Pediatric and Adolescent Seizure Detection: A Machine Learning Approach Exploring the Influence of Age and Sex in Electroencephalogram Analysis †
Abstract
1. Introduction
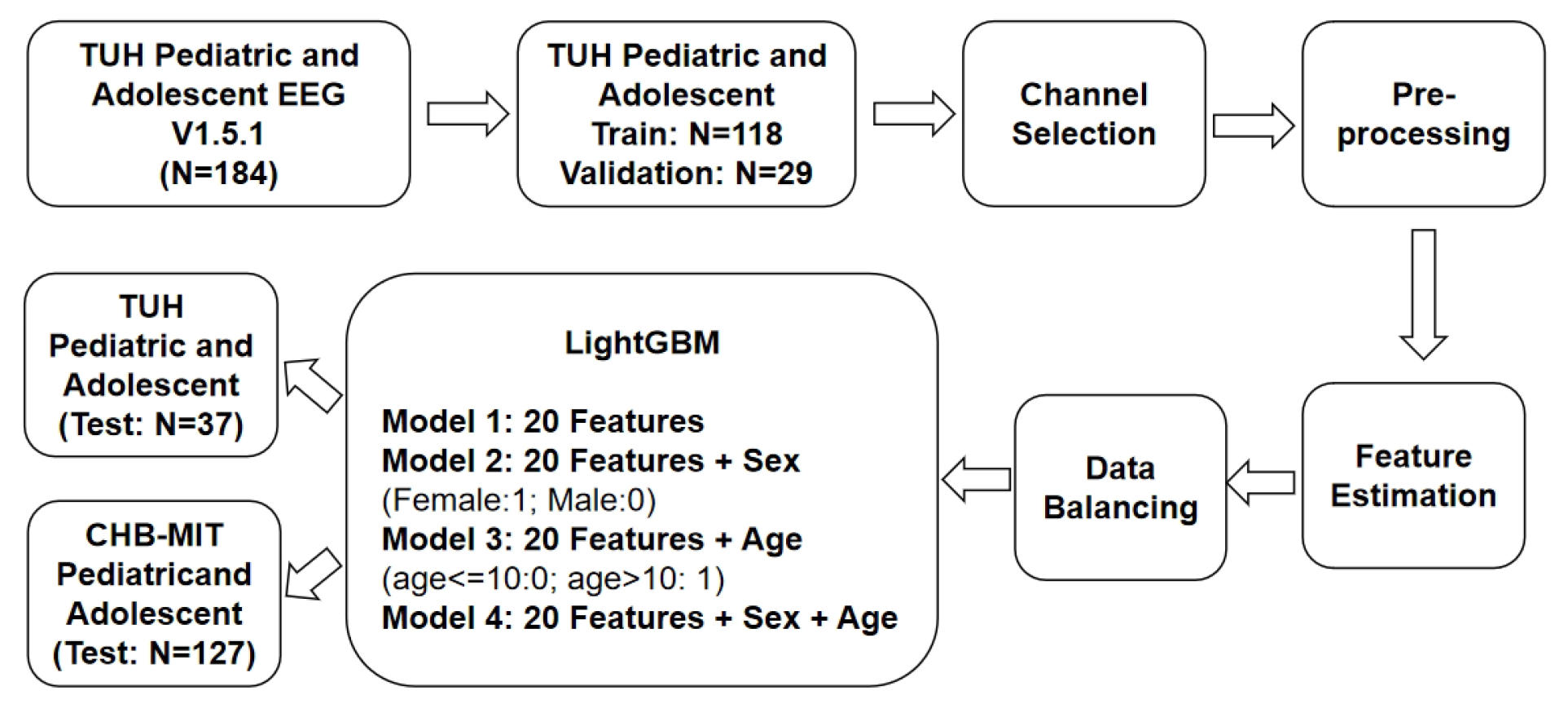
2. Data
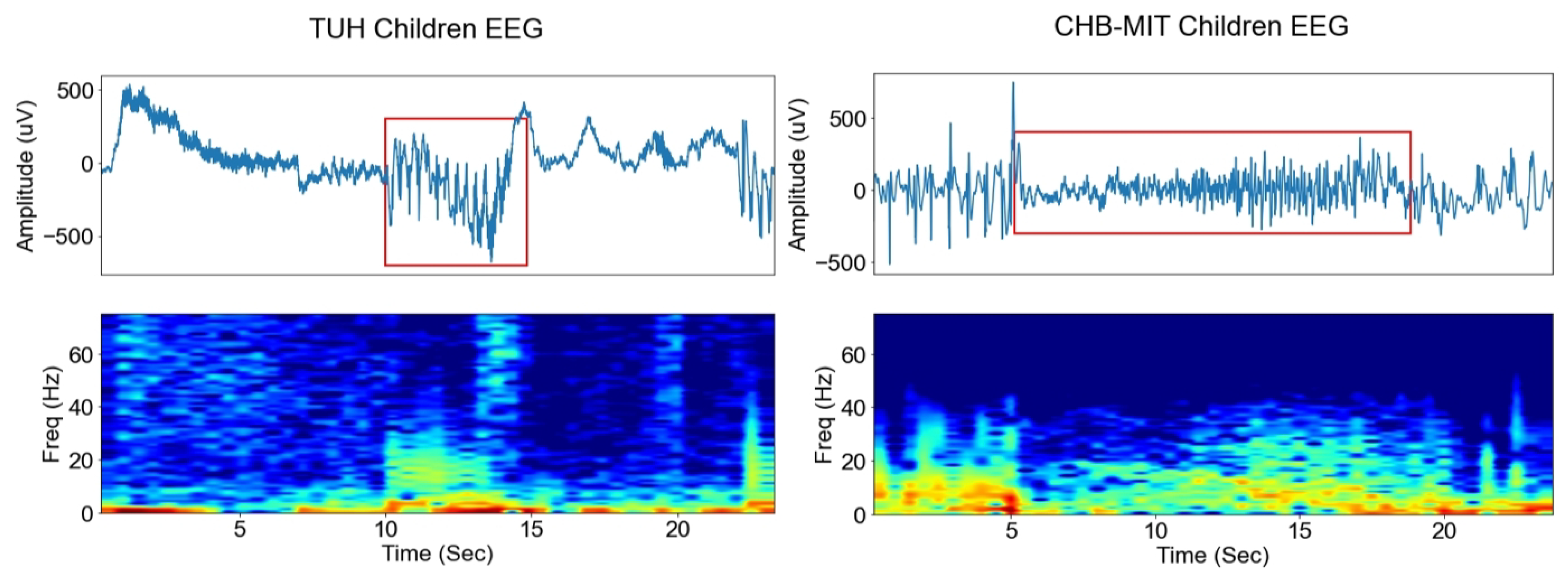
2.1. TUH EEG Dataset
2.2. CHB-MIT EEG Dataset
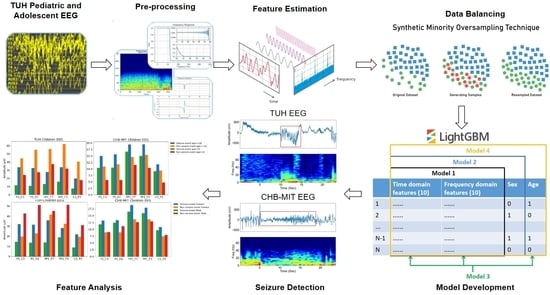
3. Methodology
3.1. Channel Selection
3.2. Data Pre-Processing
3.3. Feature Estimation
- Mean value of the pre-processed absolute amplitude of EEG recordings;
- Standard deviation of the pre-processed absolute amplitude of EEG recordings;
- Skewness of the pre-processed absolute amplitude of EEG recordings;
- Signal envelope of the pre-processed absolute amplitude of EEG recordings;
- Kurtosis of the pre-processed absolute amplitude of EEG recordings;
- Complexity of the pre-processed absolute amplitude of EEG recordings;
- Mobility of the pre-processed absolute amplitude of EEG recordings;
- Teager–Kaiser energy operator (TKEO) of the pre-processed absolute amplitude of EEG recordings;
- Variance of the pre-processed absolute amplitude of EEG recordings;
- Fractal dimension (FD) of the pre-processed absolute amplitude of EEG recordings.
- Relative band power of theta;
- Absolute band power of theta;
- Relative band power of alpha;
- Absolute band power of alpha;
- Relative band power of beta;
- Absolute band power of beta;
- Relative band power of gamma;
- Absolute band power of gamma;
- Absolute band power of the EEG amplitude;
- Sum of relative band power of beta and gamma.
- n represents the number of samples within each epoch;
- signifies the number of sign changes in the signal derivative in that epoch;
- denotes the time derivative of the pre-processed EEG signal x;
- stands for the sample, 1] refers to the ( 1)th sample and indicates the (n + 1)th sample of the pre-processed EEG signal within the epoch;
- Var (x) represents the variance of x estimated for that epoch.
3.4. Data Balancing
3.5. Classification Algorithms
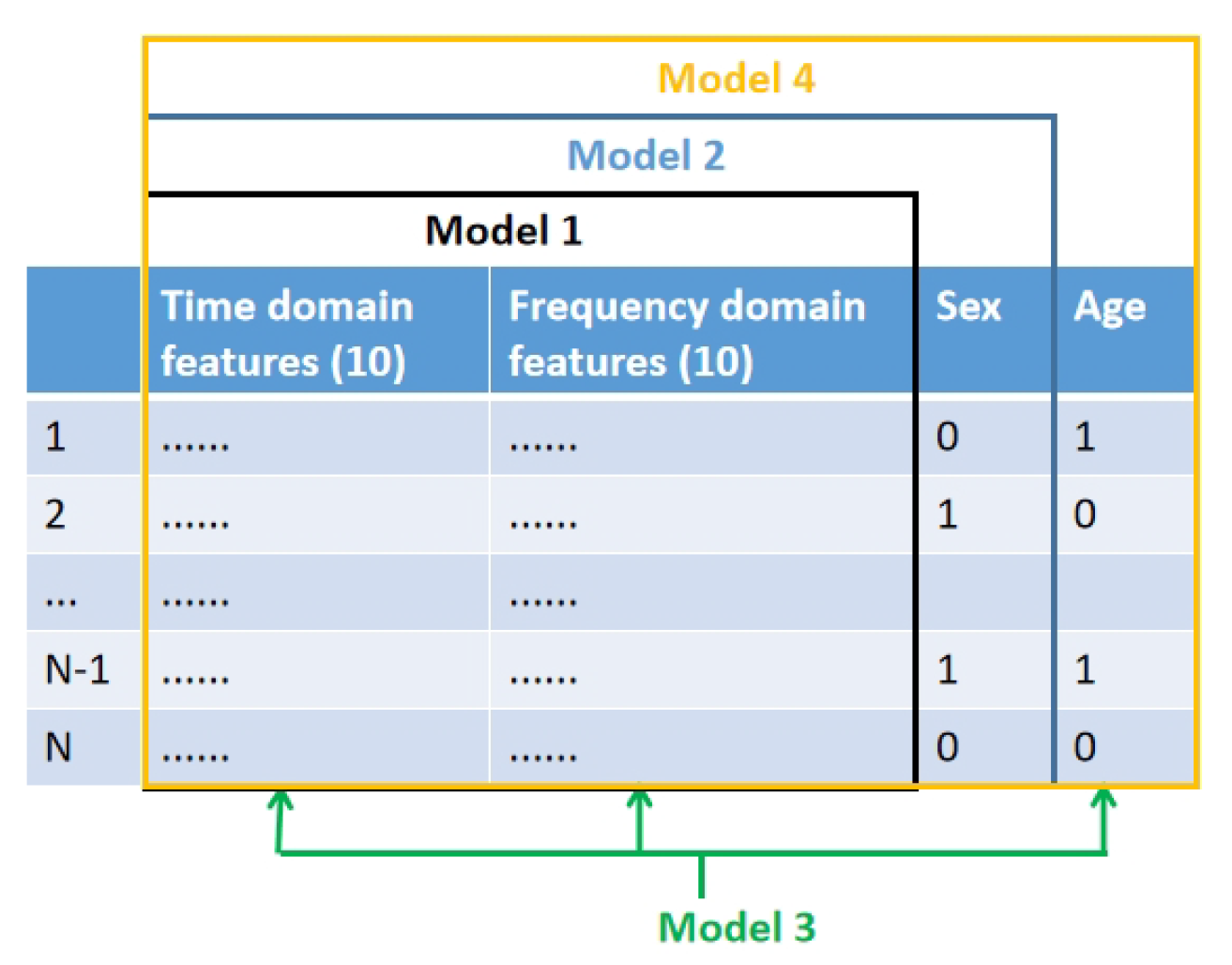
- Model 1 was trained and evaluated using the 20 features derived from both time and frequency domains as described in Section 3.3.
- Model 2 was trained and tested on the same 20 features (Section 3.3). However, it incorporated an additional feature, namely sex (male and female, where 0 denotes female and 1 denotes male), resulting in a total of 21 features.
- Model 3 was trained and tested using the original 20 features (Section 3.3) and added age group (where 0 represents children aged 10 or younger, and 1 represents children older than 10) as an additional feature, this totaled 21 features in the model.
- Model 4 was trained and tested on the same 20 features (Section 3.3), but integrated both sex and age group as additional features, thus employing a total of 22 features in its development.
3.6. Performance Evaluation
- True Positives (TP): the number of seizures correctly predicted as seizures.
- False Positives (FP): the number of non-seizures incorrectly predicted as seizures.
- True Negatives (TN): the number of non-seizures correctly predicted as non-seizures.
- False Negatives (FN): the number of seizures incorrectly predicted as non-seizures.
4. Results
4.1. Feature Importance
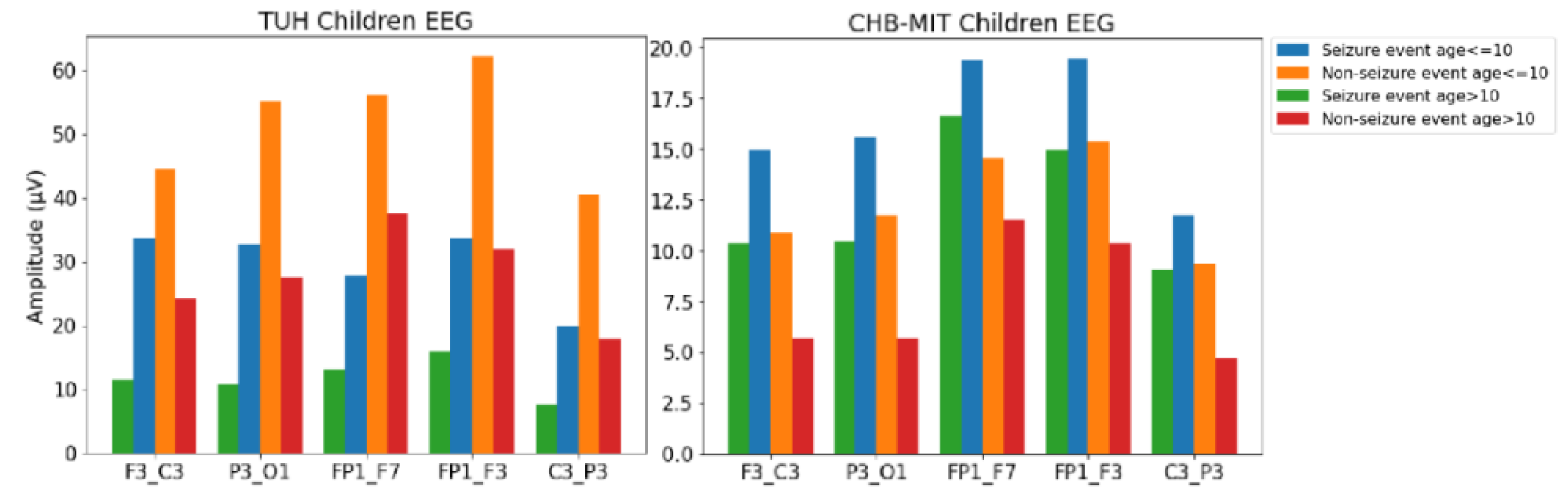
4.2. Feature Analysis
4.3. Previous Work on Seizure Detection Using TUH and CHB-MIT EEGs
4.4. Performance on TUH and CHB-MIT EEG
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Guerrini, R. Epilepsy in children. Lancet 2006, 367, 499–524. [Google Scholar] [CrossRef]
- Fountain, N.B.; Freeman, J.M. EEG is an essential clinical tool: Pro and con. Epilepsia 2006, 47, 23–25. [Google Scholar] [CrossRef]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Elsevier: Copenhagen, Denmark, 2020; pp. 25–60. [Google Scholar]
- Rajpurkar, P.; Irvin, J.; Ball, R.L.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Langlotz, C.P.; et al. Deep learning for chest radiograph diagnosis: A retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. 2018, 15, e1002686. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Ziyabari, S.; Shah, V.; Obeid, I.; Picone, J. Deep architectures for spatio-temporal modeling: Automated seizure detection in scalp EEGs. In Proceedings of the 2018 17th IEEE International Conference on Machine Learning and Applications (ICMLA), Orlando, FL, USA, 17–20 December 2018; pp. 745–750. [Google Scholar]
- Shah, V.; Golmohammadi, M.; Ziyabari, S.; Von Weltin, E.; Obeid, I.; Picone, J. Optimizing channel selection for seizure detection. In Proceedings of the 2017 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 2 December 2017; pp. 1–5. [Google Scholar]
- Ziyabari, S.; Shah, V.; Golmohammadi, M.; Obeid, I.; Picone, J. Objective evaluation metrics for automatic classification of EEG events. arXiv 2017, arXiv:1712.10107. [Google Scholar]
- Albaqami, H.; Hassan, G.M.; Datta, A. Automatic detection of abnormal eeg signals using wavenet and lstm. Sensors 2023, 23, 5960. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Delving deep into rectifiers: Surpassing human-level performance on imagenet classification. In Proceedings of the IEEE International Conference on Computer Vision, Santiago, Chile, 7–13 December 2015; pp. 1026–1034. [Google Scholar]
- Golmohammadi, M.; Ziyabari, S.; Shah, V.; Von Weltin, E.; Campbell, C.; Obeid, I.; Picone, J. Gated recurrent networks for seizure detection. In Proceedings of the 2017 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 2 December 2017; pp. 1–5. [Google Scholar]
- Vysata, O.; Kukal, J.; Prochazka, A.; Pazdera, L.; Simko, J.; Valis, M. Age-related changes in EEG coherence. Neurol. Neurochir. Pol. 2014, 48, 35–38. [Google Scholar] [CrossRef]
- Wei, L.; Mooney, C. Investigating the Need for Pediatric-Specific Machine Learning Approaches for Seizure Detection in EEG. In Proceedings of the 2023 11th International Conference on Bioinformatics and Computational Biology (ICBCB), Hangzhou, China, 21–23 April 2023; pp. 57–63. [Google Scholar]
- Wei, L.; McHugh, J.C.; Mooney, C. A Machine Learning Approach for Sex and Age Classification of Paediatric EEGs. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia,, 24–27 July 2023; pp. 1–4. [Google Scholar]
- Bresnahan, S.M.; Anderson, J.W.; Barry, R.J. Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biol. Psychiatry 1999, 46, 1690–1697. [Google Scholar] [CrossRef]
- Duffy, F.H.; Albert, M.S.; McAnulty, G.; Garvey, A.J. Age-related differences in brain electrical activity of healthy subjects. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1984, 16, 430–438. [Google Scholar] [CrossRef]
- Pierce, T.W.; Watson, T.D.; King, J.S.; Kelly, S.P.; Pribram, K.H. Age differences in factor analysis of EEG. Brain Topogr. 2003, 16, 19–27. [Google Scholar] [CrossRef]
- Marciani, M.G.; Maschio, M.; Spanedda, F.; Caltagirone, C.; Gigli, G.; Bernardi, G. Quantitative EEG evaluation in normal elderly subjects during mental processes: Age-related changes. Int. J. Neurosci. 1994, 76, 131–140. [Google Scholar] [CrossRef]
- Klass, D.W.; Brenner, R.P. Electroencephalography of the elderly. J. Clin. Neurophysiol. 1995, 12, 116–131. [Google Scholar] [CrossRef]
- Hartikainen, P.; Soininen, H.; Partanen, J.; Helkala, E.; Riekkinen, P. Aging and spectral analysis of EEG in normal subjects: A link to memory and CSF AChE. Acta Neurol. Scand. 1992, 86, 148–155. [Google Scholar] [CrossRef]
- Gasser, T.; Verleger, R.; Bächer, P.; Sroka, L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 91–99. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. Age and sex effects in the EEG: Development of the normal child. Clin. Neurophysiol. 2001, 112, 806–814. [Google Scholar] [CrossRef]
- Petersén, I.; Eeg-Olofsson, O. The development of the electroencephalogram in normal children from the age of 1 through 15 years–non-paroxysmal activity. Neuropädiatrie 1971, 2, 247–304. [Google Scholar] [CrossRef]
- Matousek, M. Frequency analysis of the EEG in normal children and adolescents. In Automation of Clinical Ectroencephalography; Raven Press: New York, NY, USA, 1973; pp. 75–102. [Google Scholar]
- Cohn, N.; Kircher, J.; Emmerson, R.; Dustman, R. Pattern reversal evoked potentials: Age, sex and hemispheric asymmetry. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1985, 62, 399–405. [Google Scholar] [CrossRef]
- Matthis, P.; Scheffner, D.; Benninger, C.; Lipinski, C.; Stolzis, L. Changes in the background activity of the electroencephalogram according to age. Electroencephalogr. Clin. Neurophysiol. 1980, 49, 626–635. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, C.; Zhang, H.; Wu, Z.; Zhang, J.; Dai, G.; Babiloni, F.; Kong, W. A lightGBM-based EEG analysis method for driver mental states classification. Comput. Intell. Neurosci. 2019, 2019, 3761203. [Google Scholar] [CrossRef]
- Chatterjee, S.; Byun, Y.C. EEG-based emotion classification using stacking ensemble approach. Sensors 2022, 22, 8550. [Google Scholar] [CrossRef]
- Aggarwal, S.; Aggarwal, L.; Rihal, M.S.; Aggarwal, S. EEG based participant independent emotion classification using gradient boosting machines. In Proceedings of the 2018 IEEE 8th International Advance Computing Conference (IACC), Greater Noida, India, 14–15 December 2018; pp. 266–271. [Google Scholar]
- Harati, A.; Lopez, S.; Obeid, I.; Picone, J.; Jacobson, M.; Tobochnik, S. The TUH EEG CORPUS: A big data resource for automated EEG interpretation. In Proceedings of the 2014 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 13 December 2014; pp. 1–5. [Google Scholar]
- Statsenko, Y.; Babushkin, V.; Talako, T.; Kurbatova, T.; Smetanina, D.; Simiyu, G.L.; Habuza, T.; Ismail, F.; Almansoori, T.M.; Gorkom, K.N.V.; et al. Automatic Detection and Classification of Epileptic Seizures from EEG Data: Finding Optimal Acquisition Settings and Testing Interpretable Machine Learning Approach. Biomedicines 2023, 11, 2370. [Google Scholar] [CrossRef]
- Shoeb, A.H. Application of Machine Learning to Epileptic Seizure Onset Detection and Treatment. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2009. [Google Scholar]
- Siuly, S.; Li, Y.; Zhang, Y. EEG signal analysis and classification. IEEE Trans. Neural Syst. Rehabilit. Eng. 2016, 11, 141–144. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. Lightgbm: A highly efficient gradient boosting decision tree. Adv. Neural Inf. Process. Syst. 2017, 30, 3149–3157. [Google Scholar]
- Li, K.; Xu, H.; Liu, X. Analysis and visualization of accidents severity based on LightGBM-TPE. Chaos Solitons Fractals 2022, 157, 111987. [Google Scholar] [CrossRef]
- Kiessner, A.K.; Schirrmeister, R.T.; Gemein, L.A.; Boedecker, J.; Ball, T. An extended clinical EEG dataset with 15,300 automatically labelled recordings for pathology decoding. NeuroImage Clin. 2023, 39, 103482. [Google Scholar] [CrossRef]
- Wei, L.; Mooney, C. Epileptic seizure detection in clinical EEGs using an XGBoost-based method. In Proceedings of the 2020 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 5 December 2020; pp. 1–6. [Google Scholar]
- Fergus, P.; Hussain, A.; Hignett, D.; Al-Jumeily, D.; Abdel-Aziz, K.; Hamdan, H. A machine learning system for automated whole-brain seizure detection. Appl. Comput. Inform. 2016, 12, 70–89. [Google Scholar] [CrossRef]
- Wei, L.; Mooney, C. Transfer Learning-based Seizure Detection on Multiple Channels of Paediatric EEGs. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023; pp. 1–4. [Google Scholar]
- Zabihi, M.; Kiranyaz, S.; Ince, T.; Gabbouj, M. Patient-specific epileptic seizure detection in long-term EEG recording in paediatric patients with intractable seizures. In Proceedings of the IET Intelligent Signal Processing Conference 2013 (ISP 2013), London, UK, 2–3 December 2013. [Google Scholar]
- Hu, X.; Yuan, S.; Xu, F.; Leng, Y.; Yuan, K.; Yuan, Q. Scalp EEG classification using deep Bi-LSTM network for seizure detection. Comput. Biol. Med. 2020, 124, 103919. [Google Scholar] [CrossRef]
- Sopic, D.; Aminifar, A.; Atienza, D. e-glass: A wearable system for real-time detection of epileptic seizures. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018; pp. 1–5. [Google Scholar]
- Wei, L.; Boutouil, H.; Gerbatin, R.R.; Mamad, O.; Heiland, M.; Reschke, C.R.; Del Gallo, F.; Fabene, P.F.; Henshall, D.C.; Lowery, M.; et al. Detection of spontaneous seizures in EEGs in multiple experimental mouse models of epilepsy. J. Neural Eng. 2021, 18, 056060. [Google Scholar] [CrossRef]
- Wei, L.; Mooney, C. Investigating the Need for Pediatric-Specific Automatic Seizure Detection. In Proceedings of the 2022 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Philadelphia, PA, USA, 3 December 2022; pp. 1–3. [Google Scholar]
- World Health Organization. Adolescent Health. Available online: https://www.who.int/health-topics/adolescent-health#tab=tab_1 (accessed on 12 December 2023).
- Kałwak, K.; Porwolik, J.; Mielcarek, M.; Gorczyńska, E.; Owoc-Lempach, J.; Ussowicz, M.; Dyla, A.; Musiał, J.; Paździor, D.; Turkiewicz, D.; et al. Higher CD34+ and CD3+ cell doses in the graft promote long-term survival, and have no impact on the incidence of severe acute or chronic graft-versus-host disease after in vivo T cell-depleted unrelated donor hematopoietic stem cell transplantation in children. Biol. Blood Marrow Transplant. 2010, 16, 1388–1401. [Google Scholar]
- Reddy, D.S.; Thompson, W.; Calderara, G. Molecular mechanisms of sex differences in epilepsy and seizure susceptibility in chemical, genetic and acquired epileptogenesis. Neurosci. Lett. 2021, 750, 135753. [Google Scholar] [CrossRef]
- Carlson, C.; Dugan, P.; Kirsch, H.E.; Friedman, D.; EPGP Investigators. Sex differences in seizure types and symptoms. Epilepsy Behav. 2014, 41, 103–108. [Google Scholar] [CrossRef]
- Fogarasi, A.; Tuxhorn, I.; Janszky, J.; Janszky, I.; Rásonyi, G.; Kelemen, A.; Halász, P. Age-dependent seizure semiology in temporal lobe epilepsy. Epilepsia 2007, 48, 1697–1702. [Google Scholar] [CrossRef]
- Greene, B.R.; Faul, S.; Marnane, W.; Lightbody, G.; Korotchikova, I.; Boylan, G.B. A comparison of quantitative EEG features for neonatal seizure detection. Clin. Neurophysiol. 2008, 119, 1248–1261. [Google Scholar] [CrossRef]
- Alharthi, M.K.; Moria, K.M.; Alghazzawi, D.M.; Tayeb, H.O. Epileptic Disorder Detection of Seizures Using EEG Signals. Sensors 2022, 22, 6592. [Google Scholar] [CrossRef]


| TUH EEG Files | CHB-MIT EEG Files | |
|---|---|---|
| Total file number | 184 | 127 |
| Total seizure duration(s) | 7602.0 | 11,117 |
| Total non-seizure duration(s) | 68,069.5 | 624,279.5 |
| Patient number | 184 | 22 |
| Age range | 1–20 | 1–22 |
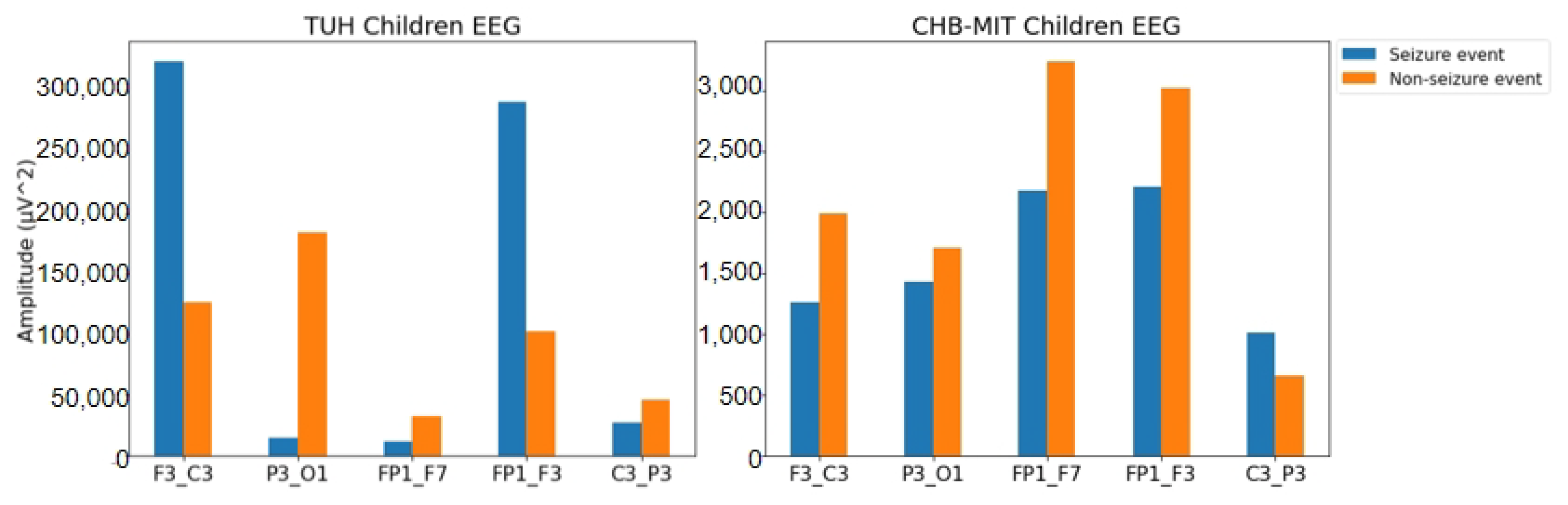
| F3-C3 | P3-O1 | FP1-F7 | FP1-F3 | C3-P3 | ||
|---|---|---|---|---|---|---|
| TUH | Seizure | 325,324.41 | 14,638.42 | 10,969.92 | 292,015.61 | 26,648.72 |
| Non-seizure | 125,824.93 | 183,742.85 | 31,594.45 | 102,562.60 | 45,582.13 | |
| CHB-MIT | Seizure | 1255.52 | 1422.89 | 2180.98 | 2209.52 | 1004.26 |
| Non-seizure | 1991.31 | 1707.90 | 3242.40 | 3021.55 | 657.07 |
| Reference | Method | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|---|
| [8] | CNN + LSTM | 30% | - | - | |
| [13] | Convolutional LSTM | 30% | - | - | |
| [9] | 2D CNN | 39.2% | 90.4% | - | |
| TUH | [10] | CNN + MLP | 31.58% | ||
| [11] | WaveNet + LSTM | 88.76% | |||
| [39] | CNN | - | - | 86.59% | |
| [15] | Random forest | 67.5% | 71.1% | - | |
| [40] | XGBoost | 20% | - | - | |
| [41] | KNN | 88% | 88% | 93% | |
| [42] | VGG16 | 85.94% | - | 85.41% | |
| CHB-MIT | [43] | SVM | 90.62% | 99.32% | - |
| [44] | Bi-LSTM | 93.61% | 91.85% | - | |
| [45] | Random forest | 93.60% | 93.37% | - |
| Dataset | Database | Sensitivity (%) | Specificity (%) | Accuracy (%) | Balanced Accuracy (%) | |
|---|---|---|---|---|---|---|
| Model 1 | Train | TUH Children | 95.18 | 96.85 | 96.68 | 96.01 |
| Validation | TUH Children | 94.97 | 96.47 | 96.32 | 95.72 | |
| Test | TUH Children | 73.15 | 95.72 | 98.68 | 86.15 | |
| Test | CHB-MIT | 58.82 | 62.15 | 62.09 | 60.48 | |
| Model 2 | Train | TUH Children | 95.55 | 96.93 | 96.79 | 96.24 |
| Validation | TUH Children | 94.89 | 96.53 | 96.37 | 95.71 | |
| Test | TUH Children | 75.07 | 99.25 | 98.82 | 87.16 | |
| Test | CHB-MIT | 62.31 | 57.56 | 57.65 | 59.93 | |
| Model 3 | Train | TUH Children | 95.51 | 96.91 | 96.77 | 96.21 |
| Validation | TUH Children | 95.23 | 96.57 | 96.44 | 95.90 | |
| Test | TUH Children | 74.28 | 99.40 | 98.95 | 86.84 | |
| Test | CHB-MIT | 59.08 | 64.92 | 64.82 | 62.00 | |
| Model 4 | Train | TUH Children | 95.57 | 97.05 | 96.90 | 96.31 |
| Validation | TUH Children | 95.20 | 96.69 | 96.54 | 95.95 | |
| Test | TUH Children | 73.50 | 99.39 | 98.93 | 86.45 | |
| Test | CHB-MIT | 59.58 | 62.81 | 62.76 | 61.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Mooney, C. Pediatric and Adolescent Seizure Detection: A Machine Learning Approach Exploring the Influence of Age and Sex in Electroencephalogram Analysis. BioMedInformatics 2024, 4, 796-810. https://doi.org/10.3390/biomedinformatics4010044
Wei L, Mooney C. Pediatric and Adolescent Seizure Detection: A Machine Learning Approach Exploring the Influence of Age and Sex in Electroencephalogram Analysis. BioMedInformatics. 2024; 4(1):796-810. https://doi.org/10.3390/biomedinformatics4010044
Chicago/Turabian StyleWei, Lan, and Catherine Mooney. 2024. "Pediatric and Adolescent Seizure Detection: A Machine Learning Approach Exploring the Influence of Age and Sex in Electroencephalogram Analysis" BioMedInformatics 4, no. 1: 796-810. https://doi.org/10.3390/biomedinformatics4010044
APA StyleWei, L., & Mooney, C. (2024). Pediatric and Adolescent Seizure Detection: A Machine Learning Approach Exploring the Influence of Age and Sex in Electroencephalogram Analysis. BioMedInformatics, 4(1), 796-810. https://doi.org/10.3390/biomedinformatics4010044