Thermodynamically Informed Nuclear Fuel Codes—A Review and Perspectives
Abstract
:1. Introduction
2. Class I: Research and Development Fuel Codes
2.1. comsol
2.2. amp
2.3. bison
2.3.1. bison Simulations of UO Oxide Fuel
2.3.2. bison Simulations of U-Pu-Zr Metallic Fuel
2.4. marmot
2.5. alcyone
2.6. germinal
3. Class II: Industrial Nuclear Fuel Performance and Safety Codes
3.1. Cubicciotti
3.2. victoria
3.3. mfpr
3.4. melcor
3.5. astec
3.6. source
4. Discussion and Perspectives
4.1. Applications
4.2. Thermodynamic Databases
4.3. Thermodynamic Calculations
4.4. Quality Assurance
4.4.1. Verification
4.4.2. Validation
4.4.3. Software Quality Assurance
5. Conclusions
- Class I codes have demonstrated some useful progress in thermodynamically informed nuclear fuel codes, such as helping interpret experimental findings, capability development, and knowledge gap identification.
- Class II codes have demonstrated a widespread utility of thermodynamically informed calculations under SA conditions whereby the added value is mainly in predicting the release of low-volatile fission products and fuel volatilization.
- There have not been any reports of Class II codes being informed by thermodynamic calculations under NOC conditions. The value in doing so is not as clear, which is mainly because of differences in intended applications.
- Thermodynamic databases of irradiated UO and (U,Pu)O (MOX) fuel have significantly evolved and benefited from a plethora of experimental data. International co-operation has proven effective in database development.
- Thermodynamic databases of irradiated non-conventional fuels (e.g., U-C-O, U-C-N, molten salts) are not as well developed and require further investigations.
- Significant improvements in simulation fidelity have been reported with direct coupling of nuclear fuel codes with a thermodynamic code, such as fission product solubility, fuel oxidation, etc. Yet the added simulation fidelity of code coupling may not always be warranted given the potential for a large increase in computational expense. The increase in computational expense can be large for FEA codes but modest for integral system level codes.
- There have not been any concerted discussions or reports in the open literature related to SQA of thermodynamic equilibrium codes. While this may be acceptable for use with Class I codes at this time, this is an area that may require careful attention for Class II codes moving forward.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaufman, L.; Bernstein, H. Computer Calculation of Phase Diagrams with Special Reference to Refractory Metals; Academic Press: Cambridge, MA, USA, 1970. [Google Scholar]
- White, W.; Johnson, S.; Dantzig, G. Chemical Equilibrium in Complex Mixtures. J. Chem. Phys. 1958, 28, 751–755. [Google Scholar] [CrossRef]
- Bale, C.; Bélisle, E.; Chartrand, P.; Decterov, S.; Eriksson, G.; Gheribi, A.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Sundman, B.; Jansson, B.; Andersson, J.O. The Thermo-Calc Databank System. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Otis, R.; Liu, Z.K. pycalphad: CALPHAD-based computational thermodynamics in python. Open Res. Softw. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Sundman, B.; Lu, X.G.; Ohtani, H. The implementation of an algorithm to calculate thermodynamic equilibria for multi-component systems with non-ideal phases in a free software. Comput. Mater. Sci. 2015, 101, 127–137. [Google Scholar] [CrossRef]
- Piro, M.; Simunovic, S.; Besmann, T.; Lewis, B.; Thompson, W. The Thermochemistry Library thermochimica. Comput. Mater. Sci. 2013, 67, 266–272. [Google Scholar] [CrossRef]
- Lukas, H.; Fries, S.; Sundman, B. Computational Thermodynamics: The Calphad Method; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Hillert, M. Phase Equilibria, Phase Diagrams and Phase Transformations–Their Thermodynamic Basis, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Liu, Z.K.; Wang, Y. Computational Thermodynamics of Materials; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Pelton, A. Phase Diagrams and Thermodynamic Modeling of Solutions; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Guéneau, C.; Dumas, J.; Piro, M. In-reactor behavior. In Advances in Nuclear Fuel Chemistry; Piro, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 11; pp. 419–467. [Google Scholar]
- Guéneau, C.; Asta, M.; Sundman, B. Computational thermodynamics: Application to Nuclear Materials. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R., Stoller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, Chapter 26; pp. 814–849. [Google Scholar]
- Michel, B.; Ramìere, I.; Viallard, I.; Introini, C.; Lainet, M.; Chauvin, N.; Marelle, V.; Boulore, A.; Helfer, T.; Masson, R.; et al. Chapter 9-Two fuel performance codes of the PLEIADES platform: ALCYONE and GERMINAL. In Nuclear Power Plant Design and Analaysis Codes; Wang, J., Li, X., Allison, C., Hohorst, J., Eds.; Woodhead: Sawston, UK, 2021. [Google Scholar]
- Clarno, K.; Philip, B.; Cochran, W.; Sampath, R.; Allu, S.; Barai, P.; Simunovic, S.; Berrill, M.; Ott, L.; Pannala, S.; et al. The AMP (Advanced Multi-Physics) Nuclear Fuel Performance code. Nucl. Eng. Des. 2012, 252, 108–120. [Google Scholar] [CrossRef]
- Baurens, B.; Sercombe, J.; Riglet-Martial, C.; Desgranges, L.; Trotignon, L.; Maugis, P. 3D thermo-chemical-mechanical simulation of power ramps with ALCYONE fuel code. J. Nucl. Mater. 2014, 452, 578–594. [Google Scholar] [CrossRef] [Green Version]
- Cantrel, L.; Cousin, F.; Chevalier-Jabet, L.; Marchetto, C. ASTEC V2 severe accident integral code: Fission product modelling and validation. Nucl. Eng. Des. 2014, 272, 195–206. [Google Scholar] [CrossRef]
- Williamson, R.; Hales, J.; Novascone, S.; Tonks, M.; Gaston, D.; Permann, C.; Andrs, D.; Martineau, R. Multidimensional multiphysics simulation of nuclear fuel behavior. J. Nucl. Mater. 2012, 423, 149–163. [Google Scholar] [CrossRef]
- Eriksson, G.; Königsberger, E. FactSage and ChemApp: Two tools for the prediction of multiphase chemical equilibria in solutions. Pure Appl. Chem. 2008, 80, 1293–1302. [Google Scholar] [CrossRef] [Green Version]
- COMSOL Multiphysics Reference Manual; Technical Report CM020005; COMSOL; COMSOL Inc.: Stockholm, Sweden, 2019.
- Tonks, M.; Gaston, D.; Millett, P.; Andrs, D.; Talbot, P. An object-oriented finite element framework for multiphysics phase field simulations. J. Nucl. Mater. 2012, 51, 20–29. [Google Scholar] [CrossRef]
- Humpries, L.; Gauntt, R. MELCOR 2.2 severe accident analysis code–current status and plans for future. In IAEA Technical Meeting on the Status and Evaluation of Severe Accident Simulation Codes for Water Cooled Reactors; International Atomic Energy Agency: Vienna, Austria, 2017. [Google Scholar]
- Veshchunov, M.; Ozrin, V.; Shestak, V.; Tarasov, V.; Dubourg, R.; Nicaise, G. Development of the Mechanistic code MFPR for Modelling Fisison-Product Release from Irradiated UO2 Fuel. Nucl. Eng. Des. 2006, 236, 179–200. [Google Scholar] [CrossRef]
- Eriksson, G.; Rosen, E. General equations for the calculation of equilibria in multiphase systems. Chem. Scr. 1973, 4, 193–194. [Google Scholar]
- Barber, D.; Iglesias, F.; Hoang, Y.; Dickson, L.; Dickson, R.; Richards, M.; Gibb, R. SOURCE IST 2.0: Development and beta testing. In Proceedings of the CANDU Fuel Conference, Niagara Falls, ON, Canada, 26–30 September 1999. [Google Scholar]
- Heames, T.; Williams, D.; Bixler, N.; Grimley, A.; Wheatley, C.; Johns, N.; Domagala, P.; Dickson, L.; Alexander, C.; Osborn-Lee, I.; et al. VICTORIA: A Mechanistic Model of Radionuclide Behavior in the Reactor Coolant System under Severe Accident Conditions; Technical Report SAND90-0756; Sandia National Laboratories: Albuquerque, NM, USA, 1992. [Google Scholar]
- Higgs, J.; Lewis, B.; Thompson, W.; He, Z. A Conceptual Model for the Fuel Oxidation of Defective Fuel. J. Nucl. Mater. 2007, 366, 99–128. [Google Scholar] [CrossRef]
- Lindemer, T.; Besmann, T. Chemical Thermodynamic Representation of UO2±x. J. Nucl. Mater. 1985, 130, 473–488. [Google Scholar] [CrossRef] [Green Version]
- Welland, M.; Thompson, W.; Lewis, B.; Manara, D. Computer Simulations of Non-Congruent Melting of Hyperstoichiometric Uranium Dioxide. J. Nucl. Mater. 2009, 385, 358–363. [Google Scholar] [CrossRef]
- Manara, D.; Ronchi, C.; Sheindlin, M.; Konings, R. On the present state of investigation of thermodynamic properties of solid and liquid UO2+x. J. Nucl. Mater. 2007, 362, 14–18. [Google Scholar] [CrossRef]
- Piro, M. Computation of Thermodynamic Equilibria Pertinent to Nuclear Materials in Multi-Physics Codes. Ph.D. Thesis, Royal Military College of Canada, Kingston, ON, Canada, 2011. [Google Scholar]
- Böhler, R.; Welland, M.; Prieur, D.; Cakir, P.; Vitova, T.; Pruessmann, T.; Pidchenko, I.; Hennig, C.; Guéneau, C.; Konings, R.; et al. Recent advances in the study of the UO2-PuO2 phase diagram at high temperatures. J. Nucl. Mater. 2014, 448, 330–339. [Google Scholar] [CrossRef]
- Manara, D.; Seibert, A.; Gouder, T.; Beneš, O.; Martel, L.; Colle, J.Y.; Griveau, J.C.; Walter, O.; Cambriani, A.; Blanco, O.; et al. Experimental Methods. In Advances in Nuclear Fuel Chemistry; Piro, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 2; pp. 89–158. [Google Scholar]
- Gauld, I.; Hermann, O.; Westfall, R. ORIGEN Scale System Module to Calculate Fuel Depletion, Actinide Transmutation, Fission Product Buildup and Decay, and Associated Radiation Terms; Technical Report ORNL/TM-2005/39, Version 6; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2005; Volume II, Section F7. [Google Scholar]
- Piro, M.; Banfield, J.; Clarno, K.; Simunovic, S.; Besmann, T.; Lewis, B.; Thompson, W. Coupled thermochemical, isotopic evolution and heat transfer simulations in highly irradiated UO2 nuclear fuel. J. Nucl. Mater. 2013, 441, 240–251. [Google Scholar] [CrossRef]
- Wieselquist, W.; Lefebvre, R.; Jessee, M. SCALE Code System, Version 6.2.4; Technical Report ORNL/TM-2005/39; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2020. [Google Scholar]
- Lewis, B.; Thompson, W.; Iglesias, F. Fission Product Chemistry in Oxide Fuels. In Comprehensive Nuclear Materials; Konings, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2.20, pp. 515–546. [Google Scholar]
- Piro, M.; Sunderland, D.; Livingstone, S.; Sercombe, J.; Revie, R.; Quastel, A.; Terrani, K.; Judge, C. Pellet-clad interaction behavior in zirconium alloy fuel cladding. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R., Stoller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 2.09; pp. 248–306. [Google Scholar]
- Gaston, D.; Newman, C.; Hansen, G.; Lebrun-Grandié, D. MOOSE: A parallel computational framework for coupled systems of nonlinear equations. Nucl. Eng. Des. 2009, 239, 1768–1778. [Google Scholar] [CrossRef] [Green Version]
- Simunovic, S.; Besmann, T.; Moore, E.; Poschmann, M.; Piro, M.; Clarno, K.; McMurray, J.; Wieselquist, W. Modeling and simulation of oxygen transport in high burnup LWR fuel. J. Nucl. Mater. 2020, 538, 152194. [Google Scholar] [CrossRef]
- Poschmann, M.; Piro, M.; Simunovic, S. Acceleration of Thermochimica Calculations in BISON; Technical Report ORNL/TM-2020/1473; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2020. [Google Scholar]
- Poschmann, M.; Bajpai, P.; Fitzpatrick, B.; Piro, M. Recent developments for molten salt systems in Thermochimica. Calphad 2021, 75, 102341. [Google Scholar] [CrossRef]
- Poschmann, M.; Piro, M.; Besmann, T.; Simunovic, S. Thermochemically-informed mass transport model for interdiffusion of U and Zr in irradiated U-Pu-Zr fuel with fission products. J. Nucl. Mater. 2021, 554, 153089. [Google Scholar] [CrossRef]
- Kim, Y.; Hofman, G.; Hayes, S.; Sohn, Y. Constituent redistribution in U-Pu-Zr fuel during irradiation. J. Nucl. Mater. 2004, 327, 27–36. [Google Scholar] [CrossRef]
- Hirschhorn, J.; Tonks, M.; Matthews, C. A CALPHAD-informed approach to modeling constituent redistribution in Zr-based metallic fuels using BISON. J. Nucl. Mater. 2021, 544, 152657. [Google Scholar] [CrossRef]
- Bai, X.M.; Tonks, M.; Zhang, Y.; Hales, J. Multiscale modeling of thermal conductivity of high burnup structures in UO2 fuels. J. Nucl. Mater. 2016, 470, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Aagesen, L.; Andersson, D.; Beeler, B.; Cooper, M.; Gamble, K.; Miao, Y.; Pastore, G.; Tonks, M. Phase-field simulations of intergranular fission gas bubble behavior in U3Si2 nuclear fuel. J. Nucl. Mater. 2020, 541, 152415. [Google Scholar] [CrossRef]
- Tonks, M.; Cheniour, A.; Aagesen, L. How to apply the phase field method to model radiation damage. Comput. Mater. Sci. 2018, 147, 353–362. [Google Scholar] [CrossRef]
- Tonks, M.; Andersson, D.; Phillpot, S.; Zhang, Y.; Williamson, R.; Stanek, C.; Uberuaga, B.; Hayes, S. Mechanistic materials modeling for nuclear fuel performance. Ann. Nucl. Energy 2017, 105, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Schwen, D.; Aagesen, L.; Peterson, J.; Tonks, M. Rapid multiphase-field model development using a modular free energy based approach with automatic differentiation in MOOSE/MARMOT. Comput. Mater. Sci. 2017, 132, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Guéneau, C.; Dupin, N.; Sundman, B.; Martial, C.; Dumas, J.C.; Gossé, S.; Chatain, S.; Bruycker, F.D.; Manara, D.; Konings, R.J. Thermodynamic modelling of advanced oxide and carbide nuclear fuels: Description of the U-Pu-O-C systems. J. Nucl. Mater. 2011, 419, 145–167. [Google Scholar] [CrossRef]
- Bajpai, P.; Poschmann, M.; Andrs, D.; Bhave, C.; Tonks, M.; Piro, M. Development of a new thermochemistry solver for multiphysics simulations of nuclear materials. In TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1013–1025. [Google Scholar]
- Michel, B.; Sercombe, J.; Nonon, C.; Fandeur, O. Modeling of pellet cladding interaction. In Comprehensive Nuclear Materials; Konings, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 3, pp. 677–712. [Google Scholar]
- Marelle, V.; Goldbronn, P.; Bernaud, S.; Castelier, E.; Julien, J.; Nkonga, K.; Noirot, L.; Ramiere, I. New developments in ALCYONE 2.0 fuel performance code. In Proceedings of the Top Fuel 2016-Light Water Reactor (LWR) Fuel Performance Meeting, Boise, ID, USA, 11–15 September 2016. [Google Scholar]
- Lemoine, F.; Bernard, D.; Federici, E. Validation assessment of neutron calculations for radial and azimuthal distributions of actinides and fission products in PWR rods. In Proceedings of the Water Reactor Fuel Performance Meeting, Chengdu, China, 11–14 September 2011. [Google Scholar]
- Noirot, L. MARGARET: A comprehensive code for the description of fission gas behavior. Nucl. Eng. Des. 2011, 241, 2099–2118. [Google Scholar] [CrossRef]
- Baron, D.; Thevenin, P.; Largenton, R.; Masson, R.; Pujet, S.; Arnaud, R. CYRANO3 an EDF nuclear fuel performance code designed for engineering applications. In Proceedings of the 10th International Conference on CANDU Fuel, Toronto, ON, Canada, 5–8 October 2008. [Google Scholar]
- GALILEO Fuel Rod Thermal-Mechanical Methodology for Pressurized Water Reactors; Rev1 ANP-10323NP; Framatome: La Défense, Courbevoie, France, 2018.
- Eriksson, G. Thermodynamic studies of high temperature equilibria: XII. SOLGASMIX, A computer program for calculation of equilibrium compositions in multiphase systems. Chem. Scr. 1975, 8, 100–103. [Google Scholar]
- Cordfunke, E.; Konings, R. Thermochemical data for reactor materials and fission products: The ECN database. J. Phase Equilibria 1993, 14, 457–464. [Google Scholar] [CrossRef]
- Schram, R.; Konings, R.; Rijnsburger, W. TBASE CONSULT Manual; Technical Report; The Netherlands Energy Research Foundation: Petten, The Netherlands, 2002. [Google Scholar]
- Introïni, C.; Sercombe, J.; Sundman, B. Development of a robust, accurate and efficient coupling between PLEIADES/ALCYONE 2.1 fuel performance code and the OpenCalphad thermo-chemical solver. Nucl. Eng. Des. 2020, 369, 110818. [Google Scholar] [CrossRef]
- Guéneau, C.; Dupin, N.; Kjellqist, L.; Geiger, E.; Kurata, M.; Gossé, S.; Corcoran, E.; Quaini, A.; Hania, R.; Smith, A.; et al. TAF-ID: An international thermodynamic database for nuclear fuels and applications. Calphad 2021, 72, 102212. [Google Scholar] [CrossRef]
- Lainet, M. GERMINAL, a fuel performance code of the PLEIADES platform to simulate the in-pile behavior of mixed oxide fuel pins for sodium-cooled fast reacors. J. Nucl. Mater. 2019, 516, 30–53. [Google Scholar] [CrossRef]
- Guérin, Y. Fuel performance of fast spectrum oxide fuel. In Comprehensive Nuclear Materials; Konings, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, pp. 547–578. [Google Scholar]
- Samuelsson, K.; Dumas, J.; Sundman, B.; Lainet, M. An improved method to evaluate the ‘Joint Oxyde-Gaine’ formation in (U,Pu)O2 irradiated fuels using the Germinal V2 code coupled to Calphad thermodynamic computations. EPJ Nucl. Sci. Technol. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Kurata, M.; Osaka, M.; Jacquemain, D.; Barrachin, M.; Haste, T. Advances in fuel chemistry during a severe accident. In Advances in Nuclear Fuel Chemistry; Piro, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 14; pp. 555–625. [Google Scholar]
- Van Uffelen, P.; Hales, J.; Li, W.; Rossiter, G.; Williamson, R. A review of fuel performance modelling. J. Nucl. Mater. 2019, 516, 373–412. [Google Scholar] [CrossRef]
- Van Uffelen, P.; Pastore, G. Oxide fuel performance modeling and simulation. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R., Stoller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, Chapter 13; pp. 363–416. [Google Scholar]
- Ogata, T.; Kim, Y.; Yacout, A. Metal fuel performance modeling and simulation. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R., Stoller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, Chapter 2; pp. 43–87. [Google Scholar]
- Verfondern, K.; Nabielek, H. TRISO fuel performance modeling and simulation. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R., Stoller, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, Chapter 11; pp. 361–397. [Google Scholar]
- Cubicciotti, D. Thermodynamics of vaporization of fission products and materials under severe reactor accident conditions: Analysis of molten core/concrete chemistry. J. Nucl. Mater. 1985, 130, 3–17. [Google Scholar] [CrossRef]
- Cubicciotti, D. Vapor transport of fission products under nuclear accident conditions. J. Nucl. Mater. 1988, 154, 53–61. [Google Scholar] [CrossRef]
- Olander, D.; Mubayi, V. Review of the Materials-Chemistry Models in the VICTORIA Code. J. Nucl. Mater. 1999, 270, 1–10. [Google Scholar] [CrossRef]
- Veshchunov, M.; Dubourg, R.; Ozrin, V.; Shestak, V.; Tarasov, V. Mechanistic modelling of urania fuel evolution and fission product migration during irradiation and heating. J. Nucl. Mater. 2007, 362, 327–335. [Google Scholar] [CrossRef]
- Ducros, G.; Malgouyres, P.; Kissane, M.; Boulaud, D.; Durin, M. Fission product release under severe accidental conditions: General presentation of the program and synthesis of VERCORS 1–6 results. Nucl. Eng. Des. 2001, 208, 191–203. [Google Scholar] [CrossRef]
- Dubourg, R.; Barrachin, M.; Ducher, R.; Gavillet, D.; Bremaecker, A.D. Fuel and fission product behaviour in early phases of a severe accident. Part II: Interpretation of the experimental results of the PHEBUS FPT2 test. J. Nucl. Mater. 2014, 453, 355–374. [Google Scholar] [CrossRef]
- Powers, D.; Brockmann, J.; Shiver, A. VANESA: A Mechanistic Model of Radionuclide Release and Aerosol Generation during Core Debris Interactions with Concrete; Technical Report SAND85-1370; Sandia National Laboratories: Albuquerque, NM, USA, 1985. [Google Scholar]
- Piro, M. Computational Thermochemistry of Nuclear Fuel. In Advances in Nuclear Fuel Chemistry; Piro, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 4; pp. 159–182. [Google Scholar]
- Brillant, G.; Marchetto, C.; Plumecocq, W. Fission product release from nuclear fuel I. Physical modelling in the ASTEC code. Ann. Nucl. Energy 2013, 61, 88–95. [Google Scholar] [CrossRef]
- Brillant, G. Models for the release from fuel of Ce, La, Sr, and Eu in accident conditions. Prog. Nucl. Energy 2011, 53, 125–131. [Google Scholar] [CrossRef]
- Brito, A.; Iglesias, F.; Liu, Y.; Petrilli, M.; Richards, M.; Gibb, R.; Reid, P. SOURCE 2.0: A computer program to calculation fission product release from multiple fuel elements for accident scenarios. In Proceedings of the CANDU Fuel Conference, Pembroke, ON, Canada, 1–4 October 1995. [Google Scholar]
- Barber, D.; Parlatan, Y.; Dickson, L.; Corse, B.; Kaye, M.; Lewis, B.; Thompson, W.; Colins, K.; Dickson, R.; Hoang, Y.; et al. SOURCE IST 2.0: Fission product release code. In Proceedings of the CANDU Fuel Conference, Belleville, ON, Canada, 18–21 September 2005. [Google Scholar]
- Barber, D. Implementation of a Gibbs energy minimizer in a fission-product release computer program. AECL Nucl. Rev. 2013, 2, 39–48. [Google Scholar] [CrossRef]
- Corse, B. Form 2.0: Fuel Oxidation and Release Model. A Computer Code to Predict the Low Volatile Fission-Product Release and Fuel Volatilization from Uranium Dioxide Fuel Under Severe Reactor Accident Conditions. Ph.D. Thesis, Royal Military College of Canada, Kingston, ON, Canada, 1997. [Google Scholar]
- Lewis, B.; Corse, B.; Thompson, W.; Kaye, M.; Iglesias, F.; Elder, P.; Dickson, R.; Liu, Z. Low volatile fission-product release and fuel volatilization during severe reactor accident conditions. J. Nucl. Mater. 1998, 252, 235–256. [Google Scholar] [CrossRef]
- Kaye, M.; Lewis, B.; Thompson, W. Thermodynamic treatment of noble metal fission products in nuclear fuel. J. Nucl. Mater. 2007, 366, 8–27. [Google Scholar] [CrossRef]
- Corcoran, E.; Lewis, B.; Thompson, W.; Mouris, J.; He, Z. Controlled Oxidation Experiments of Simulated Irradiated UO2 Fuel in Relation to Thermochemical Modelling. J. Nucl. Mater. 2010, 414, 73–82. [Google Scholar] [CrossRef]
- Thompson, W.; Lewis, B.; Corcoran, E.; Kaye, M.; White, S.; Akbari, F.; He, Z.; Verrall, R.; Higgs, J.; Thompson, D.; et al. Thermodynamic Treatment of Uranium Dioxide Based Nuclear Fuel. Int. J. Mater. Res. 2007, 98, 1004–1011. [Google Scholar] [CrossRef]
- Piro, M. Thermodynamic Predictions of CANDU Fuel Volatilization and Fission Product Behaviour Under Severe Accident Conditions. J. Nucl. Mater. in-review.
- Geiger, E.; Guéneau, C.; Corcoran, E.; Piro, M. Thermodynamic investigations of fuel-cladding chemical interaction in U-5Fs and U-10Zr metallic fuels with the TAF-ID. J. Nucl. Mater. 2021, 551, 152981. [Google Scholar] [CrossRef]
- Magni, A.; Nevo, A.D.; Luzzi, L.; Rozzia, D.; Adorni, M.; Schubert, A.; Uffelen, P.V. The TRANSURANUS fuel performance code. In Nuclear Power Plant Design and Analaysis Codes; Wang, J., Li, X., Allison, C., Hohorst, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 8; pp. 161–205. [Google Scholar]
- Kleykamp, H. The chemical state of the fission products in oxide fuels. J. Nucl. Mater. 1985, 131, 221–246. [Google Scholar] [CrossRef]
- Cheynet, B.; Fischer, E. MEPHISTA: A Thermodynamic Database for New Generation Nuclear Fuels; Technical Report hal-00222025; Thermodata-INPG-CNRS: Saint Martin d’Hères, France, 2007. [Google Scholar]
- McMurray, J.; Besmann, T.; Ard, J.; Fitzpatrick, B.; Piro, M.; Jerden, J.; Williamson, M.; Collins, B.; Betzler, B.; Qualls, A. Multi-Physics Simulations for Molten Salt Reactor Evaluation: Chemistry Modeling and Database Development; Technical Report ORNL/SPR-2018/864; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2018. [Google Scholar]
- Piro, M.; Simunovic, S. Performance Enhancing Algorithms for Computing Thermodynamic Equilibria. Calphad 2012, 39, 104–110. [Google Scholar] [CrossRef]
- Piro, M.; Simunovic, S. Global optimization algorithms to compute thermodynamic equilibria in large complex systems with performance considerations. Comput. Mater. Sci. 2016, 118, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Ni, X. Nuclear engineering software quality assurance. In Nuclear Power Plant Design and Analaysis Codes; Wang, J., Li, X., Allison, C., Hohorst, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 3; pp. 55–74. [Google Scholar]
- Piro, M.; Besmann, T.; Simunovic, S.; Lewis, B.; Thompson, W. Numerical verification of equilibrium thermodynamic computations in nuclear fuel performance codes. J. Nucl. Mater. 2011, 414, 399–407. [Google Scholar] [CrossRef]
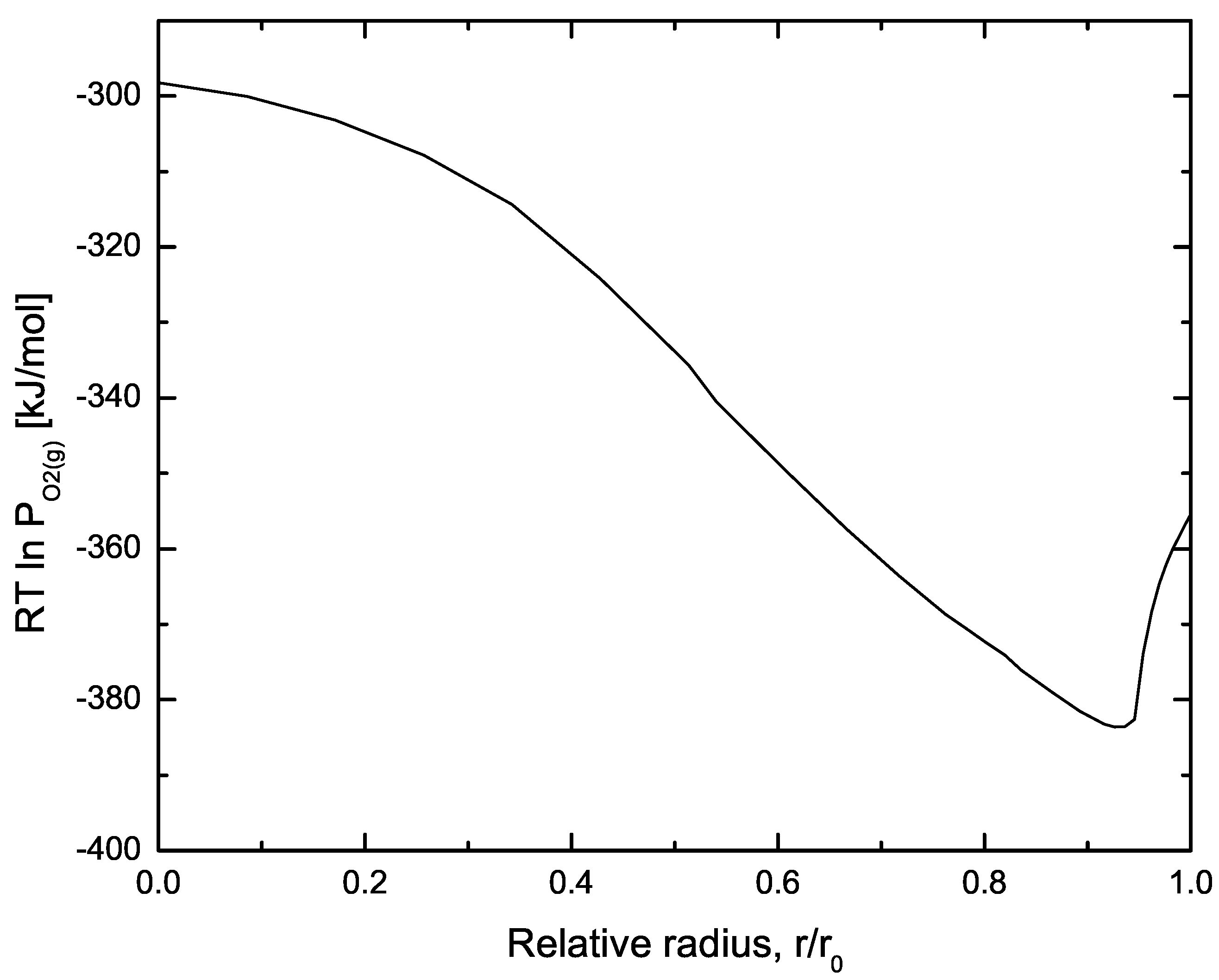
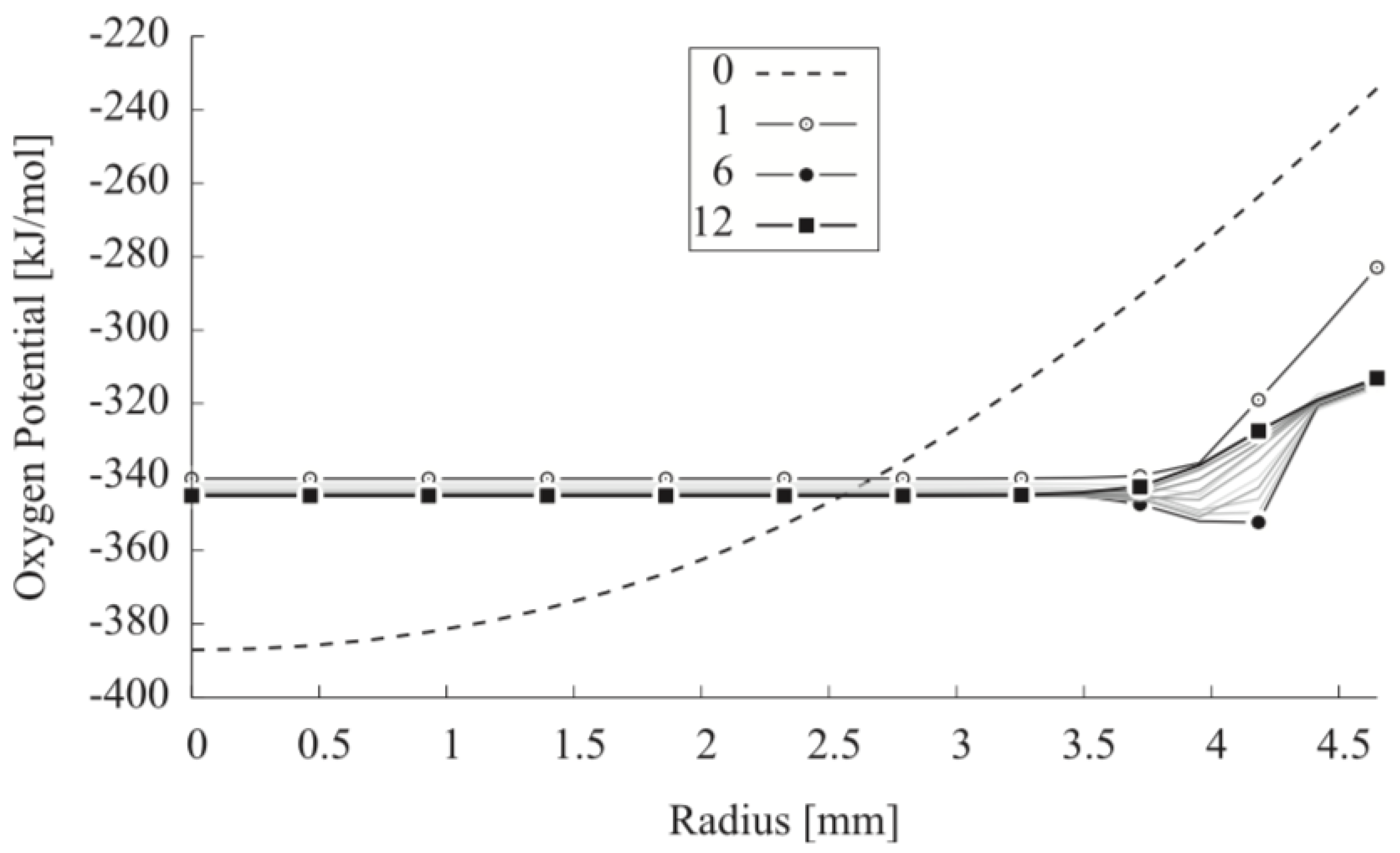
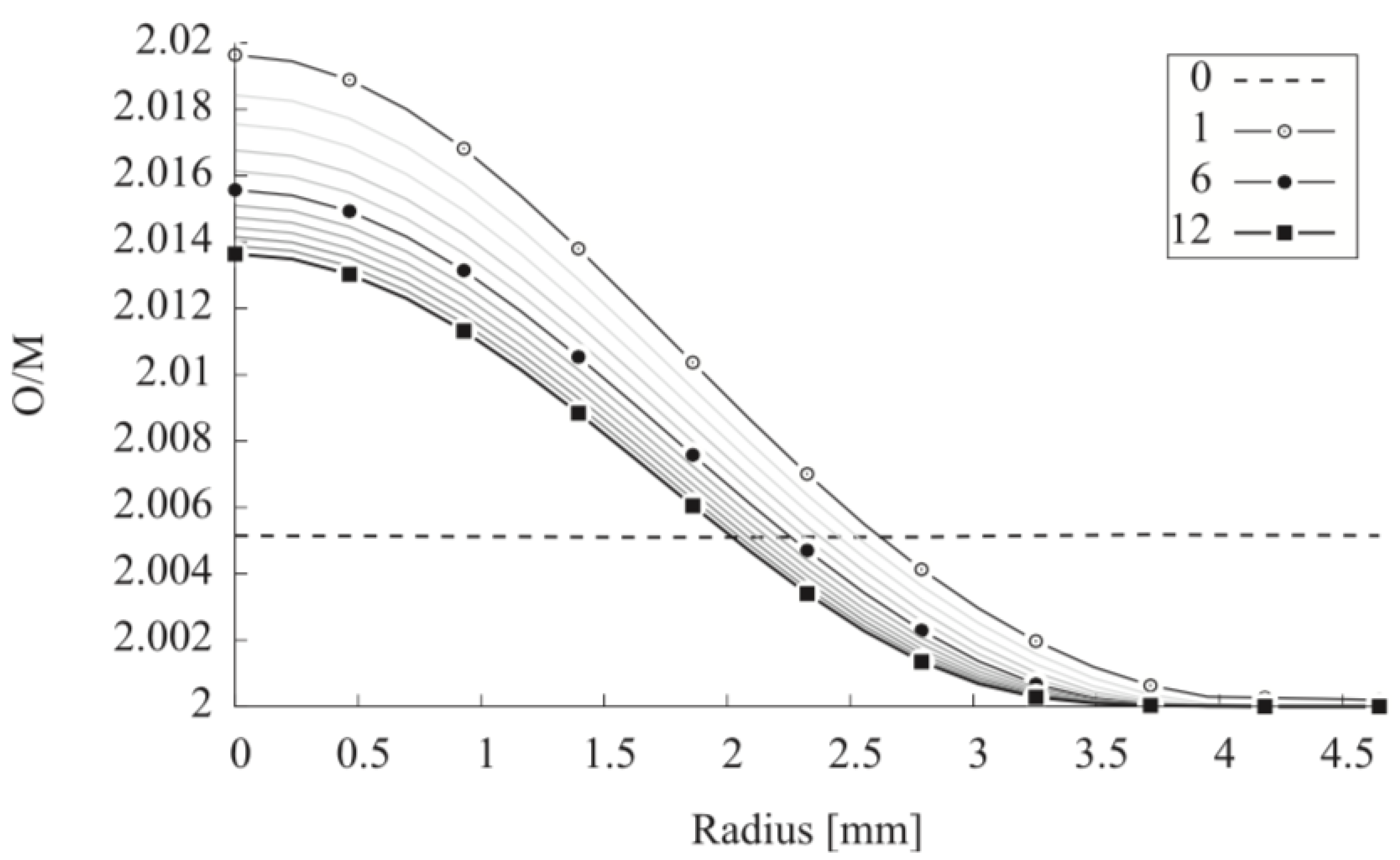
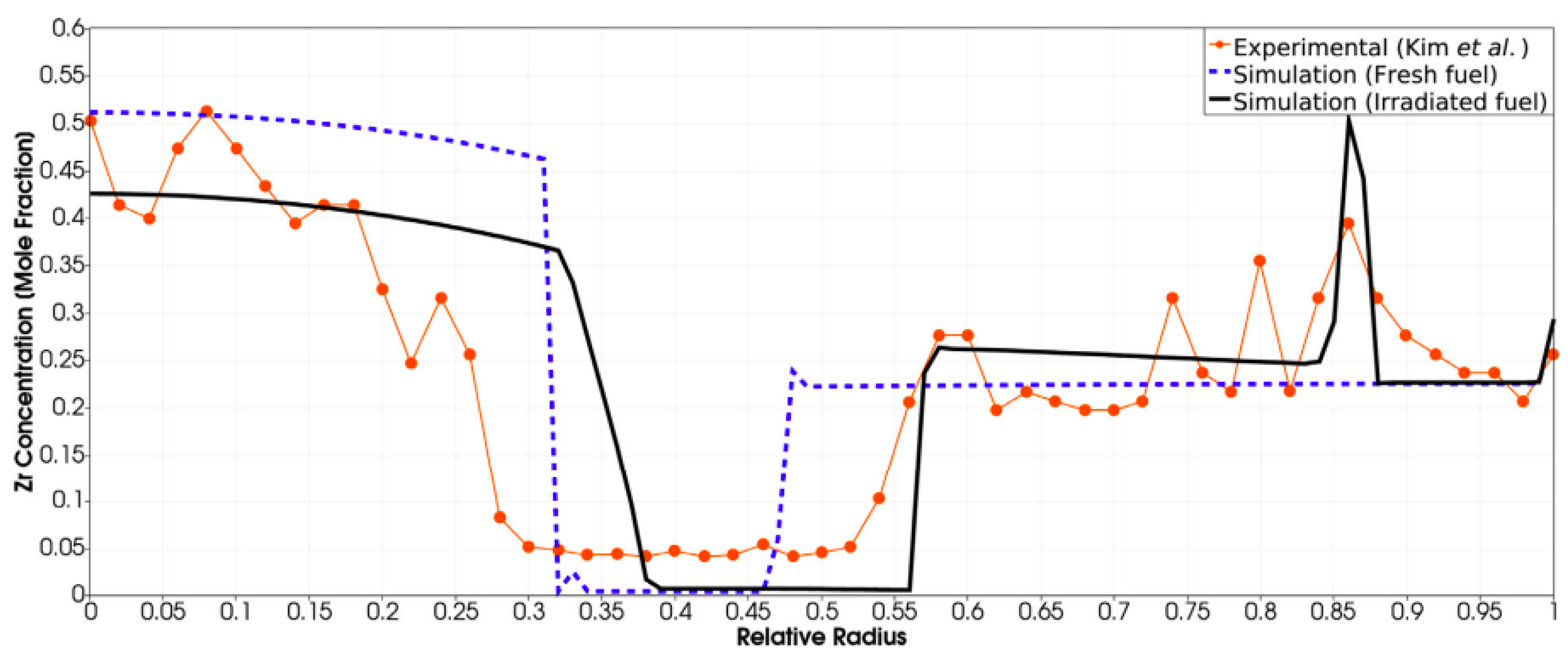
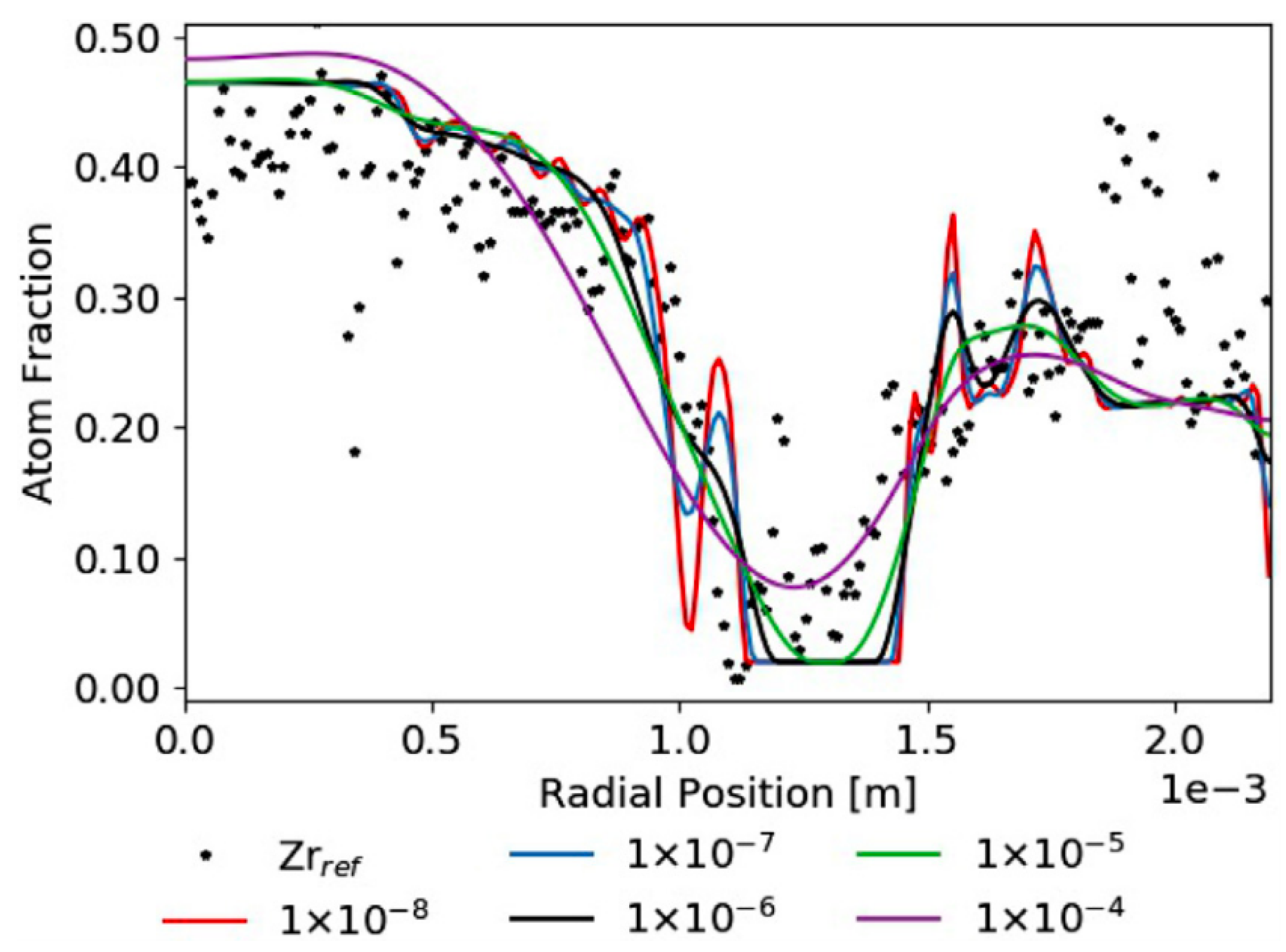
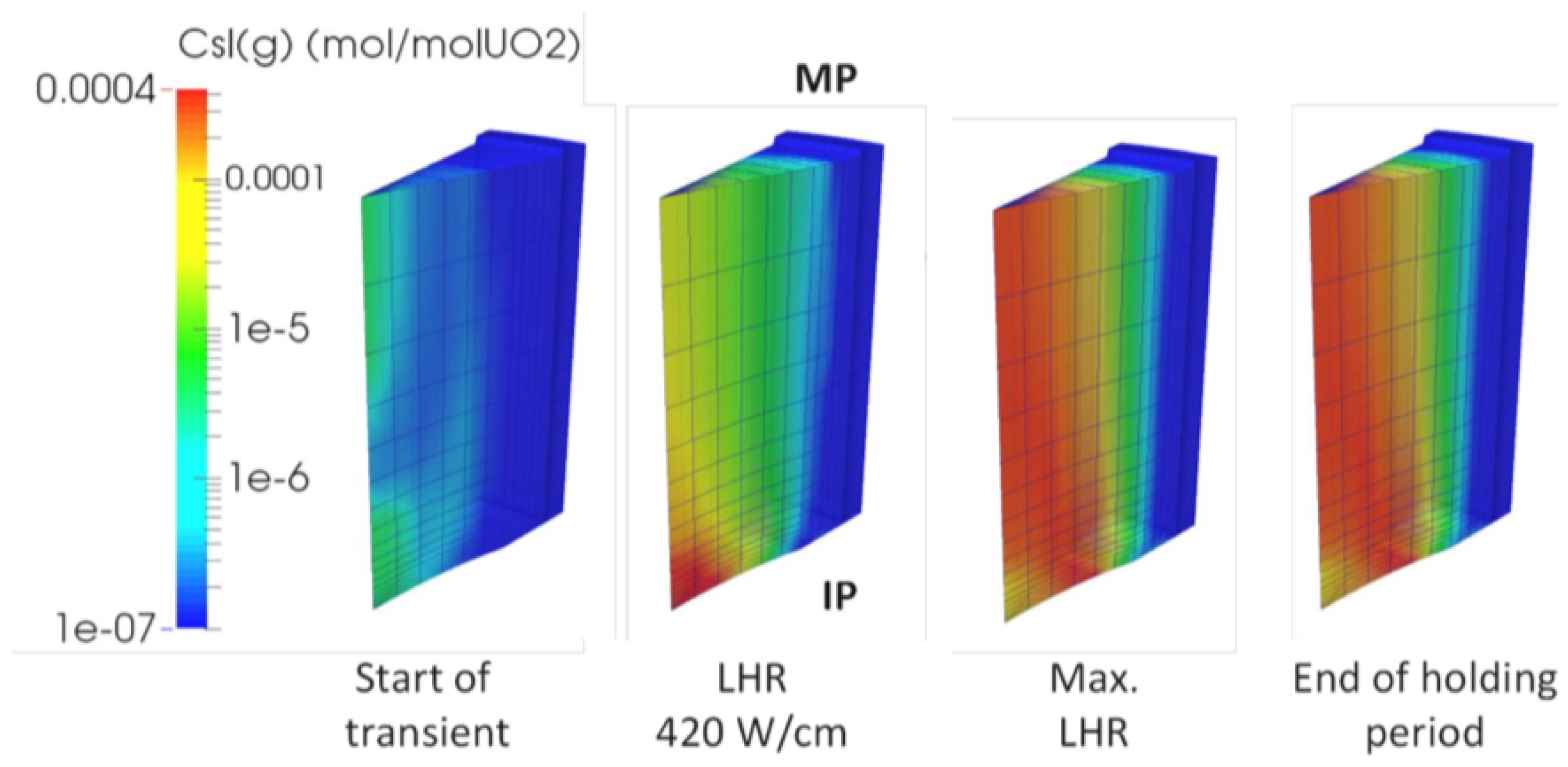
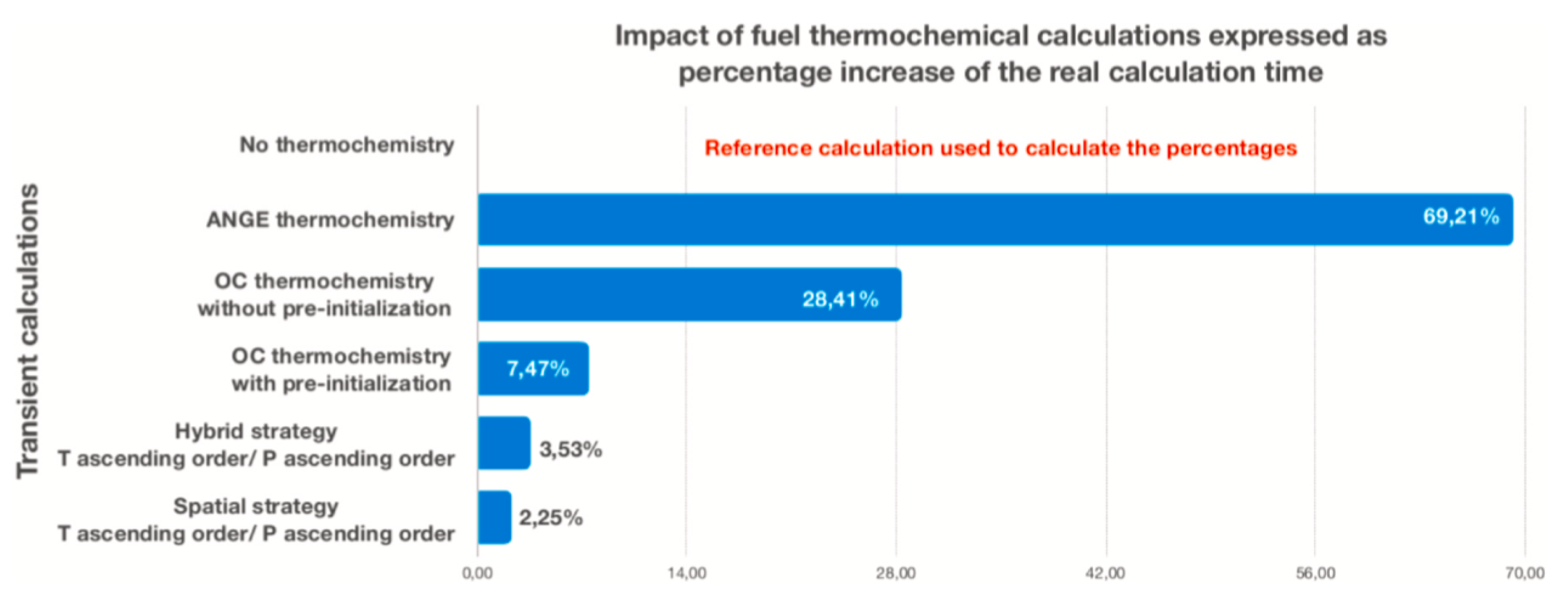
| Code Name | Primary Application | Coupling Method | Ref. |
|---|---|---|---|
| alcyone | Class I | ange, OpenCalphad | [14] |
| amp | Class I | thermochimica | [15] |
| ange | GEM | [16] | |
| astec | Class II | Empirical correlations | [17] |
| bison | Class I | thermochimica | [18] |
| ChemApp | GEM | [19] | |
| comsol | Class I | Simplified model | [20] |
| FactSage | GEM | [3] | |
| germinal | Class I | ange, OpenCalphad | [14] |
| marmot | Class I | Simplified model; Look-up table | [21] |
| melcor | Class II | GEM (unknown) | [22] |
| mfpr | Class II | Unknown | [23] |
| OpenCalphad | GEM | [6] | |
| pyCalphad | GEM | [5] | |
| solgasmix | GEM | [24] | |
| source | Class II | Empirical correlations | [25] |
| ThermoCalc | GEM | [4] | |
| thermochimica | GEM | [7] | |
| victoria | Class II | Unknown | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piro, M.H.A. Thermodynamically Informed Nuclear Fuel Codes—A Review and Perspectives. Thermo 2021, 1, 262-285. https://doi.org/10.3390/thermo1020018
Piro MHA. Thermodynamically Informed Nuclear Fuel Codes—A Review and Perspectives. Thermo. 2021; 1(2):262-285. https://doi.org/10.3390/thermo1020018
Chicago/Turabian StylePiro, Markus H. A. 2021. "Thermodynamically Informed Nuclear Fuel Codes—A Review and Perspectives" Thermo 1, no. 2: 262-285. https://doi.org/10.3390/thermo1020018
APA StylePiro, M. H. A. (2021). Thermodynamically Informed Nuclear Fuel Codes—A Review and Perspectives. Thermo, 1(2), 262-285. https://doi.org/10.3390/thermo1020018





