Characterizing the Excited States and Electronic Absorption Spectra of Small Alkylperoxy (RO2•) and Hydroperoxy (•QOOH) Radicals
Abstract
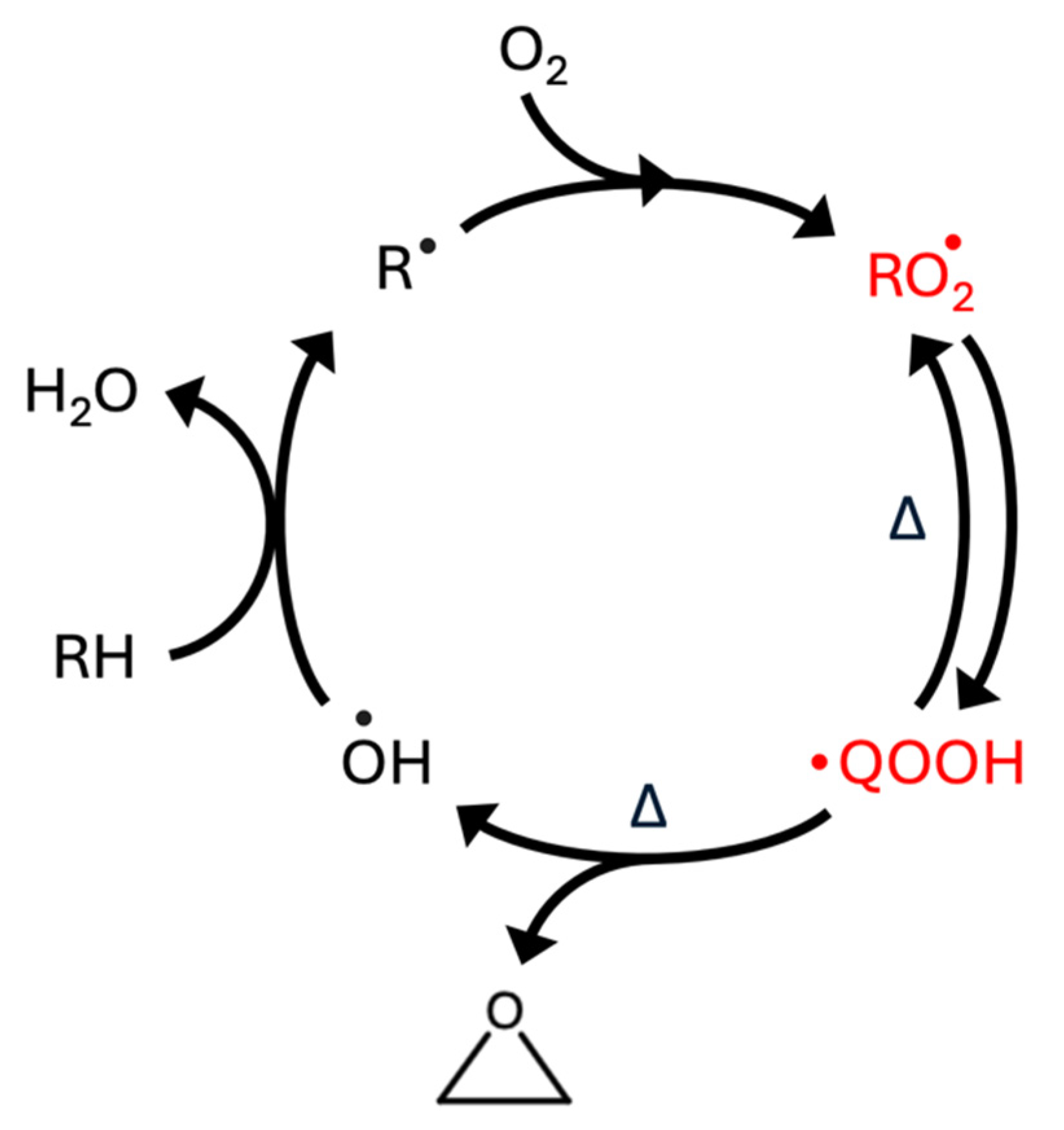
1. Introduction
2. Methodology
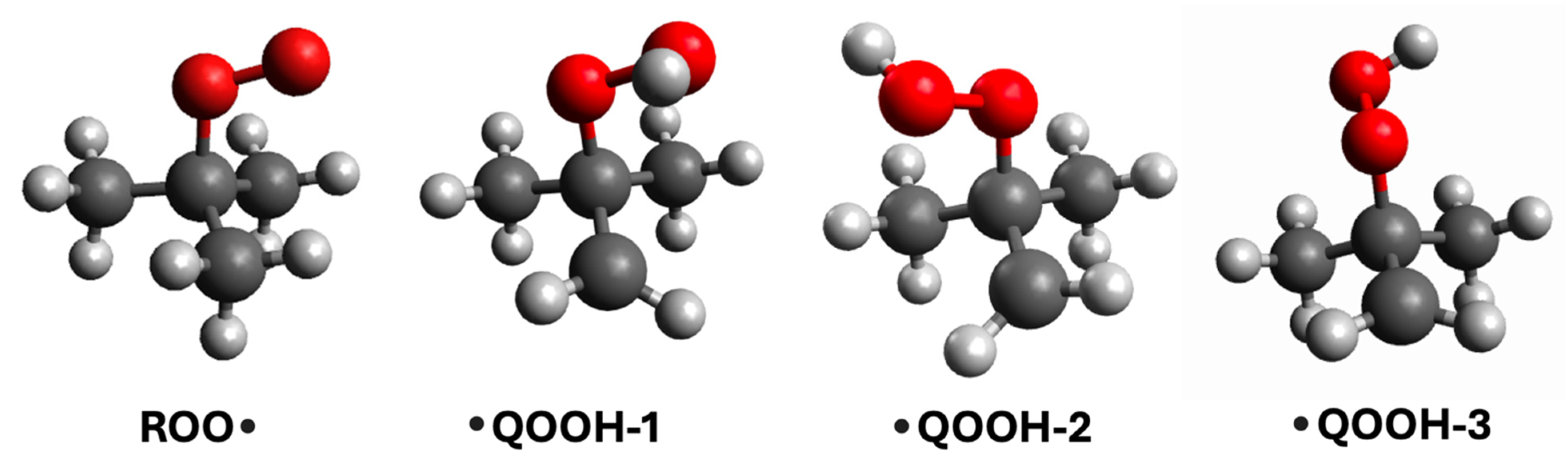
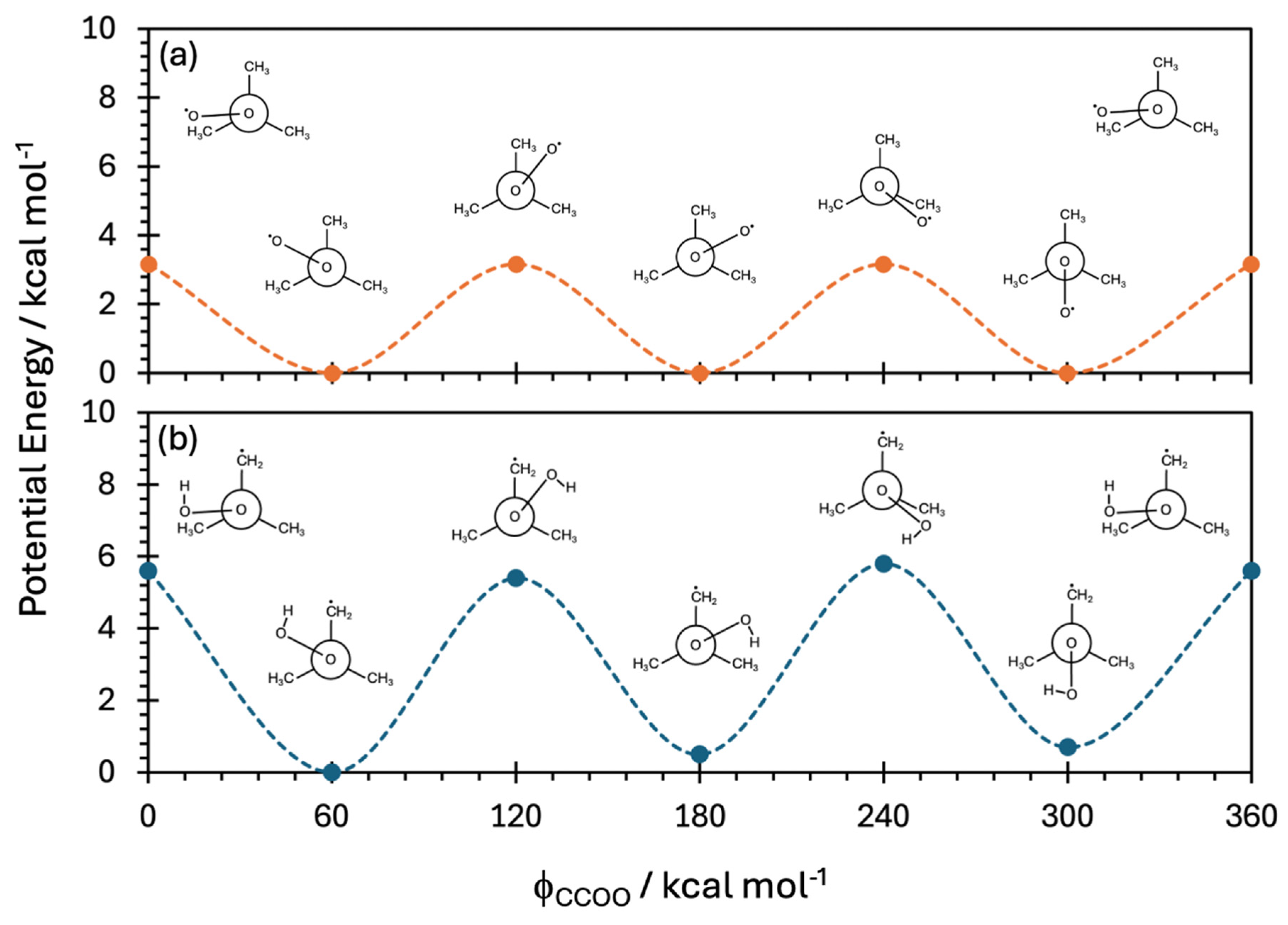
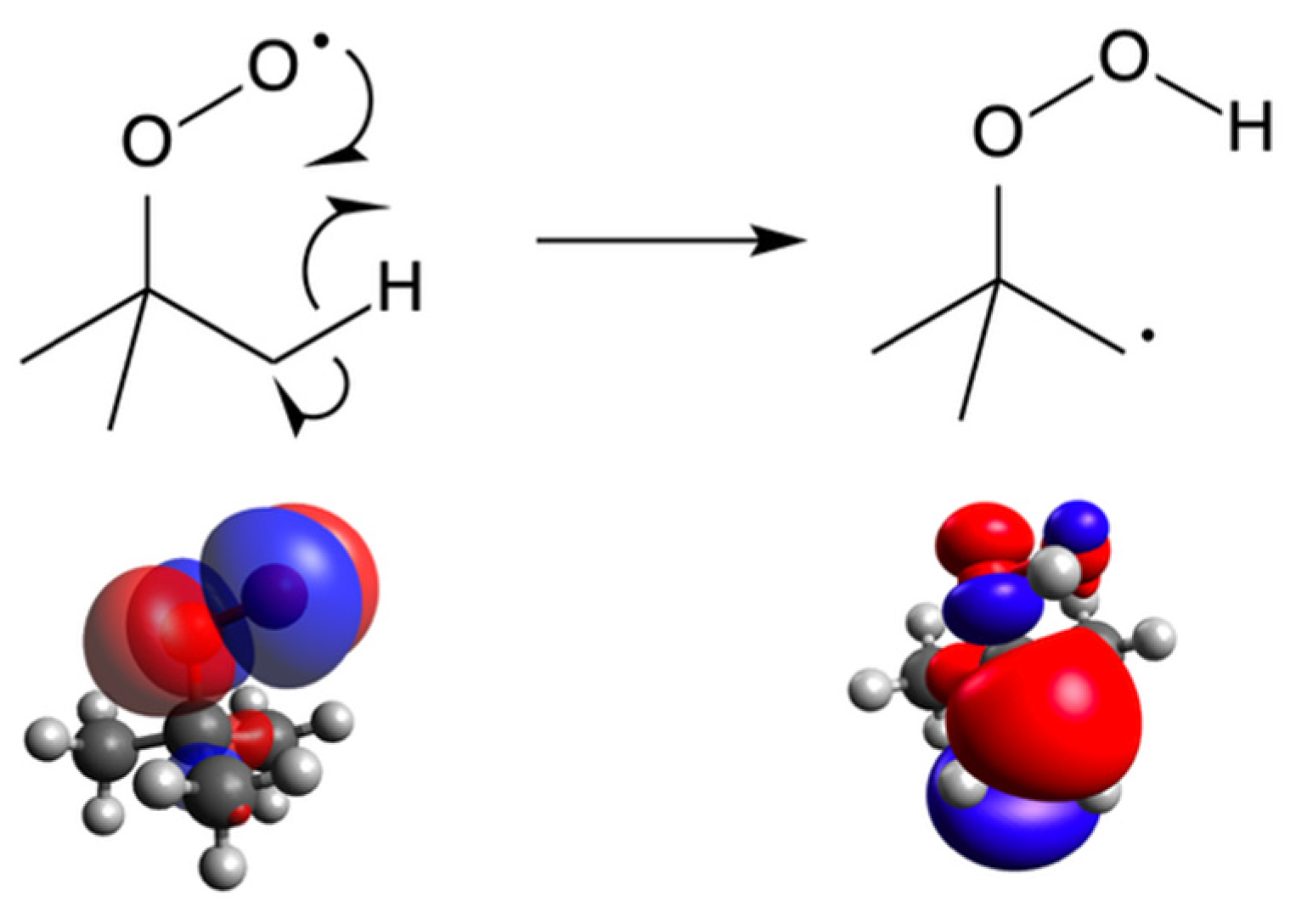
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srinivasan, N.K.; Harding, L.B.; Klippenstein, S.J. Experimental and theoretical rate constants for CH4 + O2 → CH3 + HO2. Combust. Flame 2007, 149, 104–111. [Google Scholar] [CrossRef]
- Osborn, D.L. Reaction Mechanisms on Multiwell Potential Energy Surfaces in Combustion (and Atmospheric) Chemistry. Annu. Rev. Phys. Chem. 2017, 68, 233–260. [Google Scholar] [CrossRef]
- Zádor, J.; Taatjes, C.A.; Fernandes, R.X. Kinetics of elementary reactions in low-temperature autoignition chemistry. Prog. Energy Combust. Sci. 2011, 37, 371–421. [Google Scholar] [CrossRef]
- Bianchi, F.; Kurtén, T.; Riva, M.; Mohr, C.; Rissanen, M.P.; Roldin, P.; Berndt, T.; Crounse, J.D.; Wennberg, P.O.; Mentel, T.F.; et al. Highly Oxygenated Organic Molecules (HOM) from Gas-Phase Autoxidation Involving Peroxy Radicals: A Key Contributor to Atmospheric Aerosol. Chem. Rev. 2019, 119, 3472–3509. [Google Scholar] [CrossRef]
- Praske, E.; Otkjær, R.V.; Crounse, J.D.; Hethcox, J.C.; Stoltz, B.M.; Kjaergaard, H.G.; Wennberg, P.O. Atmospheric autoxidation is increasingly important in urban and suburban North America. Proc. Natl. Acad. Sci. USA 2018, 115, 64–69. [Google Scholar] [CrossRef]
- Crounse, J.D.; Nielsen, L.B.; Jørgensen, S.; Kjaergaard, H.G.; Wennberg, P.O. Autoxidation of Organic Compounds in the Atmosphere. J. Phys. Chem. Lett. 2013, 4, 3513–3520. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Moshammer, K.; Popolan-Vaida, D.M.; Shankar, V.S.B.; Lucassen, A.; Hemken, C.; Taatjes, C.A.; Leone, S.R.; Kohse-Höinghaus, K.; et al. Additional chain-branching pathways in the low-temperature oxidation of branched alkanes. Combust. Flame 2016, 164, 386–396. [Google Scholar] [CrossRef]
- Wang, Z.; Sarathy, S.M. Third O2 addition reactions promote the low-temperature auto-ignition of n-alkanes. Combust. Flame 2016, 165, 364–372. [Google Scholar] [CrossRef]
- Schervish, M.; Donahue, N.M. Peroxy radical chemistry and the volatility basis set. Atmos. Chem. Phys. 2020, 20, 1183–1199. [Google Scholar] [CrossRef]
- Savee, J.D.; Papajak, E.; Rotavera, B.; Huang, H.; Eskola, A.J.; Welz, O.; Sheps, L.; Taatjes, C.A.; Zádor, J.; Osborn, D.L. Direct observation and kinetics of a hydroperoxyalkyl radical (QOOH). Science 2015, 347, 643–646. [Google Scholar] [CrossRef]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipilä, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A large source of low-volatility secondary organic aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Kalafut-Pettibone, A.J.; Klems, J.P.; Burgess, D.R.; McGivern, W.S. Alkylperoxy Radical Photochemistry in Organic Aerosol Formation Processes. J. Phys. Chem. A 2013, 117, 14141–14150. [Google Scholar] [CrossRef]
- Paulot, F.; Crounse, J.D.; Kjaergaard, H.G.; Kürten, A.; Clair, J.M.S.; Seinfeld, J.H.; Wennberg, P.O. Unexpected Epoxide Formation in the Gas-Phase Photooxidation of Isoprene. Science 2009, 325, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of Secondary Organic Aerosols Through Photooxidation of Isoprene. Science 2004, 303, 1173–1176. [Google Scholar] [CrossRef]
- Crounse, J.D.; Paulot, F.; Kjaergaard, H.G.; Wennberg, P.O. Peroxy radical isomerization in the oxidation of isoprene. Phys. Chem. Chem. Phys. 2011, 13, 13607–13613. [Google Scholar] [CrossRef] [PubMed]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Vereecken, L.; Aumont, B.; Barnes, I.; Bozzelli, J.W.; Goldman, M.J.; Green, W.H.; Madronich, S.; Mcgillen, M.R.; Mellouki, A.; Orlando, J.J.; et al. Perspective on Mechanism Development and Structure-Activity Relationships for Gas-Phase Atmospheric Chemistry. Int. J. Chem. Kinet. 2018, 50, 435–469. [Google Scholar] [CrossRef]
- Atkinson, R. Kinetics of the gas-phase reactions of OH radicals with alkanes and cycloalkanes. Atmos. Chem. Phys. 2003, 3, 2233–2307. [Google Scholar] [CrossRef]
- Klems, J.P.; Lippa, K.A.; McGivern, W.S. Quantitative Evidence for Organic Peroxy Radical Photochemistry at 254 nm. J. Phys. Chem. A 2015, 119, 344–351. [Google Scholar] [CrossRef]
- Orlando, J.J.; Tyndall, G.S. Laboratory studies of organic peroxy radical chemistry: An overview with emphasis on recent issues of atmospheric significance. Chem. Soc. Rev. 2012, 41, 6294–6317. [Google Scholar] [CrossRef]
- Boamah, M.D.; Sullivan, K.K.; Shulenberger, K.E.; Soe, C.M.; Jacob, L.M.; Yhee, F.C.; Atkinson, K.E.; Boyer, M.C.; Haines, R.; Arumainayagam, C.R. Low-energy electron-induced chemistry of condensed methanol: Implications for the interstellar synthesis of prebiotic molecules. Faraday Discuss. 2014, 168, 249–266. [Google Scholar] [CrossRef]
- Assaf, E.; Tanaka, S.; Kajii, Y.; Schoemaecker, C.; Fittschen, C. Rate constants of the reaction of C2–C4 peroxy radicals with OH radicals. Chem. Phys. Lett. 2017, 684, 245–249. [Google Scholar] [CrossRef]
- Assaf, E.; Schoemaecker, C.; Vereecken, L.; Fittschen, C. Experimental and theoretical investigation of the reaction of RO2 radicals with OH radicals: Dependence of the HO2 yield on the size of the alkyl group. Int. J. Chem. Kinet. 2018, 50, 670–680. [Google Scholar] [CrossRef]
- Biggs, P.; Canosa-Mass, C.E.; Fracheboud, J.-M.; Shallcross, D.E.; Wayne, R.P. Rate constants for the reactions of C2H5, C2H5O and C2H5O2 radicals with NO3 at 298 K and 2.2 torr. J. Chem. Soc. Faraday Trans. 1995, 91, 817–825. [Google Scholar] [CrossRef]
- Ziemann, P.J.; Atkinson, R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef]
- Eberhard, J.; Howard, C.J. Rate Coefficients for the Reactions of Some C3 to C5 Hydrocarbon Peroxy Radicals with NO. J. Phys. Chem. A 1997, 101, 3360–3366. [Google Scholar] [CrossRef]
- Peng, Z.; Jimenez, J.L. Radical chemistry in oxidation flow reactors for atmospheric chemistry research. Chem. Soc. Rev. 2020, 49, 2570–2616. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, M.E.; Valorso, R.; Aumont, B.; Rickard, A.R. Estimation of rate coefficients and branching ratios for reactions of organic peroxy radicals for use in automated mechanism construction. Atmos. Chem. Phys. 2019, 19, 7691–7717. [Google Scholar] [CrossRef]
- Berndt, T.; Scholz, W.; Mentler, B.; Fischer, L.; Herrmann, H.; Kulmala, M.; Hansel, A. Accretion Product Formation from Self- and Cross-Reactions of RO2 Radicals in the Atmosphere. Angew. Chem. Int. Ed. 2018, 57, 3820–3824. [Google Scholar] [CrossRef] [PubMed]
- Archibald, A.T.; Petit, A.S.; Percival, C.J.; Harvey, J.N.; Shallcross, D.E. On the importance of the reaction between OH and RO2 radicals. Atmos. Sci. Lett. 2009, 10, 102–108. [Google Scholar] [CrossRef]
- Chen, L.W.; Hung, C.M.; Matsui, H.; Lee, Y.P. New experimental evidence to support roaming in the reaction Cl+ isobutene (i-C4 H8). Sci. Rep. 2017, 7, 40105. [Google Scholar]
- Yan, C.; Kocevska, S.; Krasnoperov, L.N. Kinetics of the Reaction of CH3O2 Radicals with OH Studied over the 292–526 K Temperature Range. J. Phys. Chem. A 2016, 120, 6111–6121. [Google Scholar] [CrossRef]
- Assaf, E.; Sheps, L.; Whalley, L.; Heard, D.; Tomas, A.; Schoemaecker, C.; Fittschen, C. The Reaction between CH3O2 and OH Radicals: Product Yields and Atmospheric Implications. Environ. Sci. Technol. 2017, 51, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.A.; Villenave, E.; Lesclaux, R. Structure–reactivity relationships for the self- reactions of linear secondary alkylperoxy radicals: An experimental investigation. Int. J. Chem. Kinet. 1999, 31, 37–46. [Google Scholar] [CrossRef]
- Eberhard, J.; Howard, C.J. Temperature-dependent kinetics studies of the reactions of C2H5O2 and n-C3H7O2 radicals with NO. Int. J. Chem. Kinet. 1996, 28, 731–740. [Google Scholar] [CrossRef]
- Faragó, E.P.; Schoemaecker, C.; Viskolcz, B.; Fittschen, C. Experimental determination of the rate constant of the reaction between C2H5O2 and OH radicals. Chem. Phys. Lett. 2015, 619, 196–200. [Google Scholar] [CrossRef]
- Jokinen, T.; Sipilä, M.; Richters, S.; Kerminen, V.-M.; Paasonen, P.; Stratmann, F.; Worsnop, D.; Kulmala, M.; Ehn, M.; Herrmann, H.; et al. Rapid autoxidation forms highly oxidized RO2 radicals in the atmosphere. Angew. Chem. Int. Ed. Engl. 2014, 53, 14596–14600. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, P.D.; Cox, R.A.; Crowley, J.N.; Destriau, M.; Hayman, G.D.; Jenkin, M.E.; Moortgat, G.K.; Zabel, F. Organic peroxy radicals: Kinetics, spectroscopy and tropospheric chemistry. Atmos. Environ. Part A General. Top. 1992, 26, 1805–1961. [Google Scholar] [CrossRef]
- Pilling, M.J. Basic chemistry of combustion. In Low-Temperature Combustion and Autoignition; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Kaiser, E.W. Temperature and Pressure Dependence of the C2H4 Yield from the Reaction C2H5 + O2. J. Phys. Chem. 1995, 99, 707–711. [Google Scholar] [CrossRef]
- Kaiser, E.W. Formation of C3H6 from the Reaction C3H7 + O2 between 450 and 550 K. J. Phys. Chem. A 1998, 102, 5903–5906. [Google Scholar] [CrossRef]
- Kaiser, E.W.; Wallington, T.J. Formation of C3H6 from the Reaction C3H7 + O2 and C2H3Cl from C2H4Cl + O2 at 297 K. J. Phys. Chem. 1996, 100, 18770–18774. [Google Scholar] [CrossRef]
- Plumb, I.C.; Ryan, K.R. Kinetic studies of the reaction of C2H5 with O2 at 295 K. Int. J. Chem. Kinet. 1981, 13, 1011–1028. [Google Scholar] [CrossRef]
- Sharma, S.; Raman, S.; Green, W.H. Intramolecular Hydrogen Migration in Alkylperoxy and Hydroperoxyalkylperoxy Radicals: Accurate Treatment of Hindered Rotors. J. Phys. Chem. A 2010, 114, 5689–5701. [Google Scholar] [CrossRef]
- DeSain, J.D.; Taatjes, C.A. Infrared Frequency-Modulation Probing of Product Formation in Alkyl + O2 Reactions: III. The Reaction of Cyclopentyl Radical (c-C5H9) with O2 between 296 and 723 K. J. Phys. Chem. A 2001, 105, 6646–6654. [Google Scholar] [CrossRef]
- Clifford, E.P.; Farrell, J.T.; DeSain, J.D.; Taatjes, C.A. Infrared Frequency-Modulation Probing of Product Formation in Alkyl + O2 Reactions: I. The Reaction of C2H5 with O2 between 295 and 698 K. J. Phys. Chem. A 2000, 104, 11549–11560. [Google Scholar] [CrossRef]
- DeSain, J.D.; Clifford, E.P.; Taatjes, C.A. Infrared Frequency-Modulation Probing of Product Formation in Alkyl + O2 Reactions: II. The Reaction of C3H7 with O2 between 296 and 683 K. J. Phys. Chem. A 2001, 105, 3205–3213. [Google Scholar] [CrossRef]
- DeSain, J.D.; Taatjes, C.A.; Miller, J.A.; Klippenstein, S.J.; Hahn, D.K. Infrared frequency-modulation probing of product formation in alkyl + O2 reactions. Part IV. Reactions of propyl and butyl radicals with O2. Faraday Discuss. 2002, 119, 101–120. [Google Scholar] [CrossRef]
- Kaiser, E.W.; Lorkovic, I.M.; Wallington, T.J. Pressure dependence of the ethene yield from the reaction ethyl radical + oxygen. J. Phys. Chem. 1990, 94, 3352–3354. [Google Scholar] [CrossRef]
- Estupiñán, E.G.; Smith, J.D.; Tezaki, A.; Klippenstein, S.J.; Taatjes, C.A. Measurements and Modeling of DO2 Formation in the Reactions of C2D5 and C3D7 Radicals with O2. J. Phys. Chem. A 2007, 111, 4015–4030. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, C.K. Chemical kinetics of hydrocarbon ignition in practical combustion systems. Proc. Combust. Inst. 2000, 28, 1563–1577. [Google Scholar] [CrossRef]
- Miller, J.A.; Klippenstein, S.J.; Robertson, S.H. A theoretical analysis of the reaction between ethyl and molecular oxygen. Proc. Combust. Inst. 2000, 28, 1479–1486. [Google Scholar] [CrossRef]
- Rienstra-Kiracofe, J.C.; Allen, W.D.; Schaefer, H. The C2H5 + O2 Reaction Mechanism: High-Level ab Initio Characterizations. J. Phys. Chem. A 2000, 104, 9823–9840. [Google Scholar] [CrossRef]
- Estupiñán, E.G.; Klippenstein, S.J.; Taatjes, C.A. Measurements and Modeling of HO2 Formation in the Reactions of n-C3H7 and i-C3H7 Radicals with O2. J. Phys. Chem. B 2005, 109, 8374–8387. [Google Scholar] [CrossRef]
- Kaiser, E.W. Mechanism of the Reaction C2H5 + O2 from 298 To 680 K. J. Phys. Chem. A 2002, 106, 1256–1265. [Google Scholar] [CrossRef]
- DeSain, J.D.; Klippenstein, S.J.; Miller, J.A.; Taatjes, C.A. Measurements, Theory, and Modeling of OH Formation in Ethyl + O2 and Propyl + O2 Reactions. J. Phys. Chem. A 2003, 107, 4415–4427. [Google Scholar] [CrossRef]
- Sheng, C.Y.; Bozzelli, J.W.; Dean, A.M.; Chang, A.Y. Detailed Kinetics and Thermochemistry of C2H5 + O2: Reaction Kinetics of the Chemically-Activated and Stabilized CH3CH2OO• Adduct. J. Phys. Chem. A 2002, 106, 7276–7293. [Google Scholar] [CrossRef]
- Quelch, G.E.; Gallo, M.M.; Schaefer, H.F. Aspects of the reaction mechanism of ethane combustion. Conformations of the ethylperoxy radical. J. Am. Chem. Soc. 1992, 114, 8239–8247. [Google Scholar] [CrossRef]
- Quelch, G.E.; Gallo, M.M.; Shen, M.; Xie, Y.; Schaefer, H.F.; Moncrieff, D. Aspects of the Reaction Mechanism of Ethane Combustion. 2. Nature of the Intramolecular Hydrogen Transfer. J. Am. Chem. Soc. 1994, 116, 4953–4962. [Google Scholar] [CrossRef]
- Miller, J.A.; Klippenstein, S.J. The reaction between ethyl and molecular oxygen II: Further analysis. Int. J. Chem. Kinet. 2001, 33, 654–668. [Google Scholar] [CrossRef]
- Chen, C.-J.; Bozzelli, J.W. Analysis of Tertiary Butyl Radical + O2, Isobutene + HO2, Isobutene + OH, and Isobutene−OH Adducts + O2: A Detailed Tertiary Butyl Oxidation Mechanism. J. Phys. Chem. A 1999, 103, 9731–9769. [Google Scholar] [CrossRef]
- DeSain, J.D.; Klippenstein, S.J.; Taatjes, C.A. Time-resolved measurements of OH and HO2 product formation in pulsed-photolytic chlorine atom initiated oxidation of neopentane. Phys. Chem. Chem. Phys. 2003, 5, 1584–1592. [Google Scholar] [CrossRef]
- Petway, S.V.; Ismail, H.; Green, W.H.; Estupiñán, E.G.; Jusinski, L.E.; Taatjes, C.A. Measurements and Automated Mechanism Generation Modeling of OH Production in Photolytically Initiated Oxidation of the Neopentyl Radical. J. Phys. Chem. A 2007, 111, 3891–3900. [Google Scholar] [CrossRef]
- Sun, H.; Bozzelli, J.W. Thermochemical and Kinetic Analysis on the Reactions of Neopentyl and Hydroperoxy-Neopentyl Radicals with Oxygen: Part I. OH and Initial Stable HC Product Formation. J. Phys. Chem. A 2004, 108, 1694–1711. [Google Scholar] [CrossRef]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A Comprehensive Modeling Study of n-Heptane Oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A comprehensive modeling study of iso-octane oxidation. Combust. Flame 2002, 129, 253–280. [Google Scholar] [CrossRef]
- Zhu, L.; Bozzelli, J.W.; Kardos, L.M. Thermochemical Properties, ΔfH°(298), S°(298), and Cp°(T), for n-Butyl and n-Pentyl Hydroperoxides and the Alkyl and Peroxy Radicals, Transition States, and Kinetics for Intramolecular Hydrogen Shift Reactions of the Peroxy Radicals. J. Phys. Chem. A 2007, 111, 6361–6377. [Google Scholar] [CrossRef] [PubMed]
- Merle, J.K.; Hayes, C.J.; Zalyubovsky, S.J.; Glover, B.G.; Miller, T.A.; Hadad, C.M. Theoretical Determinations of the Ambient Conformational Distribution and Unimolecular Decomposition of n-Propylperoxy Radical. J. Phys. Chem. A 2005, 109, 3637–3646. [Google Scholar] [CrossRef] [PubMed]
- Sirjean, B.; Glaude, P.A.; Ruiz-Lòpez, M.F.; Fournet, R. Theoretical kinetic study of the reactions of cycloalkylperoxy radicals. J. Phys. Chem. A 2009, 113, 6924–6935. [Google Scholar] [CrossRef]
- Silke, E.J.; Pitz, W.J.; Westbrook, C.K.; Ribaucour, M. Detailed Chemical Kinetic Modeling of Cyclohexane Oxidation†. J. Phys. Chem. A 2007, 111, 3761–3775. [Google Scholar] [CrossRef]
- Knepp, A.M.; Meloni, G.; Jusinski, L.E.; Taatjes, C.A.; Cavallotti, C.; Klippenstein, S.J. Theory, measurements, and modeling of OH and HO2 formation in the reaction of cyclohexyl radicals with O2. Phys. Chem. Chem. Phys. 2007, 9, 4315–4331. [Google Scholar] [CrossRef]
- Whelan, C.A.; Blitz, M.A.; Shannon, R.; Onel, L.; Lockhart, J.P.; Seakins, P.W.; Stone, D. Temperature and Pressure Dependent Kinetics of QOOH Decomposition and Reaction with O2: Experimental and Theoretical Investigations of QOOH Radicals Derived from Cl + (CH3)3COOH. J. Phys. Chem. A 2019, 123, 10254–10262. [Google Scholar] [CrossRef]
- Zádor, J.; Huang, H.; Welz, O.; Zetterberg, J.; Osborn, D.L.; Taatjes, C.A. Directly measuring reaction kinetics of ˙QOOH—A crucial but elusive intermediate in hydrocarbon autoignition. Phys. Chem. Chem. Phys. 2013, 15, 10753–10760. [Google Scholar] [CrossRef]
- Bugler, J.; Power, J.; Curran, H.J. A theoretical study of cyclic ether formation reactions. Proc. Combust. Inst. 2017, 36, 161–167. [Google Scholar] [CrossRef]
- Bhagde, T.; Hansen, A.S.; Chen, S.; Walsh, P.J.; Klippenstein, S.J.; Lester, M.I. Energy-resolved and time-dependent unimolecular dissociation of hydroperoxyalkyl radicals (˙QOOH). Faraday Discuss. 2022, 238, 575–588. [Google Scholar] [CrossRef]
- Hansen, A.S.; Bhagde, T.; Moore, K.B.; Moberg, D.R.; Jasper, A.W.; Georgievskii, Y.; Vansco, M.F.; Klippenstein, S.J.; Lester, M.I. Watching a hydroperoxyalkyl radical (•QOOH) dissociate. Science 2021, 373, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Roy, T.K.; Jasper, A.W.; Sojdak, C.A.; Kozlowski, M.C.; Klippenstein, S.J.; Lester, M.I. Isomer-resolved unimolecular dynamics of the hydroperoxyalkyl intermediate (•QOOH) in cyclohexane oxidation. Proc. Natl. Acad. Sci. USA 2024, 121, e2401148121. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.K.; Qian, Y.; Sojdak, C.A.; Kozlowski, M.C.; Klippenstein, S.J.; Lester, M.I. Infrared signature of the hydroperoxyalkyl intermediate (•QOOH) in cyclohexane oxidation: An isomer-resolved spectroscopic study. J. Chem. Phys. 2024, 161, 034302. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Roy, T.K.; Valente, D.S.; Cruz, E.M.; Kozlowski, M.C.; Della Libera, A.; Klippenstein, S.J.; Lester, M.I. Infrared Fingerprint and Unimolecular Decay Dynamics of the Hydroperoxyalkyl Intermediate (•QOOH) in Cyclopentane Oxidation. J. Phys. Chem. A 2024, 128, 9240–9250. [Google Scholar] [CrossRef]
- Wallington, T.J.; Dagaut, P.; Kurylo, M.J. UV absorption cross sections and reaction kinetics and mechanisms for peroxy radicals in the gas phase. Chem. Rev. 1992, 92, 667–710. [Google Scholar] [CrossRef]
- Glover, B.G.; Miller, T.A. Near-IR Cavity Ringdown Spectroscopy and Kinetics of the Isomers and Conformers of the Butyl Peroxy Radical. J. Phys. Chem. A 2005, 109, 11191–11197. [Google Scholar] [CrossRef]
- Frost, G.J.; Ellison, G.B.; Vaida, V. Organic Peroxyl Radical Photolysis in the Near-Infrared: Effects on Tropospheric Chemistry. J. Phys. Chem. A 1999, 103, 10169–10178. [Google Scholar] [CrossRef]
- Zhang, C.; Shamas, M.; Assali, M.; Tang, X.; Zhang, W.; Pillier, L.; Schoemaecker, C.; Fittschen, C. Absolute Absorption Cross-Section of the Ã←X˜ Electronic Transition of the Ethyl Peroxy Radical and Rate Constant of Its Cross Reaction with HO2. Photonics 2021, 8, 296. [Google Scholar] [CrossRef]
- Atkinson, D.B.; Spillman, J.L. Alkyl Peroxy Radical Kinetics Measured Using Near-infrared CW−Cavity Ring-down Spectroscopy. J. Phys. Chem. A 2002, 106, 8891–8902. [Google Scholar] [CrossRef]
- Melnik, D.; Chhantyal-Pun, R.; Miller, T.A. Measurements of the Absolute Absorption Cross Sections of the Ã←X̃ Transition in Organic Peroxy Radicals by Dual-Wavelength Cavity Ring-Down Spectroscopy. J. Phys. Chem. A 2010, 114, 11583–11594. [Google Scholar] [CrossRef] [PubMed]
- Pushkarsky, M.B.; Zalyubovsky, S.J.; Miller, T.A. Detection and characterization of alkyl peroxy radicals using cavity ringdown spectroscopy. J. Chem. Phys. 2000, 112, 10695–10698. [Google Scholar] [CrossRef]
- Nichols, B.; Sullivan, E.N.; Ryazanov, M.; Hong, C.M.; Neumark, D.M. Investigation of the two- and three-fragment photodissociation of the tert-butyl peroxy radical at 248 nm. J. Chem. Phys. 2017, 147, 134304. [Google Scholar] [CrossRef]
- Franke, P.R.; Moore, K.B.; Schaefer, H.F.; Douberly, G.E. tert-Butyl peroxy radical: Ground and first excited state energetics and fundamental frequencies. Phys. Chem. Chem. Phys. 2019, 21, 9747–9758. [Google Scholar] [CrossRef] [PubMed]
- Copan, A.V.; Schaefer III, H.F.; Agarwal, J. Examining the ground and first excited states of methyl peroxy radical with high-level coupled-cluster theory. Mol. Phys. 2015, 113, 2992–2998. [Google Scholar] [CrossRef]
- Vansco, M.F.; Zuraski, K.; Winiberg, F.A.F.; Au, K.; Trongsiriwat, N.; Walsh, P.J.; Osborn, D.L.; Percival, C.J.; Klippenstein, S.J.; Taatjes, C.A.; et al. Functionalized hydroperoxide formation from the reaction of methacrolein-oxide, an isoprene-derived criegee intermediate, with formic acid: Experiment and theory. Molecules 2021, 26, 3058. [Google Scholar] [CrossRef]
- Poirier, C.A.; Guidry, L.M.; Ratliff, J.M.; Esposito, V.J.; Marchetti, B.; Karsili, T.N.V. Modeling the Ground- and Excited-State Unimolecular Decay of the Simplest Fluorinated Criegee Intermediate, HFCOO, Formed from the Ozonolysis of Hydrofluoroolefin Refrigerants. J. Phys. Chem. A 2023, 127, 6377–6384. [Google Scholar] [CrossRef]
- Guidry, L.M.; Bardash, L.A.; Yigiter, A.; Ravi, S.; Marchetti, B.; Karsili, T.N.V. The role of solar photolysis in the atmospheric removal of methacrolein oxide and the methacrolein oxide—Water van-der Waals complex in pristine environments. Photochem. Photobiol. 2024, 101, 423–433. [Google Scholar] [CrossRef]
- Vansco, M.F.; Caravan, R.L.; Zuraski, K.; Winiberg, F.A.F.; Au, K.; Trongsiriwat, N.; Walsh, P.J.; Osborn, D.L.; Percival, C.J.; Khan, M.A.H.; et al. Experimental Evidence of Dioxole Unimolecular Decay Pathway for Isoprene-Derived Criegee Intermediates. J. Phys. Chem. A 2020, 124, 3542–3554. [Google Scholar] [CrossRef]
- Roy, T.K.; Qian, Y.; Karlsson, E.; Rabayah, R.; Sojdak, C.A.; Kozlowski, M.C.; Karsili, T.N.V.; Lester, M.I. Vibrational spectroscopy and dissociation dynamics of cyclohexyl hydroperoxide. Chem. Sci. 2024, 15, 6160–6167. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M. Molpro: A general-purpose quantum chemistry program package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 242–253. [Google Scholar] [CrossRef]
- Werner, H.-J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. MOLPRO, Version 2018.1; a Package of ab Initio Programs; Institute for Theoretical Chemistry, University of Stuttgart: Stuttgart, Germany, 2018.
- Frisch, D.J.F.M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016.
- McCoy, J.C.; Marchetti, B.; Thodika, M.; Karsili, T.N.V. A Simple and Efficient Method for Simulating the Electronic Absorption Spectra of Criegee Intermediates: Benchmarking on CH2OO and CH3CHOO. J. Phys. Chem. A 2021, 125, 4089–4097. [Google Scholar] [CrossRef]
- McCoy, J.C.; Léger, S.J.; Frey, C.F.; Vansco, M.F.; Marchetti, B.; Karsili, T.N.V.; Marchetti, B.; Karsili, T.N.V. Modeling the Conformer-Dependent Electronic Absorption Spectra and Photolysis Rates of Methyl Vinyl Ketone Oxide and Methacrolein Oxide. J. Phys. Chem. A 2022, 126, 485–496. [Google Scholar] [CrossRef]
- Wang, G.; Liu, T.; Zou, M.; Sojdak, C.A.; Kozlowski, M.C.; Karsili, T.N.; Lester, M.I. Electronic Spectroscopy and Dissociation Dynamics of Vinyl-Substituted Criegee Intermediates: 2-Butenal Oxide and Comparison with Methyl Vinyl Ketone Oxide and Methacrolein Oxide Isomers. J. Phys. Chem. A 2022, 127, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.; Rabayah, R.; Liu, T.; Cruz, E.M.; Kozlowski, M.C.; Karsili, T.N.V.; Lester, M.I. Electronic Spectroscopy of the Halogenated Criegee Intermediate, ClCHOO: Experiment and Theory. J. Phys. Chem. A 2024, 128, 10949–10956. [Google Scholar] [CrossRef] [PubMed]
- Prlj, A.; Hollas, D.; Curchod, B.F.E. Deciphering the Influence of Ground-State Distributions on the Calculation of Photolysis Observables. J. Phys. Chem. A 2023, 127, 7400–7409. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.S.; Bhagde, T.; Qian, Y.; Cavazos, A.; Huchmala, R.M.; Boyer, M.A.; Gavin-Hanner, C.F.; Klippenstein, S.J.; McCoy, A.B.; Lester, M.I. Infrared spectroscopic signature of a hydroperoxyalkyl radical (•QOOH). J. Chem. Phys. 2021, 156, 14301. [Google Scholar] [CrossRef]
- Beames, J.M.; Liu, F.; Lu, L.; Lester, M.I. UV spectroscopic characterization of an alkyl substituted Criegee intermediate CH3CHOO. J. Chem. Phys. 2013, 138, 244307. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fang, Y.; Beames, J.M.; Lester, M.I. Velocity map imaging of O-atom products from UV photodissociation of the CH2OO Criegee intermediate. J. Chem. Phys. 2015, 142, 214312. [Google Scholar] [CrossRef]
- Wang, G.; Liu, T.; Zou, M.; Karsili, T.N.V.; Lester, M.I. UV photodissociation dynamics of the acetone oxide Criegee intermediate: Experiment and theory. Phys. Chem. Chem. Phys. 2023, 25, 7453–7465. [Google Scholar] [CrossRef]
- Esposito, V.J.; Liu, T.; Wang, G.; Caracciolo, A.; Vansco, M.F.; Marchetti, B.; Karsili, T.N.V.; Lester, M.I. Photodissociation Dynamics of CH2OO on Multiple Potential Energy Surfaces: Experiment and Theory. J. Phys. Chem. A 2021, 125, 6571–6579. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, T.; Caracciolo, A.; Vansco, M.F.; Trongsiriwat, N.; Walsh, P.J.; Marchetti, B.; Karsili, T.N.V.; Lester, M.I. Photodissociation dynamics of methyl vinyl ketone oxide: A four-carbon unsaturated Criegee intermediate from isoprene ozonolysis. J. Chem. Phys. 2021, 155, 174305. [Google Scholar] [CrossRef] [PubMed]
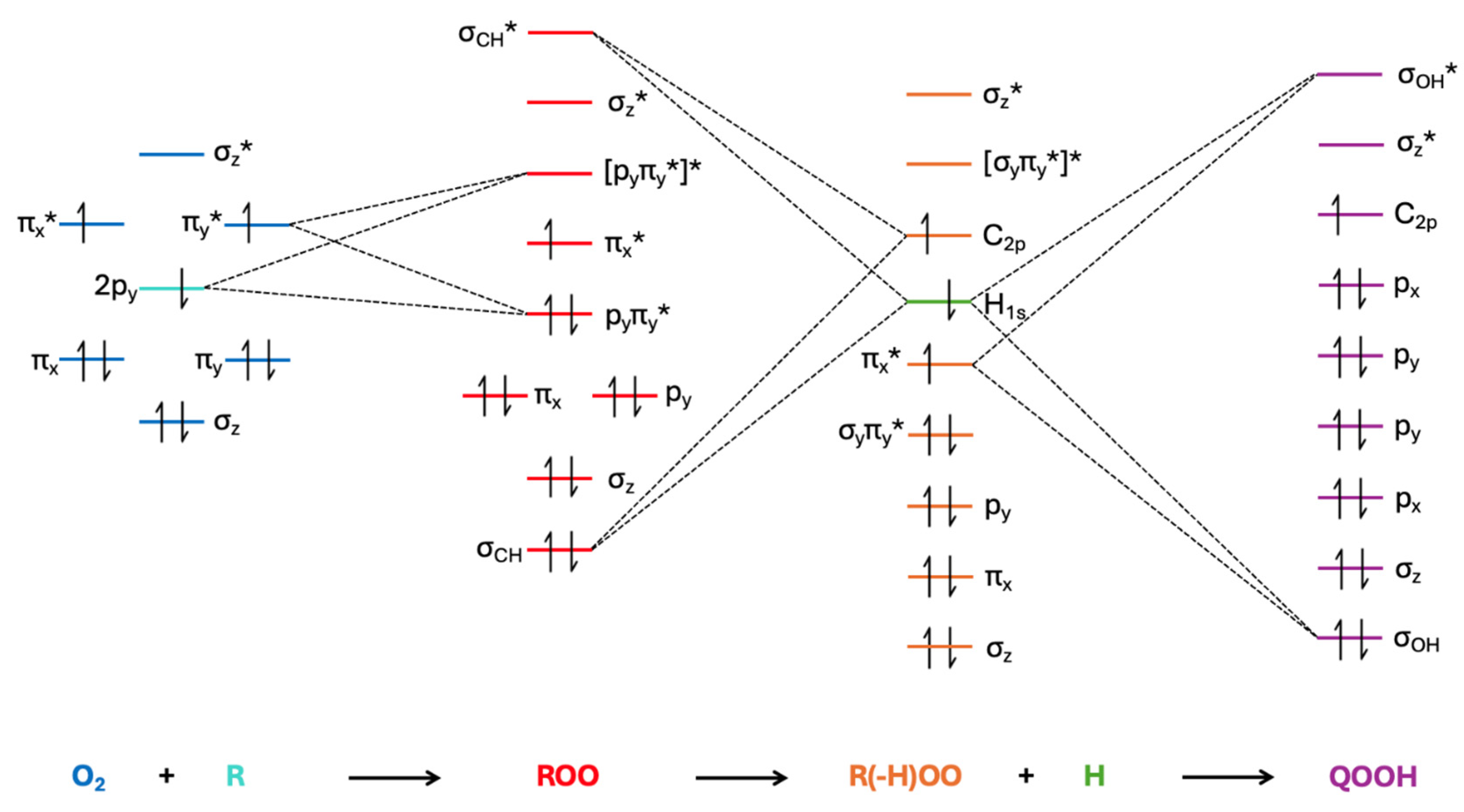
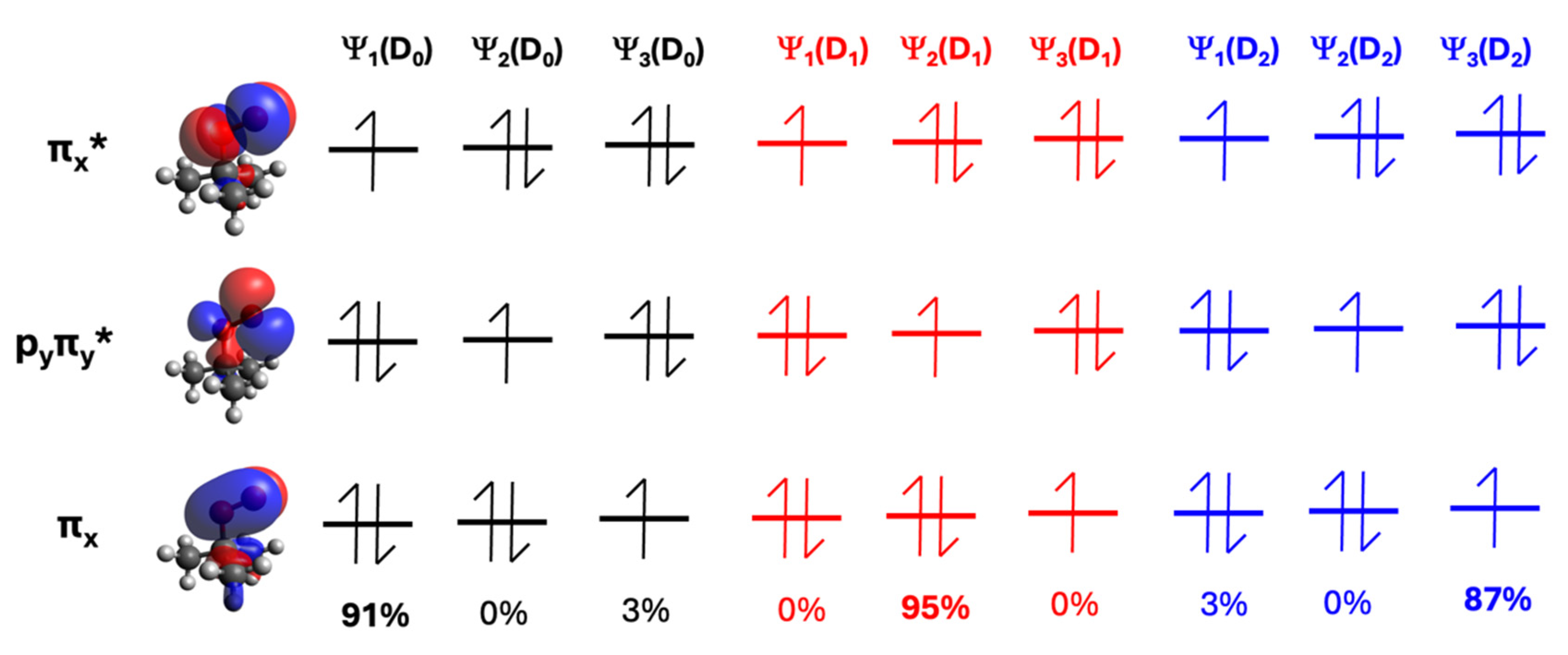

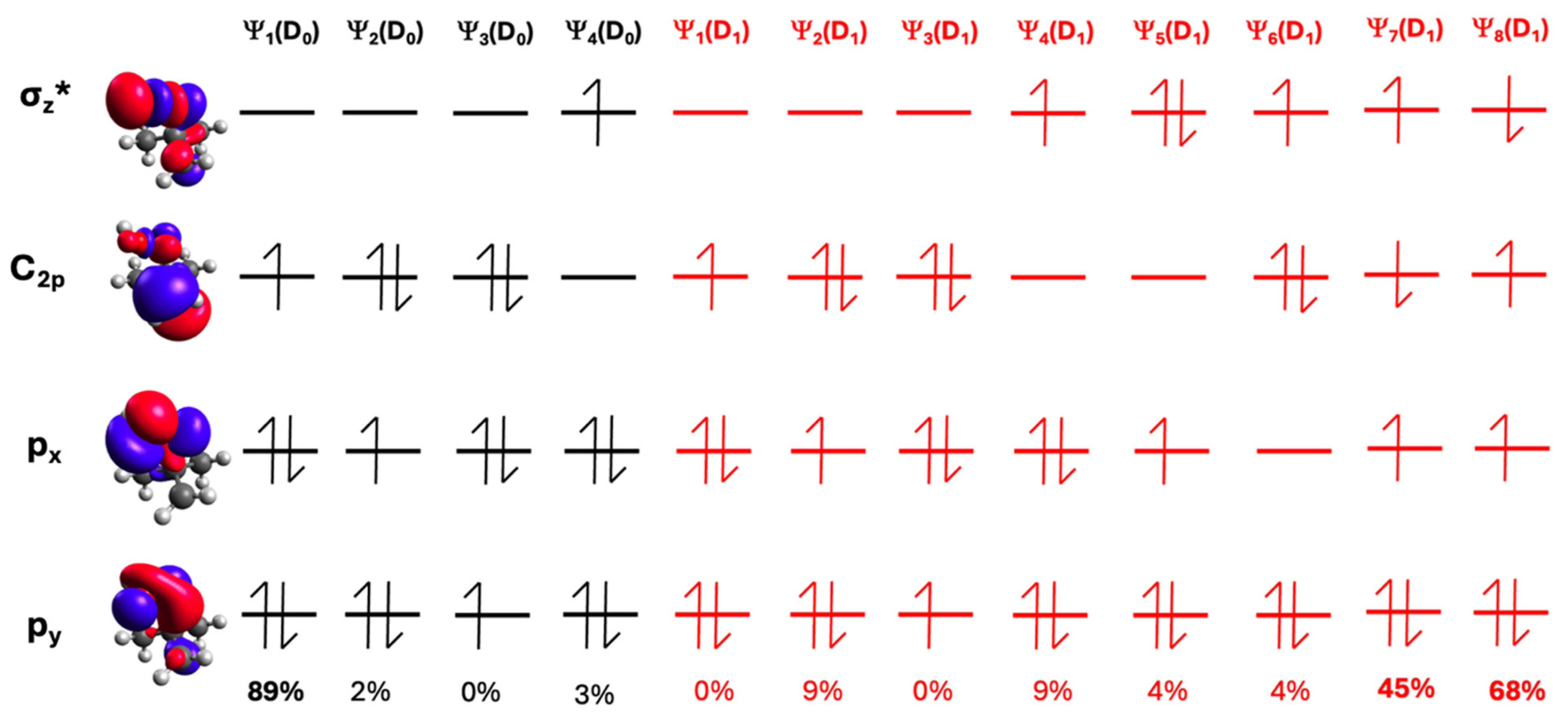

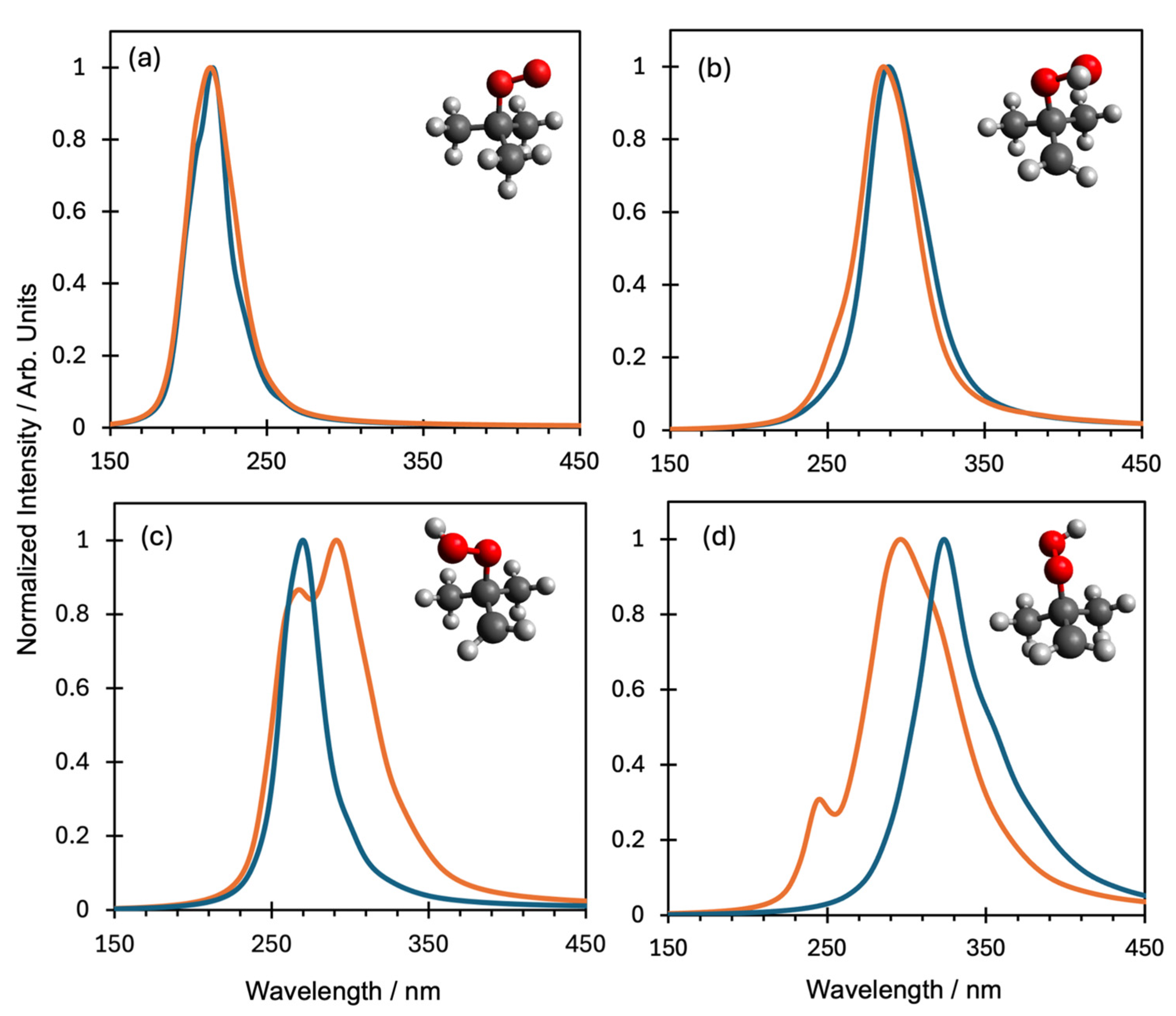
| Species | D1 ← D0 Transition | D2 ← D0 Transition | ||
|---|---|---|---|---|
| VEE | Oscillator Strength | VEE | Oscillator Strength | |
ROO | 1.20 eV (1033.2 nm) | 0.0000 | 5.70 eV (217.5 nm) | 0.5259 |
QOOH (conformer 1) | 4.55 eV (290 nm) | 0.0183 | 5.37 (234 nm) | 0.0110 |
QOOH (conformer 2) | 4.63 eV (268 nm) | 0.0000 | 5.72 eV (217 nm) | 0.0008 |
QOOH (conformer 3) | 3.52 eV (352 nm) | 0.0003 | 4.55 eV (272 nm) | 0.0001 |
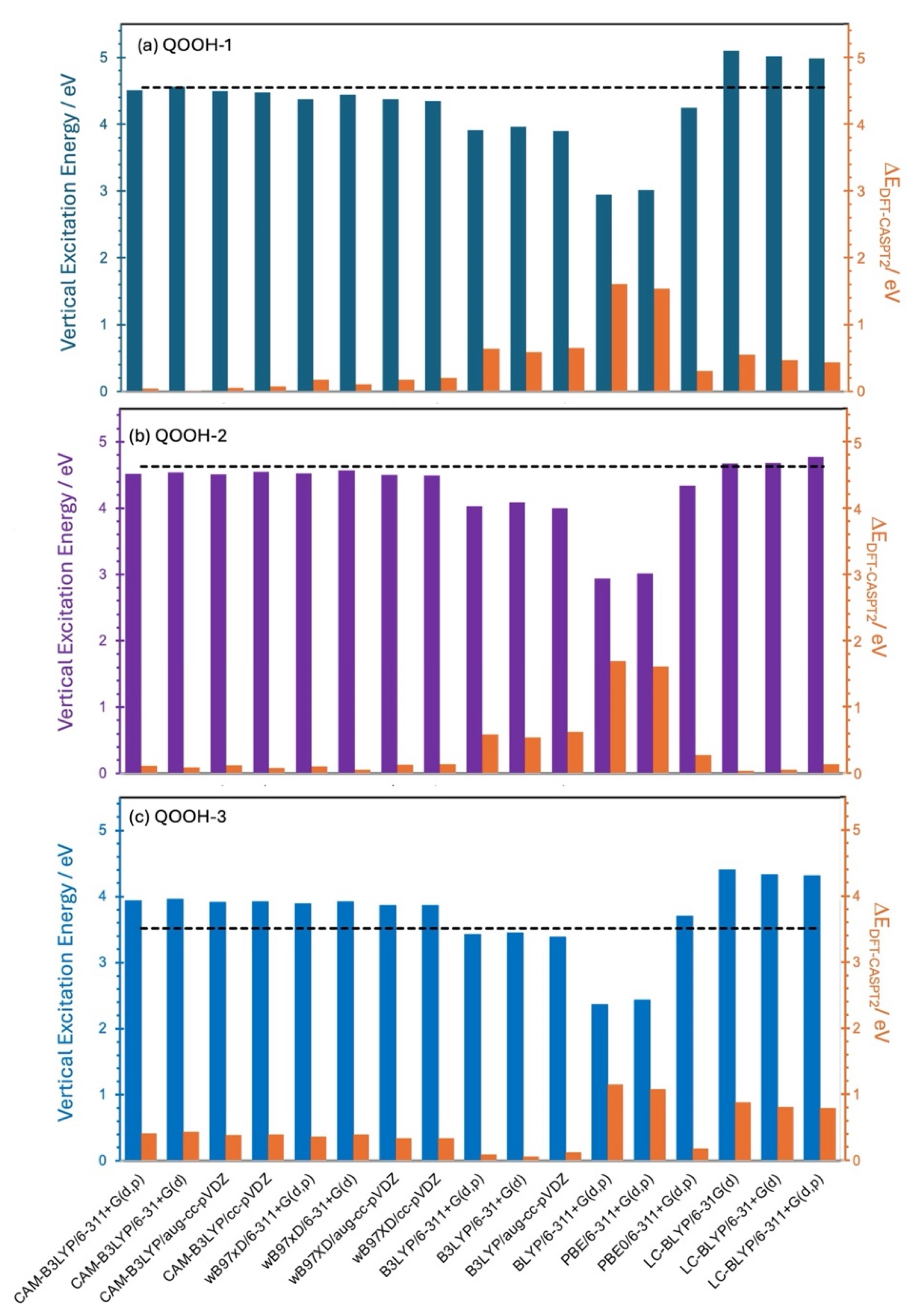
| Level of Theory | ROO | QOOH-1 | QOOH-2 | QOOH-3 | ||||
|---|---|---|---|---|---|---|---|---|
| ΔE | δ | ΔE | δ | ΔE | δ | ΔE | δ | |
| CASPT2/AVTZ | 5.70 | - | 4.55 | - | 4.63 | - | 3.52 | - |
| CAM-B3LYP/6-311+G(d,p) | 5.30 | 0.40 | 4.51 | 0.04 | 4.51 | 0.12 | 3.93 | 0.42 |
| CAM-B3LYP/6-31+G(d) | 5.31 | 0.39 | 4.56 | 0.01 | 4.54 | 0.09 | 3.95 | 0.44 |
| CAM-B3LYP/AVDZ | 5.28 | 0.42 | 4.50 | 0.05 | 4.50 | 0.12 | 3.91 | 0.39 |
| CAM-B3LYP/VDZ | 5.52 | 0.18 | 4.48 | 0.07 | 4.54 | 0.08 | 3.92 | 0.40 |
| ωB97xD/6-311+G(d,p) | 5.27 | 0.43 | 4.38 | 0.17 | 4.52 | 0.11 | 3.89 | 0.37 |
| ωB97xD/6-31+G(d) | 5.28 | 0.42 | 4.44 | 0.11 | 4.57 | 0.06 | 3.92 | 0.40 |
| ωB97xD/AVDZ | 5.26 | 0.44 | 4.37 | 0.18 | 4.50 | 0.13 | 3.87 | 0.35 |
| ωB97xD/VDZ | 5.47 | 0.23 | 4.35 | 0.20 | 4.49 | 0.14 | 3.86 | 0.35 |
| B3LYP/6-311+G(d,p) | 5.04 | 0.66 | 3.91 | 0.64 | 4.04 | 0.59 | 3.42 | 0.10 |
| B3LYP/6-31+G(d) | 5.05 | 0.65 | 3.96 | 0.59 | 4.08 | 0.54 | 3.45 | 0.07 |
| B3LYP/AVDZ | 5.03 | 0.67 | 3.90 | 0.65 | 4.00 | 0.63 | 3.39 | 0.13 |
| PBE/6-311+G(d,p) | 4.31 | 1.39 | 3.01 | 1.54 | 3.01 | 1.69 | 2.43 | 1.09 |
| PBE0/6-311+G(d,p) | 5.15 | 0.55 | 4.24 | 0.31 | 4.35 | 1.61 | 3.70 | 0.18 |
| LC-BLYP/6-31G(d) | 5.69 | 0.01 | 5.10 | 0.55 | 4.67 | 0.28 | 4.41 | 0.89 |
| LC-BLYP/6-31+G(d) | 5.50 | 0.20 | 5.02 | 0.47 | 4.68 | 0.04 | 4.33 | 0.82 |
| LC-BLYP/6-311+G(d,p) | 5.49 | 0.21 | 4.99 | 0.44 | 4.77 | 0.06 | 4.32 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidry, L.M.; Guidry, S.E.; Barua, T.; Marchetti, B.; Vansco, M.F.; Karsili, T.N.V. Characterizing the Excited States and Electronic Absorption Spectra of Small Alkylperoxy (RO2•) and Hydroperoxy (•QOOH) Radicals. Photochem 2025, 5, 26. https://doi.org/10.3390/photochem5030026
Guidry LM, Guidry SE, Barua T, Marchetti B, Vansco MF, Karsili TNV. Characterizing the Excited States and Electronic Absorption Spectra of Small Alkylperoxy (RO2•) and Hydroperoxy (•QOOH) Radicals. Photochem. 2025; 5(3):26. https://doi.org/10.3390/photochem5030026
Chicago/Turabian StyleGuidry, Lily M., Sofia E. Guidry, Tanima Barua, Barbara Marchetti, Michael F. Vansco, and Tolga N. V. Karsili. 2025. "Characterizing the Excited States and Electronic Absorption Spectra of Small Alkylperoxy (RO2•) and Hydroperoxy (•QOOH) Radicals" Photochem 5, no. 3: 26. https://doi.org/10.3390/photochem5030026
APA StyleGuidry, L. M., Guidry, S. E., Barua, T., Marchetti, B., Vansco, M. F., & Karsili, T. N. V. (2025). Characterizing the Excited States and Electronic Absorption Spectra of Small Alkylperoxy (RO2•) and Hydroperoxy (•QOOH) Radicals. Photochem, 5(3), 26. https://doi.org/10.3390/photochem5030026







