Alterations of a CaCl2 Alginate Composite for Thermochemical Heat Storage during the Hydration in a 1 L Packed Bed Laboratory Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
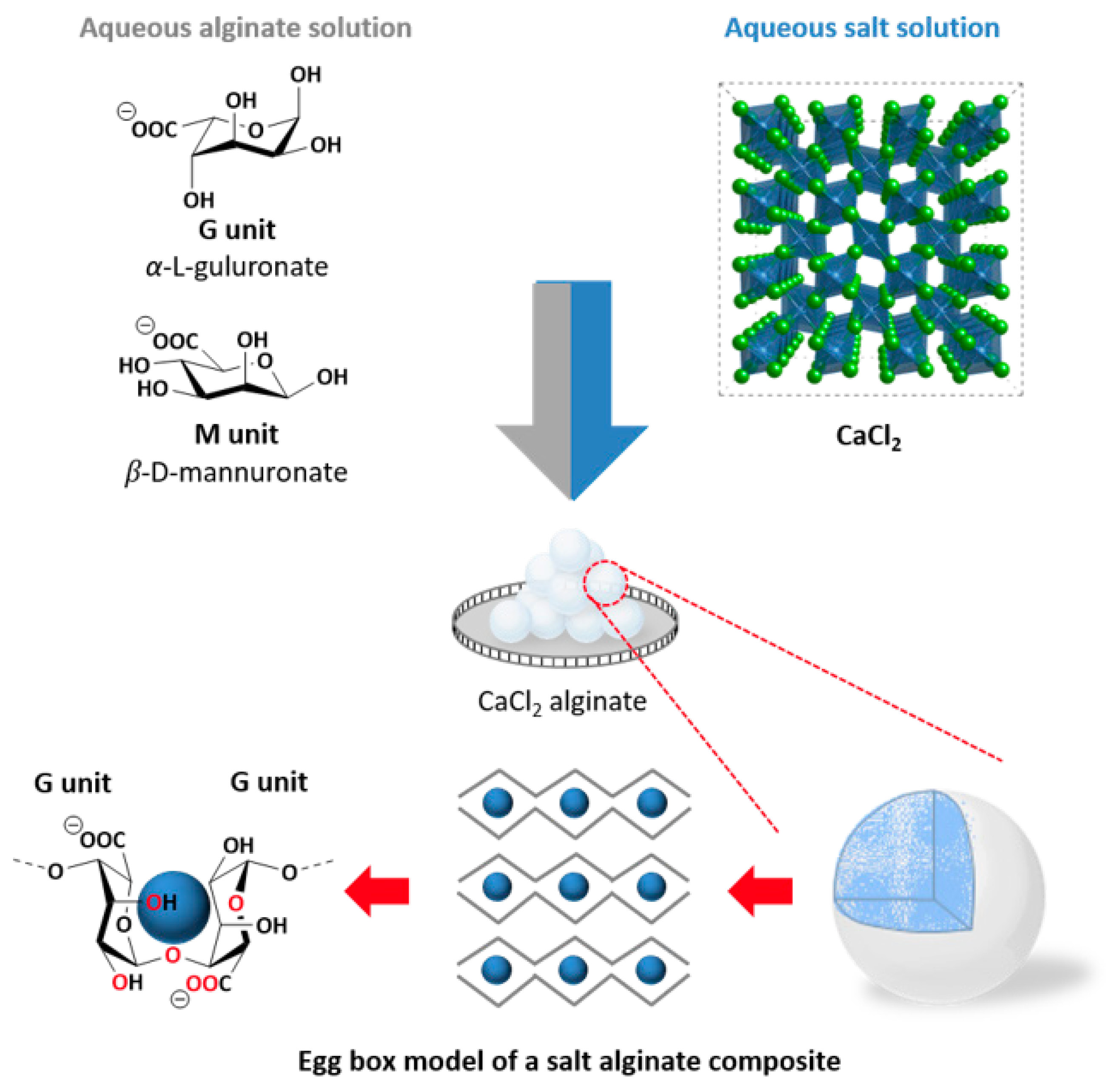
2.2. Preparation of the Calcium Chloride Alginate Composite
2.3. Characterization of the Material
2.4. Calorimetric Investigations
2.5. Thermogravimetry
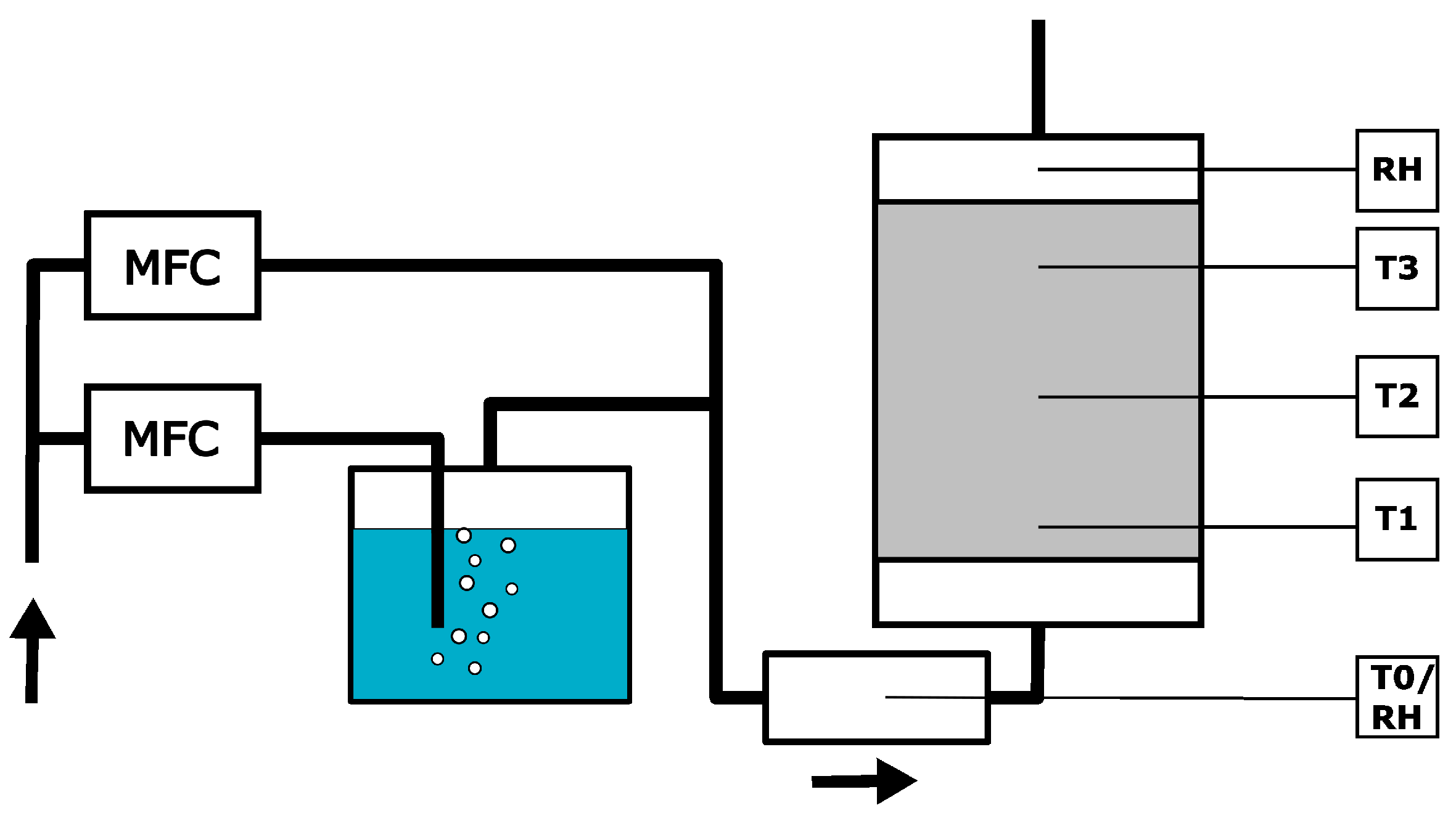
2.6. Experiments in a 1 L Reactor
3. Results and Discussion
3.1. Material Properties
3.2. Calorimetry
3.3. Thermogravimetry
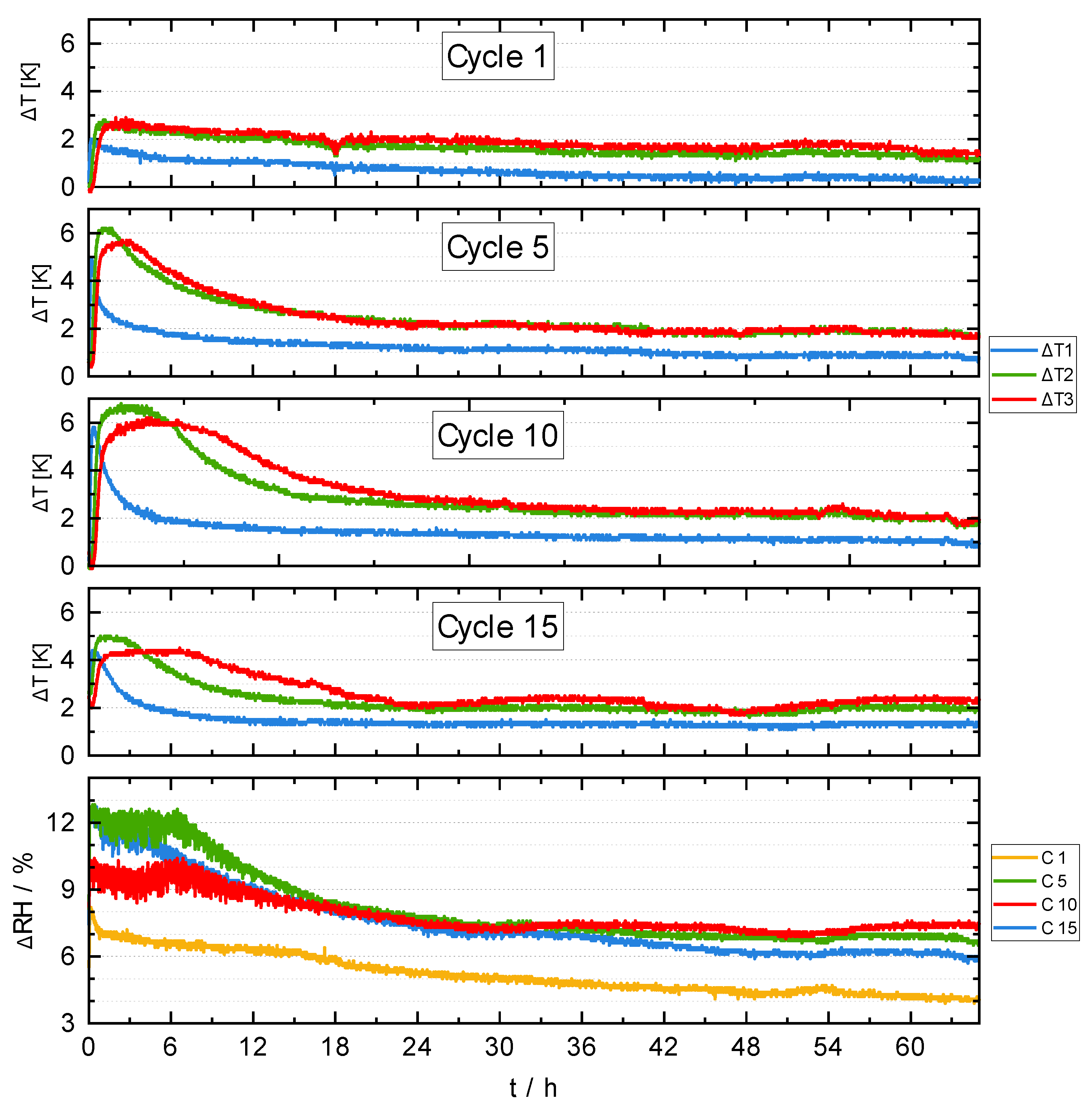
3.4. Reactor Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CCA-raw | calcium chloride alginate composite uncycled |
| CCA-CXX | calcium chloride alginate composite after XX cycles |
| CCA-K | calcium chloride alginate composite uncycled of literature source [24] |
| dav | average pore diameter |
| RH | relative humidity |
| S | specific surface area |
| T | temperature |
| t | time |
| wCaCl2 | mass fraction of CaCl2 |
| Greek symbols | |
| ρ | bulk density |
| ρ0 | raw density |
| Φ | porosity |
| Subscrips | |
| 0 | Raw (0 cycles) |
| BET | Brunauer-Emmett-Teller |
| cal | calculated |
| MIP | mercury intrusion porosimetry |
References
- Energien-Statistik Arbeitsgruppe Erneuerbare. Erneuerbare Energien in Deutschland Daten zur Entwicklung im Jahr 2021. Available online: https://www.umweltbundesamt.de/publikationen/erneuerbare-energien-in-deutschland-0 (accessed on 29 September 2023).
- Imbery, F.; Friedrich, K.; Kaspar, F.; Fleckenstein, R.; Lengefeld, K.; Bissolli, P.; Daßler, J. Klimatologische Einordnung des Jahres 2021. Available online: https://www.dwd.de/DE/klimaumwelt/aktuelle_meldungen/220105/deutschland_klimarueckblick_2021.html (accessed on 29 September 2023).
- Rundel, P.; Meyer, B.; Meiller, M.; Meyer, I.; Daschner, R.; Jakuttis, M.; Franke, M.; Binder, S.; Hornung, A. Studie Speicher fuer die Energiewende. Sulzbach-Rosenberg. 2013. Available online: https://www.umsicht-suro.fraunhofer.de/content/dam/umsicht-suro/de/documents/studien/studie_speicher_energiewende.pdf (accessed on 29 September 2023).
- Mette, B.; Kerskes, H.; Drück, H.; Müller-Steinhagen, H. Experimental and numerical investigations on the water vapor adsorption isotherms and kinetics of binderless zeolite 13X. Int. J. Heat. Mass. Transf. 2014, 71, 555–561. [Google Scholar] [CrossRef]
- Núñez, T.; Mittelbach, W.; Henning, H.-M. Development of an adsorption chiller and heat pump for domestic heating and air-conditioning applications. Appl. Therm. Eng. 2007, 27, 2205–2212. [Google Scholar] [CrossRef]
- Kaneko, K. Micropore filling mechanism in inorganic sorbents. Stud. Surf. Sci. Catal. 1996, 99, 573–598. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Liu, H.; Le Pierrès, N.; Luo, L. A review on long-term sorption solar energy storage. Renew. Sustain. Energy Rev. 2009, 13, 2385–2396. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Schmidt, T.; Rammelberg, H.U.; Watts, B.A.; Ruck, W.K.L. A systematic multi-step screening of numerous salt hydrates for low temperature thermochemical energy storage. Appl. Energy 2014, 124, 1–16. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Cacciola, G.; Restuccia, G. Selective water sorbents for multiple applications, 1. CaCl2 confined in mesopores of silica gel: Sorption properties. React. Kinet. Catal. Lett. 1996, 59, 325–333. [Google Scholar] [CrossRef]
- Jänchen, J.; Ackermann, D.; Weiler, E.; Stach, H.; Brösicke, W. Calorimetric investigation on zeolites, AlPO4’s and CaCl2 impregnated attapulgite for thermochemical storage of heat. Thermochim. Acta 2005, 434, 37–41. [Google Scholar] [CrossRef]
- Tso, C.Y.; Chao, C.Y.H. Activated carbon, silica-gel and calcium chloride composite adsorbents for energy efficient solar adsorption cooling and dehumidification systems. Int. J. Refrig. 2012, 35, 1626–1638. [Google Scholar] [CrossRef]
- van Essen, V.M.; Zondag, H.A.; Gores, J.C.; Bleijendaal, L.P.J.; Bakker, M.; Schuitema, R.; van Helden, W.G.J.; He, Z.; Rindt, C.C.M. Characterization of MgSO4 Hydrate for Thermochemical Seasonal Heat Storage. J. Sol. Energy Eng. 2009, 131, 4. [Google Scholar] [CrossRef]
- Posern, K.; Kaps, C. Humidity controlled calorimetric investigation of the hydration of MgSO4 hydrates. J. Therm. Anal. Calorim. 2008, 92, 905–909. [Google Scholar] [CrossRef]
- Posern, K.; Kaps, C. Calorimetric studies of thermochemical heat storage materials based on mixtures of MgSO4 and MgCl2. Thermochim. Acta 2010, 502, 73–76. [Google Scholar] [CrossRef]
- Ferchaud, C.J.; Zondag, H.A.; de Boer, R. Material Research on Salt Hydrates for Seasonal Heat Storage Application in a Residential Environment. In Proceedings of the International Symposium on Innovative Materials for Processes in Energy System (IMPRESS 2013), Fukuoka, Japan, 4–6 September 2013. [Google Scholar]
- van Essen, V.M.; Cot Gores, J.; Bleijendaal, L.P.J.; Zondag, H.A.; Schuitema, R.; Bakker, M.; van Helden, W.G.J. Characterization of Salt Hydrates for Compact Seasonal Thermochemical Storage. In Proceedings of the ASME 2009 3rd International Conference on Energy Sustainability collocated with the Heat Transfer and InterPACK09 Conferences, San Francisco, CA, USA, 19–23 July 2009; ASMEDC: Houston, TX, USA, 2009; Volume 2, pp. 825–826. [Google Scholar]
- Steiger, M. Thermodynamic properties of SrCl2(aq) from 252 K to 524 K and phase equilibria in the SrCl2–H2O system: Implications for thermochemical heat storage. J. Chem. Thermodyn. 2018, 120, 106–115. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Rammelberg, H.U.; Lele, A.F.; Korhammer, K.; Watts, B.A.; Schmidt, T.; Ruck, W.K.L. A review on the use of calcium chloride in applied thermal engineering. Appl. Therm. Eng. 2015, 75, 513–531. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Restuccia, G.; Freni, A.; Aristov, Y.I. Water sorption on composites “LiBr in a porous carbon”. Fuel Process. Technol. 2002, 79, 225–231. [Google Scholar] [CrossRef]
- Simonova, I.A.; Freni, A.; Restuccia, G.; Aristov, Y.I. Water sorption on composite “silica modified by calcium nitrate”. Microporous Mesoporous Mater. 2009, 122, 223–228. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Tokarev, M.M.; Parmon, V.N.; Aristov, Y.I. Selective water sorbents for multiple application, 6. Freshwater production from the atmosphere. React. Kinet. Catal. Lett. 1998, 65, 153–159. [Google Scholar] [CrossRef]
- Gaeini, M.; Rouws, A.L.; Salari, J.W.O.; Zondag, H.A.; Rindt, C.C.M. Characterization of microencapsulated and impregnated porous host materials based on calcium chloride for thermochemical energy storage. Appl. Energy 2018, 212, 1165–1177. [Google Scholar] [CrossRef]
- Reynolds, J.; Williams, R.; Elvins, J.; Jewell, E.; Searle, J.; Ke, X. Development and characterisation of an alginate and expanded graphite based composite for thermochemical heat storage. J. Mater. Sci. 2023, 58, 5610–5624. [Google Scholar] [CrossRef]
- Kallenberger, P.A.; Posern, K.; Linnow, K.; Brieler, F.J.; Steiger, M.; Fröba, M. Alginate-Derived Salt/Polymer Composites for Thermochemical Heat Storage. Adv. Sustain. Syst. 2018, 2, 1700160. [Google Scholar] [CrossRef]
- DIN 66137-2: 2019-03; Bestimmung der Dichte fester Stoffe_-Teil_2: Gaspyknometrie. Beuth Verlag GmbH: Berlin, Germany, 2019.
- DIN ISO 9277:2014-01; Bestimmung der spezifischen Oberfläche von Festkörpern mittels Gasadsorption_-BET-Verfahren (ISO_9277:2010). Beuth Verlag GmbH: Berlin, Germany, 2014.
- DIN ISO 15901-1: 2019-03; Bewertung der Porengrößenverteilung und Porosität von Feststoffen mittels Quecksilberporosimetrie und Gasadsorption_- Teil_1: Quecksilberporosimetrie (ISO_15901-1:2016). Beuth Verlag GmbH: Berlin, Germany, 2019.
- Stach, H.; Mugele, J.; Jänchen, J.; Weiler, E. Influence of Cycle Temperatures on the Thermochemical Heat Storage Densities in the Systems Water/Microporous and Water/Mesoporous Adsorbents. Adsorption 2005, 11, 393–404. [Google Scholar] [CrossRef]
- Pátek, J.; Klomfar, J.; Součková, M. Solid−Liquid Equilibrium in the System of CaCl2 −H2O with Special Regard to the Transition Points. J. Chem. Eng. Data 2008, 53, 2260–2271. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Simon, T.; Dohrmann, M.; Steiger, M.; Fröba, M. Calcium Chloride Alginate Composites as Thermochemical Heat Storage Media and the Influence of Different Alginate Species on the Behavior of the Composites. in preperation.
- Carlsson, B.; Stymne, H.; Wettermark, G. An incongruent heat-of-fusion system—CaCl2·6H2O—Made congruent through modification of the chemical composition of the system. Sol. Energy 1979, 23, 343–350. [Google Scholar] [CrossRef]
- Kerskes, H.; Mette, B.; Bertsch, F.; Asenbeck, S.; Drück, H. Chemical energy storage using reversible solid/gas-reactions (CWS—Results of the research project. Energy Procedia 2012, 30, 294–304. [Google Scholar] [CrossRef]
- Kerskes, H.; Sommer, K.; Müller-Steinhagen, H.M. Final Report: Integrales Konzept zur solarthermischen Gebäudeheizung mit Sorptionswärmespeicher. MonoSorp (BWK25006). Stuttgart. 2007. Available online: https://pudi.lubw.de/detailseite/-/publication/77219-Monosorp_-_Integrales_Konzept_zur_solarthermischen_Geb%C3%A4udeheizung_mit_Sorptionsw%C3%A4rmespeicher.pdf (accessed on 29 August 2023).



| Cycle | wCaCl2 (wt%) | ρ0 (g/cm3) | ρ (g/cm3) | ρMIP (g/cm3) | ϕMIP (%) | ϕcal (%) | SBET (m2/g) | SMIP (m2/g) | dav (nm) |
|---|---|---|---|---|---|---|---|---|---|
| raw | 69.0 | 2.00 | 0.97 | 0.96 | 28 | 53 | 1.4 | 36 | 61 |
| 5 | - | - | 0.86 | 0.84 | 14 | 57 | 1.1 | 17 | 60 |
| 10 | - | - | 1.21 | 1.52 | 14 | 40 | 1.0 | 15 | 43 |
| 15 | - | - | 1.09 | 1.57 | 10 | 45 | 1.0 | 8 | 52 |
| Temperature [°C]/ Relative Humidity [%] | Heat of Reaction [kJ/kg] | Storage Density [kJ/cm³] ([kWh/m³]) | Water Uptake [gH2O/gsample dry] | |
|---|---|---|---|---|
| CCA-raw | 25/20 | 1324 | 1.28 (357) | 0.35 |
| 25/30 | 1478 | 1.39 (387) | 0.64 | |
| CCA-C05 | 25/20 | 1343 | 1.13 (313) | 0.39 |
| 25/30 | 1790 | 1.50 (418) | 0.61 | |
| CCA-C10 | 25/20 | 1036 | 1.57 (437) | 0.33 |
| 25/30 | 1271 | 1.93 (537) | 0.32 | |
| CCA-C15 | 25/20 | 1347 | 2.11 (587) | 0.47 |
| 25/30 | 1419 | 2.23 (619) | 0.48 | |
| CCA-K [24] | 30/20 | 1018 | 1.10 (306) | 0.64 |
| 30/30 | 1206 | 1.50 (417) | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heitmann, S.; Simon, T.; Osburg, A.; Fröba, M. Alterations of a CaCl2 Alginate Composite for Thermochemical Heat Storage during the Hydration in a 1 L Packed Bed Laboratory Reactor. Thermo 2023, 3, 593-604. https://doi.org/10.3390/thermo3040035
Heitmann S, Simon T, Osburg A, Fröba M. Alterations of a CaCl2 Alginate Composite for Thermochemical Heat Storage during the Hydration in a 1 L Packed Bed Laboratory Reactor. Thermo. 2023; 3(4):593-604. https://doi.org/10.3390/thermo3040035
Chicago/Turabian StyleHeitmann, Stephan, Tamás Simon, Andrea Osburg, and Michael Fröba. 2023. "Alterations of a CaCl2 Alginate Composite for Thermochemical Heat Storage during the Hydration in a 1 L Packed Bed Laboratory Reactor" Thermo 3, no. 4: 593-604. https://doi.org/10.3390/thermo3040035
APA StyleHeitmann, S., Simon, T., Osburg, A., & Fröba, M. (2023). Alterations of a CaCl2 Alginate Composite for Thermochemical Heat Storage during the Hydration in a 1 L Packed Bed Laboratory Reactor. Thermo, 3(4), 593-604. https://doi.org/10.3390/thermo3040035







