1. Introduction
Parkinsonism encompasses a range of neurodegenerative disorders characterized by common motor symptoms such as tremors, rigidity, bradykinesia, and postural instability. Parkinson’s Disease (PD) is the most prevalent form, whereas Atypical Parkinsonism (APD), which includes conditions such as Multiple System Atrophy (MSA), Progressive Supranuclear Palsy (PSP), Corticobasal Degeneration (CBD), and vascular Parkinsonism (VaP) presents with additional symptoms and generally progresses more rapidly [
1]. Accurate differentiation between PD and APD is critical due to their distinct pathophysiologies, treatment responses, and prognoses [
1,
2]. Traditional diagnostic methods largely rely on clinical observations, including early falls, marked cognitive decline, and poor response to dopaminergic therapies in APD. However, these methods can be subjective, which often leads to misdiagnoses [
1,
3].
Recent advancements in neuroimaging and genetic testing provide promising tools to improve the differentiation of typical Parkinson’s Disease (PD) from Atypical Parkinsonian Disorders (APDs). Techniques like neuromelanin MRI and diffusion tensor imaging help identify brain degeneration patterns specific to PD, while genetic markers, such as LRRK2 and GBA mutations, link to distinct PD subtypes with varying symptom profiles (see [
4] for a review).
Dysarthria, a motor speech disorder affecting muscle control in speech, is commonly observed in Parkinsonian patients. Recently, speech analysis has emerged as a valuable tool in distinguishing between Parkinson’s Disease (PD) and Atypical Parkinsonian Syndromes (APSs). For example, a quantitative speech analysis study was 95% accurate in differentiating APSs from PD, and 75% accurate in distinguishing Progressive Supranuclear Palsy (PSP) from Multiple System Atrophy (MSA), highlighting its diagnostic potential [
5]. In the study, which included 15 PD, 12 PSP, and 13 MSA patients, and 37 healthy controls, dysarthria was present in all Parkinsonian patients. However, the varying combinations of hypokinetic, spastic, and ataxic dysarthria reflect the distinct underlying pathologies of these conditions. Specifically, PD was characterized by hypokinetic dysarthria, marked by reduced loudness and monopitch. In contrast, MSA displayed ataxic dysarthria, noted for excess pitch and intensity fluctuations, as well as vocal tremors, while PSP presented a combination of hypokinetic and spastic dysarthria, characterized by a strained–strangled voice and a slow speaking rate. These findings underscore the utility of quantitative speech analysis in distinguishing between different forms of Parkinsonism.
Articulatory imprecision is another hallmark feature of Parkinson’s Disease (PD), where speech becomes less distinct due to reduced precision in the movement of the articulators. Even in mild cases of PD, imprecise vowel articulation has been observed, highlighting the early impact of motor control impairments characteristic of the disease [
6,
7]. However, research on the specific speech characteristics that differentiate PD from Progressive Supranuclear Palsy (PSP) and Multiple System Atrophy (MSA) remains limited, with many studies focusing on timing variations in articulation. For instance, ref. [
8] evaluated consonant articulation deficits, particularly in voiced and voiceless stops, across participants with PD, PSP, and MSA, as well as in healthy controls, using both acoustic and perceptual methods. Voiced and voiceless stops differ in terms of the timing of the onset of voicing (voice onset time, VOT). Imprecise consonant articulation was observed across all Parkinsonian groups. Notably, PSP and MSA exhibited prolonged VOT for voiceless plosives compared to PD, reflecting greater dysarthria severity and slower articulation. In MSA, the VOT for voiced plosives was significantly shorter, likely due to cerebellar damage. Specifically, the shortening of the negative VOT (voicing lead or negative VOT) caused the voicing to disappear, leaving only a short burst, which contributed to an increased number of voiced plosives being misclassified as voiceless during perceptual evaluation. These timing variations in articulation may offer valuable insights into the underlying disease mechanisms.
However, studies on VOT in individuals with PD have produced inconsistent results. While some studies report longer VOT durations in PD [
9,
10], others have found no significant changes or even shorter VOT [
11,
12,
13]. These discrepancies may be due to variations in speaking rate [
14]. Although the VOT ratio, a rate-independent measure, has been used to clarify these inconsistencies, it has not fully resolved the conflicting findings [
10,
13].
Despite promising insights, research on acoustic biomarkers for Parkinson’s Disease and atypical Parkinsonian disorders often faces methodological limitations including small sample sizes which restrict the generalizability of findings, highlighting the need for larger, more diverse cohorts to validate speech deterioration patterns across PD and APD subtypes. Additionally, inconsistencies in assessment protocols introduce variability, impacting reliability and replicability. Ref. [
15] underscored the importance of standardized, objective speech assessment measures in Alzheimer’s Disease and Mild Cognitive Impairment, noting the impact of biases stemming from variable recording conditions and methods—a recommendation also applicable to neuromotor disorders such as Parkinsonism.
In response to these methodological needs, ref. [
16] introduced the SMARTSPEECH protocol, employing a smartphone application to systematically investigate speech biomarkers for PD and other synucleinopathies. This approach aims to create accessible, diagnostically valuable data collection methods. Furthermore, ref. [
17] provided comprehensive guidelines for recording and analyzing speech in movement disorders, establishing protocols for recording environments, vocal tasks, and acoustic features to standardize data collection. This foundational work addresses methodological inconsistencies, enhancing both the replicability and clinical relevance of speech biomarker research.
Although not fully diagnostic alone, speech-based biomarkers offer significant value in settings with limited access to advanced testing, complementing neuroimaging and genetic tools for early diagnosis and personalized treatment strategies in PD and APDs. In contrast to prior studies emphasizing articulation timing, such as VOT or other commonly investigated speech characteristics like fundamental frequency (F0), speech rate, intensity variability, rhythmic patterns, and vowel articulation, the current study examined consonant imprecision related to the ability to achieve complete closure in the vocal tract during stop consonant articulation. This analysis prioritizes articulatory precision over frequency, intensity, or timing-related factors, affecting both voiced and voiceless stops in Parkinsonian speech. The distinct lenition patterns observed between PD and APD suggest that treatment approaches may need to be tailored to address the unique articulation challenges of each group. Using Phonet, a machine learning model [
18], we aimed to assess whether stop consonant weakening could reliably distinguish PD from APD. By analyzing these subtle phonological patterns, the study seeks to contribute to early diagnosis, track disease progression, and improve treatment strategies for both PD and APD.
6. Discussion and Summary
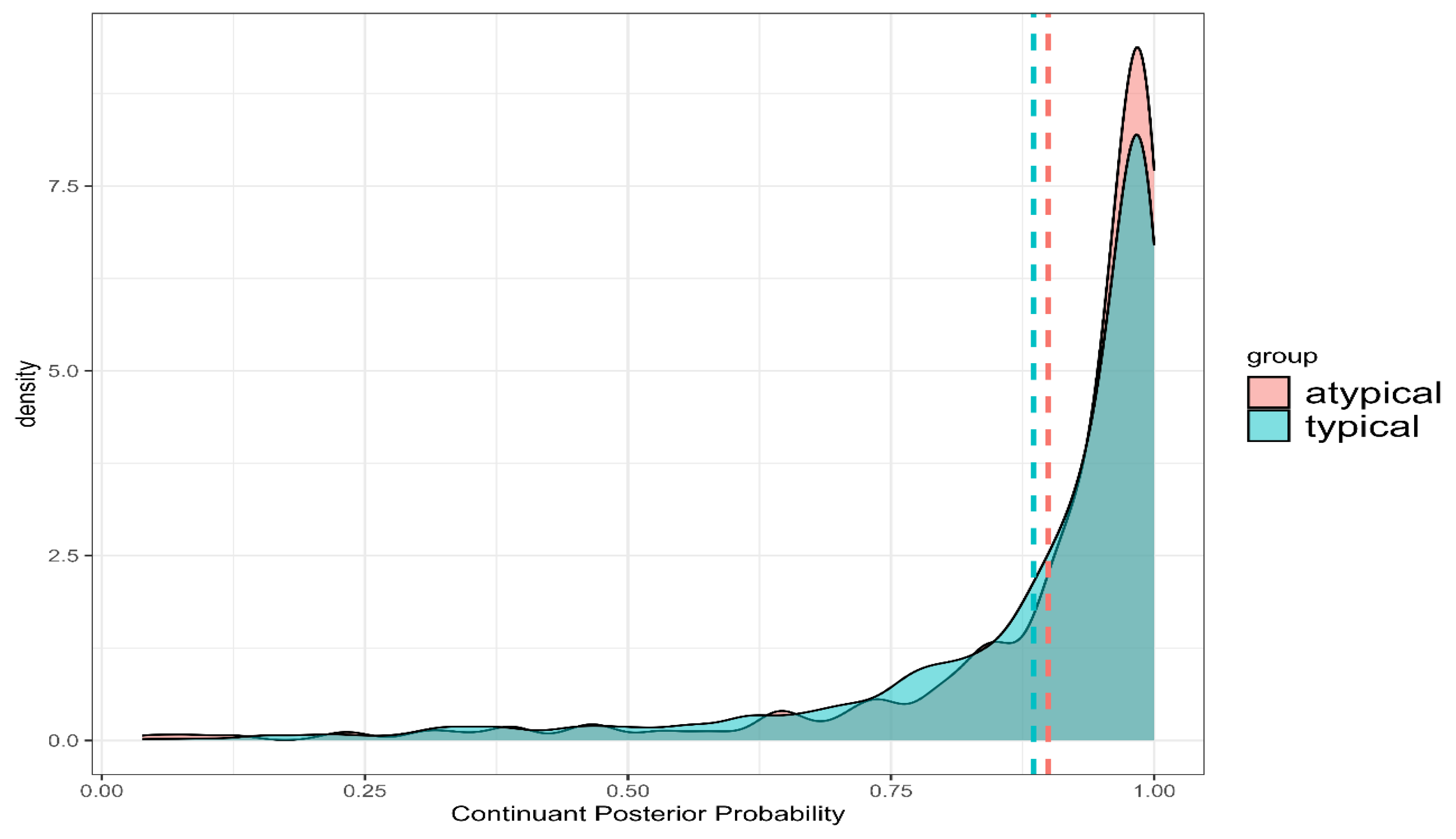
The findings from this study highlight the potential of lenition, quantified through posterior probabilities of phonological features, as a diagnostic marker for distinguishing Parkinson’s Disease (PD) from Atypical Parkinsonism (APD). Although no significant differences were observed in the overall posterior probabilities of continuant and sonorant features, critical distinctions emerged through deviation analysis. Specifically, PD patients exhibited less variability and more stable articulatory patterns, while APD patients demonstrated greater variability, indicating less precise motor control. These findings suggest that lenition is more systematic and controlled in PD, whereas APD is characterized by more erratic and unpredictable articulatory patterns.
The lack of significant overall group differences could result from several factors. Variation in disease severity among participants may play a role, as more advanced stages of APD typically involve greater motor control deficits. Dopaminergic treatment effects, particularly in the PD group, could also mask articulatory differences by mitigating symptom severity. Additionally, variability in individual characteristics, such as age and general health, may reduce statistical power, highlighting the importance of future studies to clarify articulatory patterns through these variables.
Moreover, the broader neurodegenerative impact of APD likely contributes to the increased variability in articulation, affecting motor control more severely and broadly than in PD. While PD primarily affects the substantia nigra and its dopaminergic pathways, APD involves more widespread neural disruptions, including in the cerebellum, basal ganglia, and brainstem [
2]. These wider disruptions align with observed lenition patterns in APD, with motor control deficits in this population being more generalized than those typically seen in PD.
A key finding in this study was the interaction between group and voicing. In PD patients, voiced stops exhibited higher continuant and sonorant posterior probabilities than voiceless stops. This suggests that, although PD patients struggle to form complete oral closures for both voiced and voiceless stops, they maintain finer control over the oral aperture, preserving the contrast between voiced and voiceless stops. In contrast, APD patients showed the distinction between voiced and voiceless stops in sonorant posterior probabilities but failed to maintain this distinction in continuant probabilities, indicating a breakdown in their ability to control smaller oral aperture variations, though they could manage larger oral openings. These findings align with [
8], which reported a similar breakdown in voicing distinctions in APD. Specifically, [
8] found that APD patients were unable to produce prevoicing (lead voicing or negative VOT), which caused voiced stops to be perceived as voiceless stops. This inability is likely due to increased supraglottal pressure during oral closure, which makes it more difficult to initiate and sustain vocal fold vibration. This increased pressure counteracts the subglottal pressure needed for voicing, exacerbating motor execution deficits and complex vocal fold movements. The more extensive neural degeneration in APD likely contributes to this reduced capacity for maintaining contrastive features. Regarding place of articulation, PD patients demonstrated less lenition in alveolar stops compared to velar stops, consistent with aerodynamic principles [
48,
49], which predict greater lenition for sounds articulated further back in the vocal tract. APD patients, however, showed no such distinction, suggesting a generalized motor deficit that affects stops across all places of articulation equally, reinforcing the hypothesis of widespread neural damage in APD.
The higher variability in speech production observed in APD, particularly in maintaining phonological contrasts, suggests a more significant disruption of the fine motor control necessary for articulation. By contrast, PD patients exhibited more stable articulatory patterns, consistent with the more localized neurodegenerative impact of the disease. This pattern is consistent with the findings of [
5], who noted that dysarthria in Progressive Supranuclear Palsy (PSP) and Multiple System Atrophy (MSA) differed from that of PD due to its greater severity and the presence of spastic and ataxic components. Specifically, PSP was marked by increased dysfluency, slower speaking rates, inappropriate silences, and vowel articulation deficits, while MSA was characterized by vocal tremors, pitch fluctuations, and prolonged phonemes. The broader motor impairments seen in APD are reflected in the more frequent and severe lenition observed in this study, consistent with the findings of [
4].
These findings highlight crucial differences in how PD and APD patients manage articulation, especially in sustaining distinctions between voiced and voiceless sounds. PD patients, while experiencing some articulatory limitations, retain a degree of control over the oral aperture and are able to achieve the transglottal pressure necessary for voicing. This suggests a relatively preserved coordination of laryngeal and respiratory functions, supporting the maintenance of voicing contrasts. Conversely, the extensive neural degeneration in APD, particularly affecting the cerebellum and basal ganglia, disrupts finer control over both oral and laryngeal articulation, leading to greater challenges in sustaining voicing distinctions and contributing to reduced intelligibility in APD patients.
The findings also suggest the need for differential therapeutic interventions for PD and APD to address these specific articulatory challenges. For PD, exercises that strengthen respiratory and phonatory functions—such as those in the Lee Silverman Voice Treatment (LSVT LOUD)—are highly beneficial. These exercises focus on enhancing respiratory support and improving vocal fold adduction, both crucial for maintaining voiced-voiceless distinctions. LSVT LOUD, in particular, has shown efficacy in increasing vocal loudness and improving speech clarity, which are key to sustaining voicing contrasts in PD [
28]. Additionally, respiratory support exercises that focus on breath control and coordination with phonation can mitigate supraglottal pressure issues, potentially promoting more stable voicing onset and maintenance [
50].
Targeted tongue exercises, as described by [
51], designed to strengthen the suprahyoid muscles and improve swallowing, may also enhance muscle control and support oral closure. This improvement can help PD patients produce clearer stop consonants and maintain voiced–voiceless distinctions, ultimately boosting articulatory precision.
For APD patients, techniques like exaggerated articulation—employed in the Be Clear program [
52]—and pacing strategies can improve overall speech intelligibility by stabilizing timing and reducing the slurred speech common in ataxic dysarthria [
53]. The Be Clear program, an intensive treatment for non-progressive dysarthria, incorporates these techniques to improve intelligibility in individuals with similar impairments following traumatic brain injury (TBI). Through the repeated practice of exaggerated articulation, it has shown promising results for speech clarity, supporting gains in intelligibility, vowel space, and articulatory precision.
Beyond speech-specific interventions, non-pharmacological management strategies can enhance functional communication and quality of life for APD patients. These include physical therapy and balance training for motor symptoms, occupational therapy for fine motor skills essential to daily tasks, and cognitive therapy for addressing potential cognitive deficits. Together, these approaches offer comprehensive support for APD patients, enhancing both voicing control and functional communication [
54]. Taken together, these results suggest that lenition, particularly in articulatory variability and the maintenance of voicing contrasts, could serve as a valuable diagnostic marker for distinguishing PD from APD. The more severe and unpredictable lenition in APD, along with the breakdown in the ability to differentiate voiced and voiceless stops, reflects the broader neurodegenerative changes that affect motor control in this population. Future research should explore additional phonological features, such as nasality and frication, to further refine the differential diagnosis of Parkinsonian Syndromes.
8. Limitations and Future Research
While this study provides valuable insights into the potential of using lenition as a diagnostic marker for Parkinson’s Disease (PD) and Atypical Parkinsonism (APD), several limitations should be considered. First, the relatively small and imbalanced sample size—particularly the smaller number of APD participants compared to PD participants—may limit the generalizability of the results. A larger and more balanced dataset, including a broader range of APD subtypes, is needed to confirm these findings and strengthen the validity of the diagnostic patterns observed.
Second, the variability in disease stage and severity among participants is a significant factor to consider. While our analysis provides valuable insights into articulatory patterns in both PD and APD, the clinical utility of our method would be most beneficial for early-stage diagnosis, where distinguishing between typical and atypical Parkinsonism is often most challenging. However, our study includes participants across a range of disease stages, which may affect the generalizability of our findings if severity levels differ significantly between groups. For example, comparing more advanced stages of atypical Parkinsonism to early stages of typical Parkinsonism could impact the observed differences in articulatory patterns.
Third, the analysis relied on a logistic regression model, which assumes a linear relationship between the predictors and the log odds of group classification. This approach may oversimplify the complex relationships inherent in articulatory deviations between PD and APD, potentially overlooking non-linear patterns or interactions. Although non-linear or machine learning methods could capture these complexities more effectively, we chose logistic regression for its interpretability and to provide clear, coefficient-based insights in this exploratory study. Future studies could investigate non-linear models to determine if they enhance classification accuracy in distinguishing PD from APD.
Fourth, the study focused exclusively on a limited set of phonological features—continuant and sonorant probabilities—within a narrow set of phonemes. While these features are crucial for analyzing lenition, the speech of individuals with Parkinsonism is affected by a wide array of articulatory and phonological factors. Future research should explore additional phonological features, such as nasality, stridency (frication), and phonation (e.g., breathiness and hoarseness), to provide a more comprehensive understanding of how speech changes in PD and APD.
Moreover, the sentences used in the data collection process were limited in variety and complexity. A greater range of linguistic contexts, including spontaneous speech and conversational data, could reveal more about how lenition manifests in natural communication. Since lenition is often gradient and context-dependent, evaluating speech across different types of discourse (e.g., formal vs. informal) and in more natural settings would offer richer insights into the full extent of speech impairments in Parkinsonism. Another limitation concerns the use of the Phonet model, which, while effective in this study, may be sensitive to variability in language and dialect. The model was trained on Spanish phonological features, which may not fully align with the phonetic and phonological characteristics of English, the language used in this study. Future studies should explore how models trained on different languages perform in similar diagnostic tasks and whether language-specific models provide better diagnostic accuracy for speakers of various languages [
30].
In terms of future research directions, additional studies could investigate whether the patterns of lenition observed in this study are consistent across different stages of PD and APD. By tracking speech changes longitudinally, researchers could assess whether lenition patterns become more pronounced as these diseases progress. This could contribute to the development of speech-based biomarkers not only for diagnosis but also for monitoring disease progression.
Furthermore, future work could integrate speech analysis with other diagnostic tools, such as neuroimaging or physiological assessments, to develop more comprehensive models of disease detection and progression. Multimodal approaches may reveal correlations between speech impairments and neural degeneration, thereby providing deeper insights into the mechanisms behind speech deficits in neurodegenerative disorders.
Additionally, a focused evaluation of early-stage PD and APD patients, paired with the longitudinal tracking of lenition patterns and integration with neuroimaging and genetic testing, holds considerable potential to advance early differential diagnosis and reveal progression-specific changes in articulatory control. Such comprehensive longitudinal data could establish lenition as a reliable biomarker for tracking disease progression, directly informing and refining treatment strategies to be more responsive to the evolving needs of patients.
Lastly, exploring the effects of therapeutic interventions, such as speech therapy or pharmacological treatments, on lenition patterns in PD and APD patients would be valuable. Understanding how treatment impacts articulatory precision and lenition could lead to improved therapeutic strategies that better address the specific motor speech deficits experienced by individuals with these conditions.
Author Contributions
Conceptualization, R.W., K.T. and K.W.H.; methodology, K.T. and R.W.; software, K.T.; validation, K.T., R.R. and R.W.; formal analysis, R.W., R.R. and R.M.; investigation, R.W., K.T. and K.W.H.; resources, K.W.H., K.T. and R.W.; data curation, K.W.H.; writing—original draft preparation, R.W., R.R. and R.M.; writing—review and editing, R.W., R.M. and K.T.; visualization, R.M.; supervision, R.W., K.T. and K.W.H.; project administration, R.W., K.T. and K.W.H.; funding acquisition, R.W., K.T. and K.W.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation (award 852 2037266—SenSE) and a Research Opportunity Seed Fund from the University of Florida.
Institutional Review Board Statement
The data were obtained from the database of a different study which was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (or Ethics Committee) of the University of Florida for studies involving humans. We were approved by the UFIRB to access and use the data for this study (UF IRB201602473).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study from which the data were obtained.
Data Availability Statement
Data are available upon request to the authors. The data are not publicly available due to the sensitive information contained therein.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Williams, D.R.; Litvan, I. Parkinsonian syndromes. Contin. Lifelong Learn. Neurol. 2013, 19, 1189–1212. [Google Scholar] [CrossRef]
- Litvan, I. Atypical parkinsonian disorders. Contin. Lifelong Learn. Neurol. 2004, 10, 42–64. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Rusz, J.; Bonnet, C.; Klempíř, J.; Tykalová, T.; Baborová, E.; Novotný, M.; Rulsh, A.; Růžička, E. Speech disorders reflect differing pathophysiology in Parkinson’s disease, progressive supranuclear palsy, and multiple system atrophy. J. Neurol. 2015, 262, 992–1001. [Google Scholar] [CrossRef]
- Skodda, S.; Visser, W.; Schlegel, U. Vowel articulation in Parkinson’s disease. J. Voice 2011, 25, 467–472. [Google Scholar] [CrossRef]
- Rusz, J.; Cmejla, R.; Tykalova, T.; Ruzickova, H.; Klempir, J.; Majerova, V.; Picmausova, J.; Roth, J.; Ruzicka, E. Imprecise vowel articulation as a potential early marker of Parkinson’s disease: Effect of speaking task. J. Acoust. Soc. Am. 2013, 134, 2171–2181. [Google Scholar] [CrossRef]
- Tykalova, T.; Rusz, J.; Klempir, J.; Cmejla, R.; Ruzicka, E. Distinct patterns of imprecise consonant articulation among Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Brain Lang. 2017, 165, 1–9. [Google Scholar] [CrossRef]
- Forrest, K.; Weismer, G.; Turner, G.S. Kinematic, acoustic, and perceptual analyses of connected speech produced by Parkinsonian and normal geriatric adults. J. Acoust. Soc. Am. 1989, 85, 2608–2622. [Google Scholar] [CrossRef]
- Novotný, M.; Rusz, J.; Čmejla, R.; Růžička, E. Automatic evaluation of articulatory disorders in Parkinson’s disease. IEEE/ACM Trans. Audio Speech Lang. Process. 2014, 22, 1366–1378. [Google Scholar] [CrossRef]
- Flint, A.J.; Black, S.E.; Campbell-Taylor, I.; Gailey, G.F.; Levinton, C. Acoustic analysis in the differentiation of Parkinson’s disease and major depression. J. Psycholinguist. Res. 1992, 21, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, S.M. Dissociating the performance of cortical and subcortical patients on phonemic tasks. Brain Cogn. 2003, 53, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Goberman, A.M. Voice onset time in Parkinson disease. J. Commun. Disord. 2010, 43, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Volaitis, L.E.; Miller, J.L. Phonetic prototypes: Influence of place of articulation and speaking rate on the internal structure of voicing categories. J. Acoust. Soc. Am. 1992, 92, 723–735. [Google Scholar] [CrossRef]
- Martínez-Nicolás, I.; Llorente, T.E.; Martínez-Sánchez, F.; Meilán, J.J.G. Ten years of research on automatic voice and speech analysis of people with Alzheimer’s disease and mild cognitive impairment: A systematic review article. Front. Psychol. 2021, 12, 620251. [Google Scholar] [CrossRef]
- Kouba, T.; Illner, V.; Rusz, J. Study protocol for using a smartphone application to investigate speech biomarkers of Parkinson’s disease and other synucleinopathies: SMARTSPEECH. BMJ Open 2022, 12, e059871. [Google Scholar] [CrossRef]
- Rusz, J.; Tykalova, T.; Ramig, L.O.; Tripoliti, E. Guidelines for speech recording and acoustic analyses in dysarthrias of movement disorders. Mov. Disord. 2021, 36, 803–814. [Google Scholar] [CrossRef]
- Vásquez-Correa, J.C.; Klumpp, P.; Orozco-Arroyave, J.R.; Nöth, E. Phonet: A tool based on gated recurrent neural networks to extract phonological posteriors from speech. Interspeech 2019, 2019, 549–553. [Google Scholar] [CrossRef]
- Hualde, J.I. The Sounds of Spanish; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Hualde, J.I.; Eager, C.D. Final devoicing and deletion of/-d/in Castilian Spanish. Stud. Hisp. Lusoph. Linguist. 2016, 9, 329–353. [Google Scholar] [CrossRef]
- Marotta, G. Lenition in Tuscan Italian (gorgia toscana). In Lenition and Fortition; De Gruyter Mouton: Berlin, Germany, 2008; pp. 235–270. [Google Scholar] [CrossRef]
- Hualde, J.I.; Nadeu, M. Lenition and phonemic overlap in Rome Italian. Phonetica 2012, 68, 215–242. [Google Scholar] [CrossRef]
- Katz, J.; Pitzanti, G. The phonetics and phonology of lenition: A Campidanese Sardinian case study. Lab. Phonol. J. Assoc. Lab. Phonol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Forrest, K.; Weismer, G. Dynamic aspects of lower lip movement in Parkinsonian and neurologically normal geriatric speakers’ production of stress. J. Speech Hear. Res. 1995, 38, 260–272. [Google Scholar] [CrossRef]
- Walsh, B.M. Speech Production in Individuals with Parkinson’s Disease: Basic Kinematic Parameters and Effects of Increased Linguistic Demands on Interarticulatory Coordination. Doctoral Dissertation, Purdue University, West Lafayette, IN, USA, 2007. [Google Scholar]
- Kleinow, J.; Smith, A.; Ramig, L.O. Speech motor stability in IPD: Effects of rate and loudness manipulations. J. Speech Lang. Hear. Res. 2001, 44, 1041–1051. [Google Scholar] [CrossRef]
- Cai, W.; Young, C.B.; Yuan, R.; Lee, B.; Ryman, S.; Kim, J.; Yang, L.; Levine, T.F.; Henderson, V.W.; Poston, K.L.; et al. Subthalamic nucleus—Language network connectivity predicts dopaminergic modulation of speech function in Parkinson’s disease. Proc. Natl. Acad. Sci. 2024, 121, e2316149121. [Google Scholar] [CrossRef]
- Ramig, L.O.; Sapir, S.; Fox, C.; Countryman, S. Intensive voice treatment (LSVT®) for patients with Parkinson’s disease: A 2-year follow-up. J. Neurol. Neurosurg. Psychiatry 2001, 71, 493–498. [Google Scholar] [CrossRef]
- Sapir, S.; Ramig, L.O.; Fox, C.M. Intensive voice treatment in Parkinson’s disease: Lee Silverman voice treatment. Expert Rev. Neurother. 2011, 11, 815–830. [Google Scholar] [CrossRef]
- Fox, C.; Ebersbach, G.; Ramig, L.; Sapir, S. LSVT LOUD and LSVT BIG: Behavioral treatment programs for speech and body movement in Parkinson’s disease. Park. Dis. 2012, 2012, 391946. [Google Scholar] [CrossRef]
- Creer, S.; Enderby, P.; Judge, S.; John, A. Prevalence of people who could benefit from augmentative and alternative communication (AAC) in the UK: Determining the need. Int. J. Lang. Commun. Disord. 2016, 51, 639–653. [Google Scholar] [CrossRef]
- Elsahar, Y.; Hu, S.; Bouazza-Marouf, K.; Kerr, D.; Mansor, A. Augmentative and alternative communication (AAC) advances: A review of configurations for individuals with a speech disability. Sensors 2019, 19, 1911. [Google Scholar] [CrossRef]
- Tjaden, K. Speech and swallowing in Parkinson’s disease. Top. Geriatr. Rehabil. 2008, 24, 115–126. [Google Scholar] [CrossRef]
- Tang, K.; Wayland, R.; Wang, F.; Vellozzi, S.; Altmann, L. From sonority hierarchy to posterior probability as a measure of lenition: The case of Spanish stops. J. Acoust. Soc. Am. 2023, 153, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Wayland, R.; Tang, K.; Wang, F.; Vellozzi, S.; Sengupta, R. Quantitative acoustic versus deep learning metrics of lenition. Languages 2023, 8, 98. [Google Scholar] [CrossRef]
- Tang, K.; Wayland, R.; Wang, F.; Vellozzi, S.; Sengupta, R. Evaluating the consistency of lenition measures: Neural networksʹ posterior probability, intensity velocity, and duration. J. Acoust. Soc. Am. 2024, 156, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Wayland, R.; Tang, K.; Wang, F.; Vellozzi, S.; Meyer, R.; Sengupta, R. Neural network-based measure of consonant lenition in Parkinson’s Disease. In Proceedings of the Meetings on Acoustics; AIP Publishing: College Park, Maryland, USA, 2023; Volume 52. [Google Scholar] [CrossRef]
- Wayland, R.; Tang, K.; Vellozzi, S.; Wang, F.; Sengupta, R. Measuring gradient effects of alcohol on speech with neural networks’ posterior probability of phonological features. In Proceedings of the 20th International Congress of Phonetic Sciences, Prague, Czech Republic, 7–11 August 2023. [Google Scholar]
- Wayland, R.; Tang, K.; Wang, F.; Vellozzi, S.; Sengupta, R. Neural networks’ posterior probability as measure of effects of alcohol on speech. Proc. Mtgs. Acoust. 2023, 51, 060001. [Google Scholar] [CrossRef]
- Wayland, R.; Meyer, R.; Vellozzi, S.; Tang, K. Lenition in L2 Spanish: The Impact of Study Abroad on Phonological Acquisition. Brain Sci. 2024, 14, 946. [Google Scholar] [CrossRef]
- Meyer, R.; Wayland, R.; Tang, K.; Vellozzi, S.; Sengupta, R. Measuring second language acquisition of spanish lenition. In Proceedings of the Society for Computation in Linguistics (SCiL), Irvine, CA, USA, 27–29 June 2024; pp. 297–301. [Google Scholar]
- Hosseini-Kivanani, N.; Vasquez, J.C.; Schommer, C.; Noeth, E. Exploring the use of phonological features for Parkinson’s disease detection. In Proceedings of the 20 th International Congress of Phonetic Sciences (ICPhS 2023), Prague, Czech Republic, 7–11 August 2023; pp. 3897–3901. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Research. 2011, 12, 2825–2830. [Google Scholar]
- McAuliffe, M.; Socolof, M.; Mihuc, S.; Wagner, M.; Sonderegger, M. Montreal Forced Aligner: Trainable Text-Speech Alignment Using Kaldi. 2017. Available online: https://github.com/MontrealCorpusTools/Montreal-Forced-Aligner (accessed on 19 October 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Software 2014, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 18 November 2024).
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.10.5. 2024. Available online: https://doi.org/10.32614/CRAN.package.emmeans (accessed on 19 October 2024).
- Javkin, H. Towards a phonetic explanation for universal preferences in implosives and ejectives. In Proceedings of the 3rd Annual Meeting of the Berkeley Linguistics Society, Berkeley, CA, USA, 19–21 February 1977; pp. 559–565. [Google Scholar]
- Ohala, J.J. A mathematical model of speech aerodynamics. Annu. Rep. Inst. Phon. Univ. Copenhagen. 1974, 8, 11–22. [Google Scholar] [CrossRef]
- Sapienza, C.; Hoffman, B. Respiratory Muscle Strength Training; Plural Publishing: San Diego, CA, USA, 2020. [Google Scholar]
- Plaza, E.; Busanello-Stella, A.R. Effects of a tongue training program in Parkinson’s disease: Analysis of electrical activity and strength of suprahyoid muscles. J. Electromyogr. Kinesiol. 2022, 63, 102642. [Google Scholar] [CrossRef]
- Park, S.; Theodoros, D.; Finch, E.; Cardell, E. Be Clear, an intensive treatment for non-progressive dysarthria: A case report. In Clinical Cases in Dysarthria; Routledge: Abingdon, UK, 2021; pp. 57–71. [Google Scholar]
- McHenry, M.A. The effect of pacing strategies on the variability of speech movement sequences in dysarthria. J. Speech Lang. Hear. Res. 2003, 46, 702–710. [Google Scholar] [CrossRef]
- Constantinides, V.C.; Giagkou, N.; Brinia, M.E.; Koros, C.; Stefanis, L.; Stamelou, M. Management Strategies for Atypical Parkinsonism. Curr. Treat. Options Neurol. 2024, 26, 169–187. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).