Stimuli-Responsive Luminescence of an Amphiphilic Flavin Derivative via Thermodynamic and Kinetic Aggregation in Water
Abstract
1. Introduction
2. Materials and Methods
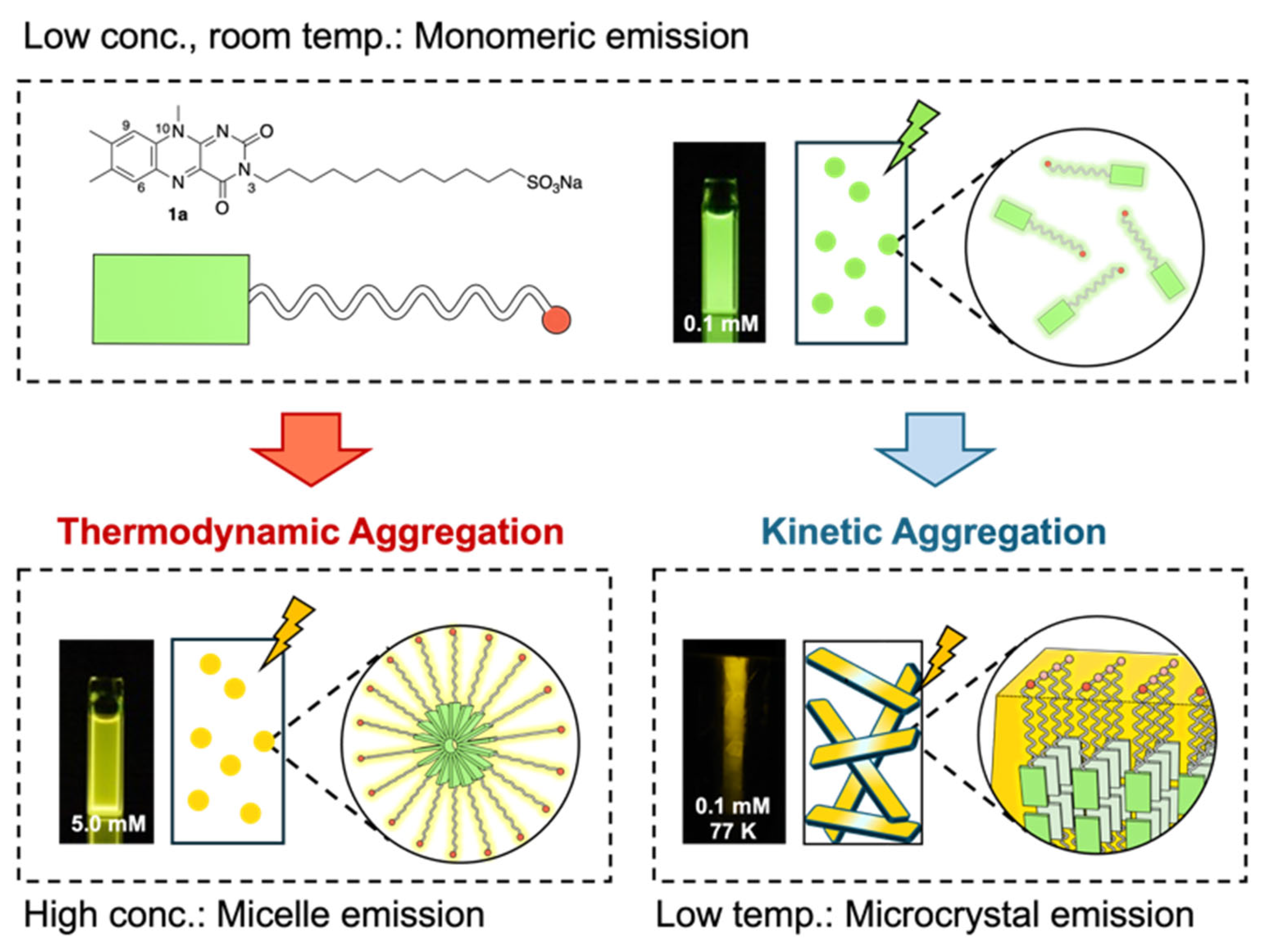
3. Results and Discussion
3.1. Stimuli-Responsive Photoluminescence Properties
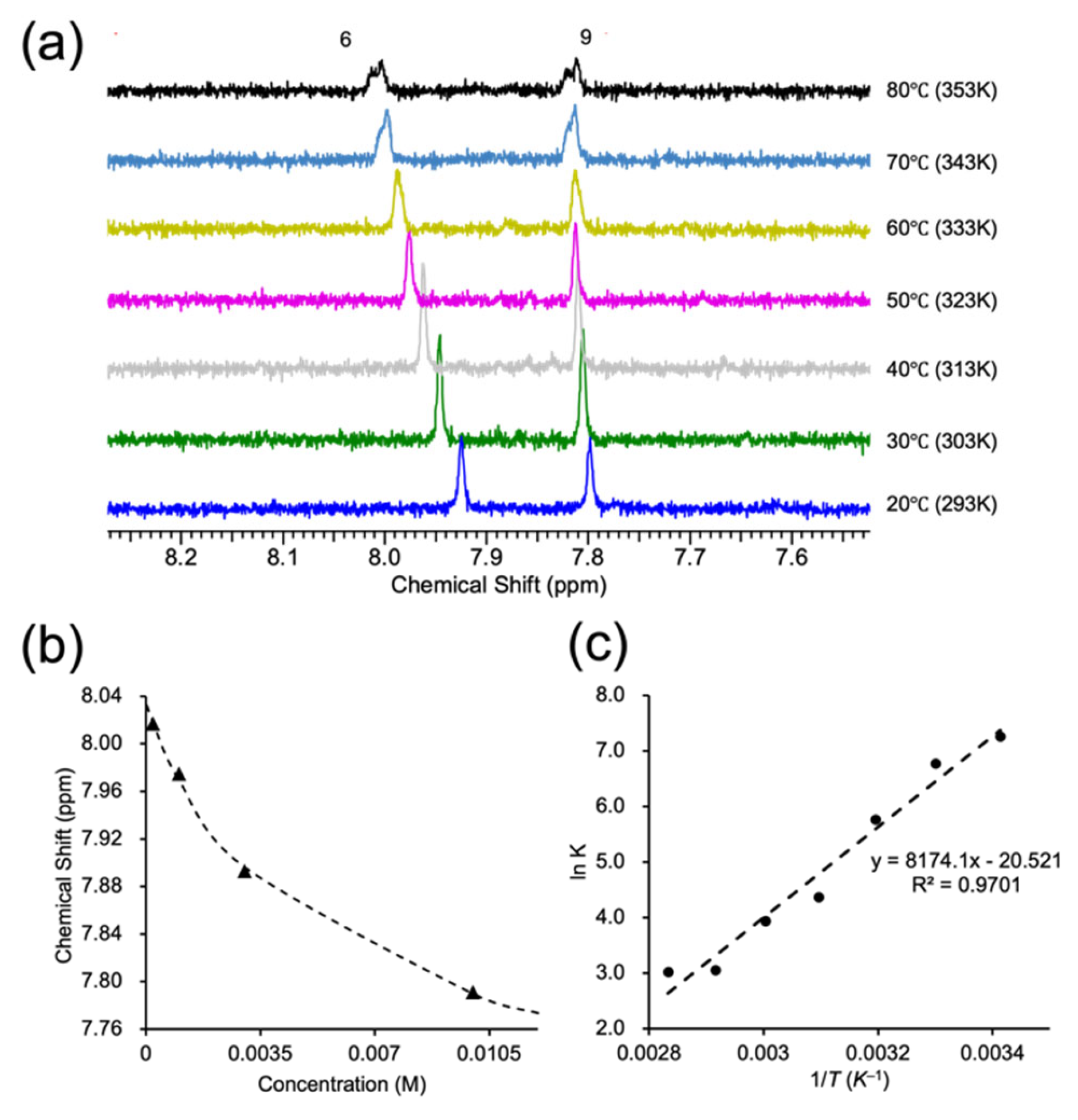
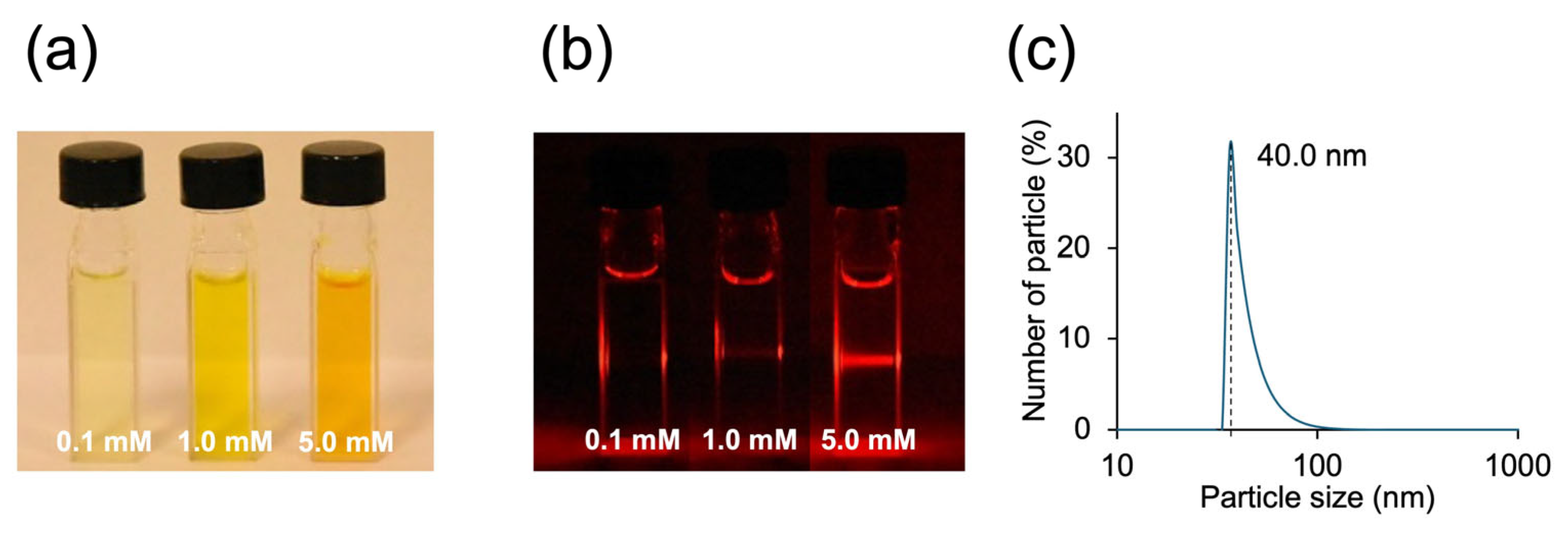
3.2. Thermodynamic Self-Assembly: Micelle-like Aggregation
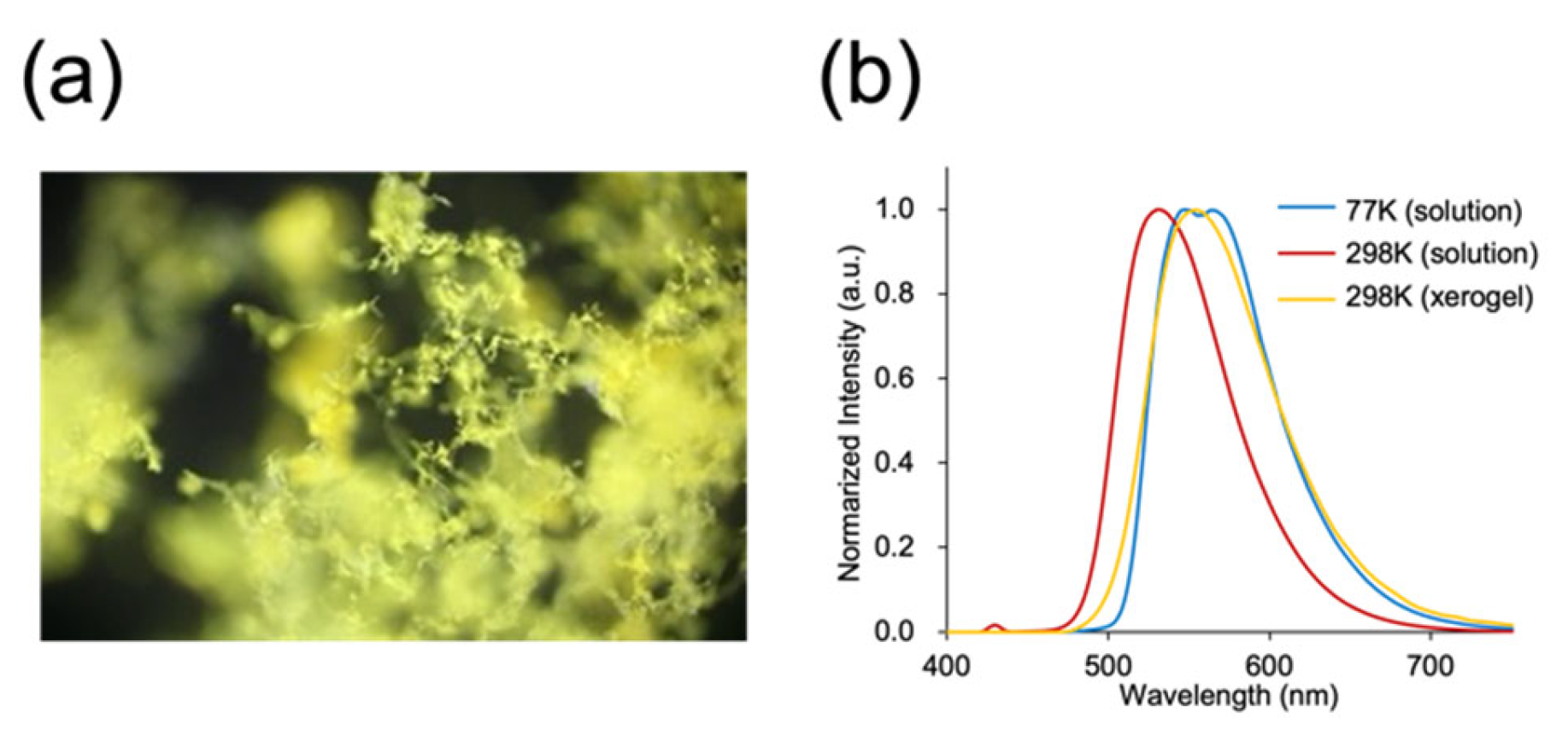
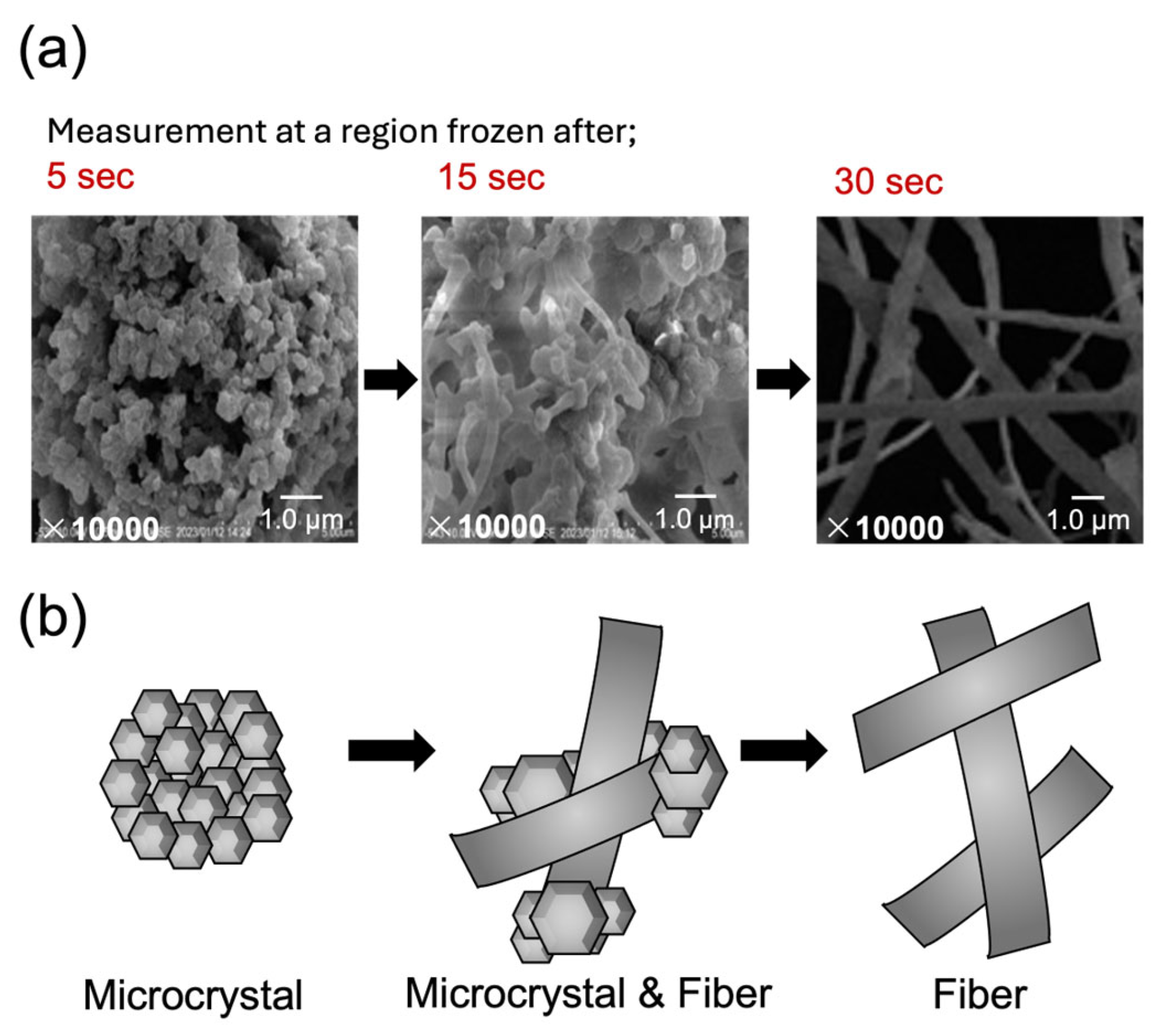
3.3. Kinetic Self-Assembly: Fiber-like Aggregation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, A.; Kobayashi, H. (Eds.) Responsive Materials and Methods: State-of-the-Art Stimuli-Responsive Materials and Their Applications; Advance Materials Series; Wiley: Beverly, MA, USA, 2014. [Google Scholar]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design Strategies for Water-Soluble Small Molecular Chromogenic and Fluorogenic Probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives. ACS Sens. 2017, 2, 1382–1399. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, F.J.M.; Herz, L.M.; Daniel, C.; Jonkheijm, P.; Schenning, A.P.H.J.; Silva, C.; Meskers, S.C.J.; Beljonne, D.; Phillips, R.T.; Friend, R.H.; et al. Efficient Energy Transfer in Mixed Columnar Stacks of Hydrogen-Bonded Oligo(p-phenylene Vinylene)s in Solution. Angew. Chem. Int. Ed. 2004, 43, 1976–1979. [Google Scholar] [CrossRef] [PubMed]
- Yagai, S.; Seki, T.; Karatsu, T.; Kitamura, A.; Würthner, F. Transformation from H- to J-Aggregated Perylene Bisimide Dyes by Complexation with Cyanurates. Angew. Chem. Int. Ed. 2008, 47, 3367–3371. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Zhang, X.; Xu, B.; Zhou, X.; Ma, C.; Zhang, Y.; Liu, S.; Xu, J. Recent Advances in Organic Mechanofluorochromic Materials. Chem. Soc. Rev. 2012, 41, 3878. [Google Scholar] [CrossRef]
- Kartha, K.K.; Babu, S.S.; Srinivasan, S.; Ajayaghosh, A. Attogram Sensing of Trinitrotoluene with a Self-Assembled Molecular Gelator. J. Am. Chem. Soc. 2012, 134, 4834–4841. [Google Scholar] [CrossRef]
- Basak, S.; Nanda, J.; Banerjee, A. Assembly of Naphthalenediimide Conjugated Peptides: Aggregation Induced Changes in Fluorescence. Chem. Commun. 2013, 49, 6891. [Google Scholar] [CrossRef]
- Samanta, S.K.; Bhattacharya, S. Excellent Chirality Transcription in Two-Component Photochromic Organogels Assembled through J-Aggregation. Chem. Commun. 2013, 49, 1425. [Google Scholar] [CrossRef]
- Praveen, V.K.; Ranjith, C.; Bandini, E.; Ajayaghosh, A.; Armaroli, N. Oligo(Phenylenevinylene) Hybrids and Self-Assemblies: Versatile Materials for Excitation Energy Transfer. Chem. Soc. Rev. 2014, 43, 4222–4242. [Google Scholar] [CrossRef]
- Yagai, S.; Okamura, S.; Nakano, Y.; Yamauchi, M.; Kishikawa, K.; Karatsu, T.; Kitamura, A.; Ueno, A.; Kuzuhara, D.; Yamada, H.; et al. Design Amphiphilic Dipolar π-Systems for Stimuli-Responsive Luminescent Materials Using Metastable States. Nat. Commun. 2014, 5, 4013. [Google Scholar] [CrossRef]
- Cao, X.; Meng, L.; Li, Z.; Mao, Y.; Lan, H.; Chen, L.; Fan, Y.; Yi, T. Large Red-Shifted Fluorescent Emission via Intermolecular π–π Stacking in 4-Ethynyl-1,8-Naphthalimide-Based Supramolecular Assemblies. Langmuir 2014, 30, 11753–11760. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, X.; Hu, R.; Xu, J.; Wang, S.; Li, S.; Li, Y.; Yang, G. Intracellular Fluorescent Temperature Probe Based on Triarylboron Substituted Poly N -Isopropylacrylamide and Energy Transfer. Anal. Chem. 2015, 87, 3694–3698. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Masuda, T.; Takayama, M.; Adachi, H.; Haino, T. Solvent-Induced Emission of Organogels Based on Tris(Phenylisoxazolyl)Benzene. Org. Biomol. Chem. 2016, 14, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Kwon, J.E.; Kim, H.-J.; Whang, D.R.; Park, S.Y. Smart Fluorescent Nanoparticles in Water Showing Temperature-Dependent Ratiometric Fluorescence Color Change. ACS Appl. Mater. Interfaces 2017, 9, 2883–2890. [Google Scholar] [CrossRef]
- Choudhury, P.; Das, K.; Das, P.K. l-Phenylalanine-Tethered, Naphthalene Diimide-Based, Aggregation-Induced, Green-Emitting Organic Nanoparticles. Langmuir 2017, 33, 4500–4510. [Google Scholar] [CrossRef]
- Das, P.; Kumar, A.; Chowdhury, A.; Mukherjee, P.S. Aggregation-Induced Emission and White Luminescence from a Combination of π-Conjugated Donor–Acceptor Organic Luminogens. ACS Omega 2018, 3, 13757–13771. [Google Scholar] [CrossRef]
- Liang, S.; Wang, Y.; Wu, X.; Chen, M.; Mu, L.; She, G.; Shi, W. An Ultrasensitive Ratiometric Fluorescent Thermometer Based on Frustrated Static Excimers in the Physiological Temperature Range. Chem. Commun. 2019, 55, 3509–3512. [Google Scholar] [CrossRef]
- Yadav, P.; Gond, S.; Singh, A.K.; Singh, V.P. A Pyrene-Thiophene Based Probe for Aggregation Induced Emission Enhancement (AIEE) and Naked-Eye Detection of Fluoride Ions. J. Lumin. 2019, 215, 116704. [Google Scholar] [CrossRef]
- Xiao, T.; Ren, D.; Tang, L.; Wu, Z.; Wang, Q.; Li, Z.-Y.; Sun, X.-Q. A Temperature-Responsive Artificial Light-Harvesting System in Water with Tunable White-Light Emission. J. Mater. Chem. A 2023, 11, 18419–18425. [Google Scholar] [CrossRef]
- Tang, L.; Wu, Z.; Zhang, Q.; Hu, Q.; Dang, X.; Cui, F.; Tang, L.; Xiao, T. A Sequential Light-Harvesting System with Thermosensitive Colorimetric Emission in Both Aqueous Solution and Hydrogel. Chem. Commun. 2024, 60, 4719–4722. [Google Scholar] [CrossRef]
- Chapman, S.K.; Perham, R.N.; Scrutton, N.S. Flavins and flavoproteins 2002. In Proceedings of the Fourteenth International Symposium, St. John’s College, University of Cambridge, Cambridge, UK, 14–18 July 2002. [Google Scholar]
- Weber, S.; Schleicher, E. (Eds.) Flavins and Flavoproteins: Methods and Protocols; Humana Press: New York, NY, USA, 2014. [Google Scholar]
- Ghisla, S.; Massey, V. Mechanisms of Flavoprotein-catalyzed Reactions. Euro. J. Biochem. 1989, 181, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Massey, V. The Chemical and Biological Versatility of Riboflavin. Biochem. Soc. Trans. 2000, 28, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Imada, Y.; Naota, T. Flavins as Organocatalysts for Environmentally Benign Molecular Transformations. Chem. Rec. 2007, 7, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Gelalcha, F.G. Heterocyclic Hydroperoxides in Selective Oxidations. Chem. Rev. 2007, 107, 3338–3361. [Google Scholar] [CrossRef]
- Imada, Y.; Iida, H.; Kitagawa, T.; Naota, T. Aerobic Reduction of Olefins by In Situ Generation of Diimide with Synthetic Flavin Catalysts. Chem. Euro. J. 2011, 17, 5908–5920. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Fraaije, M.W. Recent Developments in Flavin-based Catalysis. ChemCatChem 2013, 5, 403–415. [Google Scholar] [CrossRef]
- Imada, Y.; Kugimiya, Y.; Iwata, S.; Komiya, N.; Naota, T. Non-Covalently Dendronized Flavins as Organocatalysts for Aerobic Reduction of Olefins. Tetrahedron 2013, 69, 8572–8578. [Google Scholar] [CrossRef]
- Cibulka, R. Artificial Flavin Systems for Chemoselective and Stereoselective Oxidations. Eur. J. Org. Chem. 2015, 2015, 915–932. [Google Scholar] [CrossRef]
- Imada, Y.; Osaki, M.; Noguchi, M.; Maeda, T.; Fujiki, M.; Kawamorita, S.; Komiya, N.; Naota, T. Flavin-Functionalized Gold Nanoparticles as an Efficient Catalyst for Aerobic Organic Transformations. ChemCatChem 2015, 7, 99–106. [Google Scholar] [CrossRef]
- Kawamorita, S.; Fujiki, M.; Li, Z.; Kitagawa, T.; Imada, Y.; Naota, T. Aggregation-induced Substrate Specificity in Aerobic Reduction of Olefins with Ultrasound Gel Catalyst of Synthetic Flavin. ChemCatChem 2019, 11, 878–884. [Google Scholar] [CrossRef]
- Heelis, P.F. The Photophysical and Photochemical Properties of Flavins (Isoalloxazines). Chem. Soc. Rev. 1982, 11, 15–39. [Google Scholar] [CrossRef]
- Manna, S.; Saha, A.; Nandi, A.K. A Two Component Thermoreversible Hydrogel of Riboflavin and Melamine: Enhancement of Photoluminescence in the Gel Form. Chem. Commun. 2006, 4285. [Google Scholar] [CrossRef]
- Daďová, J.; Kümmel, S.; Feldmeier, C.; Cibulková, J.; Pažout, R.; Maixner, J.; Gschwind, R.M.; König, B.; Cibulka, R. Aggregation Effects in Visible-Light Flavin Photocatalysts: Synthesis, Structure, and Catalytic Activity of 10-Arylflavins. Chem. Eur. J. 2013, 19, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Mataranga-Popa, L.N.; Torje, I.; Ghosh, T.; Leitl, M.J.; Späth, A.; Novianti, M.L.; Webster, R.D.; König, B. Synthesis and Electronic Properties of π-Extended Flavins. Org. Biomol. Chem. 2015, 13, 10198–10204. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Inoue, R.; Kawamorita, S.; Komiya, N.; Imada, Y.; Naota, T. Highly Fluorescent Flavins: Rational Molecular Design for Quenching Protection Based on Repulsive and Attractive Control of Molecular Alignment. Chem. Eur. J. 2015, 21, 9171–9178. [Google Scholar] [CrossRef] [PubMed]
- Reiffers, A.; Torres Ziegenbein, C.; Engelhardt, A.; Kühnemuth, R.; Gilch, P.; Czekelius, C. Impact of Mono-Fluorination on the Photophysics of the Flavin Chromophore. Photochem. Photobiol. 2018, 94, 667–676. [Google Scholar] [CrossRef]
- Vinod Mouli, M.S.S.; Mishra, A.K. Flavin Based Supramolecular Gel Displaying Multi-Stimuli Triggered Sol–Gel Transition. Org. Biomol. Chem. 2023, 21, 5622–5628. [Google Scholar] [CrossRef]
- Čubiňák, M.; Varma, N.; Oeser, P.; Pokluda, A.; Pavlovska, T.; Cibulka, R.; Sikorski, M.; Tobrman, T. Tuning the Photophysical Properties of Flavins by Attaching an Aryl Moiety via Direct C–C Bond Coupling. J. Org. Chem. 2023, 88, 218–229. [Google Scholar] [CrossRef]
- Guo, H.; Liu, S.; Liu, X.; Zhang, L. Lightening Flavin by Amination for Fluorescent Sensing. Phys. Chem. Chem. Phys. 2024, 26, 19554–19563. [Google Scholar] [CrossRef]
- Agrawal, H.G.; Raha, S.; Mishra, A.K. Subtle Chemical Modification around the Flavin Core to Create Diverse Morphological Assembly and Sensing Application. J. Mol. Struct. 2025, 1319, 139229. [Google Scholar] [CrossRef]
- Gopal Agrawal, H.; Dubey, A.; Mondal, D.; Kumar Mishra, A. Subtle Molecular Engineering around Flavin Core for Stimuli-Responsive Solid-State Luminophore. Chem. Asian J. 2025, 20, e202401099. [Google Scholar] [CrossRef]
- Shuttleworth, C.W.; Brennan, A.M.; Connor, J.A. NAD(P)H Fluorescence Imaging of Postsynaptic Neuronal Activation in Murine Hippocampal Slices. J. Neurosci. 2003, 23, 3196–3208. [Google Scholar] [CrossRef]
- Shibuki, K.; Hishida, R.; Murakami, H.; Kudoh, M.; Kawaguchi, T.; Watanabe, M.; Watanabe, S.; Kouuchi, T.; Tanaka, R. Dynamic Imaging of Somatosensory Cortical Activity in the Rat Visualized by Flavoprotein Autofluorescence. J. Physiol. 2003, 549, 919–927. [Google Scholar] [CrossRef]
- Murakami, H.; Kamatani, D.; Hishida, R.; Takao, T.; Kudoh, M.; Kawaguchi, T.; Tanaka, R.; Shibuki, K. Short-term Plasticity Visualized with Flavoprotein Autofluorescence in the Somatosensory Cortex of Anaesthetized Rats. Eur. J. Neurosci. 2004, 19, 1352–1360. [Google Scholar] [CrossRef]
- Weber, B.; Burger, C.; Wyss, M.T.; Von Schulthess, G.K.; Scheffold, F.; Buck, A. Optical Imaging of the Spatiotemporal Dynamics of Cerebral Blood Flow and Oxidative Metabolism in the Rat Barrel Cortex. Eur. J. Neurosci. 2004, 20, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hishida, R.; Kubota, Y.; Kudoh, M.; Takahashi, S.; Shibuki, K. Transcranial Fluorescence Imaging of Auditory Cortical Plasticity Regulated by Acoustic Environments in Mice. Eur. J. Neurosci. 2006, 23, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Drepper, T.; Eggert, T.; Circolone, F.; Heck, A.; Krauß, U.; Guterl, J.-K.; Wendorff, M.; Losi, A.; Gärtner, W.; Jaeger, K.-E. Reporter Proteins for in Vivo Fluorescence without Oxygen. Nat. Biotechnol. 2007, 25, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Król, J.E.; Rogers, L.M.; Krone, S.M.; Top, E.M. Dual Reporter System for In Situ Detection of Plasmid Transfer under Aerobic and Anaerobic Conditions. Appl. Environ. Microbiol. 2010, 76, 4553–4556. [Google Scholar] [CrossRef]
- Landete, J.M.; Peirotén, Á.; Rodríguez, E.; Margolles, A.; Medina, M.; Arqués, J.L. Anaerobic Green Fluorescent Protein as a Marker of Bifidobacterium Strains. Int. J. Food Microbiol. 2014, 175, 6–13. [Google Scholar] [CrossRef]
- Iida, H.; Iwahana, S.; Mizoguchi, T.; Yashima, E. Main-Chain Optically Active Riboflavin Polymer for Asymmetric Catalysis and Its Vapochromic Behavior. J. Am. Chem. Soc. 2012, 134, 15103–15113. [Google Scholar] [CrossRef]
- Iida, H.; Miki, M.; Iwahana, S.; Yashima, E. Riboflavin-Based Fluorogenic Sensor for Chemo- and Enantioselective Detection of Amine Vapors. Chem. Eur. J. 2014, 20, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, S.; Hu, Y.; Pao, C.-W. Fabrication and Characterization of a Novel PMO Containing Riboflavin-5′-Phosphate Sodium Salt for Sensitive Detection of Pesticide Ferbam. Colloids Surf. A 2021, 617, 126375. [Google Scholar] [CrossRef]
- Mouli, M.S.S.V.; Mishra, A.K. Sequential Recognition Capability of a Novel Flavin-Dipicolyl Analogue toward Zinc and Phosphate Ion: A Model Capable of Selective Recognition of AMP over ADP/ATP. Dyes Pigm. 2023, 212, 111148. [Google Scholar] [CrossRef]
- Oka, M.; Kozako, R.; Teranishi, Y.; Yamada, Y.; Miyake, K.; Fujimura, T.; Sasai, R.; Ikeue, T.; Iida, H. Chiral Supramolecular Organogel Constructed Using Riboflavin and Melamine: Its Application in Photo-Catalyzed Colorimetric Chiral Sensing and Enantioselective Adsorption. Chem. Eur. J. 2024, 30, e202303353. [Google Scholar] [CrossRef]
- Kawamorita, S.; Li, Z.; Okamoto, K.; Naota, T. Multistimuli-Responsive Chromism of Vinylene-Linked Bisflavin Based on the Aggregation and Redox Properties. Chem. Eur. J. 2023, 29, e202202257. [Google Scholar] [CrossRef]
- Imada, Y.; Iida, H.; Ono, S.; Masui, Y.; Murahashi, S. Flavin-Catalyzed Oxidation of Amines and Sulfides with Molecular Oxygen: Biomimetic Green Oxidation. Chem. Asian J. 2006, 1, 136–147. [Google Scholar] [CrossRef]
- Horman, I.; Dreux, B. Estimation of Dimerisation Constants from Complexatin-Induced Displacements of1 H-NMR Chemical Shifts: Dimerisation of Caffeine. Helv. Chim. Acta 1984, 67, 754–764. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic Light Scattering and Transmission Electron Microscopy in Drug Delivery: A Roadmap for Correct Characterization of Nanoparticles and Interpretation of Results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Naito, M.; Inoue, R.; Iida, M.; Kuwajima, Y.; Kawamorita, S.; Komiya, N.; Naota, T. Control of Metal Arrays Based on Heterometallics Masquerading in Heterochiral Aggregations of Chiral Clothespin-Shaped Complexes. Chem. Eur. J. 2015, 21, 12927–12939. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamorita, S.; Okamoto, K.; Huang, S.; Naota, T. Stimuli-Responsive Luminescence of an Amphiphilic Flavin Derivative via Thermodynamic and Kinetic Aggregation in Water. Photochem 2025, 5, 25. https://doi.org/10.3390/photochem5030025
Kawamorita S, Okamoto K, Huang S, Naota T. Stimuli-Responsive Luminescence of an Amphiphilic Flavin Derivative via Thermodynamic and Kinetic Aggregation in Water. Photochem. 2025; 5(3):25. https://doi.org/10.3390/photochem5030025
Chicago/Turabian StyleKawamorita, Soichiro, Koyo Okamoto, Shufang Huang, and Takeshi Naota. 2025. "Stimuli-Responsive Luminescence of an Amphiphilic Flavin Derivative via Thermodynamic and Kinetic Aggregation in Water" Photochem 5, no. 3: 25. https://doi.org/10.3390/photochem5030025
APA StyleKawamorita, S., Okamoto, K., Huang, S., & Naota, T. (2025). Stimuli-Responsive Luminescence of an Amphiphilic Flavin Derivative via Thermodynamic and Kinetic Aggregation in Water. Photochem, 5(3), 25. https://doi.org/10.3390/photochem5030025





