Inverse Chemical Equilibrium Problem in Reacting Gaseous Mixtures: The Choice of Temperature to Maximise Product Yield
Abstract
1. Introduction
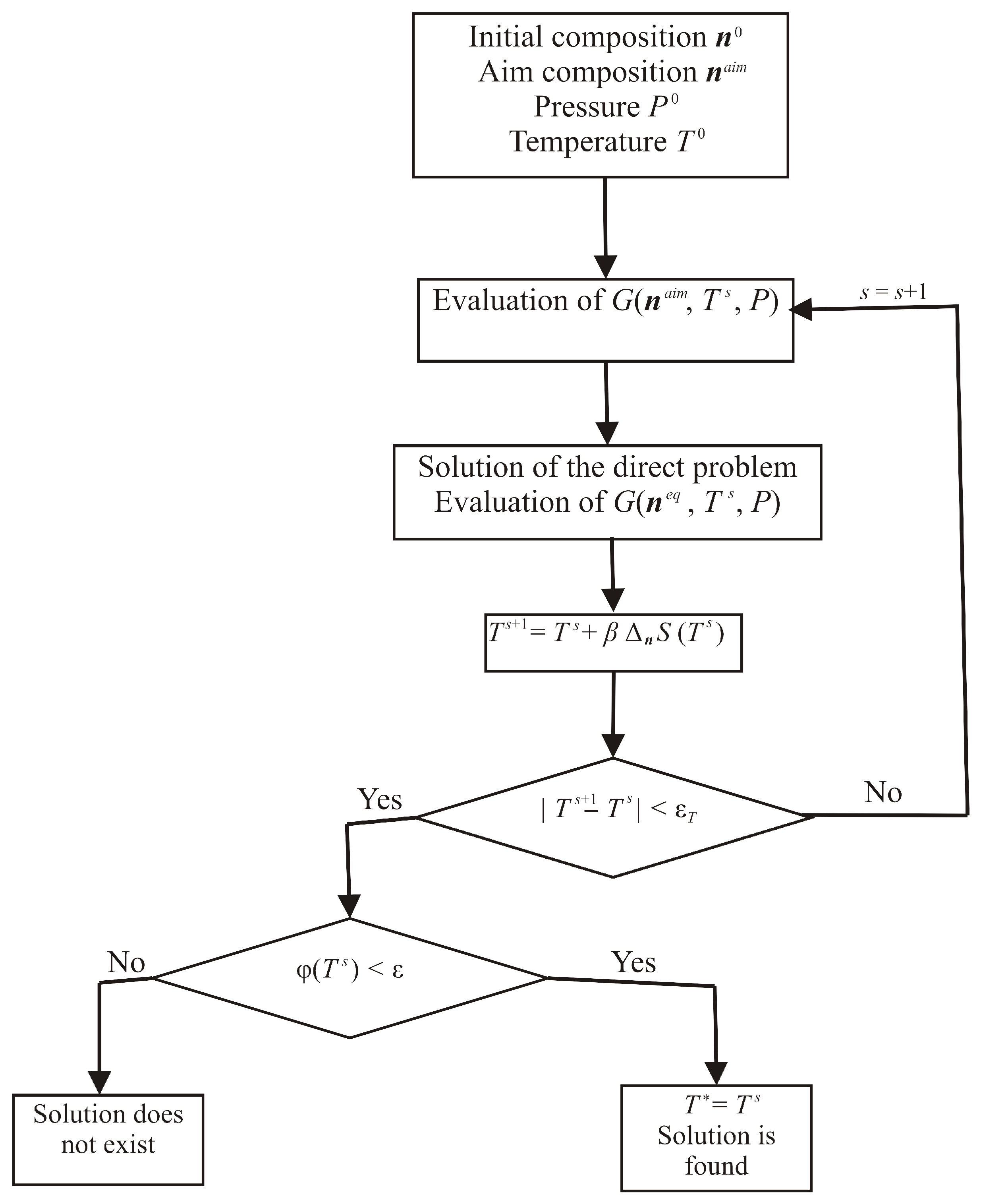
2. Inverse Problem and Auxiliary Functions
2.1. Bullseye Shot
2.2. Neighbourhood of the Point
2.3. Optimisation over Equilibria
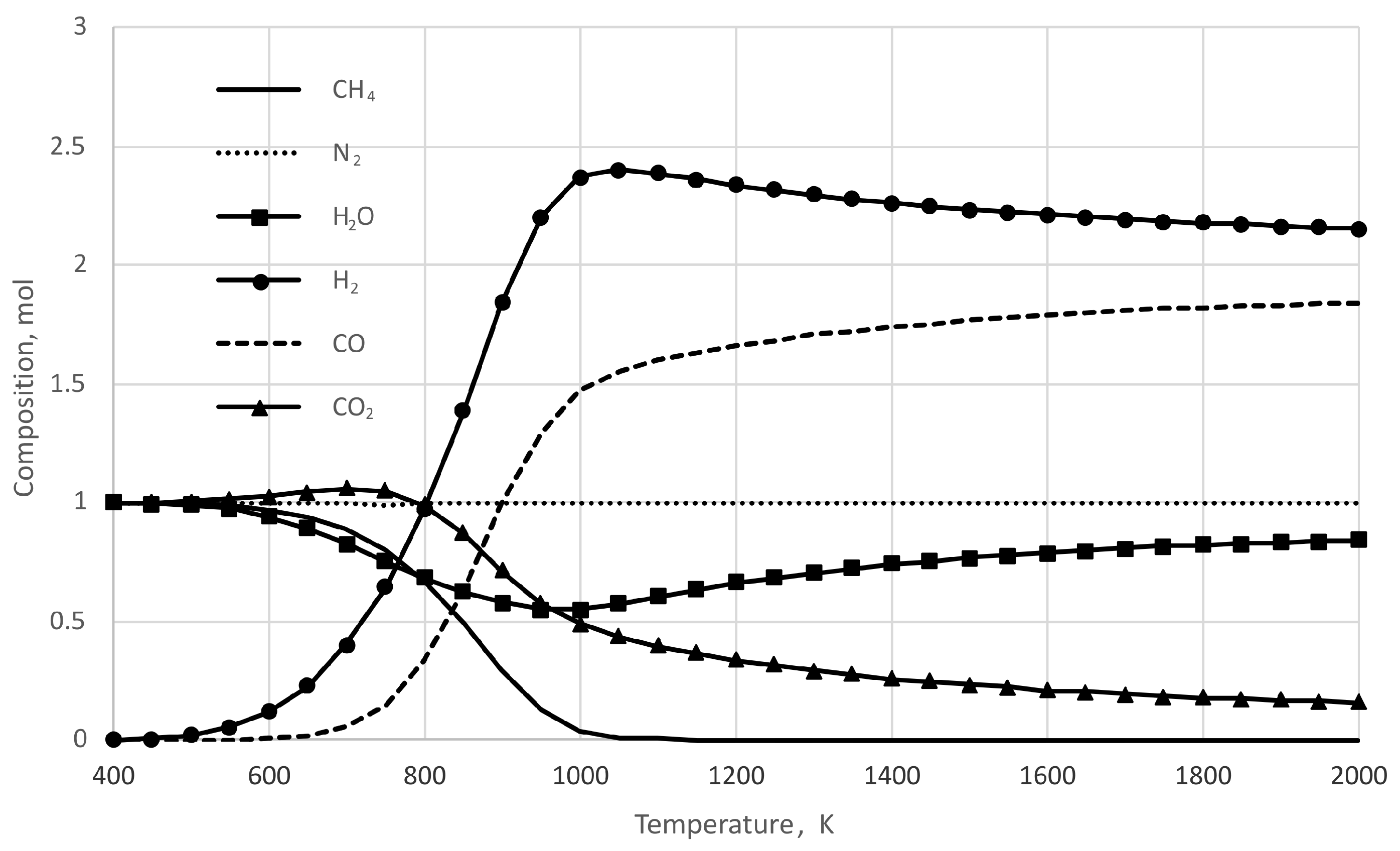
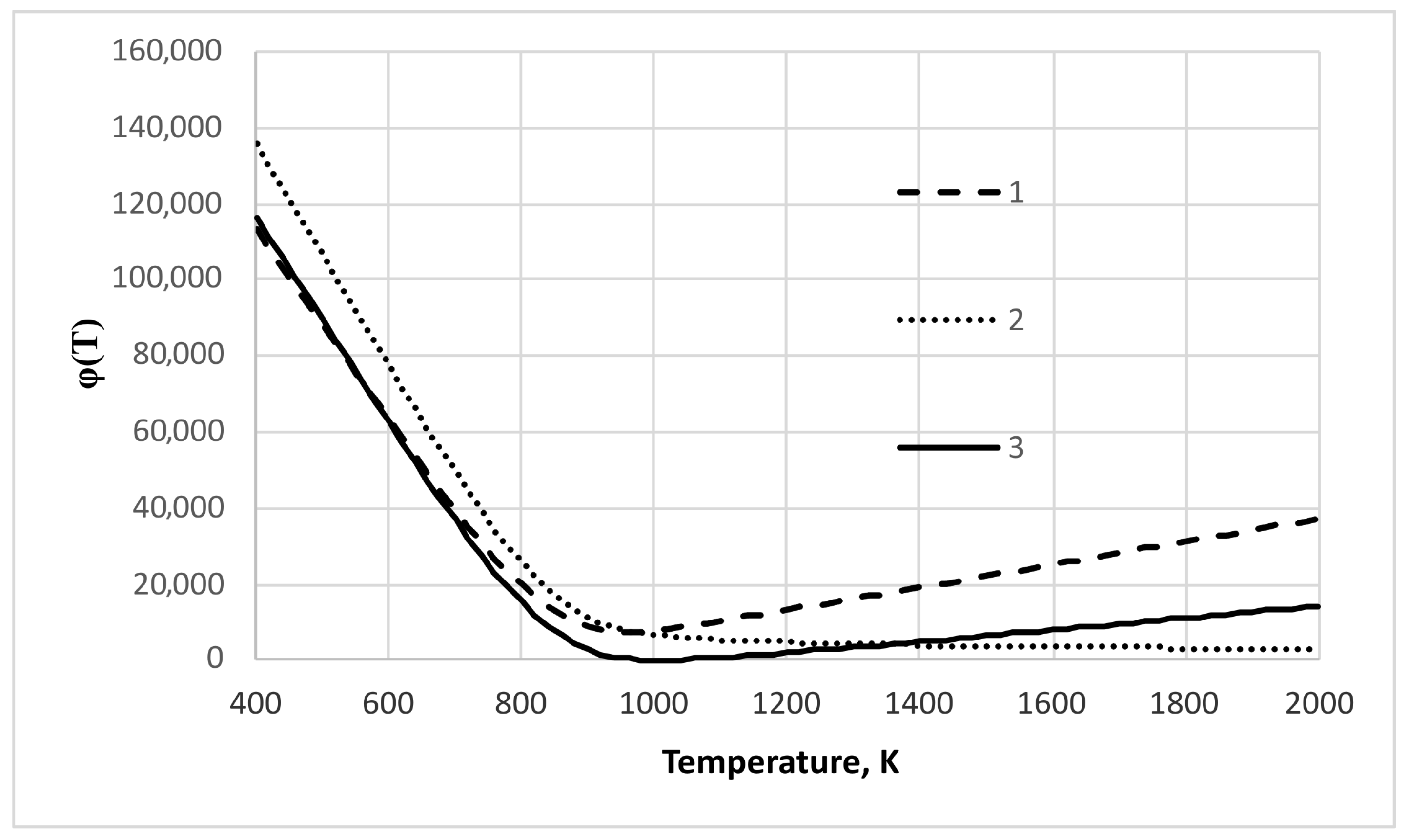
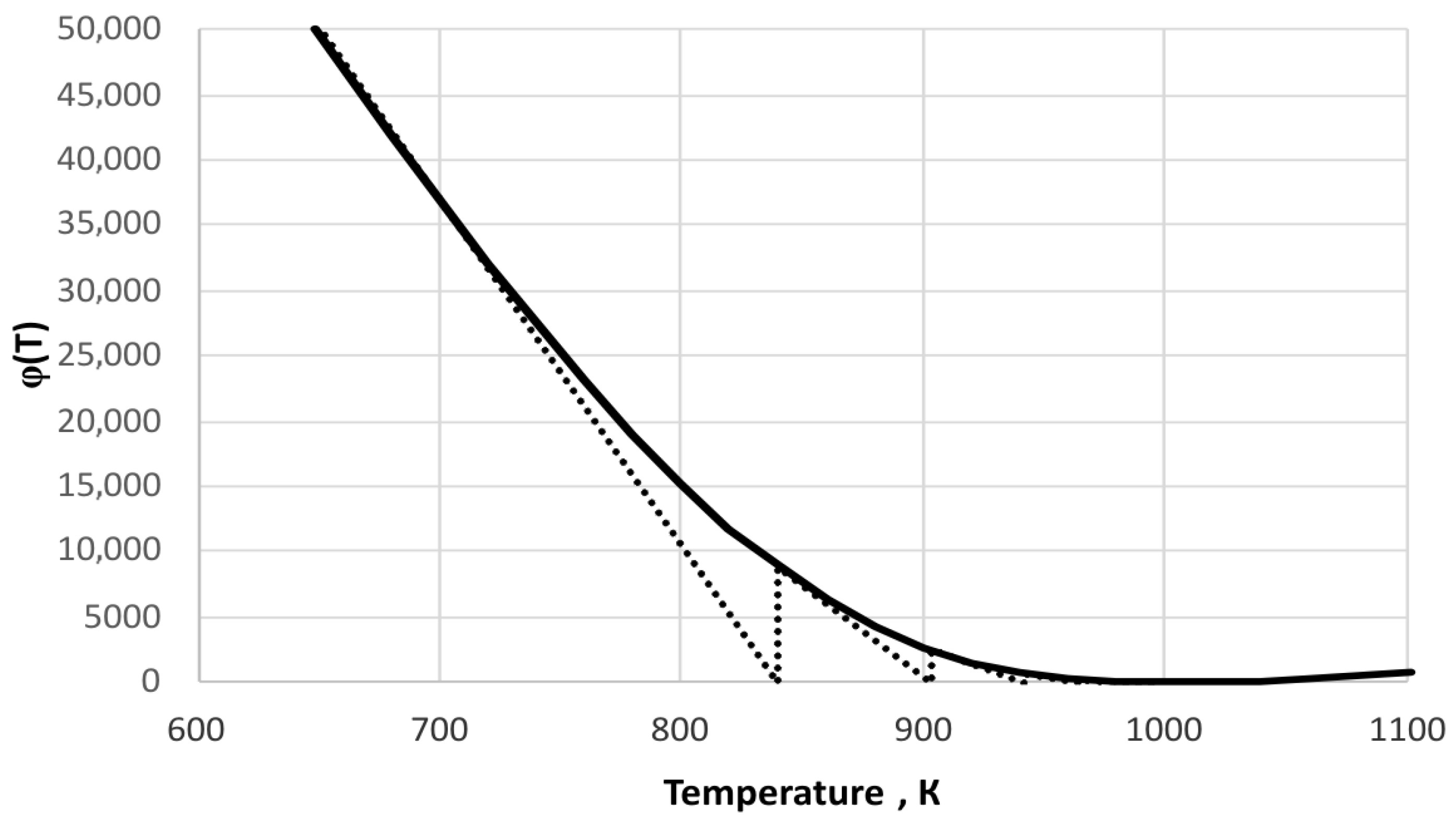
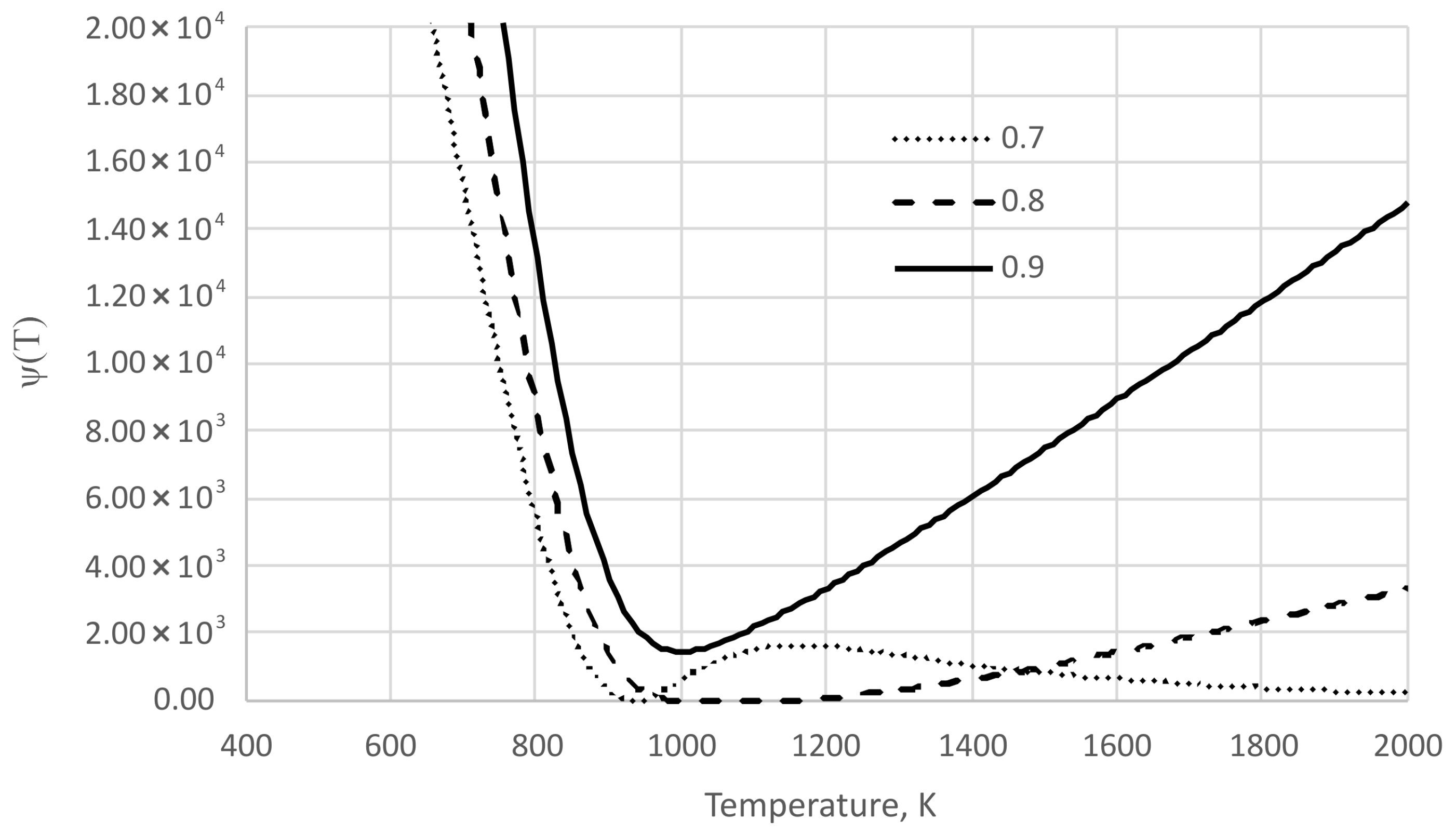
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callen, H.B. Thermodynamics and an Introduction to Thermostatics, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1985. [Google Scholar]
- Kaganovich, B.M.; Filippov, S.P.; Antsiferov, E.G. Modelling of Thermodynamic Processes; Nauka: Novosibirsk, Russia, 1993. (In Russian) [Google Scholar]
- McBride, B.J.; Zehe, M.J.; Gordon, S. NASA Glenn Coefficients for Calculating Thermodynamic Properties of Individual Species (NASA TP-2002-211556); Glenn Research Center: Cleveland, OH, USA, 2002. [Google Scholar]
- Kaganovich, B.M.; Filippov, S.P. Equilibrium Thermodynamics and Mathematical Programming; Siberian Branch of Russian Academy of Sciences: Novosibirsk, Russia, 1995. (In Russian) [Google Scholar]
- Zeldovich, Y.B. Selected Works. Volume I: Chemical Physics and Hydrodynamics; Princeton University Press: Princeton, NJ, USA, 1992. [Google Scholar]
- Marcantonio, V.; Di Paola, L.; De Falco, M.; Capocelli, M. Modeling of Biomass Gasification: From Thermodynamics to Process Simulations. Energies 2023, 16, 7042. [Google Scholar] [CrossRef]
- Moreira, P.H.L.R.; de Siqueira, R.N.C.; Vilani, C. A simple chemical equilibrium algorithm applied for single and multiple reaction systems. Comput. Appl. Eng. Educ. 2024, 32, e22728. [Google Scholar] [CrossRef]
- Shen, Z.; Li, Y.; Xu, N.; Sun, B.; Du, W.; Xu, M.; Chang, L. Investigation on the chemical equilibrium products for CnHmOlNk type fuels using equilibrium constants database. Fuel 2022, 310, 122325. [Google Scholar] [CrossRef]
- Smith, W.R.; Tahir, H.; Leal, A.M.M. Stoichiometric and non-stoichiometric methods for modeling gasification and other reaction equilibria: A review of their foundations and their interconvertibility. Renew. Sustain. Energy Rev. 2024, 189, 113935. [Google Scholar] [CrossRef]
- Brinkley, S.R., Jr. Calculation of the equilibrium composition of systems of many constituents. J. Chem. Phys. 1947, 15, 107–110. [Google Scholar] [CrossRef]
- White, W.B.; Johnson, S.M.; Dantzig, G.B. Chemical equilibrium in complex mixtures. J. Chem. Phys. 1958, 28, 751–755. [Google Scholar] [CrossRef]
- Dorn, W.S. Variational principles for chemical equilibrium. J. Chem. Phys. 1960, 32, 1490–1492. [Google Scholar] [CrossRef]
- Tsanas, C.; Stenby, E.H.; Yan, W. Calculation of simultaneous chemical and phase equilibrium by the method of Lagrange multipliers. Chem. Eng. Sci. 2017, 174, 112–126. [Google Scholar] [CrossRef]
- Zinser, A.; Rihko-Struckmann, L.; Sundmacher, K. Dynamic method for computation of chemical and phase equilibria. Comput. Chem. Eng. 2016, 89, 1–10. [Google Scholar] [CrossRef]
- Leal, A.M.M.; Blunt, M.J.; LaForce, T.C. Efficient chemical equilibrium calculations for geochemical speciation and reactive transport modelling. Geochim. Cosmochim. Acta 2014, 131, 301–322. [Google Scholar] [CrossRef]
- Bonilla-Petriciolet, A.; Rangaiah, G.P.; Segovia-Hernandez, J.G. Constrained and unconstrained Gibbs free energy minimization in reactive systems using genetic algorithm and differential evolution with tabu list. Fluid Phase Equilibria 2011, 300, 120–134. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2004. [Google Scholar]
- Kaganovich, B.M.; Keiko, A.V.; Shamansky, V.A. Equilibrium thermodynamic modeling of dissipative macroscopic systems. Adv. Chem. Eng. 2010, 39, 1–74. [Google Scholar]
- Kunze, H.; Torre, D.L.; Galan, M.R. Optimization methods in inverse problems and applications to science and engineering. Optim. Eng. 2021, 22, 2151–2158. [Google Scholar] [CrossRef]
- Chan, T.C.Y.; Mahmood, R.; Zhu, Y. Inverse Optimization: Theory and Applications. Oper. Res. 2023, 73, iii–viii. [Google Scholar] [CrossRef]
- Evseev, A.M.; Nikolaeva, L.S. Mathematical Modelling of Chemical Equilibria; Moscow University: Moscow, Russia, 1988. [Google Scholar]
- Leal, A.M.M.; Smith, W.R. Inverse chemical equilibrium problems: General formulation and algorithm. Chem. Eng. Sci. 2022, 252, 117162. [Google Scholar] [CrossRef]
- Sandu, A. Positive numerical integration methods for chemical kinetics. J. Comput. Phys. 2001, 170, 589–602. [Google Scholar] [CrossRef]
- Khamisov, O.V. Finding roots of nonlinear equations using the method of concave support functions. Math. Notes 2015, 98, 484–491. [Google Scholar] [CrossRef]
- Koukkari, P.; Pajarre, R.; Blomberg, P. Reaction rates as virtual constraints in Gibbs energy minimization. Pure Appl. Chem. 2011, 83, 1063–1074. [Google Scholar] [CrossRef]
- Pope, S.B. Gibbs function continuation for the stable computation of chemical equilibrium. Combust. Flame 2004, 139, 222–226. [Google Scholar] [CrossRef]
- Keck, J.C. Rate-controlled constrained-equilibrium theory of chemical reactions in complex systems. Prog. Energy Combust. Sci. 1990, 16, 125–154. [Google Scholar] [CrossRef]

| Component | |||
|---|---|---|---|
| 0 | 0 | 0.04 | |
| CO | 1 | 2 | 1.47 |
| 3 | 2 | 2.37 | |
| 1 | 0 | 0.49 | |
| 0 | 1 | 0.55 | |
| 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donskoy, I.; Khamisov, O. Inverse Chemical Equilibrium Problem in Reacting Gaseous Mixtures: The Choice of Temperature to Maximise Product Yield. Thermo 2025, 5, 31. https://doi.org/10.3390/thermo5030031
Donskoy I, Khamisov O. Inverse Chemical Equilibrium Problem in Reacting Gaseous Mixtures: The Choice of Temperature to Maximise Product Yield. Thermo. 2025; 5(3):31. https://doi.org/10.3390/thermo5030031
Chicago/Turabian StyleDonskoy, Igor, and Oleg Khamisov. 2025. "Inverse Chemical Equilibrium Problem in Reacting Gaseous Mixtures: The Choice of Temperature to Maximise Product Yield" Thermo 5, no. 3: 31. https://doi.org/10.3390/thermo5030031
APA StyleDonskoy, I., & Khamisov, O. (2025). Inverse Chemical Equilibrium Problem in Reacting Gaseous Mixtures: The Choice of Temperature to Maximise Product Yield. Thermo, 5(3), 31. https://doi.org/10.3390/thermo5030031







