3.1. Calculation of Dissipated Energy by the First Law of Thermodynamics
The first law of thermodynamics (or principle of conservation of energy) in its local form is stated in small deformations as follows, see e.g. [
1].
where
u stands for the internal energy density,
ρ for the material density,
r is the rate of body heating or radiation per unit mass and
q is the heat flux vector. It is recalled that
q =
q.n is the heat outflow per unit time per unit surface with normal vector
n (heat conduction). Lower case non-bold letters denote scalar quantities; lower case bold face letters denote vectors while upper case bold face letters denote tensors (with the usual notable exception of
σ and
ε for the Cauchy stress and strain tensors). The superimposed dot stands for the material time derivative and the symbol: denotes the double contraction (inner product) between two second-order tensors defined as, e.g., for the second-order tensors
A and
B where
tr stands for the trace, the superimposed
T indicates the transpose of a tensor, and
ATB indicates the product of the two tensors
AT and
B. It can be shown that in indicial notation the double contraction is given by
where the summation convention (introduced by Einstein) between the same indices (taking the values from 1 to 3) applies.
The essence of the first law of thermodynamics is that
there exists a
state function u so that Equation (4) holds at any time for all points of any material! The local form of the first law of thermodynamics (i.e., a field equation) can be derived from its global form, see e.g. [
1]. In the case of finite deformations, for the global form of the energy balance in the current configuration, one may consult [
7] and for the resulting local form (field equation) [
7,
8].
The inner product (the double contraction) between the stress and the strain rate tensor represents the mechanical power (also called deformation power or stress power) per unit volume.
Let us now consider that a material element undergoes a cyclic process, i.e., a process in which the kinematic and response functions defining the state of the representative material element under consideration, are having the same values at the beginning (time
t1) and at the end of the process (time
t2). By integrating Equation (4) from
t1 to
t2 we obtain
For small strains the mass density
ρ can be considered as constant and it can therefore be put outside of the integral. The integral from
t1 to
t2 of
is equal to
since the internal energy
u is a state function, and hence it has the same value at the end (time
t2) and the beginning (time
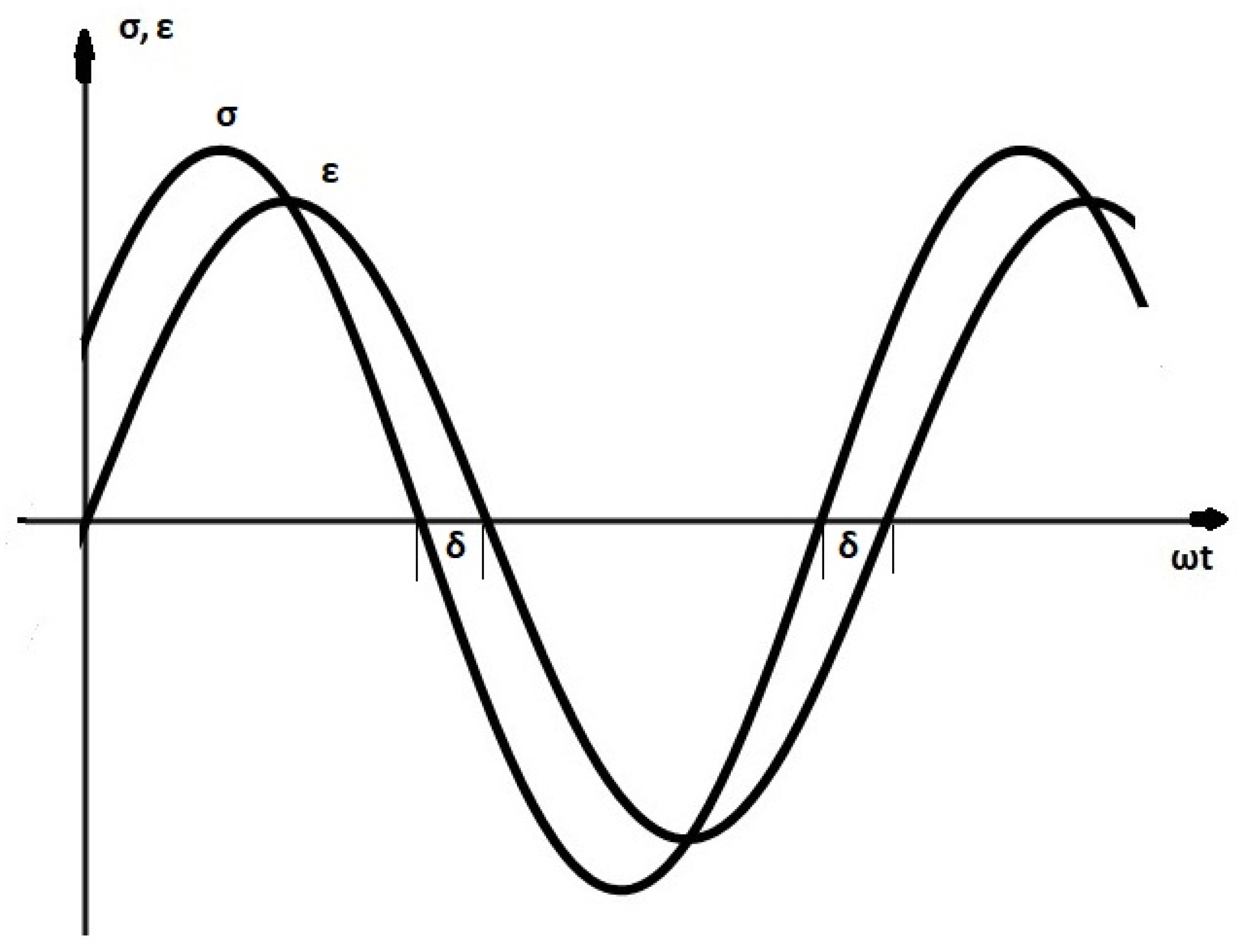
t1) of the cyclic process. The steady-state excitation, which we presented in
Section 2, is a particular case of a cyclic process. Therefore, from the above Equation (5), whose left-hand side is equal to zero, it follows that the integral of the deformation power over a period is equal to the amount of heat produced, i.e., to the mechanical energy dissipated during this time. The integral of the deformation power is equal to the area of the stress versus the strain diagram in the one-dimensional case and to the summation of the areas of the conjugate stress-strain diagrams in multidimensional cases. It can be mentioned here that Tschoegl [
9] arrives at the same result on the basis of the assumption that the kinetic energy can be neglected for a viscoelastic material. This assumption is unnecessary and is wrong in general.
The steady-state process, which we examined represents a “simple” case of loading-unloading of engineering materials and is frequently met in experimental works and in applications. If this is modeled by linear viscoelasticity the energy dissipated can be computed by the area of the stress-strain curve and by the summation of the areas of the conjugate stress-strain curves in multidimensional cases.
It should be noted that a thermodynamics framework, in addition to its other advantages, e.g., consistency between assumptions made and constitutive models, gives us the tools to identify the different mechanisms contributing to energy dissipation such as viscoelasticity and damage [
4].
3.2. Calculation of Dissipated Energy by the Second Law of Thermodynamics
We will now compute the dissipated energy by using the second law of thermodynamics for comparison purposes with the results obtained by the first law and for completeness and moreover in order to address more general material descriptions (constitutive hypotheses) and loading conditions. In particular, dissipative materials which can be described by the use of internal variables are addressed. Internal variables in mechanics and constitutive modeling is a rich and vast subject, which has been very successful. Modern inelasticity, computational inelasticity, and damage mechanics models are based on internal variables.
There are many publications on this subject and we refer here to some of them, e.g., [
1,
2,
10,
11] for nonlinear finite viscoelasticity, [
12,
13,
14,
15] for a new concept in internal variables. Within the framework of internal variables, the linear and nonlinear theories of viscoelasticity for small as well as finite deformations have been successfully formulated and applied for material modeling. The same is true for the theories of elasto-plasticity, elasto-viscoplasticity, and damage mechanics. Material models that are developed within the framework of internal variables are usually amenable to efficient implementation into computational mechanics codes, such as finite elements. Important finite element frameworks, such as ABAQUS, ANSYS, and OPENSEES to name a few use the concept of internal variables. The second law of thermodynamics may be expressed by the Clausius-Duhem inequality, which has as a result the Kelvin inequality (dissipation inequality), see e.g., [
1,
16,
17]. i.e.,
where
D is the intrinsic dissipation given by
In Equation (7)
are the “thermodynamic forces” conjugate to the internal variables
ξα, which constitute the internal variable vector
ξ. The subscript
α in the internal variables
ξ denotes their number (
α = 1
…n). The “thermodynamic forces”
are given as
where
ψ is the Helmholtz free-energy density per unit mass, see e.g., [
1,
13,
16,
17].
By employing the summation convention over the index
α and by combining Equations (7) and (8) we have the following expression for the dissipation
The decomposition of the strain tensor
ε additively, in the case of small strains, into an elastic
εe and an inelastic part
ει is equivalent to the decomposition of the free energy into elastic and inelastic components as follows [
18]
where
θ is the absolute temperature.
As is well known the free energy density
ψ serves as a potential for the (Cauchy) stress tensor
σ, i.e.,
see e.g., [
13].
Therefore, from Equations (10) and (11) we obtain the following useful expression for the stress tensor
σ since
is independent of
ε,Because of Equations (10)–(12) and by using the relation
the dissipation now (Equation (9)) reads:
where the term
represents the rate of the inelastic work per unit volume. We will now consider a cyclic process from time
t1 to time
t2 and will compute the dissipation, through this cyclic process, by integrating
D. We will proceed by first integrating the first term of
D, in Equation (13).
By using Equation (12) the second integral of the right-hand side is equal to ρ[Ψe(t2) − Ψe(t1)] and is zero for isothermal conditions. (Material density ρ has been taken out of the integral because of small strains.) This is because the elastic free energy is a function of εe and θ (see Equation (10)).
Since σ is a function of εe and θ, i.e., σ = σ(εe, θ) because of Equation (12), it holds that εe = εe(σ, θ) because of the inverse mapping theorem of advanced calculus. At times t1 and t2 the stress has the same values (cyclic process) and therefore the elastic strains also under isothermal conditions and subsequently the elastic free energy components Ψe(t1) and Ψe(t2).
Therefore, the dissipated energy in a cyclic process from
t1 to
t2 is
Strains ε and stresses σ attain the same values at the beginning and end of the cycle. If we assume that in such a cycle and for isothermal conditions the internal variable vector ξ also has the same values at the beginning and end of the cycle then the second integral of the Equation (15) becomes since Ψi = ψi(ξ, θ).
Therefore, we arrived at the same result as before, i.e., the dissipated energy is given by the area of the stress-strain diagram—in the one-dimensional case—when a complete cycle is considered, as for example in steady-state dynamic excitations over one period.
It is noted that the derivations above were carried in a direct tensorial notation.
One may want to use indicial notation and obtain the same results.
Remarks
1. From Equation (16) it is clear that in a multi-dimensional case, the dissipated energy is equal to the sum of the areas of the conjugate stress-strain diagrams. To the best of our knowledge, this is a new result, which has not been reported elsewhere.
2. It is reasonable to assume that since ε(σ, θ, ξ) and σ attain the same values at the beginning and end of the cycle then in such a process and for isothermal conditions the internal variable vector ξ also has the same value at the beginning and end of the cycle. In this case, Equation (16) follows from (15) under isothermal conditions only.
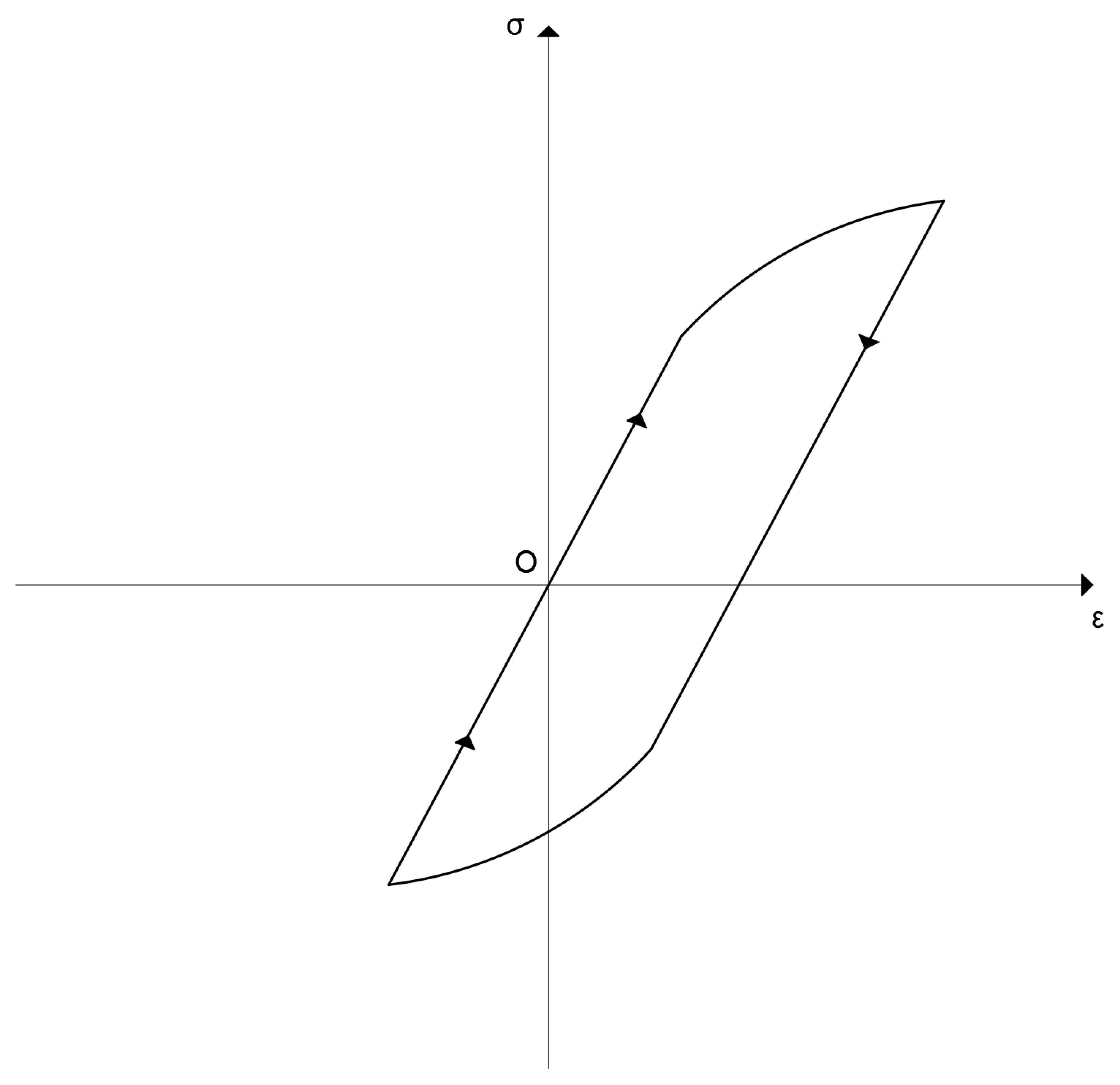
However, as pointed out by Lubliner [
1] (page 64 and figure on this page) it is possible that a situation like the following arises in an inelastic material (see the following
Figure 2, which is reproduced from Lubliner for the reader’s convenience). The loading starts from point O and returns to it. At the end of the cycle, the stress
σ has returned to its initial value, i.e., zero, as well as the total strain
ε. Nevertheless, the internal state of the material may be different from its initial state, which means that some of the internal variables introduced for the description of the material’s behavior and modeling
have not returned to their initial values at the end of the cycle.
Therefore, in such a case Equation (16) does not hold and we must use Equation (15), in order to include the contribution of the internal variables, for the computation of the dissipated energy.
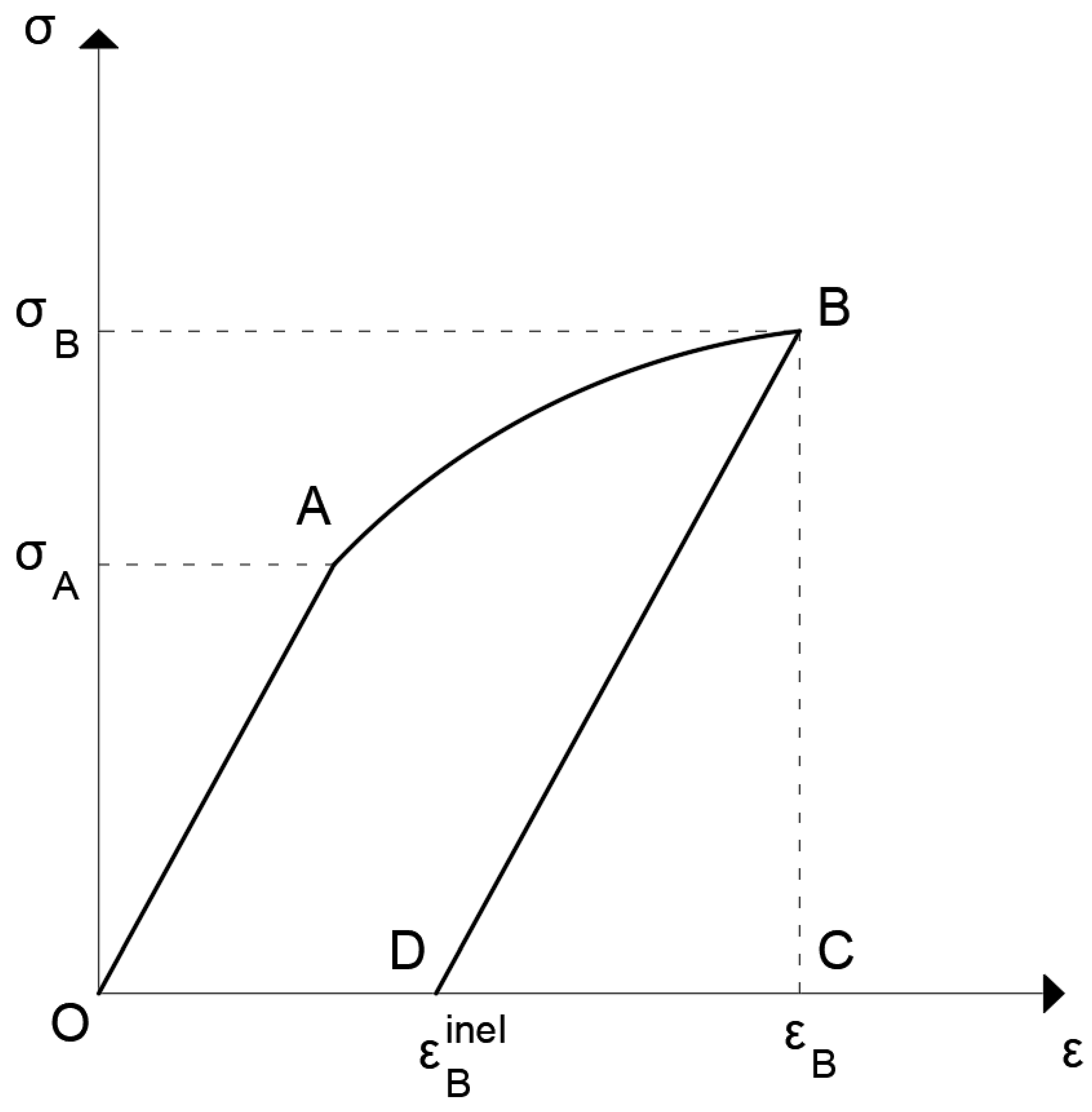
3a. In the following figure (
Figure 3) the typical one-dimensional loading-unloading curve of an elastoplastic material is shown, with a single internal variable being the
(permanent strain). If we consider the loading path from O to A to B and then start unloading from point B to point D, i.e., until the stress becomes zero and the strain is equal to
, then clearly in this path the stress returns to its initial value (zero) but neither the total strain
ε, which is equal to
does nor the internal variable, which is equal to
. It is noted that in the figure the segment DC represents the elastic strain (the recoverable strain) at point B. Therefore, in this case, even under isothermal conditions the area of the loop OABD
does not represent the dissipated energy!
It is emphasized that in publications, including papers, theses, doctoral dissertations, and even books, this is a common mistake made by their authors designating this area as dissipated energy. See, e.g., the book [
19] (Figure 3.18, p. 114).
3b. A similar mistake is found in cases of one cycle or of repeated cycles of loading- unloading. This error frequently appears again in publications, including papers, theses, doctoral dissertations, books, and even engineering codes. For example, in [
20], p. 99, Figure 7.1, (see also Figure 7.7N, p. 115) it is stated that the dissipated energy per cycle is equal to the area of the loop. This is wrong according to our development. It should have been stated that this is valid for isothermal conditions and therefore Eurocode’s 8 assertion is an assumption. Moreover, it should have been reported if the internal variables of the model used in order to obtain Figure 7.1 are returning to their initial values in each cycle. The same remarks apply to many papers in structural engineering, see e.g., [
21] among many others.
3c. Apart from structural engineering and structural mechanics, similar erroneous calculations are found in soil mechanics works. For example, such results are usually found in energy methods for the evaluation of soil liquefaction, where the area under a stress-strain curve is
assumed to represent the dissipated energy, see e.g., [
22,
23] among others.
4. In case the internal variables
do not return to their initial values in an isothermal loading process the dissipated energy is given, as we proved, by Equation (15), which is repeated below, i.e., as
Therefore, it is not given by just the sum of the areas of the conjugate stress-strain diagrams but it consists of two parts. Both parts should be taken into account for the correct computation of the dissipated energy. This result also underlines the importance of the choice of the internal variables, i.e., of the constitutive modeling.
A developer of a model knows the model’s internal variables and their evolution equations as well the form of the inelastic part of the free energy ψi. Therefore, the model’s developer knows if the internal variables return to their initial values at the end of a cycle and in case the second part of Equation (17) is needed the modeler can compute it. If this is the case, the contribution of each term of Equation (17) to the dissipated energy Wd. is calculated,
5. It is worth mentioning that there are more general cases in inelastic constitutive modeling in which the material neighborhood has structure, and its geometry should be represented by a non—Euclidean metric
g, called a material metric (or physical metric) [
15]. Modeling by material metric is within the area of internal variables. Therefore, the results presented here regarding the dissipated energy are valid for these more general cases too, with the new internal variable
g included in the relevant computations.