Application of the Triangular Spatial Relationship Algorithm in Representing and Quantifying Conformational Changes in Chlorophylls and Protein Local Environments
Abstract
1. Introduction
2. Results
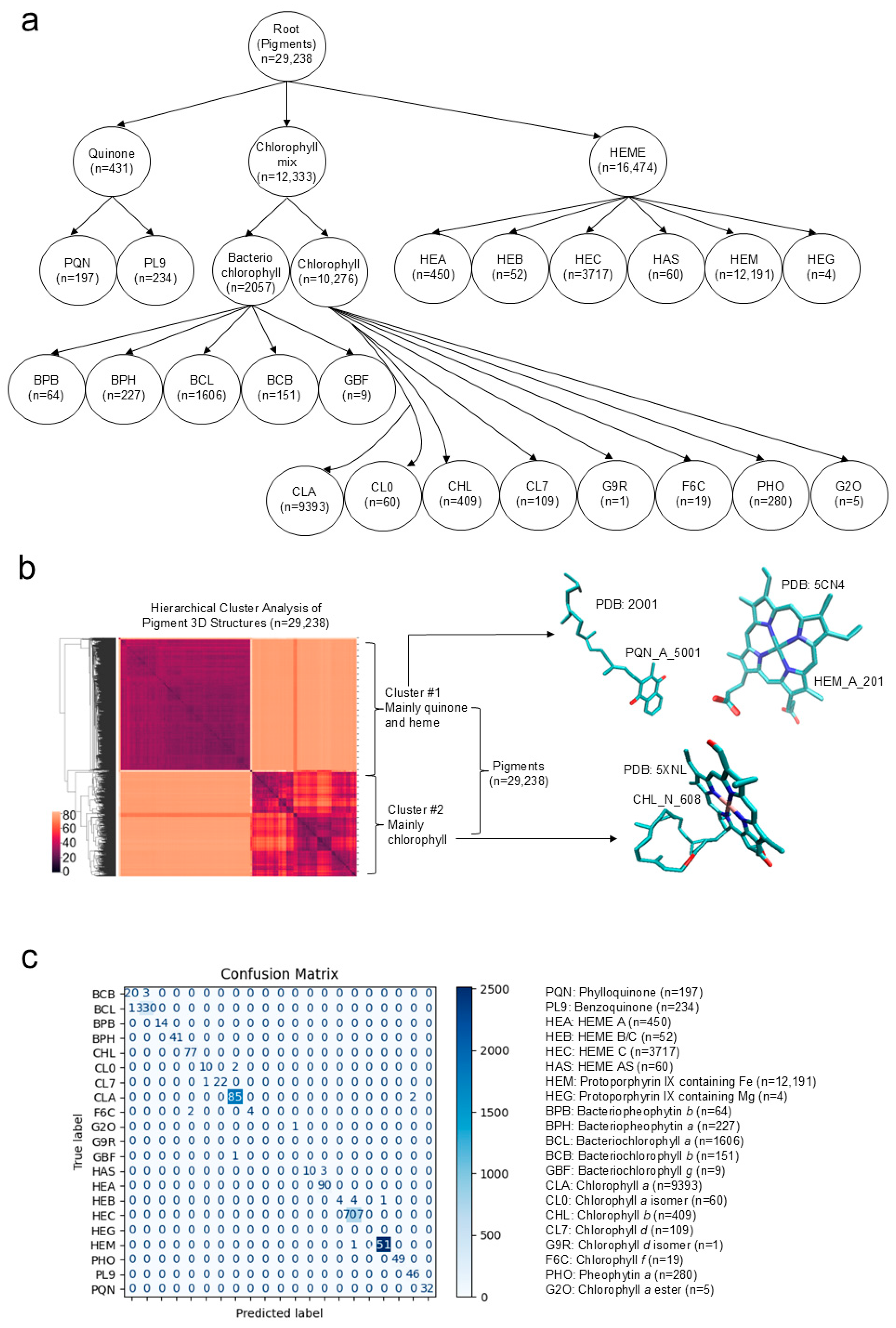
2.1. A Unique Approach to Represent 3D Structures of Pigments and an Evaluation of the Representation by Unsupervised and Supervised Machine Learning Approaches
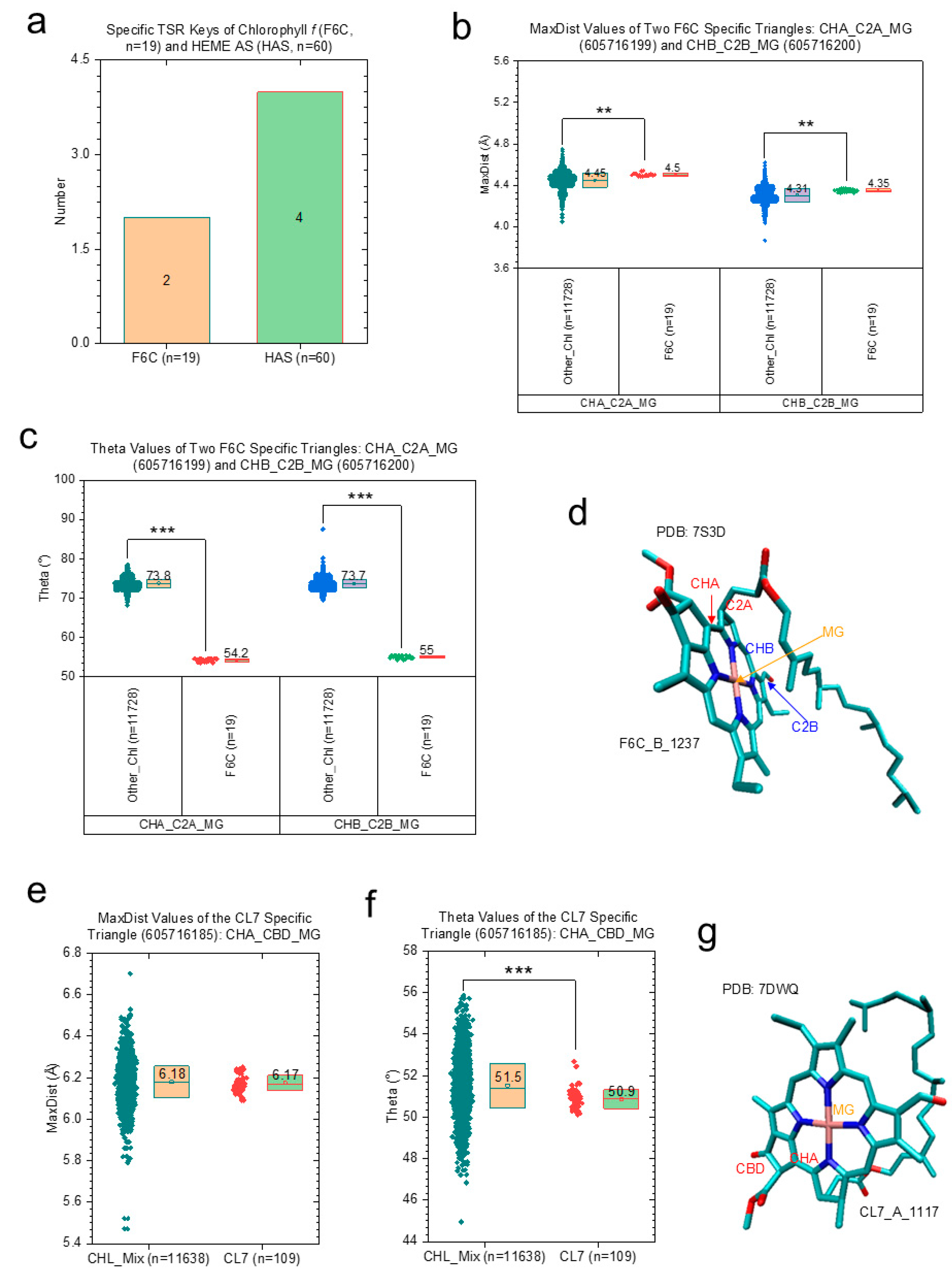
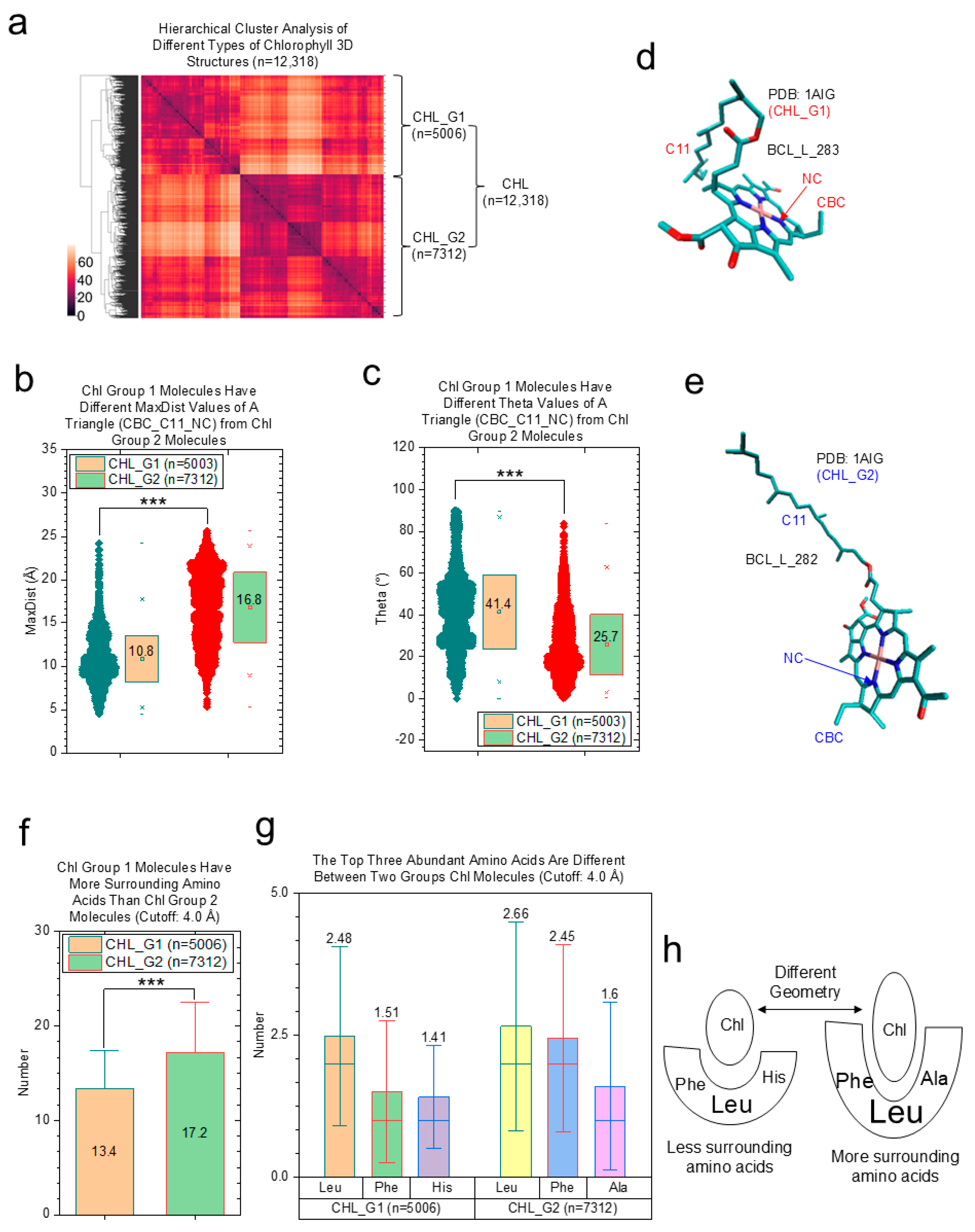
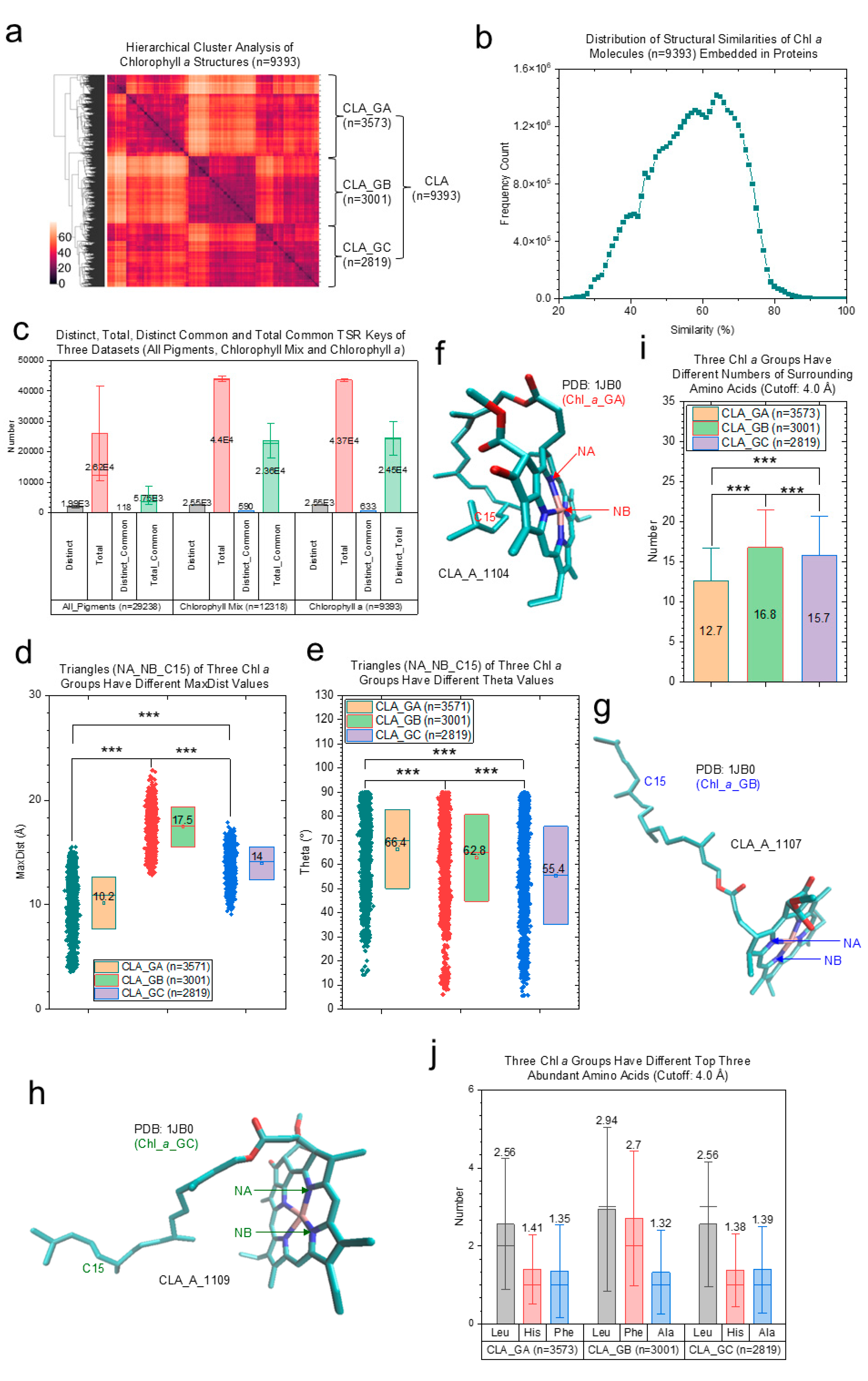
2.2. Structural Characteristics of Chl d and Chl f and Effects of Protein Environments on Different Types of Chl Molecules and Chl a Molecules
2.2.1. Structural Characteristics of Chl d and Chl f
2.2.2. Effects of Protein Environments on Various Types of Chl Molecules
2.2.3. Effects of Protein Environments on Various Types of Chl a Molecules
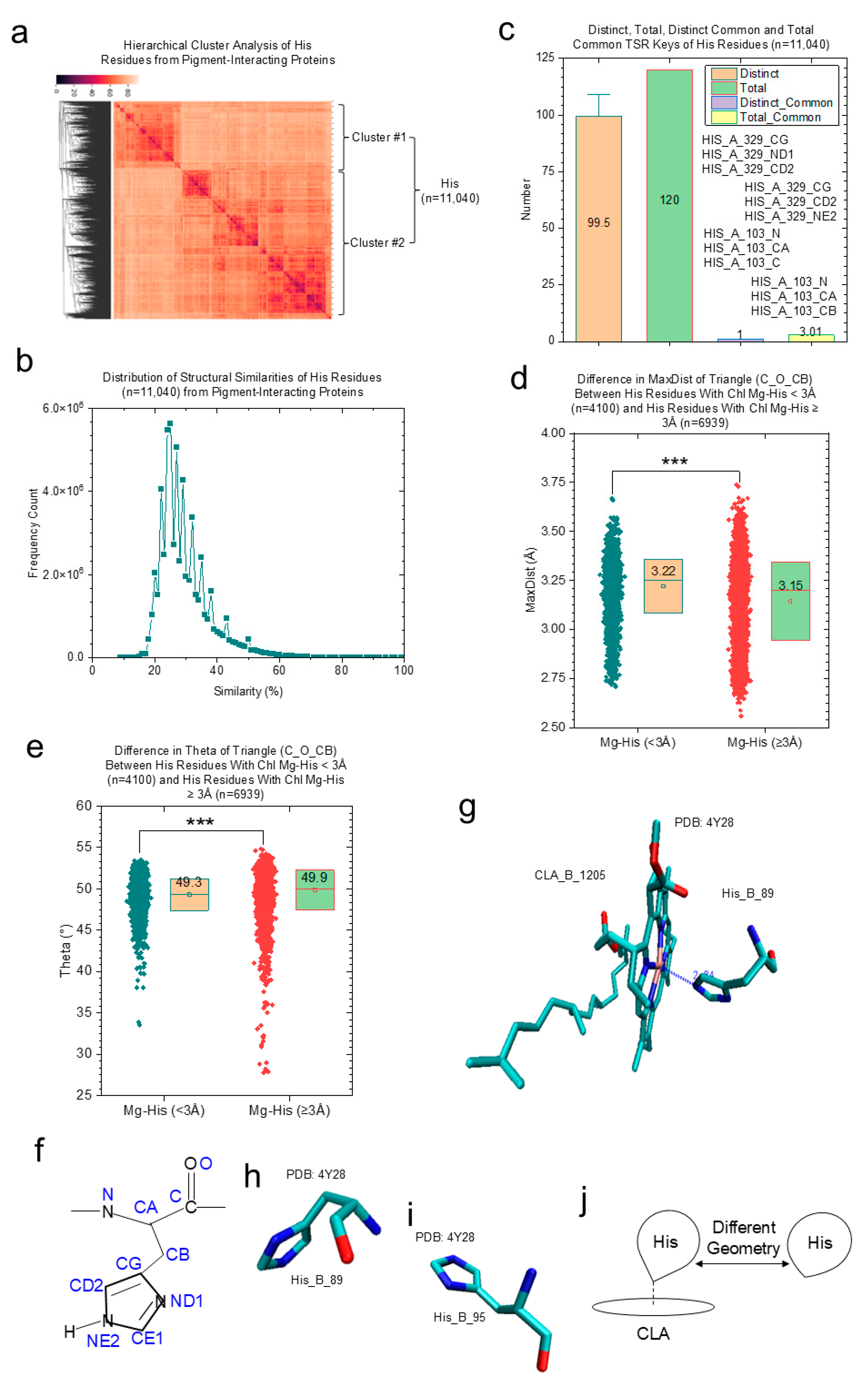
2.3. Structural Characteristics of Pigment Surrounding Protein Environments
3. Conclusions, Limitations, and Future Directions
- (i)
- A dataset containing all (B)Chl 3D structures and a histidine dataset from (B)Chl-binding proteins is prepared and annotated.
- (ii)
- Specific structural characteristics of Chls d and f were identified and are defined using the TSR keys. A total of 590 common TSR keys were identified, and those common key-associated substructures are shared by all types of Chls.
- (iii)
- Two and three clusters were found for various Chls and Chls a, respectively. The signature structures for distinguishing their corresponding two and three clusters were identified. The number of surrounding amino acids and certain amino acids play important roles in determining the conformations of Chls and Chls a.
- (iv)
- Histidine residues are used as an example for revealing structural characteristics of Chl-binding sites.
4. Methods
4.1. Key Generation
- : distance between and for triangle i;
- : distance between and for triangle i;
- : distance between and the midpoint of and for triangle i.
- m: the total number of distinct labels;
- θ: the bin value for the class in which , the angle representative, falls; to achieve discretization, we use the Adaptive Unsupervised Iterative Discretization algorithm;
- : the total number of distinct discretization levels (or bin number) for angle representative;
- d: the bin value for the class in which D, the length representative, falls; to achieve discretization, we use the Adaptive Unsupervised Iterative Discretization algorithm;
- : the total number of distinct discretization levels (or the number of bins) for length representative.
4.2. Protein Structural Similarity and Distance Calculations
4.3. Development of a Version of the TSR-Based Method for Representing and Quantifying Pigment 3D Structures
4.4. Development of a TSR Algorithm for Representing and Quantifying 3D Structures of Amino Acids
4.5. Dataset Preparation
4.5.1. Pigment Dataset Preparation
4.5.2. Histidine Dataset Preparation
4.6. Hierarchical Cluster and Classification Analyses and Visualization
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Marais, D.J. When Did Photosynthesis Emerge on Earth? Science 2000, 289, 1703–1705. [Google Scholar] [CrossRef]
- Rye, R.; Holland, H.D. Paleosols and the evolution of atmospheric oxygen: A critical review. Am. J. Sci. 1998, 298, 621–672. [Google Scholar] [CrossRef] [PubMed]
- Wientjes, E.; Roest, G.; Croce, R. From red to blue to far-red in Lhca4: How does the protein modulate the spectral properties of the pigments? Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, J.; Renger, T. How proteins trigger excitation energy transfer in the FMO complex of green sulfur bacteria. Biophys. J. 2006, 91, 2778–2797. [Google Scholar] [CrossRef]
- Diner, B.A.; Schlodder, E.; Nixon, P.J.; Coleman, W.J.; Rappaport, F.; Lavergne, J.; Vermaas, W.F.J.; Chisholm, D.A. Site-Directed Mutations at D1-His198 and D2-His197 of Photosystem II in Synechocystis PCC 6803: Sites of Primary Charge Separation and Cation and Triplet Stabilization. Biochemistry 2001, 40, 9265–9281. [Google Scholar] [CrossRef]
- Morosinotto, T.; Breton, J.; Bassi, R.; Croce, R. The Nature of a Chlorophyll Ligand in Lhca Proteins Determines the Far Red Fluorescence Emission Typical of Photosystem I *. J. Biol. Chem. 2003, 278, 49223–49229. [Google Scholar] [CrossRef]
- Kirpich, J.S.; Luo, L.; Nelson, M.R.; Agarwala, N.; Xu, W.; Hastings, G. Is the A-1 Pigment in Photosystem I Part of P700? A (P700+–P700) FTIR Difference Spectroscopy Study of A-1 Mutants. Int. J. Mol. Sci. 2024, 25, 4839. [Google Scholar] [CrossRef]
- Sturgis, J.N.; Robert, B. Pigment Binding-Site and Electronic Properties in Light-Harvesting Proteins of Purple Bacteria. J. Phys. Chem. B 1997, 101, 7227–7231. [Google Scholar] [CrossRef]
- Sturgis, J.N.; Robert, B. The role of chromophore coupling in tuning the spectral properties of peripheral light-harvesting protein of purple bacteria. Photosynth. Res. 1996, 50, 5–10. [Google Scholar] [CrossRef]
- Witt, H.; Schlodder, E.; Teutloff, C.; Niklas, J.; Bordignon, E.; Carbonera, D.; Kohler, S.; Labahn, A.; Lubitz, W. Hydrogen Bonding to P700: Site-Directed Mutagenesis of Threonine A739 of Photosystem I in Chlamydomonas reinhardtii. Biochemistry 2002, 41, 8557–8569. [Google Scholar] [CrossRef]
- McLuskey, K.; Prince, S.M.; Cogdell, R.J.; Isaacs, N.W. The Crystallographic Structure of the B800-820 LH3 Light-Harvesting Complex from the Purple Bacteria Rhodopseudomonas Acidophila Strain 7050. Biochemistry 2001, 40, 8783–8789. [Google Scholar] [CrossRef] [PubMed]
- Saer, R.; Orf, G.S.; Lu, X.; Zhang, H.; Cuneo, M.J.; Myles, D.A.A.; Blankenship, R.E. Perturbation of bacteriochlorophyll molecules in Fenna–Matthews–Olson protein complexes through mutagenesis of cysteine residues. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1857, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Khmelnitskiy, A.; Saer, R.G.; Blankenship, R.E.; Jankowiak, R. Excitonic Energy Landscape of the Y16F Mutant of the Chlorobium tepidum Fenna–Matthews–Olson (FMO) Complex: High Resolution Spectroscopic and Modeling Studies. J. Phys. Chem. B 2018, 122, 3734–3743. [Google Scholar] [CrossRef] [PubMed]
- Vrandecic, K.; Rätsep, M.; Wilk, L.; Rusevich, L.; Golub, M.; Reppert, M.; Irrgang, K.-D.; Kühlbrandt, W.; Pieper, J. Protein Dynamics Tunes Excited State Positions in Light-Harvesting Complex II. J. Phys. Chem. B 2015, 119, 3920–3930. [Google Scholar] [CrossRef]
- Llansola-Portoles, M.J.; Li, F.; Xu, P.; Streckaite, S.; Ilioaia, C.; Yang, C.; Gall, A.; Pascal, A.A.; Croce, R.; Robert, B. Tuning antenna function through hydrogen bonds to chlorophyll a. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1861, 148078. [Google Scholar] [CrossRef]
- Luo, L.; Martin, A.P.; Tandoh, E.K.; Chistoserdov, A.; Slipchenko, L.V.; Savikhin, S.; Xu, W. Impact of Peripheral Hydrogen Bond on Electronic Properties of the Primary Acceptor Chlorophyll in the Reaction Center of Photosystem I. Int. J. Mol. Sci. 2024, 25, 4815. [Google Scholar] [CrossRef]
- Damjanović, A.; Vaswani, H.M.; Fromme, P.; Fleming, G.R. Chlorophyll Excitations in Photosystem I of Synechococcus elongatus. J. Phys. Chem. B 2002, 106, 10251–10262. [Google Scholar] [CrossRef]
- Xu, W.; Chitnis, P.R.; Valieva, A.; van der Est, A.; Brettel, K.; Guergova-Kuras, M.; Pushkar, Y.N.; Zech, S.G.; Stehlik, D.; Shen, G.; et al. Electron transfer in cyanobacterial photosystem I: II. Determination of forward electron transfer rates of site-directed mutants in a putative electron transfer pathway from A0 through A1 to FX. J. Biol. Chem. 2003, 278, 27876–27887. [Google Scholar] [CrossRef]
- Lahav, Y.; Noy, D.; Schapiro, I. Spectral tuning of chlorophylls in proteins–electrostatics vs. ring deformation. Phys. Chem. Chem. Phys. 2021, 23, 6544–6551. [Google Scholar] [CrossRef]
- Shelnutt, A.J.; Song, X.-Z.; Ma, J.-G.; Jia, S.-L.; Jentzen, W.J.; Medforth, C.J.; Medforth, C. Nonplanar porphyrins and their significance in proteins. Chem. Soc. Rev. 1998, 27, 31–42. [Google Scholar] [CrossRef]
- Senge, M.O.; MacGowan, S.A.; O’Brien, J.M. Conformational control of cofactors in nature–the influence of protein-induced macrocycle distortion on the biological function of tetrapyrroles. Chem. Commun. 2015, 51, 17031–17063. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O. Exercises in molecular gymnastics—Bending, stretching and twisting porphyrins. Chem. Commun. 2006, 3, 243–256. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y.; Taylor, E.; Laujac, A.; Gao, L.; Savikhin, S.; Chitnis, P.R. Mutational analysis of photosystem I of Synechocystis sp. PCC 6803: The role of four conserved aromatic residues in the j-helix of PsaB. PLoS ONE 2011, 6, e24625. [Google Scholar] [CrossRef] [PubMed]
- Cherepanov, D.A.; Shelaev, I.V.; Gostev, F.E.; Petrova, A.; Aybush, A.V.; Nadtochenko, V.A.; Xu, W.; Golbeck, J.H.; Semenov, A.Y. Primary charge separation within the structurally symmetric tetrameric Chl2APAPBChl2B chlorophyll exciplex in photosystem I. J. Photochem. Photobiol. B Biol. 2021, 217, 112154. [Google Scholar] [CrossRef]
- Kurashov, V.; Milanovsky, G.; Luo, L.; Martin, A.; Semenov, A.Y.; Savikhin, S.; Cherepanov, D.A.; Golbeck, J.H.; Xu, W. Conserved residue PsaB-Trp673 is essential for high-efficiency electron transfer between the phylloquinones and the iron-sulfur clusters in Photosystem I. Photosynth. Res. 2021, 148, 161–180. [Google Scholar] [CrossRef]
- Kondra, S.; Sarkar, T.; Raghavan, V.; Xu, W. Development of a TSR-Based Method for Protein 3-D Structural Comparison with Its Applications to Protein Classification and Motif Discovery. Front. Chem. 2021, 8, 602291. [Google Scholar] [CrossRef]
- Milon, T.I.; Wang, Y.; Fontenot, R.L.; Khajouie, P.; Villinger, F.; Raghavan, V.; Xu, W. Development of a novel representation of drug 3D structures and enhancement of the TSR-based method for probing drug and target interactions. Comput. Biol. Chem. 2024, 112, 108117. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y. Recognizing Protein Substructure Similarity Using Segmental Threading. Structure 2010, 18, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Chen, M. Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu. Rev. Biochem. 2014, 83, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Vavilin, D.V.; Vermaas, W.F.J. Regulation of the tetrapyrrole biosynthetic pathway leading to heme and chlorophyll in plants and cyanobacteria. Physiol. Plant. 2002, 115, 9–24. [Google Scholar] [CrossRef]
- Kobayashi, K.; Masuda, T.; Tajima, N.; Wada, H.; Sato, N. Molecular Phylogeny and Intricate Evolutionary History of the Three Isofunctional Enzymes Involved in the Oxidation of Protoporphyrinogen IX. Genome Biol. Evol. 2014, 6, 2141–2155. [Google Scholar] [CrossRef]
- Mauzerall, D.C.P. Evolution of Porphyrins. Clin. Dermatol. 1998, 16, 195–201. [Google Scholar] [CrossRef]
- Kato, Y.; Nagao, R.; Noguchi, T. Redox potential of the terminal quinone electron acceptor Q<sub>B</sub> in photosystem II reveals the mechanism of electron transfer regulation. Proc. Natl. Acad. Sci. USA 2016, 113, 620–625. [Google Scholar]
- Srinivasan, N.; Golbeck, J.H. Protein–cofactor interactions in bioenergetic complexes: The role of the A1A and A1B phylloquinones in Photosystem I. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1057–1088. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.J.; Larkum, A.W.; Steel, M.; Waddell, P.J.; Penny, D. Evolution of chlorophyll and bacteriochlorophyll: The problem of invariant sites in sequence analysis. Proc. Natl. Acad. Sci. USA 1996, 93, 1930–1934. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Pandey, A.K. Chapter 19-Analysis of chlorophylls. In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 635–650. [Google Scholar]
- Hamaguchi, T.; Kawakami, K.; Shinzawa-Itoh, K.; Inoue-Kashino, N.; Itoh, S.; Ifuku, K.; Yamashita, E.; Maeda, K.; Yonekura, K.; Kashino, Y. Structure of the far-red light utilizing photosystem I of Acaryochloris marina. Nat. Commun. 2021, 12, 2333. [Google Scholar] [CrossRef]
- Swainsbury, D.J.K.; Faries, K.M.; Niedzwiedzki, D.M.; Martin, E.C.; Flinders, A.J.; Canniffe, D.P.; Shen, G.; Bryant, D.A.; Kirmaier, C.; Holten, D.; et al. Engineering of B800 bacteriochlorophyll binding site specificity in the Rhodobacter sphaeroides LH2 antenna. Biochim. Biophys. Acta. Bioenerg. 2019, 1860, 209–223. [Google Scholar] [CrossRef]
- Björn, L.O.; Papageorgiou, G.C.; Blankenship, R.E.; Govindjee. A viewpoint: Why chlorophyll a? Photosynth. Res. 2009, 99, 85–98. [Google Scholar] [CrossRef]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Kiang, N.Y.; Siefert, J.; Govindjee; Blankenship, R.E. Spectral Signatures of Photosynthesis. I. Review of Earth Organisms. Astrobiology 2007, 7, 222–251. [Google Scholar] [CrossRef] [PubMed]
- Kunugi, M.; Satoh, S.; Ihara, K.; Shibata, K.; Yamagishi, Y.; Kogame, K.; Obokata, J.; Takabayashi, A.; Tanaka, A. Evolution of Green Plants Accompanied Changes in Light-Harvesting Systems. Plant Cell Physiol. 2016, 57, 1231–1243. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Ikemoto, H.; Kurano, N.; Adachi, K.; Chihara, M.; Miyachi, S. Chlorophyll d as a major pigment. Nature 1996, 383, 402. [Google Scholar] [CrossRef]
- Tomo, T.; Allakhverdiev, S.I.; Mimuro, M. Constitution and energetics of photosystem I and photosystem II in the chlorophyll d-dominated cyanobacterium Acaryochloris marina. J. Photochem. Photobiol. B Biol. 2011, 104, 333–340. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.L.; Neilan, B.A.; Scheer, H. A red-shifted chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Kato, K.; Shinoda, T.; Nagao, R.; Akimoto, S.; Suzuki, T.; Dohmae, N.; Chen, M.; Allakhverdiev, S.I.; Shen, J.-R.; Akita, F.; et al. Structural basis for the adaptation and function of chlorophyll f in photosystem I. Nat. Commun. 2020, 11, 238. [Google Scholar] [CrossRef]
- Gisriel, C.; Shen, G.; Kurashov, V.; Ho, M.-Y.; Zhang, S.; Williams, D.; Golbeck, J.H.; Fromme, P.; Bryant, D.A. The structure of Photosystem I acclimated to far-red light illuminates an ecologically important acclimation process in photosynthesis. Sci. Adv. 2020, 6, eaay6415. [Google Scholar] [CrossRef]
- Olson, T.L.; Williams, J.C.; Allen, J.P. Influence of protein interactions on oxidation/reduction midpoint potentials of cofactors in natural and de novo metalloproteins. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1827, 914–922. [Google Scholar] [CrossRef]
- Fresch, E.; Meneghin, E.; Agostini, A.; Paulsen, H.; Carbonera, D.; Collini, E. How the Protein Environment Can Tune the Energy, the Coupling, and the Ultrafast Dynamics of Interacting Chlorophylls: The Example of the Water-Soluble Chlorophyll Protein. J. Phys. Chem. Lett. 2020, 11, 1059–1067. [Google Scholar] [CrossRef]
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; van Grondelle, R. Lessons from nature about solar light harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef]
- Bednarczyk, D.; Dym, O.; Prabahar, V.; Peleg, Y.; Pike, D.H.; Noy, D. Fine Tuning of Chlorophyll Spectra by Protein-Induced Ring Deformation. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 6901–6905. [Google Scholar] [CrossRef] [PubMed]
- Krabben, L.; Schlodder, E.; Jordan, R.; Carbonera, D.; Giacometti, G.; Lee, H.; Webber, A.N.; Lubitz, W. Influence of the Axial Ligands on the Spectral Properties of P700 of Photosystem I: A Study of Site-Directed Mutants. Biochemistry 2000, 39, 13012–13025. [Google Scholar] [CrossRef] [PubMed]
- Webber, A.N.; Su, H.; Bingham, S.E.; Käss, H.; Krabben, L.; Kuhn, M.; Jordan, R.; Schlodder, E.; Lubitz, W. Site-Directed Mutations Affecting the Spectroscopic Characteristics and Midpoint Potential of the Primary Donor in Photosystem I. Biochemistry 1996, 35, 12857–12863. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Xu, W.; Diner, B.A.; Chitnis, P.R. The two histidine axial ligands of the primary electron donor chlorophylls (P700) in photosystem I are similarly perturbed upon P700+ formation. Biochemistry 2002, 41, 11200–11210. [Google Scholar] [CrossRef]
- Redding, K.; MacMillan, F.; Leibl, W.; Brettel, K.; Hanley, J.; Rutherford, A.W.; Breton, J.; Rochaix, J.D. A systematic survey of conserved histidines in the core subunits of Photosystem I by site-directed mutagenesis reveals the likely axial ligands of P700. EMBO J. 1998, 17, 50–60. [Google Scholar] [CrossRef]
- Ramesh, V.M.; Guergova-Kuras, M.; Joliot, P.; Webber, A.N. Electron Transfer from Plastocyanin to the Photosystem I Reaction Center in Mutants with Increased Potential of the Primary Donor in Chlamydomonas reinhardtii. Biochemistry 2002, 41, 14652–14658. [Google Scholar] [CrossRef]
- Sugiura, M.; Boussac, A.; Noguchi, T.; Rappaport, F. Influence of Histidine-198 of the D1 subunit on the properties of the primary electron donor, P680, of photosystem II in Thermosynechococcus elongatus. Biochim. Biophys. Acta (BBA) Bioenerg. 2008, 1777, 331–342. [Google Scholar] [CrossRef]
- Di Donato, M.; Cohen, R.O.; Diner, B.A.; Breton, J.; van Grondelle, R.; Groot, M.L. Primary Charge Separation in the Photosystem II Core from Synechocystis: A Comparison of Femtosecond Visible/Midinfrared Pump-Probe Spectra of Wild-Type and Two P680 Mutants. Biophys. J. 2008, 94, 4783–4795. [Google Scholar] [CrossRef] [PubMed]
- Ruffle, S.V.; Wang, J.; Johnston, H.G.; Gustafson, T.L.; Hutchison, R.S.; Minagawa, J.; Crofts, A.; Sayre, R.T. Photosystem II peripheral accessory chlorophyll mutants in Chlamydomonas reinhardtii. Biochemical characterization and sensitivity to photo-inhibition. Plant Physiol. 2001, 127, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.H.; Cua, A.; Chisholm, D.A.; Diner, B.A.; Bocian, D.F.; Brudvig, G.W. Identification of Histidine 118 in the D1 Polypeptide of Photosystem II as the Axial Ligand to Chlorophyll Z. Biochemistry 1998, 37, 10040–10046. [Google Scholar] [CrossRef]
- Schlodder, E.; Coleman, W.J.; Nixon, P.J.; Cohen, R.O.; Renger, T.; Diner, B.A. Site-directed mutations at D1-His198 and D1-Thr179 of photosystem II in Synechocystis sp. PCC 6803: Deciphering the spectral properties of the PSII reaction centre. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 1197–1202. [Google Scholar] [CrossRef]
- Saito, K.; Shen, J.-R.; Ishikita, H. Influence of the Axial Ligand on the Cationic Properties of the Chlorophyll Pair in Photosystem II from Thermosynechococcus vulcanus. Biophys. J. 2012, 102, 2634–2640. [Google Scholar] [CrossRef]
- Wang, J.; Gosztola, D.; Ruffle, S.V.; Hemann, C.; Seibert, M.; Wasielewski, M.R.; Hille, R.; Gustafson, T.L.; Sayre, R.T. Functional asymmetry of photosystem II D1 and D2 peripheral chlorophyll mutants of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2002, 99, 4091–4096. [Google Scholar] [CrossRef] [PubMed]
- Kanchanawong, P.; Dahlbom, M.G.; Treynor, T.P.; Reimers, J.R.; Hush, N.S.; Boxer, S.G. Charge delocalization in the special-pair radical cation of mutant reaction centers of Rhodobacter sphaeroides from Stark spectra and nonadiabatic spectral simulations. J. Phys. Chem. B 2006, 110, 18688–18702. [Google Scholar] [CrossRef]
- Katilius, E.; Turanchik, T.; Lin, S.; Taguchi, A.K.W.; Woodbury, N.W. B-Side Electron Transfer in a Rhodobacter sphaeroides Reaction Center Mutant in Which the B-Side Monomer Bacteriochlorophyll Is Replaced with Bacteriopheophytin. J. Phys. Chem. B 1999, 103, 7386–7389. [Google Scholar] [CrossRef]
- Nabedryk, E.; Schulz, C.; Müh, F.; Lubitz, W.; Breton, J. Heterodimeric versus homodimeric structure of the primary electron donor in Rhodobacter sphaeroides reaction centers genetically modified at position M202. Photochem. Photobiol. 2000, 71, 582–588. [Google Scholar] [CrossRef]
- Bylina, E.J.; Youvan, D.C. Directed mutations affecting spectroscopic and electron transfer properties of the primary donor in the photosynthetic reaction center. Proc. Natl. Acad. Sci. USA 1988, 85, 7226–7230. [Google Scholar] [CrossRef]
- Schulz, C.; Müh, F.; Beyer, A.; Jordan, R.; Schlodder, E.; Lubitz, W. Investigation of Rhodobacter Sphaeroides Reaction Center Mutants with Changed Ligands to the Primary Donor. In Photosynthesis: Mechanisms and Effects: Volume I–V: Proceedings of the XIth International Congress on Photosynthesis, Budapest, Hungary, 17–22 August 1998; Garab, G., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 767–770. [Google Scholar]
- Fuchs, M.R.; Schnegg, A.; Plato, M.; Schulz, C.; Müh, F.; Lubitz, W.; Möbius, K. The primary donor cation P+ in photosynthetic reaction centers of site-directed mutants of Rhodobacter sphaeroides: G-tensor shifts revealed by high-field EPR at 360 GHz/12.8 T. Chem. Phys. 2003, 294, 371–384. [Google Scholar] [CrossRef]
- Vasilieva, L.G.; Fufina, T.Y.; Gabdulkhakov, A.G.; Leonova, M.M.; Khatypov, R.A.; Shuvalov, V.A. The site-directed mutation I(L177)H in Rhodobacter sphaeroides reaction center affects coordination of PA and BB bacteriochlorophylls. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Leonova, M.M.; Vasilieva, L.G.; Khatypov, R.A.; Boichenko, V.A.; Shuvalov, V.A. Properties of mutant reaction centers of Rhodobacter sphaeroides with substitutions of histidine L153, the axial Mg2+ ligand of bacteriochlorophyll BA. Biochemistry 2009, 74, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Klimov, V.V.; Klevanik, A.V.; Shuvalov, V.A.; Kransnovsky, A.A. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977, 82, 183–186. [Google Scholar] [CrossRef]
- Fowler, G.J.S.; Visschers, R.W.; Grief, G.G.; van Grondelle, R.; Hunter, C.N. Genetically modified photosynthetic antenna complexes with blueshifted absorbance bands. Nature 1992, 355, 848–850. [Google Scholar] [CrossRef]
- Olsen, J.D.; Sockalingum, G.D.; Robert, B.; Hunter, C.N. Modification of a hydrogen bond to a bacteriochlorophyll a molecule in the light-harvesting 1 antenna of Rhodobacter sphaeroides. Proc. Natl. Acad. Sci. USA 1994, 91, 7124–7128. [Google Scholar] [CrossRef] [PubMed]
- Balaban, T.S.; Braun, P.; Hättig, C.; Hellweg, A.; Kern, J.; Saenger, W.; Zouni, A. Preferential pathways for light-trapping involving β-ligated chlorophylls. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 1254–1265. [Google Scholar] [CrossRef]
- Renger, G.; Pieper, J.; Theiss, C.; Trostmann, I.; Paulsen, H.; Renger, T.; Eichler, H.J.; Schmitt, F.J. Water soluble chlorophyll binding protein of higher plants: A most suitable model system for basic analyses of pigment–pigment and pigment–protein interactions in chlorophyll protein complexes. J. Plant Physiol. 2011, 168, 1462–1472. [Google Scholar] [CrossRef]
- Messinger, J.; Alia, A.; Govindjee. Special educational issue on ‘Basics and application of biophysical techniques in photosynthesis and related processes’. Photosynth. Res. 2009, 101, 89–92. [Google Scholar] [CrossRef]
- Holm, L.; Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 1993, 233, 123–138. [Google Scholar] [CrossRef]
- Shindyalov, I.N.; Bourne, P.E. Protein structure alignment by incremental combinatorial extension (CE) of the optimal path. Protein Eng. 1998, 11, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.J.; Richards, W.G. Ultrafast shape recognition to search compound databases for similar molecular shapes. J. Comput. Chem. 2007, 28, 1711–1723. [Google Scholar] [CrossRef]
- Konc, J.; Janežič, D. ProBiS algorithm for detection of structurally similar protein binding sites by local structural alignment. Bioinformatics 2010, 26, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Kufareva, I.; Abagyan, R. Methods of protein structure comparison. Methods Mol. Biol. 2012, 857, 231–257. [Google Scholar]
- Luo, L.; Milon, T.I.; Tandoh, E.K.; Galdamez, W.J.; Chistoserdov, A.Y.; Yu, J.; Kern, J.; Wang, Y.; Xu, W. Development of a TSR-based method for understanding structural relationships of cofactors and local environments in photosystem I. BMC Bioinform. 2025, 26, 15. [Google Scholar] [CrossRef]
- Xu, W.; Xie, X.-J.; Faust, A.; Liu, M.; Li, X.; Chen, F.; Naquin, A.; Walton, A.; Kishbaugh, P.; Ji, J.-Y. All-Atomic Molecular Dynamic Studies of Human and Drosophila CDK8: Insights into Their Kinase Domains, the LXXLL Motifs, and Drug Binding Site. Int. J. Mol. Sci. 2020, 21, 7511. [Google Scholar] [CrossRef]
- Guru, D.S.; Nagabhushan, P. Triangular spatial relationship: A new approach for spatial knowledge representation. Pattern Recognit. Lett. 2001, 22, 999–1006. [Google Scholar] [CrossRef]
- Jaccard, P. Etude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull. Soc. Vaudoise Sci. Nat. 1901, 37, 547–579. [Google Scholar]
- Ackerman, M.; Ben-David, S. A characterization of linkage-based hierarchical clustering. J. Mach. Learn. Res. 2016, 17, 8182–8198. [Google Scholar]
- Kondra, S.; Chen, F.; Chen, Y.; Chen, Y.; Collette, C.J.; Xu, W. A study of a hierarchical structure of proteins and ligand binding sites of receptors using the triangular spatial relationship-based structure comparison method and development of a size-filtering feature designed for comparing different sizes of protein structures. Proteins 2022, 90, 239–257. [Google Scholar] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milon, T.I.; Orthi, K.H.; Rauniyar, K.; Renfrow, R.M.; Gallo, A.A.; Xu, W. Application of the Triangular Spatial Relationship Algorithm in Representing and Quantifying Conformational Changes in Chlorophylls and Protein Local Environments. Photochem 2025, 5, 8. https://doi.org/10.3390/photochem5010008
Milon TI, Orthi KH, Rauniyar K, Renfrow RM, Gallo AA, Xu W. Application of the Triangular Spatial Relationship Algorithm in Representing and Quantifying Conformational Changes in Chlorophylls and Protein Local Environments. Photochem. 2025; 5(1):8. https://doi.org/10.3390/photochem5010008
Chicago/Turabian StyleMilon, Tarikul I., Khairum H. Orthi, Krishna Rauniyar, Rhen M. Renfrow, August A. Gallo, and Wu Xu. 2025. "Application of the Triangular Spatial Relationship Algorithm in Representing and Quantifying Conformational Changes in Chlorophylls and Protein Local Environments" Photochem 5, no. 1: 8. https://doi.org/10.3390/photochem5010008
APA StyleMilon, T. I., Orthi, K. H., Rauniyar, K., Renfrow, R. M., Gallo, A. A., & Xu, W. (2025). Application of the Triangular Spatial Relationship Algorithm in Representing and Quantifying Conformational Changes in Chlorophylls and Protein Local Environments. Photochem, 5(1), 8. https://doi.org/10.3390/photochem5010008






