A Systems Biology- and Machine Learning-Based Study to Unravel Potential Therapeutic Mechanisms of Midostaurin as a Multitarget Therapy on FLT3-Mutated AML
Abstract
1. Introduction
2. Materials and Methods
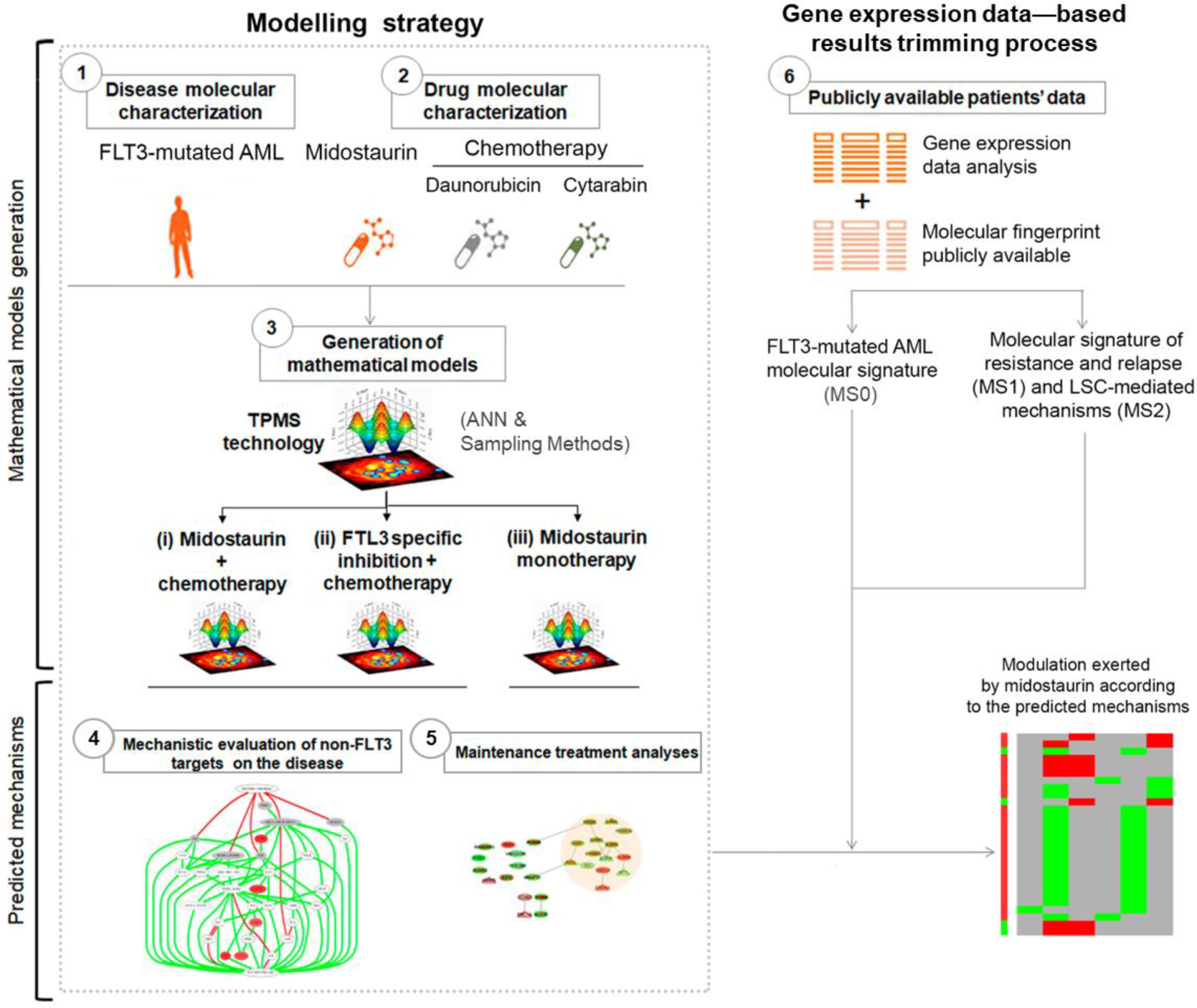
2.1. TPMS Technology: FLT3-Mutated AML Systems Biology-Based Model Creation
2.2. Molecular Characterization of FLT3-Mutated AML Pathophysiology and Drugs
2.3. TPMS Models’ Analysis
2.4. Gene Expression Data Collecting and Processing
2.5. Enrichment Analysis
3. Results
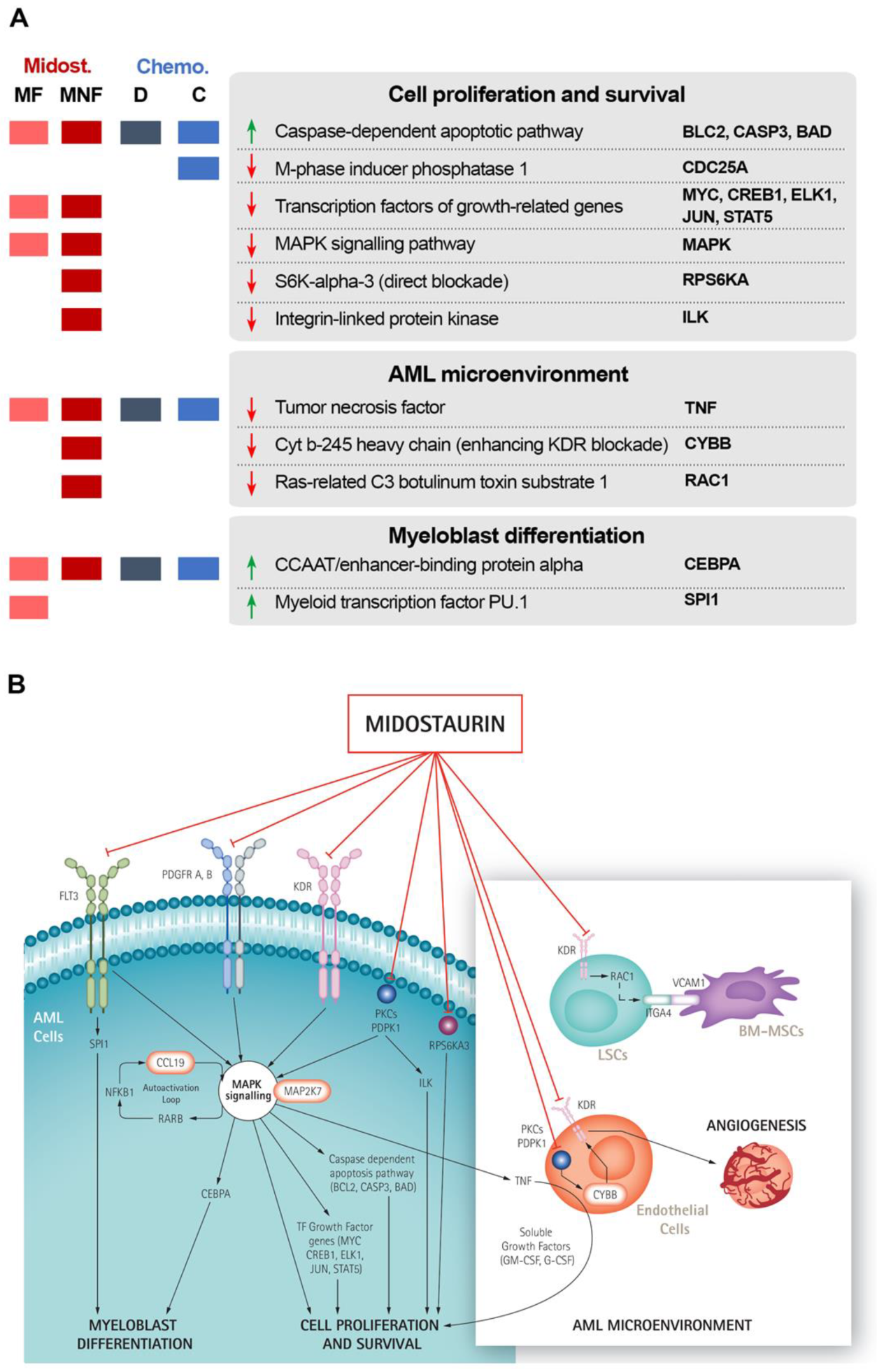
3.1. Several Non-FLT3 Midostaurin Targets May Play an Active Role in FLT3-Mutated AML
3.2. Midostaurin Targets May Act through Cell Proliferation and Survival, AML Microenvironment, and Myeloblast Diferentiation
3.3. Non-FLT3 Midostaurin Targets Affect FLT3-Mutated AML Mainly through Microenvironment Modulation
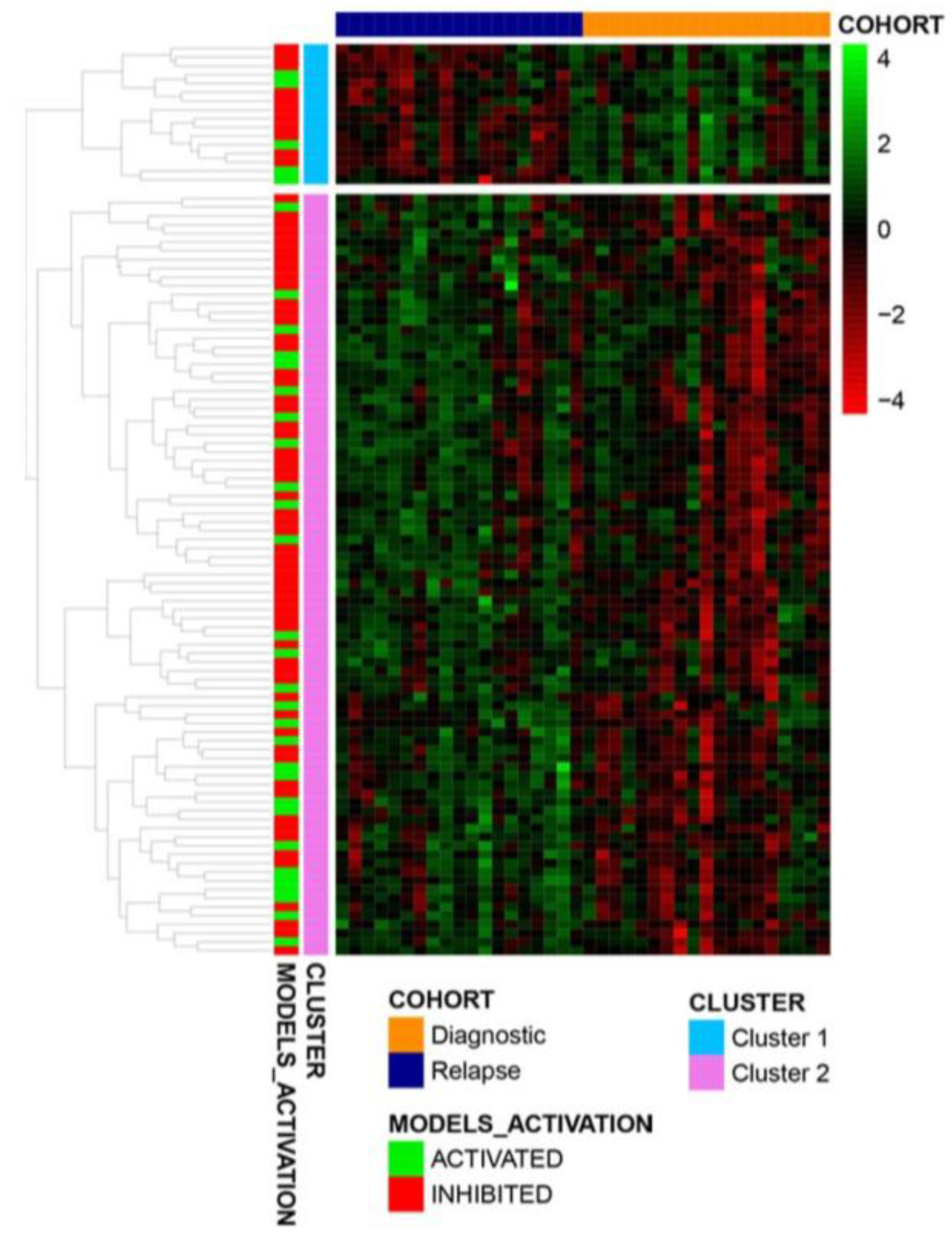
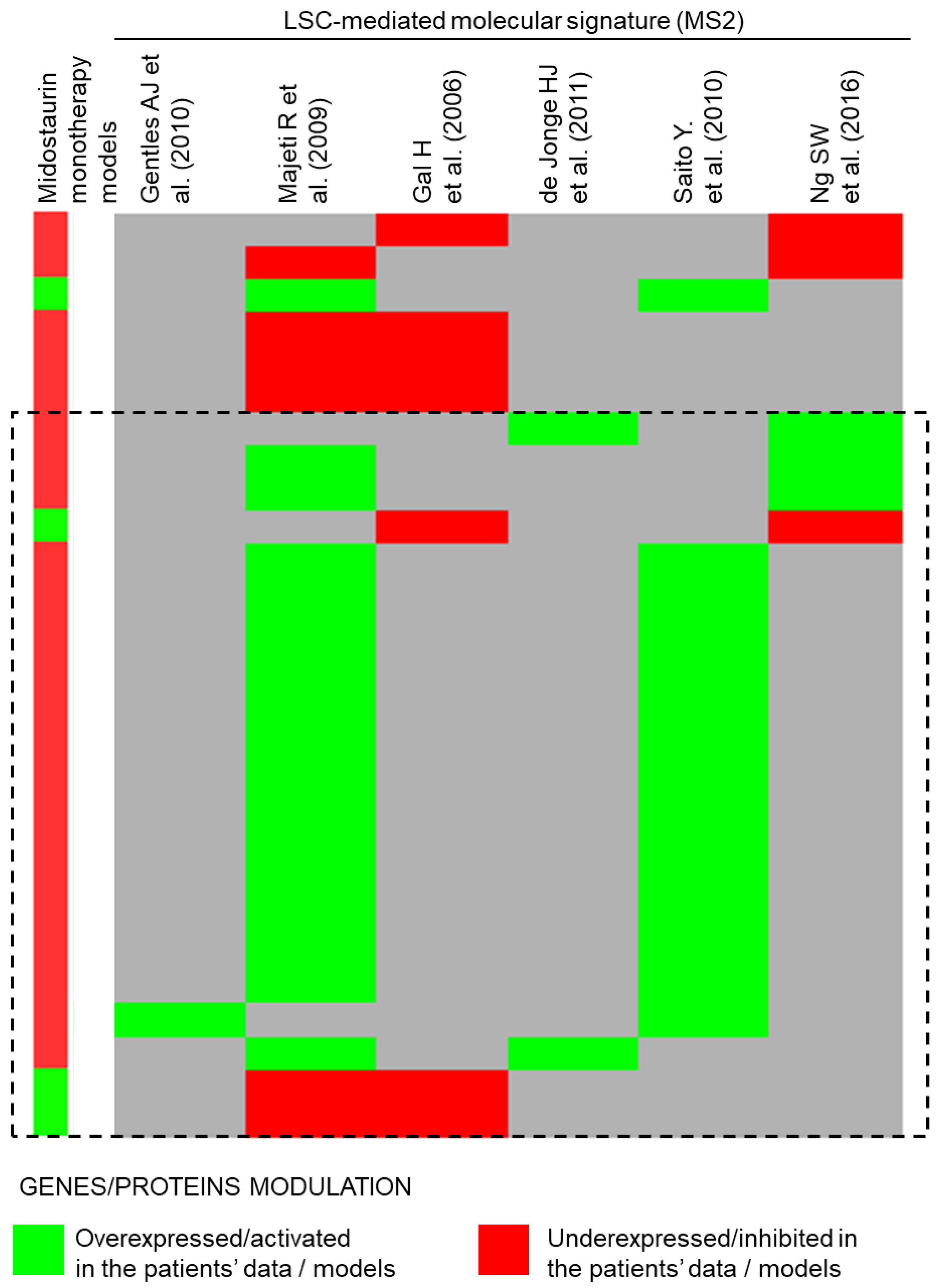
3.4. Midostaurin Monotherapy Predicted Potential Activity as Maintenance Therapy in FLT3-Mutated AML
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supplementary Methods
Appendix A1. TPMS Technology: Systems Biology-Based Model Creation and Analysis for FLT3-Mutated AML
Appendix A2. Creation of Human Biological Networks
Appendix A3. FLT3-Mutated AML Molecular Characterization
Appendix A4. MoA Mathematical Models Generation
Appendix A5. Artificial Neural Network Models Generation
References
- Dohner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Alfayez, M.; Kadia, T.; Ravandi-Kashani, F.; Daver, N. Midostaurin in Acute Myeloid Leukemia: An Evidence-Based Review And Patient Selection. Cancer Manag. Res. 2019, 11, 8817–8828. [Google Scholar] [CrossRef] [PubMed]
- Millward, M.J.; House, C.; Bowtell, D.; Webster, L.; Olver, I.N.; Gore, M.; Copeman, M.; Lynch, K.; Yap, A.; Wang, Y.; et al. The multikinase inhibitor midostaurin (PKC412A) lacks activity in metastatic melanoma: A phase IIA clinical and biologic study. Br. J. Cancer 2006, 95, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Virchis, A.; Ganeshaguru, K.; Hart, S.; Jones, D.; Fletcher, L.; Wright, F.; Wickremasinghe, R.; Man, A.; Csermak, K.; Meyer, T.; et al. A novel treatment approach for low grade lymphoproliferative disorders using PKC412 (CGP41251), an inhibitor of protein kinase C. Hematol. J. 2002, 3, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Group, C.P.S. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Investig. Ophthalmol. Vis. Sci. 2004, 45, 922–931. [Google Scholar] [CrossRef]
- Gotlib, J.; Kluin-Nelemans, H.C.; George, T.I.; Akin, C.; Sotlar, K.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Sternberg, D.W.; et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. N. Engl. J. Med. 2016, 374, 2530–2541. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Dohner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Short, N.J.; Kantarjian, H.; Ravandi, F.; Daver, N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther. Adv. Hematol. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Propper, D.J.; McDonald, A.C.; Man, A.; Thavasu, P.; Balkwill, F.; Braybrooke, J.P.; Caponigro, F.; Graf, P.; Dutreix, C.; Blackie, R.; et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J. Clin. Oncol. 2001, 19, 1485–1492. [Google Scholar] [CrossRef]
- Ikegami, Y.; Yano, S.; Nakao, K. Antitumor effect of CGP41251, a new selective protein kinase C inhibitor, on human non-small cell lung cancer cells. Jpn J. Pharmacol. 1996, 70, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Meshinchi, S.; Appelbaum, F.R. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin. Cancer Res. 2009, 15, 4263–4269. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.L.; Arenas, A.; Ortiz-Ruiz, A.; Leivas, A.; Rapado, I.; Rodriguez-Garcia, A.; Castro, N.; Zagorac, I.; Quintela-Fandino, M.; Gomez-Lopez, G.; et al. MEK inhibition enhances the response to tyrosine kinase inhibitors in acute myeloid leukemia. Sci. Rep. 2019, 9, 18630. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Levis, M.; Brown, P.; Kim, K.T.; Allebach, J.; Small, D. FLT3/ITD mutation signaling includes suppression of SHP-1. J. Biol. Chem. 2005, 280, 5361–5369. [Google Scholar] [CrossRef]
- Tabe, Y.; Konopleva, M. Role of Microenvironment in Resistance to Therapy in AML. Curr. Hematol. Malig. Rep. 2015, 10, 96–103. [Google Scholar] [CrossRef]
- Lamble, A.J.; Lind, E.F. Targeting the Immune Microenvironment in Acute Myeloid Leukemia: A Focus on T Cell Immunity. Front. Oncol. 2018, 8, 213. [Google Scholar] [CrossRef]
- Forte, D.; Krause, D.S.; Andreeff, M.; Bonnet, D.; Mendez-Ferrer, S. Updates on the hematologic tumor microenvironment and its therapeutic targeting. Haematologica 2019, 104, 1928–1934. [Google Scholar] [CrossRef]
- Cao, Z.; Ding, B.S.; Guo, P.; Lee, S.B.; Butler, J.M.; Casey, S.C.; Simons, M.; Tam, W.; Felsher, D.W.; Shido, K.; et al. Angiocrine factors deployed by tumor vascular niche induce B cell lymphoma invasiveness and chemoresistance. Cancer Cell 2014, 25, 350–365. [Google Scholar] [CrossRef]
- Jorba, G.; Aguirre-Plans, J.; Junet, V.; Segu-Verges, C.; Ruiz, J.L.; Pujol, A.; Fernandez-Fuentes, N.; Mas, J.M.; Oliva, B. In-silico simulated prototype-patients using TPMS technology to study a potential adverse effect of sacubitril and valsartan. PLoS ONE 2020, 15, e0228926. [Google Scholar] [CrossRef]
- Carcereny, E.; Fernandez-Nistal, A.; Lopez, A.; Montoto, C.; Naves, A.; Segu-Verges, C.; Coma, M.; Jorba, G.; Oliva, B.; Mas, J.M. Head to head evaluation of second generation ALK inhibitors brigatinib and alectinib as first-line treatment for ALK+ NSCLC using an in silico systems biology-based approach. Oncotarget 2021, 12, 316–332. [Google Scholar] [CrossRef]
- Romeo-Guitart, D.; Fores, J.; Herrando-Grabulosa, M.; Valls, R.; Leiva-Rodriguez, T.; Galea, E.; Gonzalez-Perez, F.; Navarro, X.; Petegnief, V.; Bosch, A.; et al. Neuroprotective Drug for Nerve Trauma Revealed Using Artificial Intelligence. Sci. Rep. 2018, 8, 1879. [Google Scholar] [CrossRef] [PubMed]
- Artigas, L.; Coma, M.; Matos-Filipe, P.; Aguirre-Plans, J.; Farres, J.; Valls, R.; Fernandez-Fuentes, N.; de la Haba-Rodriguez, J.; Olvera, A.; Barbera, J.; et al. In-silico drug repurposing study predicts the combination of pirfenidone and melatonin as a promising candidate therapy to reduce SARS-CoV-2 infection progression and respiratory distress caused by cytokine storm. PLoS ONE 2020, 15, e0240149. [Google Scholar] [CrossRef] [PubMed]
- Pierre Collet, J.-P.R. Stochastic Optimization Algorithms. In Intelligent Information Technologies: Concepts, Methodologies, Tools, and Applications; Sugumaran, V., Ed.; IGI Global: Hershey, PA, USA, 2008; pp. 1121–1137. [Google Scholar] [CrossRef]
- Iborra-Egea, O.; Galvez-Monton, C.; Roura, S.; Perea-Gil, I.; Prat-Vidal, C.; Soler-Botija, C.; Bayes-Genis, A. Mechanisms of action of sacubitril/valsartan on cardiac remodeling: A systems biology approach. NPJ Syst. Biol. Appl. 2017, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Meltzer, P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. limma: Linear Models for Microarray Data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Statistics for Biology and Health; Gentleman, R., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling The False Discovery Rate—A Practical And Powerful Approach To Multiple Testing. J. Royal Statist. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Stirewalt, D.L.; Meshinchi, S.; Kopecky, K.J.; Fan, W.; Pogosova-Agadjanyan, E.L.; Engel, J.H.; Cronk, M.R.; Dorcy, K.S.; McQuary, A.R.; Hockenbery, D.; et al. Identification of genes with abnormal expression changes in acute myeloid leukemia. Genes Chromosomes Cancer 2008, 47, 8–20. [Google Scholar] [CrossRef]
- Li, S.; Garrett-Bakelman, F.E.; Chung, S.S.; Sanders, M.A.; Hricik, T.; Rapaport, F.; Patel, J.; Dillon, R.; Vijay, P.; Brown, A.L.; et al. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat. Med. 2016, 22, 792–799. [Google Scholar] [CrossRef]
- Shlush, L.I.; Mitchell, A.; Heisler, L.; Abelson, S.; Ng, S.W.K.; Trotman-Grant, A.; Medeiros, J.J.F.; Rao-Bhatia, A.; Jaciw-Zurakowsky, I.; Marke, R.; et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 2017, 547, 104–108. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Ng, S.W.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef]
- Eppert, K.; Takenaka, K.; Lechman, E.R.; Waldron, L.; Nilsson, B.; van Galen, P.; Metzeler, K.H.; Poeppl, A.; Ling, V.; Beyene, J.; et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat. Med. 2011, 17, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Plevritis, S.K.; Majeti, R.; Alizadeh, A.A. Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA 2010, 304, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Majeti, R.; Becker, M.W.; Tian, Q.; Lee, T.L.; Yan, X.; Liu, R.; Chiang, J.H.; Hood, L.; Clarke, M.F.; Weissman, I.L. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3396–3401. [Google Scholar] [CrossRef]
- Yang, X.H.; Li, M.; Wang, B.; Zhu, W.; Desgardin, A.; Onel, K.; de Jong, J.; Chen, J.; Chen, L.; Cunningham, J.M. Systematic computation with functional gene-sets among leukemic and hematopoietic stem cells reveals a favorable prognostic signature for acute myeloid leukemia. BMC Bioinform. 2015, 16, 97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gal, H.; Amariglio, N.; Trakhtenbrot, L.; Jacob-Hirsh, J.; Margalit, O.; Avigdor, A.; Nagler, A.; Tavor, S.; Ein-Dor, L.; Lapidot, T.; et al. Gene expression profiles of AML derived stem cells; similarity to hematopoietic stem cells. Leukemia 2006, 20, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.; Yoshida, S.; Saito, Y.; Hijikata, A.; Kitamura, H.; Tanaka, S.; Nakamura, R.; Tanaka, T.; Tomiyama, H.; Saito, N.; et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat. Biotechnol. 2007, 25, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, H.J.; Woolthuis, C.M.; Vos, A.Z.; Mulder, A.; van den Berg, E.; Kluin, P.M.; van der Weide, K.; de Bont, E.S.; Huls, G.; Vellenga, E.; et al. Gene expression profiling in the leukemic stem cell-enriched CD34+ fraction identifies target genes that predict prognosis in normal karyotype AML. Leukemia 2011, 25, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Kitamura, H.; Hijikata, A.; Tomizawa-Murasawa, M.; Tanaka, S.; Takagi, S.; Uchida, N.; Suzuki, N.; Sone, A.; Najima, Y.; et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci. Transl. Med. 2010, 2, 17ra19. [Google Scholar] [CrossRef]
- Rivals, I.; Personnaz, L.; Taing, L.; Potier, M.C. Enrichment or depletion of a GO category within a class of genes: Which test? Bioinformatics 2007, 23, 401–407. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- UniProt, C. UniProt: A hub for protein information. Nucleic Acids Res 2015, 43, D204–D212. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.; Knox, C.; Lim, E.; Jewison, T.; Law, V.; Hau, D.D.; Liu, P.; Gautam, B.; Ly, S.; Guo, A.C.; et al. SMPDB: The Small Molecule Pathway Database. Nucleic Acids Res. 2010, 38, D480–D487. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Shim, H.; Shin, D.; Shim, J.E.; Ko, Y.; Shin, J.; Kim, H.; Cho, A.; Kim, E.; Lee, T.; et al. TRRUST: A reference database of human transcriptional regulatory interactions. Sci. Rep. 2015, 5, 11432. [Google Scholar] [CrossRef] [PubMed]
- Boros, K.; Puissant, A.; Back, M.; Alexe, G.; Bassil, C.F.; Sinha, P.; Tholouli, E.; Stegmaier, K.; Byers, R.J.; Rodig, S.J. Increased SYK activity is associated with unfavorable outcome among patients with acute myeloid leukemia. Oncotarget 2015, 6, 25575–25587. [Google Scholar] [CrossRef]
- Liu, D.; Mamorska-Dyga, A. Syk inhibitors in clinical development for hematological malignancies. J. Hematol. Oncol. 2017, 10, 145. [Google Scholar] [CrossRef]
- Puissant, A.; Fenouille, N.; Alexe, G.; Pikman, Y.; Bassil, C.F.; Mehta, S.; Du, J.; Kazi, J.U.; Luciano, F.; Ronnstrand, L.; et al. SYK is a critical regulator of FLT3 in acute myeloid leukemia. Cancer Cell 2014, 25, 226–242. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef]
- Bar-Sagi, D.; Hall, A. Ras and Rho GTPases: A family reunion. Cell 2000, 103, 227–238. [Google Scholar] [CrossRef]
- Aspuria, P.J.; Tamanoi, F. The Rheb family of GTP-binding proteins. Cell Signal 2004, 16, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Korn, C.; Mendez-Ferrer, S. Myeloid malignancies and the microenvironment. Blood 2017, 129, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Walter, H.S.; Dyer, M.J.S. Targeting anti-apoptotic BCL2 family proteins in haematological malignancies—From pathogenesis to treatment. Br. J. Haematol. 2017, 178, 364–379. [Google Scholar] [CrossRef]
- Carter, J.L.; Hege, K.; Yang, J.; Kalpage, H.A.; Su, Y.; Edwards, H.; Huttemann, M.; Taub, J.W.; Ge, Y. Targeting multiple signaling pathways: The new approach to acute myeloid leukemia therapy. Signal Transduct. Target Ther. 2020, 5, 288. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, A.M.; Sun, Y.; Hellmich, C.; Marlein, C.R.; Mistry, J.; Forde, E.; Piddock, R.E.; Shafat, M.S.; Morfakis, A.; Mehta, T.; et al. Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood 2019, 133, 446–456. [Google Scholar] [CrossRef]
- Ghiaur, G.; Levis, M. Mechanisms of Resistance to FLT3 Inhibitors and the Role of the Bone Marrow Microenvironment. Hematol. Oncol. Clin. N. Am. 2017, 31, 681–692. [Google Scholar] [CrossRef]
- Rashidi, A.; Uy, G.L. Targeting the microenvironment in acute myeloid leukemia. Curr. Hematol. Malig. Rep. 2015, 10, 126–131. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Zhao, R.C. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 2014, 7, 14. [Google Scholar] [CrossRef]
- Li, P.; Ji, M.; Park, J.; Bunting, K.D.; Ji, C.; Tse, W. Th17 related cytokines in acute myeloid leukemia. Front. Biosci. 2012, 17, 2284–2294. [Google Scholar] [CrossRef]
- Hou, D.; Wang, B.; You, R.; Wang, X.; Liu, J.; Zhan, W.; Chen, P.; Qin, T.; Zhang, X.; Huang, H. Stromal cells promote chemoresistance of acute myeloid leukemia cells via activation of the IL-6/STAT3/OXPHOS axis. Ann. Transl. Med. 2020, 8, 1346. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, H.J.; Konopleva, M. Targeting the CXCL12/CXCR4 axis in acute myeloid leukemia: From bench to bedside. Korean J. Intern. Med. 2017, 32, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Weber, D.; Fiedler, W.; Salih, H.R.; Wulf, G.; Salwender, H.; Schroeder, T.; Kindler, T.; Lubbert, M.; Wolf, D.; et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood 2019, 133, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Manley, P.W.; Caravatti, G.; Furet, P.; Roesel, J.; Tran, P.; Wagner, T.; Wartmann, M. Comparison of the Kinase Profile of Midostaurin (Rydapt) with That of Its Predominant Metabolites and the Potential Relevance of Some Newly Identified Targets to Leukemia Therapy. Biochemistry 2018, 57, 5576–5590. [Google Scholar] [CrossRef] [PubMed]
- Bayes-Genis, A.; Iborra-Egea, O.; Spitaleri, G.; Domingo, M.; Revuelta-Lopez, E.; Codina, P.; Cediel, G.; Santiago-Vacas, E.; Cserkoova, A.; Pascual-Figal, D.; et al. Decoding empagliflozin’s molecular mechanism of action in heart failure with preserved ejection fraction using artificial intelligence. Sci. Rep. 2021, 11, 12025. [Google Scholar] [CrossRef] [PubMed]
- Loren, V.; Garcia-Jaraquemada, A.; Naves, J.E.; Carmona, X.; Manosa, M.; Aransay, A.M.; Lavin, J.L.; Sanchez, I.; Cabre, E.; Manye, J.; et al. ANP32E, a Protein Involved in Steroid-Refractoriness in Ulcerative Colitis, Identified by a Systems Biology Approach. J. Crohns. Colitis 2019, 13, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Croft, D.; Mundo, A.F.; Haw, R.; Milacic, M.; Weiser, J.; Wu, G.; Caudy, M.; Garapati, P.; Gillespie, M.; Kamdar, M.R.; et al. The Reactome pathway knowledgebase. Nucleic Acids Res. 2014, 42, D472–D477. [Google Scholar] [CrossRef]
- Orchard, S.; Ammari, M.; Aranda, B.; Breuza, L.; Briganti, L.; Broackes-Carter, F.; Campbell, N.H.; Chavali, G.; Chen, C.; del-Toro, N.; et al. The MIntAct project--IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 2014, 42, D358–D363. [Google Scholar] [CrossRef]
- Salwinski, L.; Licata, L.; Winter, A.; Thorneycroft, D.; Khadake, J.; Ceol, A.; Aryamontri, A.C.; Oughtred, R.; Livstone, M.; Boucher, L.; et al. Recurated protein interaction datasets. Nat. Methods 2009, 6, 860–861. [Google Scholar] [CrossRef]
- Keshava Prasad, T.S.; Goel, R.; Kandasamy, K.; Keerthikumar, S.; Kumar, S.; Mathivanan, S.; Telikicherla, D.; Raju, R.; Shafreen, B.; Venugopal, A.; et al. Human Protein Reference Database—2009 update. Nucleic Acids Res. 2009, 37, D767–D772. [Google Scholar] [CrossRef]
- Segu-Verges, C.; Coma, M.; Kessel, C.; Smeets, S.; Foell, D.; Aldea, A. Application of systems biology-based in silico tools to optimize treatment strategy identification in Still’s disease. Arthritis Res. Ther. 2021, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006. [Google Scholar]
- Kirkpatrick, S.; Gelatt, C.D., Jr.; Vecchi, M.P. Optimization by simulated annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.a.P.N. Artificial Intelligence: A Modern Approach.; Prentice Hall Press: Hoboken, NJ, USA, 2009. [Google Scholar]
- Anellis, I.H. Peirce’s Truth-functional Analysis and the Origin of the Truth Table. Hist. Philos. Logic 2012, 33, 87–97. [Google Scholar] [CrossRef]
- Kuhn, M.; Letunic, I.; Jensen, L.J.; Bork, P. The SIDER database of drugs and side effects. Nucleic Acids Res. 2016, 44, D1075–D1079. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]

| Gene Symbol | Uniprot ID | Models’ Mean Signal Values | T Test | Classification Analysis | MS0-FLT3- Mutated AML vs. Healthy Samples | ||
|---|---|---|---|---|---|---|---|
| Midostaurin + Chemotherapy | FLT3-Specific Target Inhibition + Chemotherapy | Adj. p-Value | ACC | CrossVal p-Value | Differential Gene Expression (LogFC) | ||
| CYBB | P04839 | −1 | 0 | <0.05 | 100 | <0.01 | 0.07 |
| GNAO1 | P09471 | −0.89 | 0 | <0.05 | 99.99 | <0.01 | 0.09 |
| MAP2K7 | O14733 | −0.97 | 0 | <0.05 | 99.98 | <0.01 | 0.03 |
| GNG4 | P50150 | −0.87 | 0 | <0.05 | 100 | <0.01 | 0.17 |
| GNGT1 | P63211 | −0.87 | 0 | <0.05 | 100 | <0.01 | 0.3 |
| GNG3 | P63215 | −0.87 | 0 | <0.05 | 100 | <0.01 | 0.07 |
| RHEB | Q15382 | −0.98 | 0 | <0.05 | 100 | <0.01 | 0.1 |
| RASGRP3 | Q8IV61 | −0.75 | 0 | <0.05 | 99.99 | <0.01 | 1.31 |
| NLRP3 | Q96P20 | −0.78 | 0 | <0.05 | 99,99 | <0.01 | 1.21 |
| CCL19 | Q99731 | −0.86 | 0 | <0.05 | 99.98 | <0.01 | 0.13 |
| GNG13 | Q9P2W3 | −0.87 | 0 | <0.05 | 100 | <0.01 | 0.1 |
| GNG12 | Q9UBI6 | −0.89 | 0 | <0.05 | 99.99 | <0.01 | 0.1 |
| HMGCR | P04035 | 1 | 0 | <0.05 | 100 | <0.01 | −0.93 |
| Midostaurin Monotherapy | Midostaurin + Chemotherapy | FLT3-Specific Target Inhibition + Chemotherapy | Improvement by Non-FLT3 Targets | |
|---|---|---|---|---|
| (A) FTL3-AML molecular definition (whole disease) | +++ | +++ | ++ | yes |
| (B) FTL3-AML pathophysiological motives | ||||
| Epigenetic dysregulation in AML | + | + | + | no |
| Genetic predisposition to AML | + | ++ | ++ | no |
| Immunophenotypic features of AML | + | + | + | no |
| Impaired myeloblast differentiation in AML | + | + | + | no |
| Increased proliferation and survival mediated by FLT3 | +++ | +++ | +++ | no |
| Microenvironment influence in AML | +++ | +++ | + | yes |
| Gene Symbol | UniProt ID | Models’ Mean Signal Values | T Test | Classification Analysis | Protein Activation State in AML Microenvironment | ||
|---|---|---|---|---|---|---|---|
| Midostaurin + Chemotherapy | FLT3-Specific Target Inhibition + Chemotherapy | Adj. p-Value | ACC | CrossVal p-Value | |||
| TNF | P01375 | −0.97 | 0.13 | <0.05 | 91.25 | <0.01 | 1 |
| IL6 | P05231 | −0.82 | 0.26 | <0.05 | 79.66 | <0.01 | 1 |
| CXCR4 | P61073 | −0.89 | −0.11 | <0.05 | 71.61 | <0.01 | 1 |
| Gene Symbol | UniProt ID | Midostaurin Monotherapy Model Mean Signal Values | Differential Gene Expression | |
|---|---|---|---|---|
| Log2FC | Adj. p-Value | |||
| PIK3R5 | Q8WYR1 | −1.00 | 0.95 | 0.04 |
| IL4R | P24394 | −1.00 | 1.29 | 0.02 |
| RXRA | P19793 | −0.38 | 1.95 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Beyá, M.; García-Fortes, M.; Valls, R.; Artigas, L.; Gómez-Casares, M.T.; Montesinos, P.; Sánchez-Guijo, F.; Coma, M.; Vendranes, M.; Martínez-López, J. A Systems Biology- and Machine Learning-Based Study to Unravel Potential Therapeutic Mechanisms of Midostaurin as a Multitarget Therapy on FLT3-Mutated AML. BioMedInformatics 2022, 2, 375-397. https://doi.org/10.3390/biomedinformatics2030024
Díaz-Beyá M, García-Fortes M, Valls R, Artigas L, Gómez-Casares MT, Montesinos P, Sánchez-Guijo F, Coma M, Vendranes M, Martínez-López J. A Systems Biology- and Machine Learning-Based Study to Unravel Potential Therapeutic Mechanisms of Midostaurin as a Multitarget Therapy on FLT3-Mutated AML. BioMedInformatics. 2022; 2(3):375-397. https://doi.org/10.3390/biomedinformatics2030024
Chicago/Turabian StyleDíaz-Beyá, Marina, María García-Fortes, Raquel Valls, Laura Artigas, Mª Teresa Gómez-Casares, Pau Montesinos, Fermín Sánchez-Guijo, Mireia Coma, Meritxell Vendranes, and Joaquín Martínez-López. 2022. "A Systems Biology- and Machine Learning-Based Study to Unravel Potential Therapeutic Mechanisms of Midostaurin as a Multitarget Therapy on FLT3-Mutated AML" BioMedInformatics 2, no. 3: 375-397. https://doi.org/10.3390/biomedinformatics2030024
APA StyleDíaz-Beyá, M., García-Fortes, M., Valls, R., Artigas, L., Gómez-Casares, M. T., Montesinos, P., Sánchez-Guijo, F., Coma, M., Vendranes, M., & Martínez-López, J. (2022). A Systems Biology- and Machine Learning-Based Study to Unravel Potential Therapeutic Mechanisms of Midostaurin as a Multitarget Therapy on FLT3-Mutated AML. BioMedInformatics, 2(3), 375-397. https://doi.org/10.3390/biomedinformatics2030024







