image quality and model reliability. |
| Segmentation Models | Improves precision in tumor localization and classification. |
| Limitation | Details |
|---|---|
| Dataset Dependency | Reliance on small or proprietary datasets limits generalizability. |
| Overfitting | High accuracy claims may indicate overfitting to specific datasets. |
| Standardization | Lack of standardized datasets and preprocessing techniques. |
| Computational Complexity | High resource requirements hinder real-world deployment. |
| Noise Reduction | Insufficient focus on noise and image quality in some studies. |
| Study | Dataset | Model/Technique | Accuracy | Key Findings |
|---|---|---|---|---|
| Chatterjee et al. (2022) [152] | DMR-IR | VGG16 + Dragonfly Algorithm | 100% | Reduces feature dimensionality by 82%. |
| Civilibal et al. (2023) [154] | COCO | Mask R-CNN with ResNet-50 | 97.1% | Precise tumor localization and classification. |
| Mahoro et al. (2024) [156] | Not mentioned | TransUNet | 97.26% | Effective segmentation and classification. |
| Husaini et al. (2024) [157] | Not mentioned | Inception MV4 | 99.82% | Emphasizes noise mitigation and preprocessing. |
| Ref. | Year | Dataset | Instances | Proposed Model | Approach | Results |
|---|---|---|---|---|---|---|
| [162] | 2024 | WDBC | 569 | XGBoost | Preprocessing, feature engineering, feature selection using a gradient boosting regressor with Bonferroni correction, and classification. | Accuracy: 99.12% Precision: 0.9767 Recall:1.0 Specificity: 0.9861 F1-score: 0.9882. |
| [163] | 2023 | SEER | 712319 | Decision Tree | Two-step feature selection (Variance Threshold, PCA) and classification using Decision Tree | Accuracy: 98% Precision, Recall, Specificity and F1-score: 0.98 |
| [164] | 2024 | WDBC | 569 | OSELM | Data preprocessing, selection and classificaiton | Accuracy: 96.13% Precision: 94.09% Recall: 95.57% |
| Ref. | Advantages | Limitations | Future Work Recommendations |
|---|---|---|---|
| [165] | The proposed models—CRO+SVM and GA+RF—demonstrated superior performance across all three datasets: WBC (569 samples), BC-UCI (286 samples), and BCC (116 samples). Notably, CRO+SVM achieved 99.64% accuracy on the WBC dataset, while GA+RF reached 98.00% on BCC and CRO+XGBoost scored 99.12% on BC-UCI, outperforming existing models in accuracy, F1 score, precision, recall, and specificity. | The model’s performance may be influenced by class imbalance and preprocessing techniques in the datasets, potentially limiting real-world applicability. Additionally, while the study focuses on improving predictive performance, it lacks the integration of explainable AI techniques for interpretability in clinical settings. | Future work should integrate explainable AI methods to enhance trust and transparency in clinical decision-making. Collaboration with domain experts and clinicians is encouraged to develop models that are both technically robust and clinically relevant. |
| [166] | The proposed model using the Cuban dataset (n = 1697) achieved high performance with a Random Forest predictor showing 0.996 training accuracy and 0.997 AUC, surpassing traditional models. It is also cost-effective, requiring only standard hardware and easily integrable into clinical systems. | The study is limited by the size and scope of the dataset (1697 instances from one Cuban hospital), which may not fully represent the wider Cuban population. Additionally, due to the lack of historical follow-up data, the model only supports current diagnosis prediction, not long-term risk estimation. | Future work will focus on expanding data collection across multiple hospitals to enhance the generalizability and accuracy of predictions. Additionally, the integration of new risk factors such as breast density and type of occupation will be explored in collaboration with healthcare professionals. |
| [162] | Using the WDBC dataset (569 samples), the proposed eXtreme Gradient Boosting (XGBoost) model achieved 99.12% accuracy, 1.0 recall, 0.9861 specificity, and 0.9882 F1-score, outperforming state-of-the-art techniques. The model also demonstrated superior computational efficiency, being 2.11 × faster than Extra Trees in the original feature space. | The model was evaluated solely on the WDBC dataset, which may limit its generalizability to other datasets with different feature distributions or imaging data. Additionally, the dataset’s binary classification nature restricts the method’s applicability in multi-class or real-time clinical scenarios. | Future work should focus on parameter optimization to further enhance performance and adapt the model for more diverse datasets. Moreover, expanding the approach to multi-class classification and integrating real-world clinical data could significantly improve its practical utility. |
| Aspect | Details |
|---|---|
| Datasets | WDBC, SEER, BC-UCI, Breast Cancer Coimbra, Cuban |
| Models | XGBoost, Decision Tree, OSELM |
| Techniques | Feature selection, preprocessing, gradient boosting, ensemble learning |
| Highest Accuracy | 99.12% (XGBoost on WDBC) |
| Key Challenges | Dataset dependency, computational complexity, generalizability |
| Technique | Impact |
|---|---|
| Feature Selection | Reduces dimensionality and improves model performance. |
| Preprocessing | Enhances data quality and model accuracy. |
| Gradient Boosting | High accuracy and robustness in classification tasks. |
| Ensemble Learning | Combines strengths of multiple models, improving robustness and accuracy. |
| Online Learning | Efficient for real-time applications and large datasets. |
| Limitation | Details |
|---|---|
| Dataset Dependency | Reliance on limited datasets may affect generalizability. |
| Computational Complexity | High resource requirements hinder real-world deployment. |
| Standardization | Lack of standardized preprocessing techniques. |
| Generalizability | Further validation on diverse datasets is needed. |
| Real-Time Application | Computational complexity may limit real-time use. |
| Study | Dataset | Model/Technique | Accuracy | Key Findings |
|---|---|---|---|---|
| Rahman et al. (2024) [162] | WDBC | XGBoost | 99.12% | High accuracy and robustness. |
| Manikandan et al. (2023) [163] | SEER | Decision Tree | 98% | Simple architecture with competitive accuracy. |
| Albadr et al. (2024) [164] | WDBC | OSELM | 96.13% | Efficient for real-time applications. |
| Image Modality and Tabular Data | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Mammography [167,168,169] |
|
|
|
| Ultrasound [169,170] |
|
|
|
| MRI [23,170] |
|
|
|
| CT [168,171] |
|
|
|
| Histopathological [23,170] |
|
|
|
| Thermal imaging [155,172] |
|
|
|
| Tabular Data [173,174] |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Khan, M.S.H.; Watanobe, Y.; Prioty, J.T.; Annita, T.T.; Rahman, S.; Hossain, M.S.; Aitijjo, S.A.; Taskin, R.I.; Dhrubo, V.; et al. Advancements in Breast Cancer Detection: A Review of Global Trends, Risk Factors, Imaging Modalities, Machine Learning, and Deep Learning Approaches. BioMedInformatics 2025, 5, 46. https://doi.org/10.3390/biomedinformatics5030046
Rahman MA, Khan MSH, Watanobe Y, Prioty JT, Annita TT, Rahman S, Hossain MS, Aitijjo SA, Taskin RI, Dhrubo V, et al. Advancements in Breast Cancer Detection: A Review of Global Trends, Risk Factors, Imaging Modalities, Machine Learning, and Deep Learning Approaches. BioMedInformatics. 2025; 5(3):46. https://doi.org/10.3390/biomedinformatics5030046
Chicago/Turabian StyleRahman, Md. Atiqur, M. Saddam Hossain Khan, Yutaka Watanobe, Jarin Tasnim Prioty, Tasfia Tahsin Annita, Samura Rahman, Md. Shakil Hossain, Saddit Ahmed Aitijjo, Rafsun Islam Taskin, Victor Dhrubo, and et al. 2025. "Advancements in Breast Cancer Detection: A Review of Global Trends, Risk Factors, Imaging Modalities, Machine Learning, and Deep Learning Approaches" BioMedInformatics 5, no. 3: 46. https://doi.org/10.3390/biomedinformatics5030046
APA StyleRahman, M. A., Khan, M. S. H., Watanobe, Y., Prioty, J. T., Annita, T. T., Rahman, S., Hossain, M. S., Aitijjo, S. A., Taskin, R. I., Dhrubo, V., Hanip, A., & Bhuiyan, T. (2025). Advancements in Breast Cancer Detection: A Review of Global Trends, Risk Factors, Imaging Modalities, Machine Learning, and Deep Learning Approaches. BioMedInformatics, 5(3), 46. https://doi.org/10.3390/biomedinformatics5030046