Tetraphenylethylene (TPE)-Based AIE Luminogens: Recent Advances in Bioimaging Applications
Abstract
1. Introduction
2. General Mechanism of AIE
3. TPE-Based Systems for Bioimaging
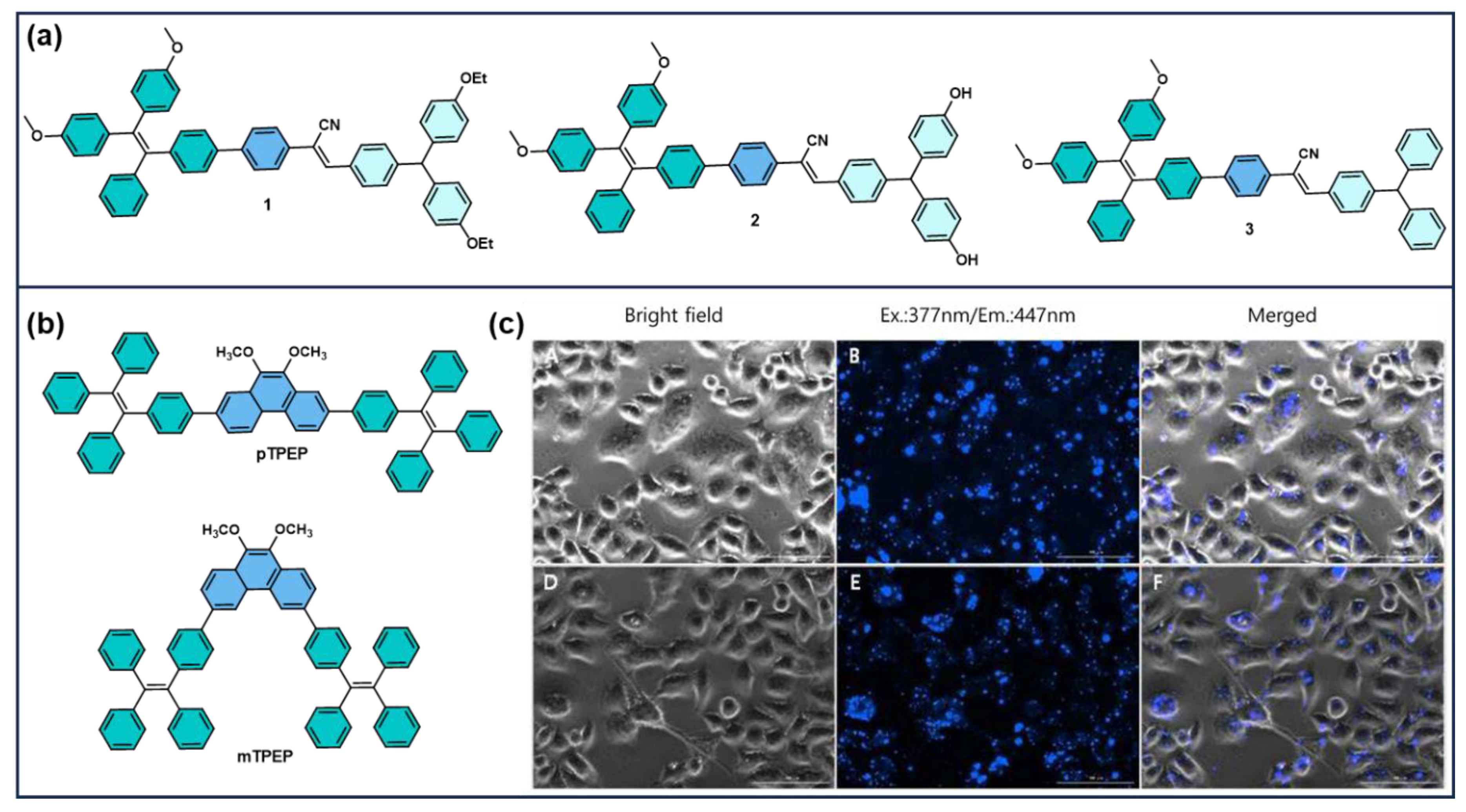
3.1. TPE-Based AIEgens for Cellular Imaging
3.2. TPE-Based AIEgens for Tissue and In Vivo Imaging
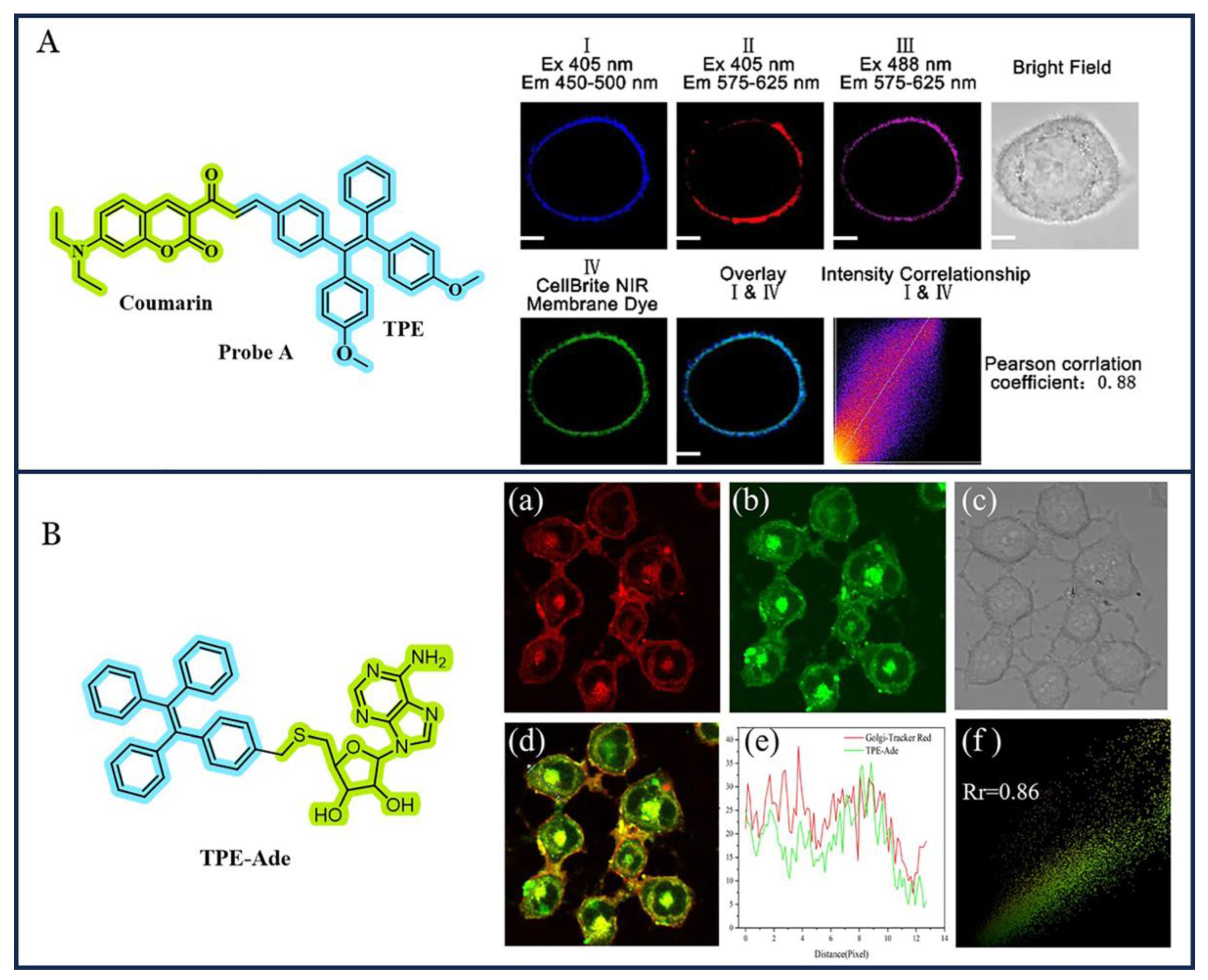
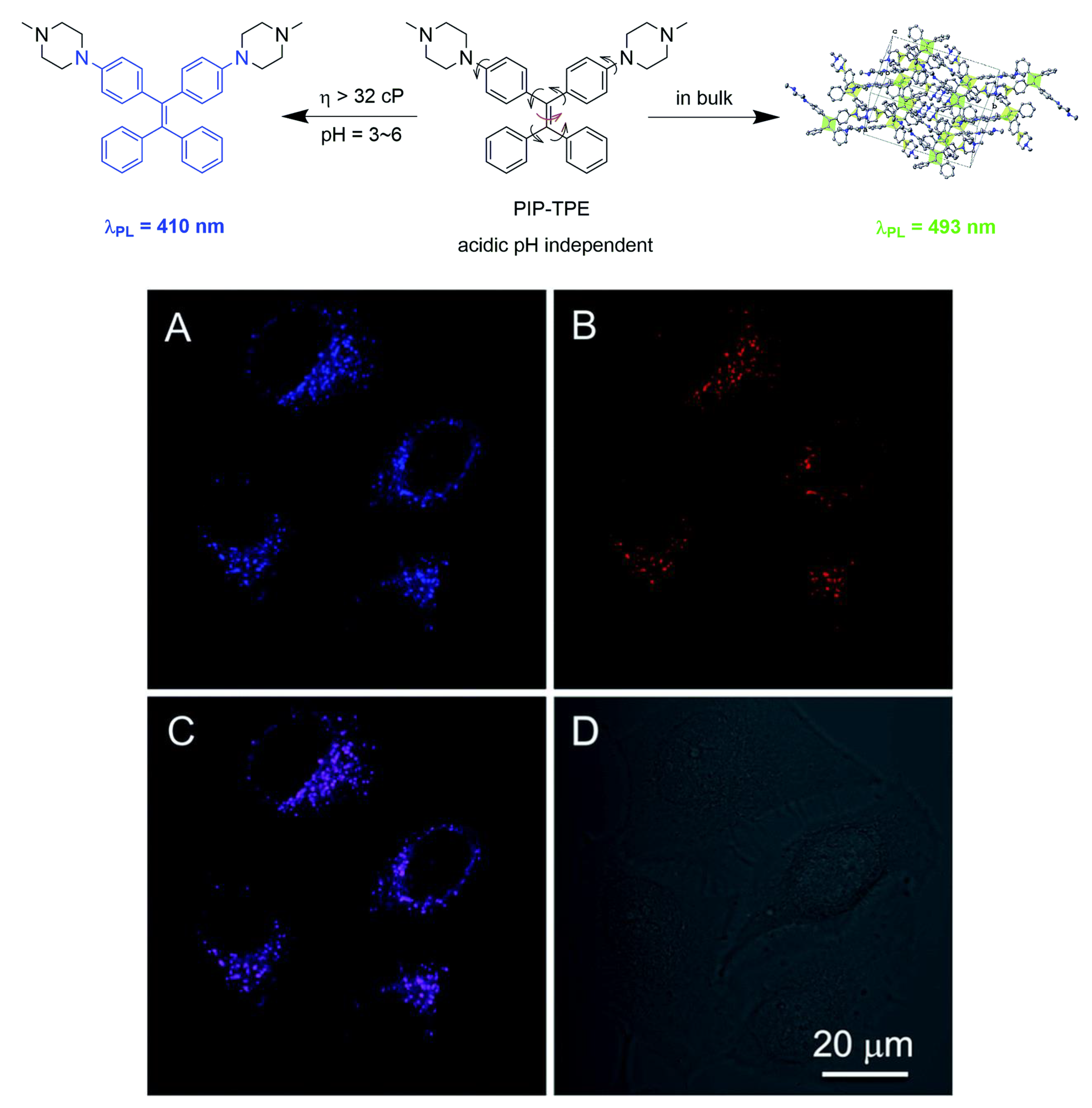
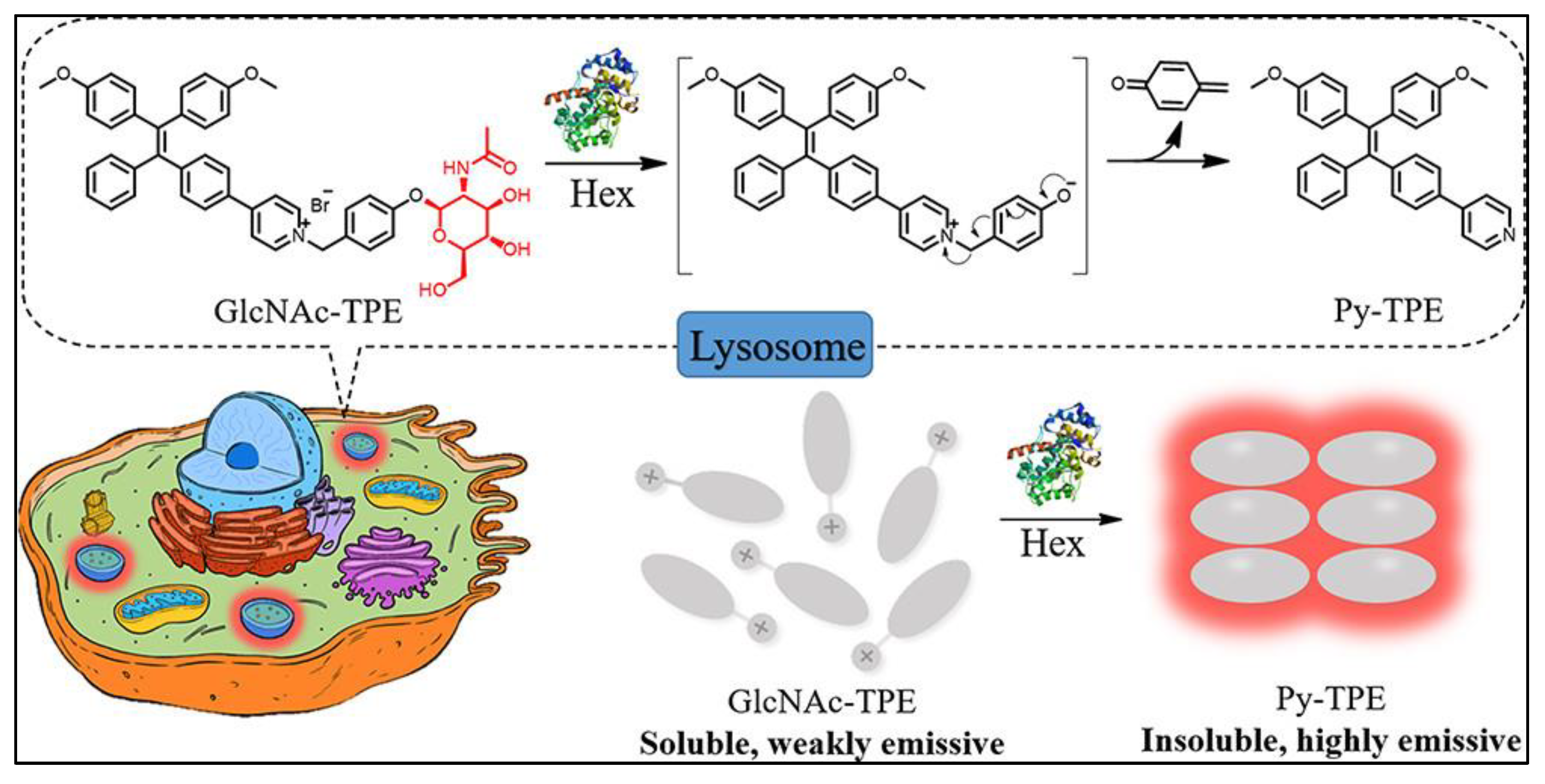
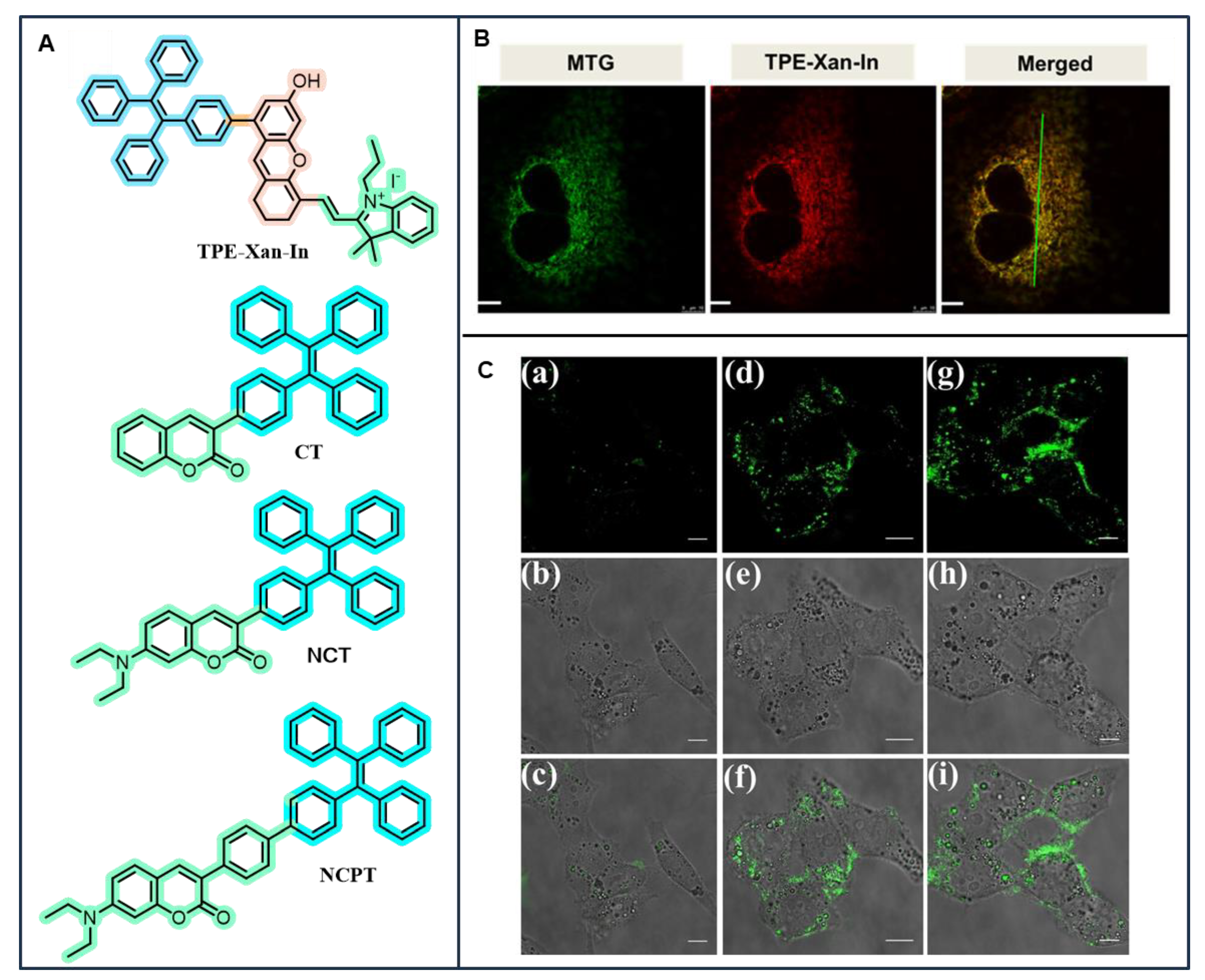
3.3. TPE-Based AIEgens for Organelle-Targeted Imaging
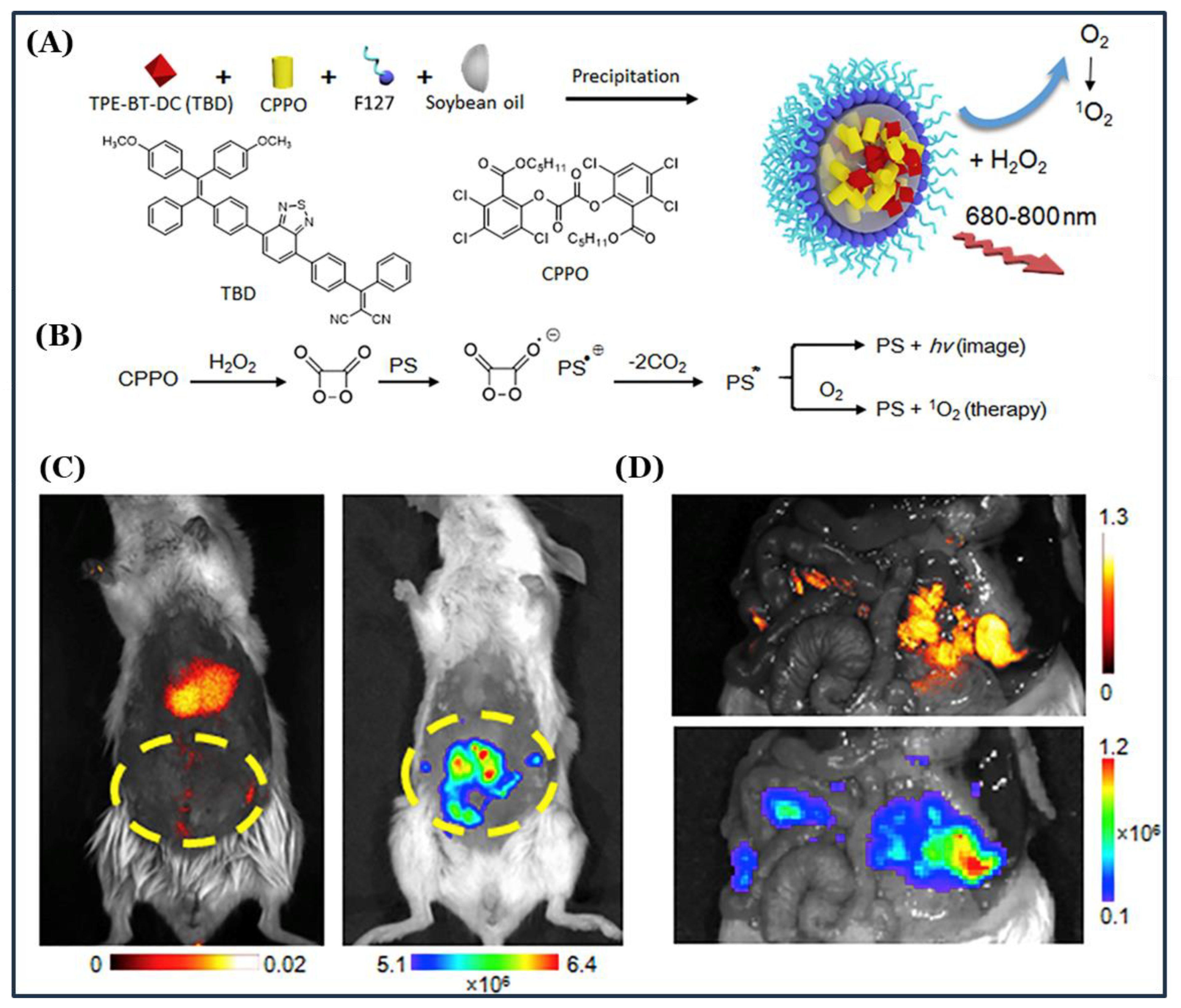
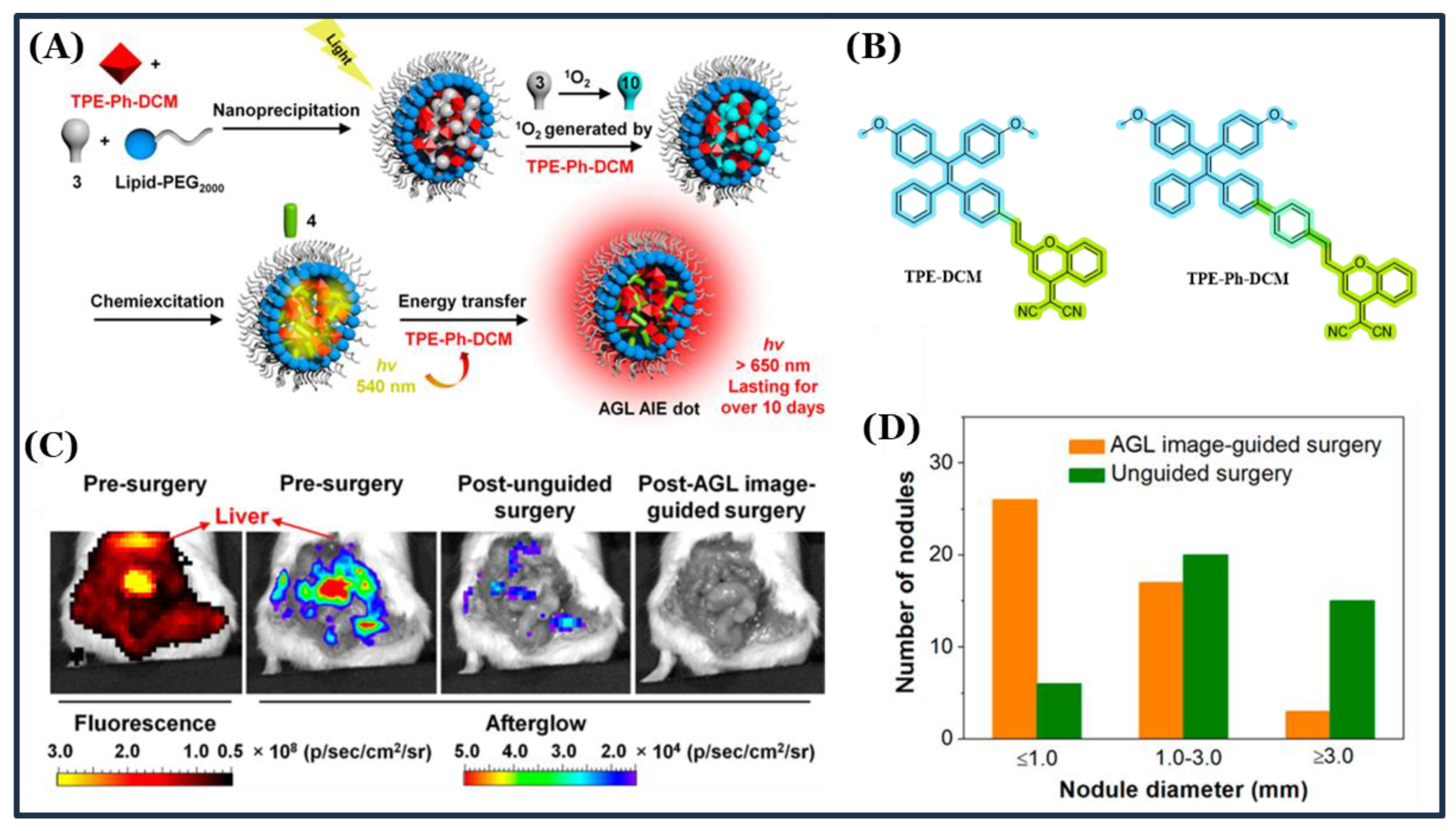
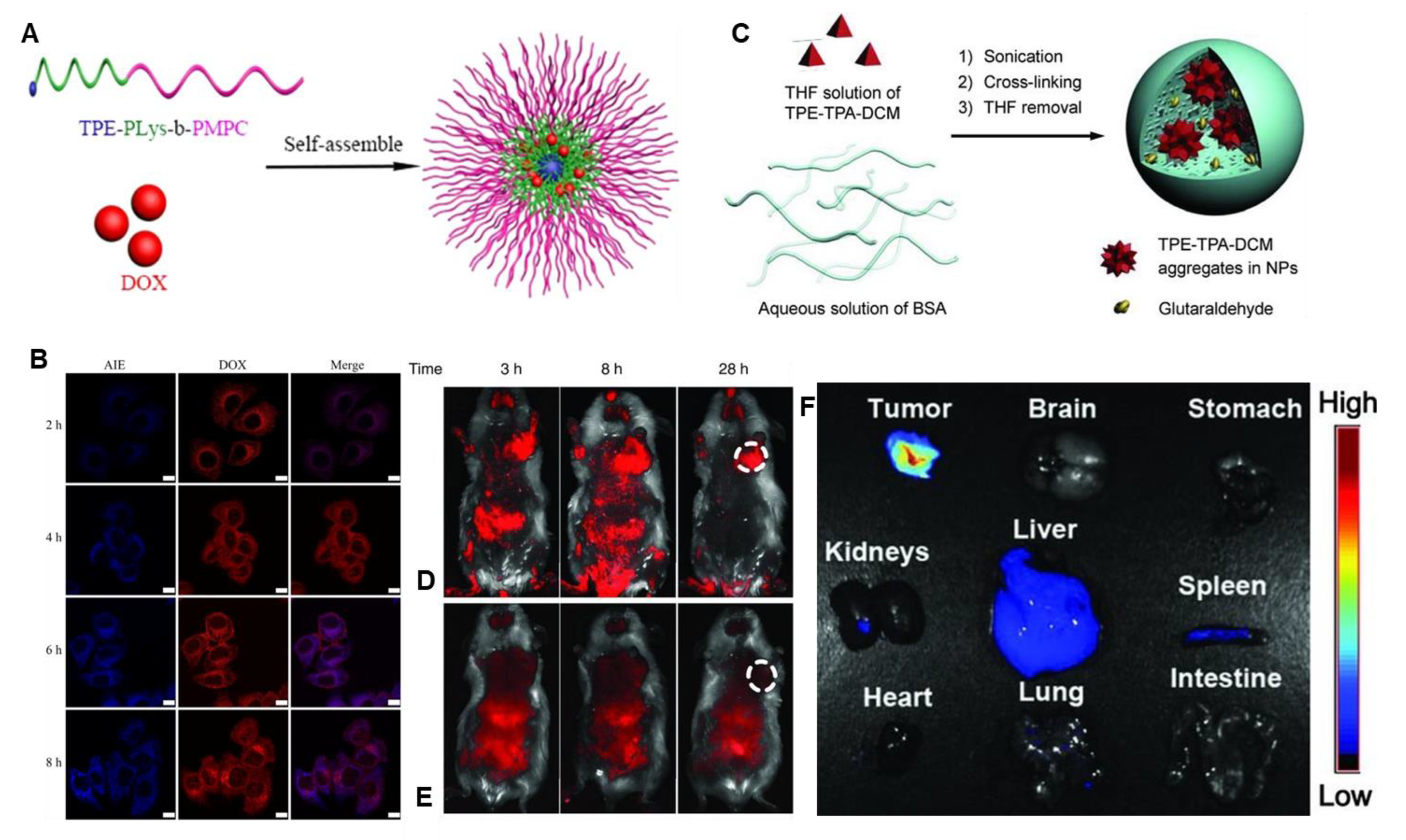
3.4. TPE-Based AIEgens for Multifunctional and Theranostic Applications
3.5. TPE-Based AIEgens for Stimuli-Responsive and Self-Assembled TPE Systems
4. Future Direction and Perspective
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, P.; Ai, J. Fluorescence Probes and Their Sensing Applications in Nanomaterials System. Talanta Open 2023, 8, 100248. [Google Scholar] [CrossRef]
- Ha, J.M.; Hur, S.H.; Pathak, A.; Jeong, J.E.; Woo, H.Y. Recent Advances in Organic Luminescent Materials with Narrowband Emission. NPG Asia Mater. 2021, 13, 53. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Xue, Y.; Zhang, J. Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives. Chemosensors 2023, 11, 226. [Google Scholar] [CrossRef]
- Keremane, K.S.; Acharya, M.G.; Naik, P.; Malakar, C.C.; Wang, K.; Poudel, B. Recent Advances in Aggregation-Induced Emission (AIE) Fluorescent Sensors for Biomolecule Detection. Chemosensors 2025, 13, 174. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, S.; Chen, F.; Ni, Y.; Wang, C.; Yang, L.; Kong, L.; Yang, J. Novel AIE-Active Tetraphenylethylene Derivatives as Multitask Smart Materials for Turn-on Mechanofluorochromism, Quantitative Sensing of Pressure and Picric Acid Detection. Dye. Pigment. 2022, 203, 110327. [Google Scholar] [CrossRef]
- Pilicode, N.; Naik, P.; Acharya, M.; Adhikari, A.V. Synthesis, Characterization and Electroluminescence Studies of Cyanopyridine-Based π-Conjugative Polymers Carrying Benzo[: C] [1,2,5]Thiadiazole and Naphtho [1,2- c:5,6- c ′]Bis([1,2,5]Thiadiazole) Units. New J. Chem. 2020, 44, 10796–10805. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, F.; Wang, J.; Zhao, Z.; Qin, A.; Yu, Z.; Tang, B.Z. Electronic Effect on the Optical Properties and Sensing Ability of AIEgens with ESIPT Process Based on Salicylaldehyde Azine. Sci. China Chem. 2018, 61, 76–87. [Google Scholar] [CrossRef]
- Blebea, N.M.; Pușcașu, C.; Vlad, R.A.; Hancu, G. Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications. Gels 2025, 11, 275. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, P.; Hu, D.; Ma, Y. Recent Progress in Hot Exciton Materials for Organic Light-Emitting Diodes. Chem. Soc. Rev. 2021, 50, 1030–1069. [Google Scholar] [CrossRef]
- Huang, J.; Yang, X.; Li, X.; Chen, P.; Tang, R.; Li, F.; Lu, P.; Ma, Y.; Wang, L.; Qin, J.; et al. Bipolar AIE-Active Luminogens Comprised of an Oxadiazole Core and Terminal TPE Moieties as a New Type of Host for Doped Electroluminescence. Chem. Commun. 2012, 48, 9586–9588. [Google Scholar] [CrossRef]
- Naik, P.; Pilicode, N.; Keremane, K.S.; Acharya, M.; Adhikari, A.V. Synthesis, Optical, Electrochemical, and Computational Investigation of New Cyanopyridine-Centered Organic Dyads. Opt. Mater. (Amst.) 2023, 142, 114002. [Google Scholar] [CrossRef]
- Harish, K.K.; Nesaragi, A.R.; Kalagatur, N.K.; Naik, P.; Madegowda, M.; Pandith, A.; Dahlous, K.A.; Mohammad, S.; Shivarudrappa, H.P.; Sharanakumar, T.M.; et al. Imidazole-Centred Cupric Ions Sensor: Experimental Validation, Theoretical Understanding, and Zebrafish Bioimaging. J. Photochem. Photobiol. A Chem. 2024, 452, 115565. [Google Scholar] [CrossRef]
- Nesaragi, A.R.; Naik, P.; Mulla, B.B.A.; Al-Zaqri, N.; Vidyasagar, C.C.; Kalagatur, N.K.; Sharanakumar, T.M.; Guddappa, H.; Sidarai, A.H.; Shivarudrappa, H.P. Selective Al3+ and Fe3+ Detection Using Imidazole–Oxadiazole Sensors: Bioimaging Evidence from Zebrafish. New J. Chem. 2025, 49, 5729–5739. [Google Scholar] [CrossRef]
- Nesaragi, A.R.; Mulla, B.B.A.; Chapi, S.; Venuprasad, K.D.; Kalagatur, N.K.; Sharanakumar, T.M.; Vidyasagar, C.C.; Guddappa, H.; Al-Zaqri, N.; Warad, I.; et al. Imidazole-Centred Oxadiazole Sensor for Detecting Al3+ and Fe3+ Cations in Living Cells: A Zebrafish Bioimaging Approach. Appl. Organomet. Chem. 2025, 39, e70087. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, R.; Wang, Z.; Zhou, Y.; Shen, Q.; Ji, W.; Dang, D.; Meng, L.; Tang, B.Z. Recent Advances in Luminescent Materials for Super-Resolution Imaging: Via Stimulated Emission Depletion Nanoscopy. Chem. Soc. Rev. 2021, 50, 667–690. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yang, X.; Qin, A.; Tang, B.Z. AIE Polymers in Sensing, Imaging and Theranostic Applications. Mater. Chem. Front. 2021, 5, 4073–4088. [Google Scholar] [CrossRef]
- Naik, P.; Elias, L.; Keremane, K.S.; Babu, D.D.; Abdellah, I.M. Metal-Free Organic Dyes for NiO-Based Dye-Sensitized Solar Cells: Recent Developments and Future Perspectives. Energy Technol. 2024, 12, 2301666. [Google Scholar] [CrossRef]
- Naik, P.; Babu, D.D.; Su, R.; El-Shafei, A.; Adhikari, A.V. Synthesis, Characterization and Performance Studies of a New Metal-Free Organic Sensitizer for DSSC Application. Mater. Today Proc. 2018, 5, 3150–3157. [Google Scholar] [CrossRef]
- Kim, T.; Baek, E.; Kim, H.; Han, J.; Lee, Y.; Oh, H.; Cho, K.H.; Kim, J. Tunable Emission Properties of Indolizine-Based Aggregation-Induced Emission Luminogens for White-Light Emission. JACS Au 2025, 5, 3262–3274. [Google Scholar] [CrossRef]
- Fan, W.; Li, Z.; Chen, K.; Wang, Z.; Yang, L. Aggregation-Induced Emission in Natural Product Research: Recent Advances and Future Perspectives. Coord. Chem. Rev. 2025, 542, 216843. [Google Scholar] [CrossRef]
- Peng, Q.; Shuai, Z. Molecular Mechanism of Aggregation-Induced Emission. Aggregate 2021, 2, e91. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Yan, D.; Wu, Q.; Song, N.; Zhang, H.; Wang, D. Aggregation-Induced Emission: A Rising Star in Chemistry and Materials Science. Chin. J. Chem. 2021, 39, 677–689. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens: Emission Brightened by Aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Zhao, W.; He, Z.; Lam, J.W.Y.; Peng, Q.; Ma, H.; Shuai, Z.; Bai, G.; Hao, J.; Tang, B.Z. Rational Molecular Design for Achieving Persistent and Efficient Pure Organic Room-Temperature Phosphorescence. Chem 2016, 1, 592–602. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; Wang, T.; Zhou, C.; Du, J.; Wang, Z.; Xu, H.; Xie, T.; Bi, G.; Jiang, J.; et al. Versatile Room-Temperature-Phosphorescent Materials Prepared from N-Substituted Naphthalimides: Emission Enhancement and Chemical Conjugation. Angew. Chem.-Int. Ed. 2016, 55, 9872–9876. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Mohanty, A.; Naik, P.; Salzner, U.; Dasgupta, J.; Patil, S. Deciphering Intramolecular Charge Transfer in Fluoranthene Derivatives. J. Mater. Chem. C Mater. 2024, 12, 9200–9209. [Google Scholar] [CrossRef]
- Sharath Kumar, K.S.; Girish, Y.R.; Ashrafizadeh, M.; Mirzaei, S.; Rakesh, K.P.; Hossein Gholami, M.; Zabolian, A.; Hushmandi, K.; Orive, G.; Kadumudi, F.B.; et al. AIE-Featured Tetraphenylethylene Nanoarchitectures in Biomedical Application: Bioimaging, Drug Delivery and Disease Treatment. Coord. Chem. Rev. 2021, 447, 214135. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, G.Q.; Khalife, S.; He, Z.H.; Xu, C.; Li, X. Self-Assembly of Emissive Metallocycles with Tetraphenylethylene, BODIPY and Terpyridine in One System. Supramol. Chem. 2019, 31, 597. [Google Scholar] [CrossRef]
- Tanaka, Y.; Machida, T.; Noumi, T.; Sada, K.; Kokado, K. Emissive Tetraphenylethylene (TPE) Derivatives in a Dissolved State Tightly Fastened by a Short Oligo(Ethylene Glycol) Chain. Org. Chem. Front. 2020, 7, 2649–2656. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Naik, P.; Palariya, A.K.; Panda, S.; Patil, S.A.; Avashti, S. Surface and Grain Boundary Passivation Using Tetraphenylethylene Derivative for High-Performance Perovskite Solar Cell. Mater. Chem. Front. 2025. [Google Scholar] [CrossRef]
- Lafzi, F.; Taskesenligil, Y.; Canlmkurbey, B.; Plravadlll, S.; Kilic, H.; Saracoglu, N. Four-Winged Propeller-Shaped Indole-Modified and Indole-Substituted Tetraphenylethylenes: Greenish-Blue Emitters with Aggregation-Induced Emission Features for Conventional Organic Light-Emitting Diodes. ACS Omega 2022, 7, 44322–44337. [Google Scholar] [CrossRef]
- Bhosale, R.S.; Aljabri, M.; La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethene Derivatives: A Promising Class of AIE Luminogens—Synthesis, Properties, and Applications. Princ. Appl. Aggreg.-Induc. Emiss. 2019, 223–264. [Google Scholar] [CrossRef]
- Duc La, D.; Anuradha, A.; Gupta, A.; Al Kobaisi, M.; Rananaware, A.; Bhosale, S.V. Supramolecular Chemistry of AIE-Active Tetraphenylethylene Luminophores. SPR Nanosci. 2017, 4, 75–107. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Mahmoudi, M.; Gupta, P.K.; Volyniuk, D.; Grazulevicius, J.V.; Misra, R. Mechanochromic Materials Based on Tetraphenylethylene-Substituted Phenothiazines: Substituent-Dependent Hypsochromic and Bathochromic Switching of Emission. J. Phys. Chem. C 2023, 127, 1643–1654. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Kong, L.; Yang, J. Mechanoresponsive Material of AIE-Active 1,4-Dihydropyrrolo [3,2-b]Pyrrole Luminophores Bearing Tetraphenylethylene Group with Rewritable Data Storage. Molecules 2018, 23, 3255. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chi, Z.; Mao, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Aldred, M.P.; Chi, Z. Recent Advances in Mechano-Responsive Luminescence of Tetraphenylethylene Derivatives with Aggregation-Induced Emission Properties. Mater. Chem. Front. 2018, 2, 861–890. [Google Scholar] [CrossRef]
- Kalva, N.; Tran, C.H.; Lee, M.W.; Augustine, R.; Lee, S.J.; Kim, I. Aggregation-Induced Emission-Active Hyperbranched Polymers Conjugated with Tetraphenylethylene for Nitroaromatic Explosive Detection. Dye. Pigment. 2021, 194, 109617. [Google Scholar] [CrossRef]
- Kokado, K.; Sada, K. Consideration of Molecular Structure in the Excited State to Design New Luminogens with Aggregation-Induced Emission. Angew. Chem. 2019, 131, 8724–8731. [Google Scholar] [CrossRef]
- Chang, C.C.; Hsieh, M.C.; Lin, J.C.; Chang, T.C. Selective Photodynamic Therapy Based on Aggregation-Induced Emission Enhancement of Fluorescent Organic Nanoparticles. Biomaterials 2012, 33, 897–906. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, B.; Geng, J.; Chang, Z.; Aparicio-Ixta, L.; Nie, H.; Goh, C.C.; Ng, L.G.; Qin, A.; Ramos-Ortiz, G.; et al. Red Emissive Biocompatible Nanoparticles from Tetraphenylethene-Decorated BODIPY Luminogens for Two-Photon Excited Fluorescence Cellular Imaging and Mouse Brain Blood Vascular Visualization. Part. Part. Syst. Charact. 2014, 31, 481–491. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wu, Z.; Sun, Y.; Fang, J.; Chen, D. Highly Efficient Luminescent Side-Chain Polymers with Short-Spacer Attached Tetraphenylethylene AIEgens via RAFT Polymerization Capable of Naked Eye Explosive Detection. Polym. Chem. 2018, 9, 4150–4160. [Google Scholar] [CrossRef]
- Geng, J.; Li, K.; Ding, D.; Zhang, X.; Qin, W.; Liu, J.; Tang, B.Z.; Liu, B. Lipid-PEG-Folate Encapsulated Nanoparticles with Aggregation Induced Emission Characteristics: Cellular Uptake Mechanism and Two-Photon Fluorescence Imaging. Small 2012, 8, 3655–3663. [Google Scholar] [CrossRef]
- Gawade, V.K.; Jadhav, R.W.; Bhosale, S.V. AIE-Based & Organic Luminescent Materials: Nanoarchitectonics and Advanced Applications. Chem. Asian J. 2024, 19, e202400682. [Google Scholar] [CrossRef]
- Serhan, M.; Jackemeyer, D.; Long, M.; Sprowls, M.; Diez Perez, I.; Maret, W.; Chen, F.; Tao, N.; Forzani, E. Total Iron Measurement in Human Serum with a Novel Smartphone-Based Assay. IEEE J. Transl. Eng. Health Med. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Zhang, D.; Zhu, D.; Tang, B.Z. Fluorescent Bio/Chemosensors Based on Silole and Tetraphenylethene Luminogens with Aggregation-Induced Emission Feature. J. Mater. Chem. 2010, 20, 1858–1867. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Tang, B.Z.; Wong, K.S. Aggregation Enhancement on Two-Photon Optical Properties of AIE-Active D-TPE-A Molecules. J. Phys. Chem. C 2014, 118, 26981–26986. [Google Scholar] [CrossRef]
- Yang, J.; Fang, M.; Li, Z. Organic Luminescent Materials: The Concentration on Aggregates from Aggregation-Induced Emission. Aggregate 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Liang, J.; Tang, B.Z.; Liu, B. Specific Light-up Bioprobes Based on AIEgen Conjugates. Chem. Soc. Rev. 2015, 44, 2798–2811. [Google Scholar] [CrossRef]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by Luminogens with Aggregation-Induced Emission Characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Xu, S.; Duan, Y.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32, 1903530. [Google Scholar] [CrossRef]
- Madushani, B.; Mamada, M.; Goushi, K.; Nguyen, T.B.; Nakanotani, H.; Kaji, H.; Adachi, C. Multiple Donor–Acceptor Design for Highly Luminescent and Stable Thermally Activated Delayed Fluorescence Emitters. Sci. Rep. 2023, 13, 7644. [Google Scholar] [CrossRef]
- Barman, D.; Narang, K.; Parui, R.; Zehra, N.; Khatun, M.N.; Adil, L.R.; Iyer, P.K. Review on Recent Trends and Prospects in π-Conjugated Luminescent Aggregates for Biomedical Applications. Aggregate 2022, 3, e172. [Google Scholar] [CrossRef]
- El-Zohry, A.M.; Orabi, E.A.; Karlsson, M.; Zietz, B. Twisted Intramolecular Charge Transfer (TICT) Controlled by Dimerization: An Overlooked Piece of the TICT Puzzle. J. Phys. Chem. A 2021, 125, 2885–2894. [Google Scholar] [CrossRef]
- Sasaki, S.; Drummen, G.P.C.; Konishi, G.I. Recent Advances in Twisted Intramolecular Charge Transfer (TICT) Fluorescence and Related Phenomena in Materials Chemistry. J. Mater. Chem. C Mater. 2016, 4, 2731–2743. [Google Scholar] [CrossRef]
- Vesga-Hernández, C.; dos Santos Carvalho, R.; Barreto, A.; Pedrozo Peñafiel, M.J.; de Noronha, J.R.; Maqueira, L.; Back, D.; Mello Nascimento, D.; Zucolotto Cocca, L.H.; Aucélio, R.Q.; et al. Aggregation-Induced Enhanced Emission and Twisted Intramolecular Charge Transfer in a Pyridylcarbodinitrile with Tunable Photoluminescence in Solution, Films, and OLEDs. J. Photochem. Photobiol. A Chem. 2025, 464, 116312. [Google Scholar] [CrossRef]
- Kuila, S.; Miranda-Salinas, H.; Eng, J.; Li, C.; Bryce, M.R.; Penfold, T.J.; Monkman, A.P. Rigid and Planar π-Conjugated Molecules Leading to Long-Lived Intramolecular Charge-Transfer States Exhibiting Thermally Activated Delayed Fluorescence. Nat. Commun. 2024, 15, 9611. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Matsubara, Y.; Ochi, T.; Wakamiya, T.; Yoshida, Z.I. How the π Conjugation Length Affects the Fluorescence Emission Efficiency. J. Am. Chem. Soc. 2008, 130, 13867–13869. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lai, H.; Gu, Y.; Wei, Z.; Chen, L.; Lai, X.; Han, L.; Tan, P.; Pu, M.; Xiao, F.; et al. The Balance Effect of π–π Electronic Coupling on NIR-II Emission and Photodynamic Properties of Highly Hydrophobic Conjugated Photosensitizers. Adv. Sci. 2024, 11, 2307569. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, H.; Gong, H.; Li, P.; Zhang, T.; Deng, Z.; Sun, W.; Tian, Y.; Fu, H. Heavy-Atom Effect Regulating Room-Temperature Phosphorescence of Organic-Inorganic Zn-Based Halides for White-Light Emission. J. Phys. Chem. Lett. 2024, 15, 4729–4736. [Google Scholar] [CrossRef]
- Hamzehpoor, E.; Ruchlin, C.; Tao, Y.; Liu, C.H.; Titi, H.M.; Perepichka, D.F. Efficient Room-Temperature Phosphorescence of Covalent Organic Frameworks through Covalent Halogen Doping. Nat. Chem. 2023, 15, 83–90. [Google Scholar] [CrossRef]
- Gu, Q.; Pandey, S.K.; Tiwari, R. A Computational Method to Estimate Spin–Orbital Interaction Strength in Solid State Systems. Comput. Mater. Sci. 2023, 221, 112090. [Google Scholar] [CrossRef]
- Fan, T.; Liu, Q.; Zhang, H.; Wang, X.; Zhang, D.; Duan, L. Enhancing Spin–Orbit Coupling in an Indolocarbazole Multiresonance Emitter by a Sulfur-Containing Peripheral Substituent for a Fast Reverse Intersystem Crossing. Adv. Mater. 2024, 36, 2408816. [Google Scholar] [CrossRef]
- Zhu, C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: A Trailblazing Journey to the Field of Biomedicine. ACS Appl. Bio Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- Ma, H.; Liu, C.; Hu, Z.; Yu, P.; Zhu, X.; Ma, R.; Sun, Z.; Zhang, C.H.; Sun, H.; Zhu, S.; et al. Propylenedioxy Thiophene Donor to Achieve NIR-II Molecular Fluorophores with Enhanced Brightness. Chem. Mater. 2020, 32, 2061–2069. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Persch, E.; Dumele, O.; Diederich, F. Molecular Recognition in Chemical and Biological Systems. Angew. Chem.-Int. Ed. 2015, 54, 3290–3327. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.I.; Pesenti, M.; Crespi, S.; Shenoy, D.S.; Penconi, M.; Bossi, A.; Pellegrino, S. Aggregation-Induced Enhanced Emission of Tetraphenylethene-Phenylalanine Hybrids: Synthesis and Characterization. J. Org. Chem. 2024, 89, 4733–4740. [Google Scholar] [CrossRef]
- Li, X.; Yin, Y.; Yan, H.; Lu, C. Aggregation-Induced Emission Characteristics of o-Carborane-Functionalized Tetraphenylethylene Luminogens: The Influence of Carborane Cages on Photoluminescence. Chem. Asian J. 2017, 12, 2207–2210. [Google Scholar] [CrossRef]
- Duo, Y.; Han, L.; Yang, Y.; Wang, Z.; Wang, L.; Chen, J.; Xiang, Z.; Yoon, J.; Luo, G.; Tang, B.Z. Aggregation-Induced Emission Luminogen: Role in Biopsy for Precision Medicine. Chem. Rev. 2024, 124, 11242–11347. [Google Scholar] [CrossRef]
- Li, W.; Ai, S.; Zhu, H.; Lin, W. Activatable Second-near-Infrared-Window Multimodal Luminogens with Aggregation-Induced-Emission and Aggregation-Caused-Quenching Properties for Step-Imaging Guided Tumor Therapy. Nat. Commun. 2025, 16, 2471. [Google Scholar] [CrossRef]
- Feng, G.; Tay, C.Y.; Chui, Q.X.; Liu, R.; Tomczak, N.; Liu, J.; Tang, B.Z.; Leong, D.T.; Liu, B. Ultrabright Organic Dots with Aggregation-Induced Emission Characteristics for Cell Tracking. Biomaterials 2014, 35, 8669–8677. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, Z.; Cai, P.; Liu, R.; Tomczak, N.; Ding, D.; Liu, J.; Qin, W.; Zhao, Z.; Hu, Y.; et al. Organic Dots with Aggregation-Induced Emission (AIE Dots) Characteristics for Dual-Color Cell Tracing. Chem. Mater. 2013, 25, 4181–4187. [Google Scholar] [CrossRef]
- Xu, J.; Yan, R.; Wang, H.; Du, Z.; Gu, J.; Cheng, X.; Xiong, J. A Novel Biocompatible Zwitterionic Polyurethane with AIE Effect for Cell Imaging in Living Cells. RSC Adv. 2018, 8, 6798–6804. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, D.T.; Ramos-Romero, S.; Shankar, B.H.; Garrido, C.; Rubio, N.; Sanchez-Cid, L.; Gómez, S.B.; Blanco, J.; Ramaiah, D. In Vitro and in Vivo Demonstration of Photodynamic Activity and Cytoplasm Imaging through TPE Nanoparticles. ACS Chem. Biol. 2016, 11, 104–112. [Google Scholar] [CrossRef]
- Ding, A.X.; Hao, H.J.; Gao, Y.G.; Shi, Y.D.; Tang, Q.; Lu, Z.L. D-A-D Type Chromophores with Aggregation-Induced Emission and Two-Photon Absorption: Synthesis, Optical Characteristics and Cell Imaging. J. Mater. Chem. C Mater. 2016, 4, 5379–5389. [Google Scholar] [CrossRef]
- Mai, D.K.; Lee, J.; Min, I.; Vales, T.P.; Choi, K.H.; Park, B.J.; Cho, S.; Kim, H.J. Aggregation-Induced Emission of Tetraphenylethene-Conjugated Phenanthrene Derivatives and Their Bio-Imaging Applications. Nanomaterials 2018, 8, 728. [Google Scholar] [CrossRef]
- Chu, N.T.; Chakravarthy, R.D.; Shih, N.C.; Lin, Y.H.; Liu, Y.C.; Lin, J.H.; Lin, H.C. Fluorescent Supramolecular Hydrogels Self-Assembled from Tetraphenylethene (TPE)/Single Amino Acid Conjugates. RSC Adv. 2018, 8, 20922–20927. [Google Scholar] [CrossRef]
- Wang, L.; Xia, Q.; Zhang, Z.; Qu, J.; Liu, R. Precise Design and Synthesis of an AIE Fluorophore with Near-Infrared Emission for Cellular Bioimaging. Mater. Sci. Eng. C 2018, 93, 399–406. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Xia, S.; Wan, S.; Steenwinkel, T.E.; Medford, J.; Durocher, E.; Luck, R.L.; Werner, T.; Liu, H. Cell Membrane-Specific Fluorescent Probe Featuring Dual and Aggregation-Induced Emissions. ACS Appl. Mater. Interfaces 2020, 12, 20172–20179. [Google Scholar] [CrossRef]
- Xing, X.; Jia, Y.; Zhang, J.; Wu, Z.; Qin, M.; Li, P.; Feng, X.; Sun, Y.; Zhao, G. A Novel Aggregation Induced Emission (AIE) Fluorescence Probe by Combining Tetraphenylethylene and 2′,3′-O-Isopropylideneadenosine for Localizing Golgi Apparatus. Sens. Actuators B Chem. 2021, 329, 129245. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, K.; Zhang, N.; Wang, Y.; Yan, J.; Wang, D.; Liu, X. Facile Synthesis of Tetraphenylethene (TPE)-Based Fluorophores Derived by π-Extended Systems: Opposite Mechanofluorochromism, Anti-Counterfeiting and Bioimaging. Chem.-A Eur. J. 2023, 29, e202203772. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, J.; Hu, Z.; Wang, Q.; Islam, M.M.; Ni, J.S.; Elsegood, M.R.J.; Lam, J.W.Y.; Zhou, E.; Tang, B.Z. Pyrene-Based Aggregation-Induced Emission Luminogens (AIEgen): Structure Correlated with Particle Size Distribution and Mechanochromism. J. Mater. Chem. C Mater. 2019, 7, 6932–6940. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, B.; Guo, J.; Cao, H.; Cheng, J.; Guo, J.; Li, D. Aggregation-Induced Emission(AIE)for next-Generation Biosensing and Imaging: A Review. Biosens. Bioelectron. 2025, 271, 117067. [Google Scholar] [CrossRef]
- Li, K.; Qin, W.; Ding, D.; Tomczak, N.; Geng, J.; Liu, R.; Liu, J.; Zhang, X.; Liu, H.; Liu, B.; et al. Photostable Fluorescent Organic Dots with Aggregation-Induced Emission (AIE Dots) for Noninvasive Long-Term Cell Tracing. Sci. Rep. 2013, 3, 1150. [Google Scholar] [CrossRef]
- Feng, G.; Liu, B. Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights. Acc. Chem. Res. 2018, 51, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xiong, L.H.; He, X. Applications of Aggregation-Induced Emission Materials in Immunology: From Diagnostics to Immunotherapy. Chemical and Biomedical Imaging 2025, 3, 499–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qian, J.; Qin, W.; Qin, A.; Tang, B.Z.; He, S. Biocompatible and Photostable AIE Dots with Red Emission for in Vivo Two-Photon Bioimaging. Sci. Rep. 2014, 4, 4279. [Google Scholar] [CrossRef]
- Leung, C.W.T.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. A Photostable AIE Luminogen for Specific Mitochondrial Imaging and Tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef]
- Tang, S.; Ye, S.; Zhang, X. When Aggregation-Induced Emission Meets Protein Aggregates. Natl. Sci. Rev. 2021, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.T.; Su, L.; Qian, J.; Zhu, Z.; Chen, G.; He, S.; Qin, A.; Xi, W.; Tang, B.Z. Long-Term Two-Photon Neuroimaging with a Photostable AIE Luminogen. Biomed. Opt. Express 2015, 6, 1477–1486. [Google Scholar] [CrossRef]
- Zhu, Z.; Leung, C.W.T.; Zhao, X.; Wang, Y.; Qian, J.; Tang, B.Z.; He, S. Using AIE Luminogen for Long-Term and Low-Background Three-Photon Microscopic Functional Bioimaging. Sci. Rep. 2015, 5, 15189. [Google Scholar] [CrossRef]
- Leung, C.W.T.; Guo, F.; Hong, Y.; Zhao, E.; Kwok, R.T.K.; Leung, N.L.C.; Chen, S.; Vaikath, N.N.; El-Agnaf, O.M.; Tang, Y.; et al. Detection of Oligomers and Fibrils of α-Synuclein by AIEgen with Strong Fluorescence. Chem. Commun. 2015, 51, 1866–1869. [Google Scholar] [CrossRef]
- Mao, D.; Wu, W.; Kong, D.; Ding, D.; Mao, D.; Wu, W.; Ji, S.; Chen, C.; Hu, F.; Kong, D.; et al. Chemiluminescence-Guided Cancer Therapy Using a Chemiexcited Photosensitizer Chemiluminescence-Guided Cancer Therapy Using a Chemiexcited Photosensitizer. Chem 2017, 3, 991–1007. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, X.; Duan, X.; Zheng, H.L.; Xue, X.S.; Ding, D. Near-Infrared Afterglow Luminescent Aggregation-Induced Emission Dots with Ultrahigh Tumor-to-Liver Signal Ratio for Promoted Image-Guided Cancer Surgery. Nano Lett. 2019, 19, 318–330. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, Y.; Chen, X.; Zheng, F.; Shen, Y.; Chen, G.; Ye, Q.; Chen, K.; Xiao, X.; Peng, Y. Tetraphenylethylene-Based AIE Nanoprobes for Labeling Lysosome by Two-Photon Imaging in Living Cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 306, 123630. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Jia, Q.; Deng, Z.; Wang, B.; Zhang, S.; Jiang, J.; Xing, G.; Wang, Z.; Qiu, Z.; Zhao, Z.; et al. Boosting Luminescence Efficiency of Near-Infrared-II Aggregation-Induced Emission Luminogens via a Mash-Up Strategy of π-Extension and Deuteration for Dual-Model Image-Guided Surgery. ACS Nano 2024, 18, 9431–9442. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yuan, W.; Chang, K.; Zhong, C.; Yuan, Y.; Li, J.; Zhang, Y.; Deng, T.; Fan, Y.; Yuan, L.; et al. Organic Materials with Ultrabright Phosphorescence at Room Temperature under Physiological Conditions for Bioimaging. Angew. Chem.-Int. Ed. 2024, 64, e202415637. [Google Scholar] [CrossRef]
- Li, Z.; Zou, J.; Chen, X. In Response to Precision Medicine: Current Subcellular Targeting Strategies for Cancer Therapy. Adv. Mater. 2023, 35, 2209529. [Google Scholar] [CrossRef]
- Saminathan, A.; Zajac, M.; Anees, P.; Krishnan, Y. Organelle-Level Precision with next-Generation Targeting Technologies. Nat. Rev. Mater. 2021, 7, 355–371. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Woo, S. Subcellular Drug Distribution: Exploring Organelle-Specific Characteristics for Enhanced Therapeutic Efficacy. Pharmaceutics 2024, 16, 1167. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, D.; Zhang, S.; Yang, Y.; Liu, J.J.; Wang, X.; Liu, C.; Milkie, D.E.; Moore, R.P.; Tulu, U.S.; et al. Visualizing Intracellular Organelle and Cytoskeletal Interactions at Nanoscale Resolution on Millisecond Timescales. Cell 2018, 175, 1430–1442.e17. [Google Scholar] [CrossRef]
- Paris, J.L.; Baeza, A.; Vallet-Regí, M. Overcoming the Stability, Toxicity, and Biodegradation Challenges of Tumor Stimuli-Responsive Inorganic Nanoparticles for Delivery of Cancer Therapeutics. Expert. Opin. Drug Deliv. 2019, 16, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Griffin, A.; Qiang, Z.; Ren, J. Organelle-Targeted Therapies: A Comprehensive Review on System Design for Enabling Precision Oncology. Signal Transduct. Target. Ther. 2022, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lin, S.; Tang, C.; Li, Y.; Yan, D.; Wang, D.; Chen, X. Dual-/Multi-Organelle-Targeted AIE Probes Associated with Oxidative Stress for Biomedical Applications. J. Mater. Chem. B 2024, 12, 8812–8824. [Google Scholar] [CrossRef]
- Lou, X.; Zhang, M.; Zhao, Z.; Min, X.; Hakeem, A.; Huang, F.; Gao, P.; Xia, F.; Tang, B.Z. A Photostable AIE Fluorogen for Lysosome-Targetable Imaging of Living Cells. J. Mater. Chem. B 2016, 4, 5412–5417. [Google Scholar] [CrossRef]
- Su, X.; Yu, Q.; Zhang, T.; Zhang, Y.M.; Yu, L.; Zhang, I.; Li, M.; Liu, Y.; Zhang, S.X.A. A Fluorescence Molecular Switch with High Contrast Multi-Emissions and ON/OFF States. RSC Adv. 2016, 6, 90305–90309. [Google Scholar] [CrossRef]
- Cai, Y.; Gui, C.; Samedov, K.; Su, H.; Gu, X.; Li, S.; Luo, W.; Sung, H.H.Y.; Lam, J.W.Y.; Kwok, R.T.K.; et al. An Acidic PH Independent Piperazine-TPE AIEgen as a Unique Bioprobe for Lysosome Tracing. Chem. Sci. 2017, 8, 7593–7603. [Google Scholar] [CrossRef]
- Wang, Q.; Li, C.; Chen, Q.; Zhang, P.; Wang, D. Lysosome-Targeting Red-Emitting Aggregation-Induced Emission Probe with Large Stokes Shift for Light-Up in Situ Visualization of β-N-Acetylhexosaminidase. Anal. Chem. 2019, 91, 12611–12614. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Yang, W.; Zhao, X.; Li, L.; Cao, Z.; Zhou, H.; Zheng, R.; Deng, Y.; Peng, C.; et al. A Novel AIE-Based Mitochondria-Targeting Fluorescent Probe for Monitoring of the Fluctuation of Endogenous Hypochlorous Acid in Ferroptosis Models. Anal Bioanal Chem 2024, 416, 4873–4885. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.; Shi, Y.; Hu, Y. Chemical Imaging for Biological Systems: Techniques, AI-Driven Processing, and Applications. J. Mater. Chem. B 2025, 13, 6916–6948. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Suhag, D.; Kaushik, S.; Taxak, V.B. Theranostics: Combining Diagnosis and Therapy. In Handbook of Biomaterials for Medical Applications, Volume 1: Fundamentals; Springer Nature: Singapore, 2024; pp. 271–295. [Google Scholar] [CrossRef]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, S.; Fan, Z.; Huang, G.; Tang, J.; Nie, L. Current Strategies of Photoacoustic Imaging Assisted Cancer Theragnostics toward Clinical Studies. ACS Photonics 2022, 9, 2555–2578. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal Therapy and Photoacoustic Imaging via Nanotheranostics in Fighting Cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Wang, W.J.; Xin, Z.Y.; Su, X.; Hao, L.; Qiu, Z.; Li, K.; Luo, Y.; Cai, X.M.; Zhang, J.; Alam, P.; et al. Aggregation-Induced Emission Luminogens Realizing High-Contrast Bioimaging. ACS Nano 2025, 19, 281–306. [Google Scholar] [CrossRef]
- Shen, H.; Xu, C.; Sun, F.; Zhao, M.; Wu, Q.; Zhang, J.; Li, S.; Zhang, J.; Lam, J.W.Y.; Tang, B.Z. Metal-Based Aggregation-Induced Emission Theranostic Systems. ChemMedChem 2022, 17, e202100578. [Google Scholar] [CrossRef]
- Burkett, B.J.; Bartlett, D.J.; McGarrah, P.W.; Lewis, A.R.; Johnson, D.R.; Berberoğlu, K.; Pandey, M.K.; Packard, A.T.; Halfdanarson, T.R.; Hruska, C.B.; et al. A Review of Theranostics: Perspectives on Emerging Approaches and Clinical Advancements. Radiol. Imaging Cancer 2023, 5, e220157. [Google Scholar] [CrossRef]
- Zhao, N.; Li, M.; Yan, Y.; Lam, J.W.Y.; Zhang, Y.L.; Zhao, Y.S.; Wongd, K.S.; Tang, B.Z. A Tetraphenylethene-Substituted Pyridinium Salt with Multiple Functionalities: Synthesis, Stimuli-Responsive Emission, Optical Waveguide and Specific Mitochondrion Imaging. J. Mater. Chem. C Mater. 2013, 1, 4640–4646. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, C.Y.Y.; Kwok, R.T.K.; Chen, Y.; Chen, S.; Lam, J.W.Y.; Tang, B.Z. Photostable AIE Fluorogens for Accurate and Sensitive Detection of S-Phase DNA Synthesis and Cell Proliferation. J. Mater. Chem. B 2015, 3, 4993–4996. [Google Scholar] [CrossRef]
- Zhuang, W.; Ma, B.; Liu, G.; Li, G.; Wang, Y. TPE-Conjugated Biomimetic and Biodegradable Polymeric Micelle for AIE Active Cell Imaging and Cancer Therapy. J. Appl. Polym. Sci. 2018, 135, 45651. [Google Scholar] [CrossRef]
- Qin, W.; Ding, D.; Liu, J.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible Nanoparticles with Aggregation-Induced Emission Characteristics as Far-Red/Near-Infrared Fluorescent Bioprobes for In Vitro and In Vivo Imaging Applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Jin, G.; He, R.; Liu, Q.; Lin, M.; Dong, Y.; Li, K.; Tang, B.Z.; Liu, B.; Xu, F. Near-Infrared Light-Regulated Cancer Theranostic Nanoplatform Based on Aggregation-Induced Emission Luminogen Encapsulated Upconversion Nanoparticles. Theranostics 2019, 9, 246–264. [Google Scholar] [CrossRef]
- Gao, X.; Mao, D.; Zuo, X.; Hu, F.; Cao, J.; Zhang, P.; Sun, J.Z.; Liu, J.; Liu, B.; Tang, B.Z. Specific Targeting, Imaging, and Ablation of Tumor-Associated Macrophages by Theranostic Mannose-AIEgen Conjugates. Anal. Chem. 2019, 91, 6836–6843. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhuang, J.; Li, N.; Li, Y.; Zhu, S.; Hao, J.; Xin, J.; Zhao, N. Efficient Near-Infrared Photosensitizer with Aggregation-Induced Emission Characteristics for Mitochondria-Targeted and Image-Guided Photodynamic Cancer Therapy. Mater. Chem. Front. 2020, 4, 2064–2071. [Google Scholar] [CrossRef]
- Situ, B.; Ye, X.; Zhao, Q.; Mai, L.; Huang, Y.; Wang, S.; Chen, J.; Li, B.; He, B.; Zhang, Y.; et al. Identification and Single-Cell Analysis of Viable Circulating Tumor Cells by a Mitochondrion-Specific AIE Bioprobe. Adv. Sci. 2020, 7, 1902760. [Google Scholar] [CrossRef]
- Wang, K.; Gao, H.; Zhang, Y.; Yan, H.; Si, J.; Mi, X.; Xia, S.; Feng, X.; Liu, D.; Kong, D.; et al. Highly Bright AIE Nanoparticles by Regulating the Substituent of Rhodanine for Precise Early Detection of Atherosclerosis and Drug Screening. Adv. Mater. 2022, 34, 2106994. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Y.; Niu, G.; Gu, D.; Wang, J.; Cao, Y.; Yin, Y.; Li, X.; Ding, D.; Xi, R.; et al. Photostable PH-Sensitive Near-Infrared Aggregation-Induced Emission Luminogen for Long-Term Mitochondrial Tracking. ACS Appl. Mater. Interfaces 2019, 11, 13134–13139. [Google Scholar] [CrossRef]
- Huang, Z.; Ding, A.; Yang, J.; Wang, C.; Tang, F. Conjugating Coumarin with Tetraphenylethylene to Achieve Dual-State Emission for Reversible Mechanofluorochromism and Live Cell Imaging. Chem.–A Eur. J. 2023, 29, e202203628. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Zhao, W.; Li, S.; Li, X.; Liu, Z.; Jiang, K.; Sun, S. Tumor Targeting PH-Triggered Fluorescence-Switchable Hyaluronic Acid-Based Micelles with Aggregation-Induced Emission Activity for Tracing Drug Release and Intelligent Drug Delivery. Int. J. Biol. Macromol. 2024, 277, 134386. [Google Scholar] [CrossRef]
- Liu, Z.; Zong, Z.; Li, X.; Sun, S. Smart Polymeric Micelles with Aggregation-Induced Emission and PH-Responsive Fluorescence Color Change Behavior for Bioimaging and Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 6654. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Ren, J.; Han, X.; Wang, Q.; Huang, K.; Du, B.; Liang, L. A Red Emission Tetraphenyl Ethylene-Fused Rhodamine Dye for Viscosity Sensing and Mitochondria-Targeted Cell Imaging. J. Mol. Struct. 2024, 1310, 138280. [Google Scholar] [CrossRef]
- Liu, M.; Huang, H.; Wang, K.; Xu, D.; Wan, Q.; Tian, J.; Huang, Q.; Deng, F.; Zhang, X.; Wei, Y. Fabrication and Biological Imaging Application of AIE-Active Luminescent Starch Based Nanoprobes. Carbohydr. Polym. 2016, 142, 38–44. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Y.; Liu, S.; Li, J.; Zhao, B.; Wang, F.; Zhao, Z.; Ni, Q.; Liu, F.; Xue, J. Simultaneous Visualization and Depletion of Peroxynitrite by a Simple Aggregation-Induced Emission Nanoprobe for Preventing Breast Cancer Metastasis after Surgery. Anal. Chem. 2024, 96, 4180–4189. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, L.; Wu, C.; Liu, L.; Li, H. Reviewing the Evolutive ACQ-to-AIE Transformation of Photosensitizers for Phototheranostics. Luminescence 2024, 39, e4655. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, X.; Liang, K.; Gao, M.; Kong, B. Frontier Luminous Strategy of Functional Silica Nanohybrids in Sensing and Bioimaging: From ACQ to AIE. Aggregate 2022, 3, e121. [Google Scholar] [CrossRef]
- Gao, J.; Tang, Y.; Martella, D.; Guo, J.; Wiersma, D.S.; Li, Q. Stimuli-Responsive Photonic Actuators for Integrated Biomimetic and Intelligent Systems. Responsive Mater. 2023, 1, e20230008. [Google Scholar] [CrossRef]
- Khalil, A.K.A.; Teow, Y.H.; Takriff, M.S.; Ahmad, A.L.; Atieh, M.A. Recent Developments in Stimuli-Responsive Polymer for Emerging Applications: A Review. Results Eng. 2025, 25, 103900. [Google Scholar] [CrossRef]
- Dolui, S.; Sahu, B.; Banerjee, S. Stimuli-Responsive Functional Polymeric Materials: Recent Advances and Future Perspectives. Macromol. Chem. Phys. 2025, 226, 2400472. [Google Scholar] [CrossRef]
- Heo, J.; Seo, S.; Yun, H.; Ku, K.H. Stimuli-Responsive Nanoparticle Self-Assembly at Complex Fluid Interfaces: A New Insight into Dynamic Surface Chemistry. Nanoscale 2024, 16, 3951–3968. [Google Scholar] [CrossRef]
- Xu, C.; Zou, H.; Zhao, Z.; Zhang, P.; Kwok, R.T.K.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Tang, B.Z. A New Strategy toward “Simple” Water-Soluble AIE Probes for Hypoxia Detection. Adv. Funct. Mater. 2019, 29, 1903278. [Google Scholar] [CrossRef]
- Xu, C.; Zou, H.; Zhao, Z.; Zheng, Z.; Kwok, R.T.K.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Chen, S.; Zheng, L.; et al. Turning on Light Emission of a Dark Pro-Aggregation-Induced Emission Luminogen in Aqueous Media Through Reductase-Modulated Derotation. Adv. Nanobiomed Res. 2021, 1, 2000080. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Hu, P.; Xu, C.; Guo, W.; Wang, Z.; Mao, Z.; Yang, Z.; Liu, C.; Shi, G.; et al. Achieving Remarkable and Reversible Mechanochromism from a Bright Ionic AIEgen with High Specificity for Mitochondrial Imaging and Secondary Aggregation Emission Enhancement for Long-Term Tracking of Tumors. Mater. Chem. Front. 2020, 4, 941–949. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, W.; Xiao, P.; Kang, M.; Yan, D.; Wen, H.; Song, N.; Wang, D.; Tang, B.Z. Molecular Engineering of High-Performance Aggregation-Induced Emission Photosensitizers to Boost Cancer Theranostics Mediated by Acid-Triggered Nucleus-Targeted Nanovectors. ACS Nano 2021, 15, 10689–10699. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, J.; Kim, Y.Y.; Lee, S.; Kim, Y. Synergistic Combination of Tamoxifen–Tetraphenylethylene Co-Assembled Micelles and Orlistat-Induced Lipid Droplet Inhibition to Overcome Tamoxifen Resistance. Adv. Heal. Mater. 2025, e02060. [Google Scholar] [CrossRef]
- Cen, P.; Huang, J.; Jin, C.; Wang, J.; Wei, Y.; Zhang, H.; Tian, M. Aggregation-Induced Emission Luminogens for in Vivo Molecular Imaging and Theranostics in Cancer. Aggregate 2023, 4, e352. [Google Scholar] [CrossRef]
- Shen, H.; Xu, C.; Ye, R.; Liu, T.M.; Zhang, J.; Kwok, R.T.K.; Lam, J.W.Y.; Guo, Z.; Sun, J.; Tang, B.Z. Water-Soluble Aggregation-Induced Emission Luminogens with Near-Infrared Emission for Advanced Phototheranostics. Small Sci. 2023, 3, 2300052. [Google Scholar] [CrossRef]
- Lan, K.; Zhang, C.; Li, Y.; Hu, C.; Su, Z.; Cheng, C. Strain Energy Prompted Tunable Aggregation-Induced Emission Property of Tetraphenylethylene. Angew. Chem.-Int. Ed. 2025, e202507763. [Google Scholar] [CrossRef]
- Zhu, F.Y.; Mei, L.J.; Tian, R.; Li, C.; Wang, Y.L.; Xiang, S.L.; Zhu, M.Q.; Tang, B.Z. Recent Advances in Super-Resolution Optical Imaging Based on Aggregation-Induced Emission. Chem. Soc. Rev. 2024, 53, 3350–3383. [Google Scholar] [CrossRef]
- Bin, Y.; Huang, L.; Qin, J.; Zhao, S.; Tian, J.; Zhang, L. Exceptional Near-Infrared II Organic Small Molecule Nanoagent for Photoacoustic/Photothermal Imaging-Guided Highly Efficient Therapy in Cancer. Bioconjugate Chem. 2025, 36, 803–814. [Google Scholar] [CrossRef]
- Huang, G.; Jiang, Y.; Yang, S.; Li, B.S.; Tang, B.Z. Multistimuli Response and Polymorphism of a Novel Tetraphenylethylene Derivative. Adv. Funct. Mater. 2019, 29, 1900516. [Google Scholar] [CrossRef]
- Qayyum, M.; Bushra, T.; Khan, Z.A.; Gul, H.; Majeed, S.; Yu, C.; Farooq, U.; Shaikh, A.J.; Shahzad, S.A. Synthesis and Tetraphenylethylene-Based Aggregation-Induced Emission Probe for Rapid Detection of Nitroaromatic Compounds in Aqueous Media. ACS Omega 2021, 6, 25447–25460. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Huang, Z.; Guo, S.; Yang, J.X.; Kong, L. A Multi-Stimuli-Responsive Tetraphenylethene Derivative with High Fluorescent Emission in Solid State. Dye. Pigment. 2022, 197, 109909. [Google Scholar] [CrossRef]
- Gao, R.; Guo, X.; Wang, L.; Yang, W. Highly Differentiated Multi-Stimuli-Responsive Fluorescence Performance of Tetraphenylethylene-Containing Styrene–Maleic Acid Copolymers Induced by Macromolecular Architecture Control. Polym. Chem. 2023, 14, 5178–5190. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Cheng, Y.; Wu, H.; Su, X.; Wang, R.; Xiao, K.; Zhang, W.; Chen, Z.; Xu, B.; et al. Hydrazone-Based Photocages for Precise Sub-Organelle Visualization and Drug Release. Commun. Chem. 2025, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Babu, D.D.; Wu, M.; Wang, Y. Synergistic Supports Beyond Carbon Black for Polymer Electrolyte Fuel Cell Anodes. ChemCatChem 2018, 10, 4497–4508. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Pan, S.; Sun, X.; Zhou, J.; Li, H. Color-Tunable Carbon Dots with Aggregation-Induced Emission Constructed by FRET between Surface Luminescence Centers. Adv. Opt. Mater. 2024, 12, 2301486. [Google Scholar] [CrossRef]
- Yang, J.C.; Chen, K.; Zhang, G.L.; Qi, C.; Feng, H.T.; Tang, B.Z. Novel Supramolecular Artificial Light-Harvesting Systems Based on AIE-Active Macrocycles for Efficient White-Light Photocatalysis in Water. Chem. Sci. 2025, 16, 4741–4748. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.C.; Peng, Z.; Wu, J.; Anandhababu, G.; Babu, D.D.; Huang, Y.; Ghausi, M.A.; Wu, M.; Wang, Y. Novel N-Mo2C Active Sites for Efficient Solar-to-Hydrogen Generation. ChemElectroChem 2018, 5, 1186–1190. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hariprasad, V.; Keremane, K.S.; Naik, P.; Babu, D.D.; Shivashankar, S.M. Tetraphenylethylene (TPE)-Based AIE Luminogens: Recent Advances in Bioimaging Applications. Photochem 2025, 5, 23. https://doi.org/10.3390/photochem5030023
Hariprasad V, Keremane KS, Naik P, Babu DD, Shivashankar SM. Tetraphenylethylene (TPE)-Based AIE Luminogens: Recent Advances in Bioimaging Applications. Photochem. 2025; 5(3):23. https://doi.org/10.3390/photochem5030023
Chicago/Turabian StyleHariprasad, Vanam, Kavya S. Keremane, Praveen Naik, Dickson D. Babu, and Sunitha M. Shivashankar. 2025. "Tetraphenylethylene (TPE)-Based AIE Luminogens: Recent Advances in Bioimaging Applications" Photochem 5, no. 3: 23. https://doi.org/10.3390/photochem5030023
APA StyleHariprasad, V., Keremane, K. S., Naik, P., Babu, D. D., & Shivashankar, S. M. (2025). Tetraphenylethylene (TPE)-Based AIE Luminogens: Recent Advances in Bioimaging Applications. Photochem, 5(3), 23. https://doi.org/10.3390/photochem5030023







