The Experiences of Older Adults with Dementia of “Balance Wise”—An Individual or Group-Delivered Exercise Programme: A Qualitative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Researcher Reflexivity
2.2. Participants
2.3. Recruitment
2.4. Intervention: Balance Wise
2.5. Data Collection
2.5.1. Demographic Data
2.5.2. Interviews
2.5.3. Data Analysis
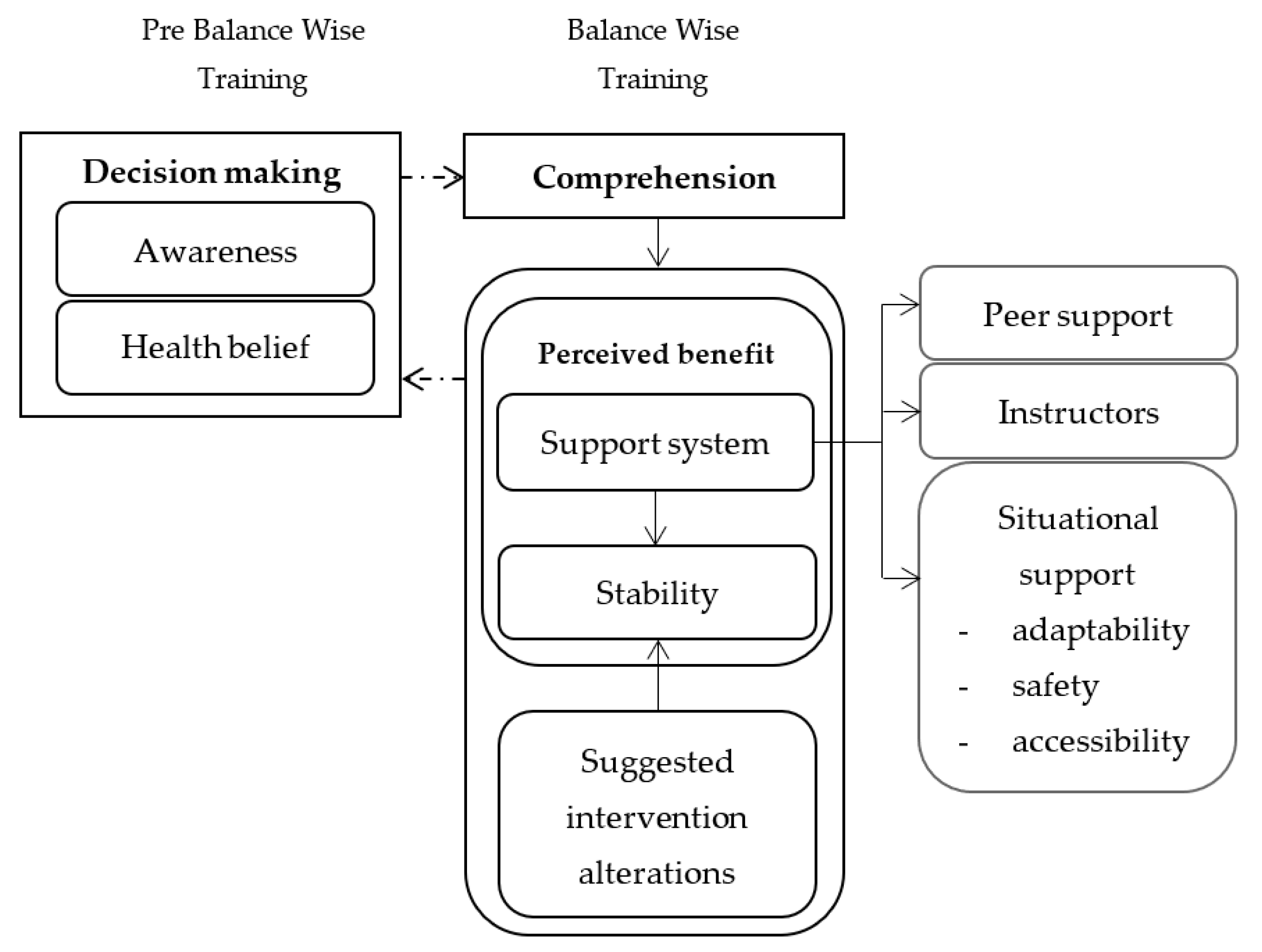
3. Results
3.1. Linkage between Themes
3.2. Decision Making
I used to go downstairs sideways, one foot at a time. But now I go down normally, but it requires more concentration now than it used to. So, from that point of view, yes. I suppose I always have concentrated, but now I realise I must concentrate a bit more. (Wilson).
If you get on the bicycle, and put the load on, pretending [cycling] up hill, putting stress on [muscle], that’s good. The same [thing] when you do stepping. You do the stepping [similar to walking] up hill, because of the slope, it [uses a lot of] energy, and you have to work at it. No pain, no gain. (Dane).
I thought ‘well, we’ll give that a try.’ It’s reasonable to say that [Zain] doesn’t make those decisions. I do, to try and keep him going. And since then, walking’s got a bit easier for you, hasn’t it, for you, since you’ve been coming to the programme. (Joana, caregiver).
3.3. Comprehension
Because you’ve got to think—they’ve got to try and think about what they’re doing. And I think it might help coordination, it might help, well, balance in particular, I think that’s what the biggest problem is for older people. They lose their balance, and I think that meant, yeah, those exercise programmes on the walkways and things I think would probably help quite significantly [challenging] for quite a few of them. (Joana, caregiver).
3.4. Perceived Benefits
3.4.1. Support System
Peer Support
Well, I enjoy the social side of it. I [found the activities were] pretty easy. [I] enjoyed and [I] like the people, very much. [The group had] a great mixture of both in personalities and the [cognitive] stage they’re at. (Hansen).
I really like the class; I met some nice people. A couple of really nice women, that I think they have asked for my contact details. I think we will keep in touch. So that was a good aspect of it. (Cole, caregiver).
Instructors’ Support
So yes, but it’s been good seeing how you handle other people. And trying to set them on the right road if they’re not doing it correctly–that’s a difficult one. [A] challenging one, actually. (Alia).
I think sometimes clear explanation beforehand, with a visual demonstration. But that might be just me, I don’t know. I think it’s good when there’s two of you because one of you is watching while the other one’s demonstrating, I think that’s probably very good, because you can see who needs a bit of help. (Megan, caregiver).
Situational Support (Adaptability, Safety, and Accessibility)
I think I [am aware] and I have to concentrate a little bit more on my balance. For instance, recently at Papatowai, going down [the trail] was very steep and [Jim] said ‘remember your exercise’, and I [walked] down without any problems. It was a real test. (Julie).
You have to think up something a little bit more strenuous on the mind or strenuous on the feet, to be able to dissociate the two, so that you did tend to lose your balance, although you can’t have people falling down all over the place. (Wilson).
Doing it in a group was probably more fun, but I was a bit restricted with time, in that I’m doing a lot of other things as well. And I just felt if I did it at home, it would be more conscientious effort on my part. Without wasting time coming and going. (Julie).
Well, he just didn’t really want to participate, but I encouraged him to continue the course. I say if you start something you [have to] finish it. But it worked out all right in the end. (Megan, caregiver).
3.4.2. Stability
I think the [activities that] associated with balance. There were mainly—some of those were on foam, and some were without. I think the balance [activities], it’s not only [that activity] involving the muscle, [it also involving] the brain and the sensory organs. That’s what I think is the most interesting part. (Dane).
Well, I know already that [exercise] helped with my walking, my balance, and [while I am] getting dressed. I have mentioned to you that I can now stand on one leg and put my trousers on. (Sue).
3.4.3. Suggested Intervention Alterations
I mean throwing somethings to one [and] another, but it’s probably a little bit lacking in [the exercise programme], [such as] an activity that involves coordination between individuals rather than coordination between hand and eye, in one individual. But coordination between- in other words, the unexpected, dealing with the unexpected. (Wilson).
You are thinking as well as physically doing something, and you [are] combining the two. You know, could have been much better if I would [have] been counting forward, but that would be too easy, wouldn’t it? (Leslie).
It is a hard [to achieve] balance, between systematically, repetitively, doing the same thing, and making it challenging and different. I think there was a balance there, but it needs to have a pattern for people who can pick that up and realise [each of the exercise]. It is because they [have difficulties in] memory, but they will still remember [the exercises] as they go, [and] at the same time you have to stimulate their interest. (Cole, caregiver).
He gets tired easily and he [takes a] rest most [of the] afternoons, because [he needs to] concentrate [while doing] something on his own, [such as] a code cracker and he rests for a while before continue doing his task. So, I guess, if [the exercise] is an hour, there’s [would] have to be spaces for rest where there was sitting time. (Megan, caregiver).
3.5. Safety and Adverse Events
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Harmer, P.; Eckstrom, E.; Ainsworth, B.E.; Fitzgerald, K.; Voit, J.; Chou, L.-S.; Welker, F.L.; Needham, S. Efficacy of exercise-based interventions in preventing falls among community-dwelling older persons with cognitive impairment: Is there enough evidence? An updated systematic review and meta-analysis. Age Ageing 2021, 50, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.d.M.; Radanovic, M.; Forlenza, O.V. Fear of falling and falls in older adults with mild cognitive impairment and Alzheimer’s disease. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2015, 22, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Speechley, M. Falls in cognitively impaired older adults: Implications for risk assessment and prevention. J. Am. Geriatr. Soc. 2018, 66, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Mesbah, N.; Perry, M.; Hill, K.D.; Kaur, M.; Hale, L. Postural stability in older adults with Alzheimer Disease. Phys. Therapy 2017, 97, 290–309. [Google Scholar]
- Fogg, C.; Griffiths, P.; Meredith, P.; Bridges, J. Hospital outcomes of older people with cognitive impairment: An integrative review. Int. J. Geriatr. Psychiatry 2018, 33, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Uriz-Otano, F.; Uriz-Otano, J.I.; Malafarina, V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence of cognitive impairment. J. Am. Med. Dir. Assoc. 2015, 16, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Shirooka, H.; Nishiguchi, S.; Fukutani, N.; Tashiro, Y.; Nozaki, Y.; Hirata, H.; Yamaguchi, M.; Tasaka, S.; Matsushita, T.; Matsubara, K.; et al. Cognitive impairment is associated with the absence of fear of falling in community-dwelling frail older adults. Geriatr. Gerontol. Int. 2017, 17, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, CD012424. [Google Scholar] [CrossRef]
- Lam, F.M.; Huang, M.Z.; Liao, L.R.; Chung, R.C.; Kwok, T.C.; Pang, M.Y. Physical exercise improves strength, balance, mobility, and endurance in people with cognitive impairment and dementia: A systematic review. J. Physiother. 2018, 64, 4–15. [Google Scholar] [CrossRef]
- Adzhar, M.A.; Manlapaz, D.; Singh, D.K.A.; Mesbah, N. Exercise to improve postural stability in older adults with Alzheimer’s disease: A systematic review of randomized control trials. Int. J. Environ. Res. Public Health 2022, 19, 10350. [Google Scholar] [CrossRef]
- Di Lorito, C.; Bosco, A.; Booth, V.; Goldberg, S.; Harwood, R.H.; Van der Wardt, V. Adherence to exercise interventions in older people with mild cognitive impairment and dementia: A systematic review and meta-analysis. Prev. Med. Rep. 2020, 19, 101139. [Google Scholar] [CrossRef]
- Taylor, M.E.; Lord, S.R.; Brodaty, H.; Kurrle, S.E.; Hamilton, S.; Ramsay, E.; Webster, L.; Payne, N.L.; Close, J.C. A home-based, carer-enhanced exercise program improves balance and falls efficacy in community-dwelling older people with dementia. Int. Psychogeriatr. 2017, 29, 81–91. [Google Scholar] [CrossRef]
- Hancox, J.E.; van der Wardt, V.; Pollock, K.; Booth, V.; Vedhara, K.; Harwood, R.H. Factors influencing adherence to home-based strength and balance exercises among older adults with mild cognitive impairment and early dementia: Promoting Activity, Independence and Stability in Early Dementia (PrAISED). PLoS ONE 2019, 14, e0217387. [Google Scholar] [CrossRef]
- Malthouse, R.; Fox, F. Exploring experiences of physical activity among people with Alzheimer’s disease and their spouse carers: A qualitative study. Physiotherapy 2014, 100, 169–175. [Google Scholar] [CrossRef]
- Hale, L.A.; Mayland, B.; Jenkins, M.L.; Buttery, Y.; Norris, P.; Butler, M.; Holland, M.; Ngocha-Chaderopa, E.; McKenzie-Green, B.; Czuba, K.; et al. Constructing normalcy in dementia care: Carers’ perceptions of their roles and the supports they need. Gerontologist 2020, 60, 905–915. [Google Scholar] [CrossRef]
- Sheehan, O.C.; Haley, W.E.; Howard, V.J.; Huang, J.; Rhodes, J.D.; Roth, D.L. Stress, burden, and well-being in dementia and nondementia caregivers: Insights from the caregiving transitions study. Gerontologist 2021, 61, 670–679, Erratum in Gerontologist 2021, 61, 804. [Google Scholar] [CrossRef]
- Brodaty, H.; Donkin, M. Family caregivers of people with dementia. Dialogues Clin. Neurosci. 2009, 11, 217–228. [Google Scholar] [CrossRef]
- Riffin, C.; Van Ness, P.H.; Wolff, J.L.; Fried, T. Family and other unpaid caregivers and older adults with and without dementia and disability. J. Am. Geriatr. Soc. 2017, 65, 1821–1828, Erratum in J. Am. Geriatr. Soc. 2017, 65, 2549.. [Google Scholar] [CrossRef]
- Cheng, S.T. Dementia caregiver burden: A research update and critical analysis. Curr. Psychiatry Rep. 2017, 19, 64. [Google Scholar] [CrossRef]
- Forbes, D.; Forbes, S.C.; Blake, C.M.; Thiessen, E.J.; Forbes, S. Exercise programs for people with dementia. Cochrane Database Syst. Rev. 2015, 4, CD006489. [Google Scholar] [CrossRef]
- Mesbah, N.; Perry, M.; Hale, L.; Hill, K.D.; Wilkinson, A. Perspectives of people with mild to moderate cognitive impairment and their caregivers about physical activity and exercise for fall prevention: A qualitative study. Disabilities 2023, 3, 255–268. [Google Scholar] [CrossRef]
- Campbell, A.J.; Robertson, M.C.; Gardner, M.M.; Norton, R.N.; Tilyard, M.W.; Buchner, D.M. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997, 315, 1065–1069. [Google Scholar] [CrossRef]
- Thomas, S.; Mackintosh, S.; Halbert, J. Does the ‘Otago exercise programme’ reduce mortality and falls in older adults?: A systematic review and meta-analysis. Age Ageing 2010, 39, 681–687. [Google Scholar] [CrossRef]
- Waters, D.; Hale, L.; Hale, B.; Robertson, L.; Herbison, P. Evaluation of a peer-led falls prevention program for older adults. Arch. Phys. Med. Rehabil. 2011, 92, 1581–1586. [Google Scholar] [CrossRef]
- Suttanon, P.; Hill, K.D.; Said, C.M.; Williams, S.B.; Byrne, K.N.; LoGiudice, D.; Lautenschlager, N.T.; Dodd, K.J. Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer’s disease: A pilot randomized controlled trial. Clin. Rehabil. 2013, 27, 427–438. [Google Scholar] [CrossRef]
- Tayabas, L.M.T.; León, T.C.; Espino, J.M. Qualitative evaluation: A critical and interpretative complementary approach to improve health programs and services. Int. J. Qual. Stud. Health Well-being 2014, 9, 1. [Google Scholar] [CrossRef]
- Thomas, D.R. A general inductive approach for analyzing qualitative evaluation data. Am. J. Eval. 2006, 27, 237–246. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- Creswell, J.; Plano Clark, V. Designing and Conducting Mixed Methods Research, 3rd ed.; Sage Publications, Inc.: Singapore, 2018. [Google Scholar]
- Blakely, T.; Tobias, M.; Robson, B.; Ajwani, S.; Bonné, M.; Woodward, A. Widening ethnic mortality disparities in New Zealand 1981–99. Soc. Sci. Med. 2005, 61, 2233–2251. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Alzheimer’s Society. Assessing the Mental Capacity of a Person with Dementia. Available online: https://www.alzheimers.org.uk/get-support/legal-financial/assessing-mental-capacity-dementia. (accessed on 12 December 2023).
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Sari, A.B.-A.; Sheldon, T.A.; Cracknell, A.; Turnbull, A. Sensitivity of routine system for reporting patient safety incidents in an NHS hospital: Retrospective patient case note review. BMJ 2007, 334, 79. [Google Scholar] [CrossRef]
- Mulhall, A. In the field: Notes on observation in qualitative research. J. Adv. Nurs. 2003, 41, 306–313. [Google Scholar] [CrossRef]
- Davis, P.; Lay-Yee, R.; Briant, R.; Ali, W.; Scott, A.; Schug, S. Adverse events in New Zealand public hospitals I: Occurrence and impact. N. Z. Med. J. 2002, 115, 1–9. [Google Scholar]
- Löckenhoff, C.E.; Carstensen, L.L. Socioemotional selectivity theory, aging, and health: The increasingly delicate balance between regulating emotions and making tough choices. J. Personal. 2004, 72, 1395–1424. [Google Scholar] [CrossRef]
- Becker, M.H. The Health Belief Model and personal health behavior. Health Educ. Monogr. 1974, 2, 324–508. [Google Scholar] [CrossRef]
- Kim, S.; Sargent-Cox, K.; Cherbuin, N.; Anstey, K.J. Development of the motivation to change lifestyle and health behaviours for dementia risk reduction scale. Dement. Geriatr. Cogn. Disord. Extra 2014, 5, 172–183. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Wang, L.; Yang, T.; Yang, Y. A health promoting-lifestyle prediction model for dementia prevention among Chinese adults: Based on the health belief model. BMC Public Health 2022, 22, 2450. [Google Scholar] [CrossRef]
- Beishon, L.; Haunton, V.; Subramaniam, H.; Mukaetova-Ladinska, E.B.; Panerai, R.B.; Robinson, T.; Evley, R. Qualitative Analysis of the Cognition and Flow (CoGFlowS) Study: An individualized approach to cognitive training for dementia is needed. J. Alzheimer’s Dis. 2021, 83, 209–225. [Google Scholar] [CrossRef]
- Goeman, D.; Renehan, E.; Koch, S. What is the effectiveness of the support worker role for people with dementia and their carers? A systematic review. BMC Health Serv. Res. 2016, 16, 285. [Google Scholar] [CrossRef]
- Keyes, S.; Clarke, C.; Wilkinson, H.; Alexjuk, J.; Wilcockson, J.; Robinson, L.; Reynolds, J.; McClelland, S.; Corner, L.; Cattan, M. “We’re all thrown in the same boat...”: A qualitative analysis of peer support in dementia care. Dementia 2016, 15, 560–577. [Google Scholar] [CrossRef]
- Clare, L.; Rowlands, J.; Quin, R. Collective strength: The impact of developing a shared social identity in early-stage dementia. Dementia 2008, 7, 9–30. [Google Scholar] [CrossRef]
- Ward, R.; Howorth, M.; Wilkinson, H.; Campbell, S.; Keady, J. Supporting the friendships of people with dementia. Dementia 2011, 11, 287–303. [Google Scholar] [CrossRef]
- Erfani, S.S.; Abedin, B.; Blount, Y. Social support, social belongingness, and psychological well-being: Benefits of online healthcare community membership. In Proceedings of the Pacific Asia Conference on Information Systems, PACIS 2016, Chiayi, Taiwan, 27 June–1 July 2016; p. 396. Available online: http://aisel.aisnet.org/pacis2016/396 (accessed on 1 October 2023).
- Beauchamp, M.R.; Carron, A.V.; McCutcheon, S.; Harper, O. Older adults’ preferences for exercising alone versus in groups: Considering contextual congruence. Ann. Behav. Med. 2007, 33, 200–206. [Google Scholar] [CrossRef]
- Burke, S.M.; Carron, A.V.; Eys, M.A.; Ntoumanis, N.; Estabrooks, P.A. Group versus individual approach? A meta-analysis of the effectiveness of interventions to promote physical activity. Sport Exerc. Psychol. Rev. 2006, 2, 19–35. [Google Scholar] [CrossRef]
- Dishman, R.K.; Buckworth, J. Increasing physical activity: A quantitative synthesis. Med. Sci. Sports Exerc. 1996, 28, 706–719. [Google Scholar] [CrossRef]
- Ripich, D.N. Functional communication with AD patients: A caregiver training program. Alzheimer Dis. Assoc. Disord. 1994, 8, 95–109. [Google Scholar] [CrossRef]
- Small, J.A.; Gutman, G.; Makela, S.; Hillhouse, B. Effectiveness of communication strategies used by caregivers of persons with Alzheimer’s disease during activities of daily living. J. Speech Lang. Hear. Res. 2003, 46, 353–367. [Google Scholar] [CrossRef]
- Small, J.A.; Kemper, S.; Lyons, K. Sentence comprehension in Alzheimer’s disease: Effects of grammatical complexity, speech rate, and repetition. Psychol. Aging 1997, 12, 3. [Google Scholar] [CrossRef]
- Savundranayagam, M.Y.; Ryan, E.B.; Anas, A.P.; Orange, J. Communication and dementia: Staff perceptions of conversational strategies. Clin. Gerontol. 2007, 31, 47–63. [Google Scholar] [CrossRef]
- Long, A.; Di Lorito, C.; Logan, P.; Booth, V.; Howe, L.; Hood-Moore, V.; van der Wardt, V. The impact of a dementia-friendly exercise class on people living with dementia: A mixed-methods study. Int. J. Environ. Res. Public Health 2020, 17, 4562. [Google Scholar] [CrossRef]
- van der Wardt, V.; Hancox, J.; Gondek, D.; Logan, P.; Nair, R.D.; Pollock, K.; Harwood, R. Adherence support strategies for exercise interventions in people with mild cognitive impairment and dementia: A systematic review. Prev. Med. Rep. 2017, 7, 38–45. [Google Scholar] [CrossRef]
- United Nations. Convention on the Rights of Persons with Disabilities. Available online: https://social.desa.un.org/issues/disability/crpd/convention-on-the-rights-of-persons-with-disabilities-crpd (accessed on 14 October 2023).
- Dick, M.B.; Hsieh, S.; Dick-Muehlke, C.; Davis, D.S.; Cotman, C.W. The variability of practice hypothesis in motor learning: Does it apply to Alzheimer’s disease? Brain Cogn. 2000, 44, 470–489. [Google Scholar] [CrossRef]
- Harrison, B.E.; Son, G.-R.; Kim, J.; Whall, A.L. Preserved implicit memory in dementia: A potential model for care. Am. J. Alzheimer’s Dis. Other Dement. 2007, 22, 286–293. [Google Scholar] [CrossRef]
- van Halteren-van Tilborg, I.A.D.A.; Scherder, E.J.A.; Hulstijn, W. Motor-skill learning in Alzheimer’s disease: A review with an eye to the clinical practice. Neuropsychol. Rev. 2007, 17, 203–212. [Google Scholar] [CrossRef]
- Hiyamizu, M.; Morioka, S.; Shomoto, K.; Shimada, T. Effects of dual task balance training on dual task performance in elderly people: A randomized controlled trial. Clin. Rehabil. 2012, 26, 58–67. [Google Scholar] [CrossRef]
- Suttanon, P.; Hill, K.D.; Said, C.M.; Byrne, K.N.; Dodd, K.J. Factors influencing commencement and adherence to a home-based balance exercise program for reducing risk of falls: Perceptions of people with Alzheimer’s disease and their caregivers. Int. Psychogeriatr. 2012, 24, 1172–1182. [Google Scholar] [CrossRef]
- de Andrade, L.P.; Madalena, N.; Coelho, F.G. de M.; Tanaka, K.; Stella, F.; Gobbi, L.T.B. Dual task and postural control in Alzheimer’s and Parkinson’s disease. Mot. Rev. Educ. Física 2014, 20, 78–84. [Google Scholar] [CrossRef]
- IJmker, T.; Lamoth, C.J.C. 2012. Gait and cognition: The relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture 2012, 35, 126–130. [Google Scholar] [CrossRef]
| Questions |
|---|
|
| G/I | Participants with Dementia | Care Partners | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Sex | Age (Year) | MMSE Score | LTCs | Name | Age (Year) | LTCs | Relationship | |
| G | Alia | F | 74 | 28 | 11 | ||||
| G | Zain | M | 74 | 21 | 1 | Joana | 70 | 0 | Wife |
| G | Dane | M | 82 | 24 | 2 | ||||
| G | Sue | F | 81 | 30 | 0 | ||||
| G | Wilson | M | 87 | 28 | 3 | ||||
| G | Mark | M | 72 | 24 | 2 | ||||
| I | Julie | F | 71 | 25 | 0 | Brown | 71 | 0 | Husband |
| G | Leslie | F | 71 | 25 | 0 | ||||
| G | Wood | M | 74 | 17 | 7 | Cole | 69 | 1 | Ex-wife |
| G | Hansen | M | 76 | 21 | 3 | Megan | 76 | 1 | Wife |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesbah, N.; Perry, M.; Hill, K.D.; Manlapaz, D.; Hale, L. The Experiences of Older Adults with Dementia of “Balance Wise”—An Individual or Group-Delivered Exercise Programme: A Qualitative Study. Disabilities 2024, 4, 11-26. https://doi.org/10.3390/disabilities4010002
Mesbah N, Perry M, Hill KD, Manlapaz D, Hale L. The Experiences of Older Adults with Dementia of “Balance Wise”—An Individual or Group-Delivered Exercise Programme: A Qualitative Study. Disabilities. 2024; 4(1):11-26. https://doi.org/10.3390/disabilities4010002
Chicago/Turabian StyleMesbah, Normala, Meredith Perry, Keith D. Hill, Donald Manlapaz, and Leigh Hale. 2024. "The Experiences of Older Adults with Dementia of “Balance Wise”—An Individual or Group-Delivered Exercise Programme: A Qualitative Study" Disabilities 4, no. 1: 11-26. https://doi.org/10.3390/disabilities4010002
APA StyleMesbah, N., Perry, M., Hill, K. D., Manlapaz, D., & Hale, L. (2024). The Experiences of Older Adults with Dementia of “Balance Wise”—An Individual or Group-Delivered Exercise Programme: A Qualitative Study. Disabilities, 4(1), 11-26. https://doi.org/10.3390/disabilities4010002








